Abstract
Staphylococcus aureus is an opportunistic pathogen that normally colonizes the human anterior nares. At the same time, this pathogen is one of the leading causes of life-threatening bloodstream infections, such as sepsis and endocarditis. In this review we will present the current understanding of the pathogenesis of these invasive infections, focusing on the mechanisms of S. aureus clearance from the bloodstream by the immune system, and how this pathogen hijacks the host defense and coagulation systems and further interacts with the blood vessel endothelium. Additionally, we will delve into the regulatory mechanisms S. aureus employs during an invasive infection. These new insights into host-pathogen interactions show promising avenues for the development of novel therapies for treating bloodstream infections.
Introduction
Staphylococcus aureus is an asymptomatic colonizer of human nares, with approximately 30% of individuals permanently colonized [1]. It is also the main cause of skin and soft tissue infections, especially in the already colonized individuals [2]. While local skin infections are mostly self-limiting, they sometime become an entryway for this pathogen into the deeper tissues and the bloodstream – skin infections are in fact the most commonly identified source for the S. aureus bacteremia [3,4]. Mechanisms of the occasional S. aureus systemic spread from skin lesions are not fully understood, though main risks factors for development of S. aureus sepsis are age (highest risk in infants and the elderly), additional comorbidities (heart diseases, diabetes, renal disease, HIV infection), presence of indwelling medical devices, intravenous drug use, and low socioeconomic status [5]. Even though majority of individuals colonized with S. aureus will not develop an invasive infection, the total number of the infected individuals places S. aureus as one of leading pathogens causing bloodstream infections [6]. These infections are characterized by high mortality rates despite proper treatment (from 20% to 50%, depending on infection severity), frequent recurrences (5–10%), and lasting impairments in over one-third of the survivors [5–7]. The incidence of S. aureus bloodstream infections has been rising in recent years in developed countries [5,6]. Severe S. aureus infections are also an overlooked, but important challenge in developing countries [8]. Overall, there is an urgent need to better understand these invasive infections, and to design new therapies for improving patient care.
Bloodstream infections: overview
The presence of S. aureus in the bloodstream (bacteremia) can lead to the development of sepsis - a systemic inflammatory response to infection. A typical feature of sepsis is the paradoxical immunosuppressive response that is sometimes concurrent with inflammation. This combination of inflammation and immunosuppression leads to collateral damage of local tissues and renders the host defenseless against the causative pathogen and secondary infections [9]. The inflammatory response shifts the balance between pro- and anti-coagulating mechanisms, potentially leading to disseminated intravascular coagulation (DIC). The DIC microthrombi develop in the vasculature, damage the endothelium and block blood flow, resulting in oxygen depletion in organs. As this systemic coagulation depletes available clotting factors, it is often followed by hemorrhages that further aggravate organ injury [9]. The endothelium lining blood vessels is an essential player in sepsis, secreting pro-inflammatory and pro-coagulant factors, but excessive inflammation is also responsible for endothelial damage, leading to vascular leakage and failure to maintain proper blood pressure [9]. This general scheme of sepsis pathology can be impacted by multiple virulence mechanisms of S. aureus, which directly targets immune responses, coagulation, and endothelium. In addition to sepsis, the presence of S. aureus in the bloodstream can also lead to endocarditis, which is an infection of the heart valves. This is often associated with the endothelial damage and/or activation, and development of infected thrombi, connecting the local pathology of the endocarditis with the more systemic mechanisms observed in sepsis.
In this review, we will draw attention to certain aspects of the S. aureus bloodstream infections that differentiate it from a more “typical” sepsis. Many virulence mechanisms of S. aureus have been identified in the past in experimental models of bloodstream infections and have been reviewed elsewhere [10–12]. In this review, we will focus on some of the most recent developments in the field – that is on how S. aureus hijacks the initial immune response for its spread, takes advantage of the interaction with coagulation and endothelium, and regulates the expression of virulence factors in the bloodstream. Finally, we will also demonstrate how these factors create challenges and opportunities for designing new therapies for treating S. aureus bloodstream infections.
Immune clearance of S. aureus from the bloodstream
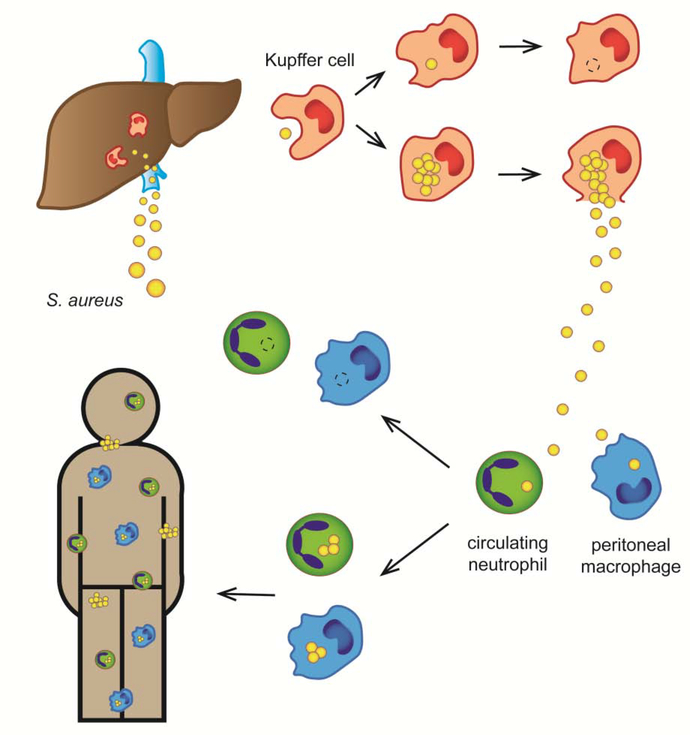
After entering the bloodstream, S. aureus is initially cleared by specialized liver macrophages called Kupffer cells [13–15] (Fig. 1). Circulating platelets aid in this process by aggregating around bacteria on the surface of the Kupffer cells, encasing S. aureus until it is properly phagocytosed [15]. Efficacy of this sequestration differs between S. aureus strains, which suggests presence of some not-yet-understood mechanisms of Kupffer cell evasion [15]. This clearance process is in many cases sufficient to prevent serious infection, but a small fraction of phagocytosed staphylococci might survive and multiply inside the Kupffer cells, eventually turning the liver into a source of the in-host dissemination [13,14,16]. As S. aureus is being released from this intracellular niche into the peritoneum and the liver circulation, it encounters peritoneal macrophages and bloodstream neutrophils acting as a second line of phagocyte defense [13,16]. However, a small fraction of ingested S. aureus can once again survive inside these phagocytes, turning them into “Trojan Horses” carrying intracellular bacteria to other body sites, and eventually causing a systemic dissemination [13,16].
Figure 1. The role of the immune system in clearance and systemic spread of S. aureus in the bloodstream.
S. aureus is initially cleared from the bloodstream by Kupffer cells (liver macrophages). While Kupffer cells can kill the majority of phagocytosed bacteria, a small fraction of S. aureus survives and proliferates intracellularly, eventually killing the cells and being released back into the bloodstream and peritoneum. Subsequently, S. aureus is phagocytosed by neutrophils in liver circulation and by peritoneal macrophages. If these host cells fail to kill bacteria, they turn into “Trojan Horses”, carrying intracellular S. aureus throughout the body and causing a disseminated infection.
As our understanding of the dynamics of S. aureus in bloodstream evolves, the importance of developing treatments that could target intracellular bacteria, and boost phagocytic killing, becomes clear [14,17]. This is further demonstrated by the increased susceptibility to S. aureus infections of individuals suffering from various innate immunity dysfunctions, especially from ones that decrease neutrophil’s ability to kill the pathogens (reviewed in [18]). The central role of phagocytosis in early stages of bacteremia also underlies the importance of anti-phagocytic immune evasion molecules produced by S. aureus (reviewed in [19,20]). However, there is still relatively little research on the possible specific mechanisms of S. aureus phagocytosis and immune evasion in Kupffer cells, as opposed to the more typical peripheral neutrophils and macrophages.
Adaptive immunity and S. aureus bloodstream infections
S. aureus infections often reoccur, typically in skin infections more than in bloodstream infections, suggesting a possible involvement of some protective memory immune mechanism in sepsis [21]. This might be especially important for the development of anti-S. aureus vaccines, but there is no clear consensus yet on the role of the adaptive immunity in S. aureus invasive infections (partly reviewed in [22]).
Effect of B cell memory on staphylococcal sepsis has not been yet exhaustively investigated in animal models, but B cell depletion did not affect severity of a mouse primary invasive infection [23]. More importantly, lack of functional B cells is not a risk factor for S. aureus sepsis in humans [22]. These suggest no role for B cells and antibodies in sepsis. There are, however, repeated observations of low levels of anti-S. aureus IgG antibodies correlating with sepsis development, and predicting worse infection outcomes [24–26]. Perhaps these contradictory results are best explained by the ability of S. aureus to evade B cell-mediated immunity, making these cells appear unimportant in cases of successful S. aureus disease [22].
While IL-17 secreting T cells are essential for defense against S. aureus skin infections, the role of T cells and their different subsets in S. aureus sepsis has been harder to establish. The IL-17 secreting γδ T cells, as well as the Th1 memory cells, appear to be protective during invasive S. aureus infections [27,28], but many contradictory results exist [11,22,29]. Part of this confusion might stem from the activity of the S. aureus superantigens, causing unspecific activation and expansion of T cells that aggravates infection [30]. Different S. aureus strains used in disease models harbor different repertoires of superantigens, and superantigen susceptibility differs among mouse strains (though always being significantly lower than in humans) [30], possibly explaining the contradictory results. In recent years more research on the role of non-conventional (non-MHC-restricted) T cells appeared, suggesting that activation by S. aureus superantigens of the invariant Natural Killer T cells (iNKT, type I NKT), and possibly Mucosal Associated Invariant T cells (MAITs) could aggravate S. aureus infection, while type II NKT cells might play a protective role [31–33], but the emerging picture is far from clear.
Coagulation and endothelium as targets of S. aureus virulence
A hallmark feature of S. aureus sepsis in both patients and animal models is the pronounced shift towards pro-coagulant activity [34–37]. This is largely due to inflammation-induced expression and release of pro-coagulant factors such as tissue factor (TF) and von Willebrand factor (vWf) by endothelium, accompanied by decreased serum activity of anti-coagulant (e.g. ADAMTS13 protease cleaving vWf) and fibrinolytic (e.g. plasminogen) factors. These changes are mostly mediated by host vascular endothelium responses to inflammatory cytokines, and they are aggravated by the presence of the bacterial pathogen and its products. For example, S. aureus-induced complement activation causes TF expression by immune cells, and S. aureus alpha-toxin induces abnormal platelet activation, leading to formation of platelet aggregates and occlusion of small vessels by microthrombi [38,39]. Moreover, S. aureus produces its own coagulases (staphylocoagulase and vWf-binding protein) and directly engages host platelets, which allows the pathogen to bypass host regulatory systems and hijack the coagulation cascade (reviewed in [40]). Investigation of animal models with altered coagulation and fibrinolysis (eg. vWf-deficient, ADAMTS13-deficient, plasminogen-deficient, or Factor V Leiden heterozygous mice) demonstrated importance of these host systems during sepsis [35,41,42]. However, attempts to identify a clear role of pre-existing coagulopathy or thrombophilia during human S. aureus sepsis are either lacking or provide mixed results [43]. This is likely because the overall aberrant coagulation and fibrinolysis during sepsis overshadows more subtle effects of its manipulation by S. aureus. Moreover, many of the typical coagulopathies have no effect on S. aureus – induced coagulation, as staphylococcal coagulases activate prothrombin irrespectively of the presence and activity of other host coagulation factors, and thus remain fully functional even in most individuals with bleeding disorders [40].
S. aureus can directly bind fibrinogen (and fibrin, generated from fibrinogen during coagulation) and use it to cross-link individual cells to form large fibrinogen-encased clumps (reviewed in [44]). Despite some insights offered by infection experiments in transgenic mice with fibrinogen which cannot polymerize into fibrin, the relative importance and the exact settings in which S. aureus interacts with un-polymerized fibrinogen versus when it interacts with fibrin remain unclear, especially during bloodstream infection [45]. Formation of clumps and encasement in fibrin layers is thought to protect bacteria from immune attacks and antibiotics, and helps establish infectious foci inside vasculature (Fig. 2). Indeed, the ability of S. aureus to induce coagulation and to clump with fibrinogen is one of its most pronounced virulence mechanisms in animal models, and removal of fibrinogen during infection decreases sepsis severity [46–50]. Moreover, production of staphylokinase (an anti-coagulant / fibrinolytic) factor by S. aureus has been linked to decreased infection severity both in humans and in mouse models [51,52], further stressing importance of excessive coagulation for pathogenesis of bloodstream infection.
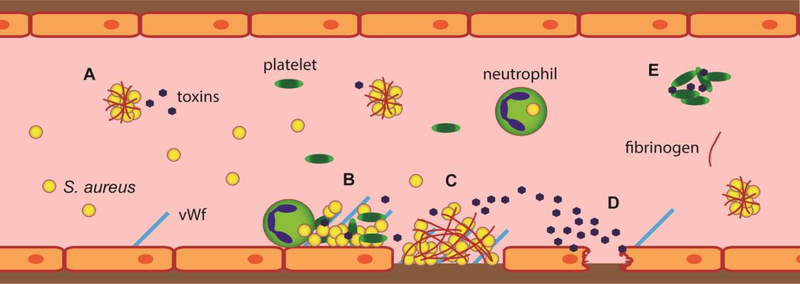
Figure 2. Virulence mechanisms of S. aureus inside the bloodstream.
After entry into the bloodstream, S. aureus hijacks the host coagulation system to create clumps of individual bacteria cross-linked by and surface coated with host fibrinogen (A). Inflammation causes endothelial cells to surface display various adhesive molecules (e.g. von Willebrand factor, vWf), which act as anchors for S. aureus aggregates with platelets and for incoming immune cells potentially carrying intracellular bacteria (B). In areas where endothelial cells are damaged, the underlying collagen-rich matrix is exposed, binding circulating vWf and initiating coagulation, which results in deposition of fibrin clots. The display of collagen, vWf and fibrin allows adhesion of circulating S. aureus cells and clumps (C). Clumps of S. aureus attached to endothelium secrete toxins (e.g. alpha-toxin and superantigens) that further activate inflammatory signaling in endothelium or cause its direct damage (D). Secreted alpha-toxin also poisons nearby platelets, causing their aggregation and formation of microthrombi, potentially clogging smaller vessels (E).
Closely related to coagulation and fibrinogen binding is S. aureus adhesion to the endothelium (Fig. 2). This has been mostly investigated in the context of endocarditis, where infected thrombi develop directly on the surface of the heart valves, but the same mechanism probably occurs in other sites in the vessel walls during sepsis. S. aureus attaches to endothelium through two distinct mechanisms [10,53]. First, when the endothelium become mechanically damaged and underlying layers of vessel wall rich in collagen are exposed, coagulation cascade is initiated and a fibrin- and vWf-containing clot develops. This provides a binding site for S. aureus, which can interact with collagen, fibrin and vWf through its surface proteins and coagulases [53–55]. In the alternative case when no mechanical damage is present, and the endothelium becomes activated in response to inflammation, endothelial cells display vWf, selectins, and other adhesion molecules on their surface. These in turn recruit platelets and immune cells. The platelets and vWf provide binding sites for S. aureus, while incoming immune cells potentially bring additional intracellular bacteria directly to the site of endothelial inflammation [53]. Irrespective of the adhesion pathway, the binding of S. aureus to the vessel wall allows the invading pathogen to directly damage the endothelium by its secreted toxins such as alpha-toxin and endothelium-activating superantigens, further augmenting the endothelial dysfunction, aberrant coagulation, and vascular leakage [56–58]. This creates local infectious foci at the vessel wall, from which S. aureus can metastasize into the surrounding organs or disseminate through bloodstream to other body sites.
Strain-specific features of S. aureus bloodstream infections
Identification of relevant virulence factors has been an important part of research on S. aureus infections. However, the virulence (that is, the ability to inflict damage on the host) emerges from a complex and unique host-pathogen interplay [59,60]. Thus, the same virulence mechanisms might be crucial in one patient, and play no role in the other, depending on presence of other virulence factors, patient’s immune state, stage of infection, etc. In the case of S. aureus, this is demonstrated by the varying virulence properties responsible for mortality in bacteremia caused by S. aureus strains from two different clonal complexes [61]. Notably, the only virulence-related properties that seemed to be important in both of the analyzed clonal complexes in one study were polymorphisms in the gene coding for capsule production [61], with capsule being an established virulence factor in S. aureus sepsis [62]. The most common S. aureus clone in North America is, however, the USA300 lineage, which does not produce capsule [63], demonstrating that common virulence features might be strain-dependent or limited to certain geographical locations. Similarly, while secreted virulence factors (alpha-toxin, staphylococcal coagulases) and iron-scavenging surface proteins have been demonstrated to be important determinants in experimental bloodstream infections, they do not play a role in sepsis in immunocompromised hosts, where cell-wall anchoring of surface proteins was defined as an essential virulence factor [64].
Interesting example of host- and strain-dependent mechanisms of virulence are the S. aureus isolates from the CC30 clonal complex. As they often lack secreted toxins and have reduced virulence in mouse and invertebrate models, they are considered “less virulent” [65,66]. On the other hand, they are also associated epidemiologically with complicated bacteremia in humans [67] and appear to be virulent in rabbit model, presumably due to their superantigen secretion [68]. This is consistent with the aforementioned study of predictors of mortality in sepsis, where levels of secreted cytotoxic toxins did not correlate with outcome of CC30 sepsis [61]. Altogether, CC30 strains seem to employ virulence mechanisms that are distinct from the toxin-based mechanisms usually noticed in mouse infection models, causing mortality not through aggressive damage to the host, but through the ability to persist. This demonstrates that different sets of virulence factors can cause disease through distinct mechanisms, not always apparent in the experimental infection models. This must be taken into account in design of new therapies, as each clonal lineage of S. aureus presents with a unique combination of virulence factors and often with different epidemiology [69]. It is probably most useful to think of different S. aureus lineages as being able to adapt to set of conditions, such as a specific host niche or environment, through particular disease mechanisms mediated by unique combinations of virulence factors. This implies that instead of a single “S. aureus bloodstream infection”, there are rather multiple types of these infections with differing pathophysiological mechanisms. This highlights the recent calls for a more “personalized medicine” approach to sepsis treatment [9,70]. An individualized approach, taking into account specifics of both the particular infecting S. aureus strain and of the patient’s changing response to infection will be necessary.
S. aureus gene regulation in the bloodstream
An essential feature of a successful infection is the coordinated and timely expression of virulence factors and other relevant genes by the pathogen. To respond to the host environmental cues, S. aureus relies on numerous regulatory systems (reviewed in [71]). Unlike individual virulence factors, often employed by only some of the S. aureus lineages, the need for tight gene regulation is universal across all strains. As in the case of individual virulence factors, the role of S. aureus regulatory systems in bloodstream infections is complex and condition dependent.
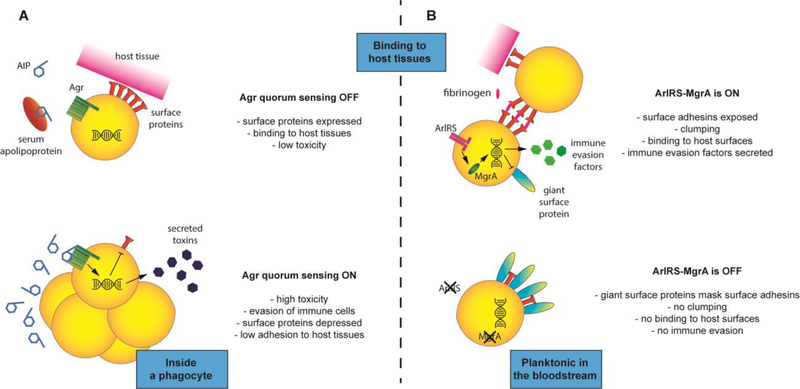
The Agr quorum-sensing system is the most studied of the S. aureus regulators, and its correct activity is required for secretion of number of toxins and other soluble virulence factors [71] (Fig. 3A). It senses an autoinducing peptide (AIP) secreted by the S. aureus itself, and when AIP reaches a critical local concentration due to high bacterial density, Agr signaling is activated [71]. Agr was one of the first regulators found to be involved in bloodstream infections [72]. The Agr-deficient isolates are, however, commonly found in patients with bloodstream infections (reviewed in [73]). Attempts to correlate Agr functionality with sepsis outcome either shows no correlation, or even an opposite effect where Agr-deficient strains are found in more severe disease cases, especially in persistent bacteremia [74–77]. These observations are attributed to a number of factors, including the pro-inflammatory properties of strains with functional Agr, the elevated adherence capacity of Agr-deficient isolates [73], the Agr-inhibitory activity of apolipoproteins in host serum [78], and the increased fitness cost of maintaining a functional Agr system [76]. At the same time, an active Agr system is essential for intracellular survival inside phagocytes [73], which is the first step in establishing a bloodstream infection. In some instances, compensatory mutations might develop during infection with the Agr-deficient isolates, overcoming their apparent “decreased” virulence [79]. But overall, the role of Agr probably depends on the phase of the infection. In early phases, when toxin secretion is needed to survive inside phagocytes and establish a niche in the host, maintaining a functional Agr system is useful. In the later stages, when persistence requires strong adhesion and low toxin production to avoid eliciting immune responses, Agr activity is unnecessary or even detrimental. Similarly, the role of Agr probably depends on the pathogen’s location: as long as it remains floating inside bloodstream, Agr activity is quenched by serum, and any produced toxins would be quickly diluted. When S. aureus finds itself in clumps or inside host cells, high bacterial density and/or lack of serum allow for Agr activation, and produced toxins can easily reach high concentrations [50].
Figure 3. S. aureus gene regulation in bloodstream infection.
When S. aureus remains planktonic in the bloodstream, the local concentration of quorum-sensing autoinducing peptide (AIP) remains low, additionally quenched by serum apolipoproteins. This keeps the Agr system turned off, resulting in high expression of surface proteins responsible for attachment to host surfaces, and a low level of toxins. When S. aureus finds itself in clumps or inside cells, the local high concentration of secreted AIP causes Agr system to activate, increasing toxin levels that target host tissues and immune cells, and decreasing surface adhesins (A). When the ArlRS – MgrA cascade remains active, it suppresses production of giant surface proteins (allowing for normal activity of surface adhesins in fibrinogen-mediated clumping and adhesion to host tissues) and drives secretion of immune-evasion factors. When the ArlRS – MgrA cascade is off, the secretion of immune evasion factors decreases, and derepressed giant surface proteins shield neighboring surface adhesins, blocking them from mediating clumping or adhesion to host tissues (B).
The ambiguous role of Agr in sepsis raises the question if its therapeutic targeting is an optimal treatment approach. One the positive, Agr regulation is similar across multiple S. aureus lineages, making it a promising universal treatment target [80], and many potent inhibitors have been developed [81]. Inhibiting the Agr system could lock S. aureus into a non-toxicogenic expression state, making it vulnerable to phagocytic killing and clearance. However, timing is important since the system activates in waves, and it is critical to target it early in the dynamic process to limit toxin production [82]. In case of bloodstream infections, the use of Agr inhibition would probably require careful monitoring of the pathogen’s genotype and stage of the infection, to ensure the treatment is used at the most opportune moment.
Another virulence regulatory system of interest in bloodstream infections is the two-component ArlRS system, and its downstream effector, the global regulator MgrA [71] (Fig. 3B). In the context of bloodstream, this cascade was shown to regulate clumping in plasma, adhesion to vessel walls, and interactions with endothelium [46,48,83,84]. ArlRS – MgrA cascade also regulates expression of several immune evasion genes [85], controls response to host antimicrobial peptides [86], and coordinates response to metabolic conditions imposed by host environment [87,88]. Most importantly, the inactivation of ArlRS or MgrA resulted in a decreased virulence in animal models of sepsis and endocarditis [46,48,86,88,89]. Unlike in the case of the Agr, there are no reports of ArlRS and MgrA mutants amongst bloodstream S. aureus isolates, making it a promising universal treatment target. However, as with every regulator controlling multiple genes, the S. aureus strain-specific effects must be considered. For example, depending on virulence factors present in the strain, inactivation of the ArlRS – MgrA cascade might either decrease or increase S. aureus attachment to host cells [86,90]. Therefore additional research on ArlRS – MgrA regulatory mechanisms and potential for therapeutic targeting across diverse S. aureus strains is needed.
Conclusions
Despite advanced understanding of pathogenesis of S. aureus sepsis and endocarditis, there are still no good preventive measures available, and treatment is still mostly limited to antibiotic administration, supportive (intensive) care, and surgical interventions. Focusing on intracellular S. aureus, and on its interactions with host coagulation system and endothelium, are among the most promising treatment avenues. However, increasing evidence points to strain- and host-dependent differences in mechanisms of S. aureus bloodstream infections. This suggests future advances will likely be in personalized and precision medicine, where interventions would be tailored to particulars of the infecting bacterial strain and host responses. Recent successful attempts to divide sepsis into several phenotypes in order to administer different treatments shows that even fairly simple subdivisions of this heterologous entity might benefit patient care [70]. This might be especially important in context of public health, individuals with low socioeconomic status, or of developing nations, where “full-scale” personalized medicine will likely remain prohibitively costly [91–93]. An alternative and promising approach to therapeutic development is to focus on the gene regulation in S. aureus. As coordinated expression of virulence genes remains necessary in all stages of infection irrespective of other strain peculiarities, targeting regulatory systems could be a potential way to design universal anti-S. aureus therapies. Additional insights will be hopefully gained thanks to increasing use of “humanized” mice models, which allow the study of human-specific S. aureus virulence factors that are inactive in normal rodent models [94].
Highlights.
Phagocytes play both protective and detrimental roles in containing bloodstream S. aureus.
S. aureus hijacks the host coagulation system and uses it to bind host endothelium.
Strategies used by S. aureus to cause host damage are highly strain-dependent.
Multiple regulatory systems control S. aureus bloodstream infections, including Agr, ArlRS and MgrA.
Acknowledgements
Authors’ work was supported by NIH NIAID grants AI083211 and AI141490 (ARH) and by American Heart Association postdoctoral fellowship 17POST33670580 (JMK).
Footnotes
Conflict of Interest
None
Declaration of Interest
None
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
Outstanding interest * *
Special interest *
- 1.Wertheim HF, Melles DC, Vos MC, van Leeuwen W, van Belkum A, Verbrugh HA, Nouwen JL: The role of nasal carriage in Staphylococcus aureus infections. Lancet Infect Dis 2005, 5:751–762. [DOI] [PubMed] [Google Scholar]
- 2.Dryden MS: Skin and soft tissue infection: microbiology and epidemiology. Int J Antimicrob Agents 2009, 34 Suppl 1:S2–7. [DOI] [PubMed] [Google Scholar]
- 3.Wilson J, Guy R, Elgohari S, Sheridan E, Davies J, Lamagni T, Pearson A: Trends in sources of methicillin-resistant Staphylococcus aureus (MRSA) bacteraemia: data from the national mandatory surveillance of MRSA bacteraemia in England, 2006–2009. J Hosp Infect 2011, 79:211–217. [DOI] [PubMed] [Google Scholar]
- 4.Yarovoy JY, Monte AA, Knepper BC, Young HL: Epidemiology of Community-Onset Staphylococcus aureus Bacteremia. West J Emerg Med 2019, 20:438–442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Asgeirsson H, Thalme A, Weiland O: Staphylococcus aureus bacteraemia and endocarditis - epidemiology and outcome: a review. Infect Dis (Lond) 2018, 50:175–192. [DOI] [PubMed] [Google Scholar]
- 6.Kern WV, Rieg S: Burden of bacterial bloodstream infection-a brief update on epidemiology and significance of multidrug-resistant pathogens. Clin Microbiol Infect 2019. [DOI] [PubMed] [Google Scholar]
- 7.Jacobsson G, Gustafsson E, Andersson R: Outcome for invasive Staphylococcus aureus infections. Eur J Clin Microbiol Infect Dis 2008, 27:839–848. [DOI] [PubMed] [Google Scholar]
- 8.Nickerson EK, West TE, Day NP, Peacock SJ: Staphylococcus aureus disease and drug resistance in resource-limited countries in south and east Asia. Lancet Infect Dis 2009, 9:130–135. [DOI] [PubMed] [Google Scholar]
- 9.Angus DC, van der Poll T: Severe sepsis and septic shock. N Engl J Med 2013, 369:840–851. [DOI] [PubMed] [Google Scholar]
- 10.Hoerr V, Franz M, Pletz MW, Diab M, Niemann S, Faber C, Doenst T, Schulze PC, Deinhardt-Emmer S, Loffler B: S. aureus endocarditis: Clinical aspects and experimental approaches. Int J Med Microbiol 2018, 308:640–652. [DOI] [PubMed] [Google Scholar]
- 11.Tarkowski A, Collins LV, Gjertsson I, Hultgren OH, Jonsson IM, Sakiniene E, Verdrengh M: Model systems: modeling human staphylococcal arthritis and sepsis in the mouse. Trends Microbiol 2001, 9:321–326. [DOI] [PubMed] [Google Scholar]
- 12.Thomer L, Schneewind O, Missiakas D: Pathogenesis of Staphylococcus aureus Bloodstream Infections. Annu Rev Pathol 2016, 11:343–364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pollitt EJG, Szkuta PT, Burns N, Foster SJ: Staphylococcus aureus infection dynamics. PLoS Pathog 2018, 14:e1007112.Through series of zebrafish and mouse experiments, demonstrates that two early waves of phagocytosis occur during S. aureus sepsis (Kupffer cells and subsequently neutrophils), and that they paradoxically contribute both to the control of the infection, and to the systemic spread.
- 14.Surewaard BG, Deniset JF, Zemp FJ, Amrein M, Otto M, Conly J, Omri A, Yates RM, Kubes P: Identification and treatment of the Staphylococcus aureus reservoir in vivo. J Exp Med 2016, 213:1141–1151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wong CH, Jenne CN, Petri B, Chrobok NL, Kubes P: Nucleation of platelets with blood-borne pathogens on Kupffer cells precedes other innate immunity and contributes to bacterial clearance. Nat Immunol 2013, 14:785–792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jorch SK, Surewaard BG, Hossain M, Peiseler M, Deppermann C, Deng J, Bogoslowski A, van der Wal F, Omri A, Hickey MJ, et al. : Peritoneal GATA6+ macrophages function as a portal for Staphylococcus aureus dissemination. J Clin Invest 2019, 129:4643–4656.Through intravital microscopy and mouse models, demonstrates how Kupffer cells serve as reservoir of intracellular S. aureus for its further systemic spread through peritoneal macrophages.
- 17.Lehar SM, Pillow T, Xu M, Staben L, Kajihara KK, Vandlen R, DePalatis L, Raab H, Hazenbos WL, Morisaki JH, et al. : Novel antibody-antibiotic conjugate eliminates intracellular S. aureus. Nature 2015, 527:323–328. [DOI] [PubMed] [Google Scholar]
- 18.Buvelot H, Posfay-Barbe KM, Linder P, Schrenzel J, Krause KH: Staphylococcus aureus, phagocyte NADPH oxidase and chronic granulomatous disease. FEMS Microbiol Rev 2017, 41:139–157. [DOI] [PubMed] [Google Scholar]
- 19.de Jong NWM, van Kessel KPM, van Strijp JAG: Immune Evasion by Staphylococcus aureus. Microbiol Spectr 2019, 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Guerra FE, Borgogna TR, Patel DM, Sward EW, Voyich JM: Epic Immune Battles of History: Neutrophils vs. Staphylococcus aureus. Front Cell Infect Microbiol 2017, 7:286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fritz SA, Tiemann KM, Hogan PG, Epplin EK, Rodriguez M, Al-Zubeidi DN, Bubeck Wardenburg J, Hunstad DA: A serologic correlate of protective immunity against community-onset Staphylococcus aureus infection. Clin Infect Dis 2013, 56:1554–1561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Karauzum H, Datta SK: Adaptive Immunity Against Staphylococcus aureus. Curr Top Microbiol Immunol 2017, 409:419–439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gjertsson I, Hultgren OH, Stenson M, Holmdahl R, Tarkowski A: Are B lymphocytes of importance in severe Staphylococcus aureus infections? Infect Immun 2000, 68:2431–2434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jacobsson G, Colque-Navarro P, Gustafsson E, Andersson R, Mollby R: Antibody responses in patients with invasive Staphylococcus aureus infections. Eur J Clin Microbiol Infect Dis 2010, 29:715–725. [DOI] [PubMed] [Google Scholar]
- 25.Michalik S, Sundaramoorthy N, Murr A, Depke M, Volker U, Broker BM, Aamot HV, Schmidt F: Early-Stage Staphylococcus aureus Bloodstream Infection Causes Changes in the Concentrations of Lipoproteins and Acute-Phase Proteins and Is Associated with Low Antibody Titers against Bacterial Virulence Factors. mSystems 2020, 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Stentzel S, Sundaramoorthy N, Michalik S, Nordengrun M, Schulz S, Kolata J, Kloppot P, Engelmann S, Steil L, Hecker M, et al. : Specific serum IgG at diagnosis of Staphylococcus aureus bloodstream invasion is correlated with disease progression. J Proteomics 2015, 128:1–7. [DOI] [PubMed] [Google Scholar]
- 27.Murphy AG, O’Keeffe KM, Lalor SJ, Maher BM, Mills KH, McLoughlin RM: Staphylococcus aureus infection of mice expands a population of memory gammadelta T cells that are protective against subsequent infection. J Immunol 2014, 192:3697–3708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Brown AF, Murphy AG, Lalor SJ, Leech JM, O’Keeffe KM, Mac Aogain M, O’Halloran DP, Lacey KA, Tavakol M, Hearnden CH, et al. : Memory Th1 Cells Are Protective in Invasive Staphylococcus aureus Infection. PLoS Pathog 2015, 11:e1005226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Broker BM, Mrochen D, Peton V: The T Cell Response to Staphylococcus aureus. Pathogens 2016, 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tuffs SW, Haeryfar SMM, McCormick JK: Manipulation of Innate and Adaptive Immunity by Staphylococcal Superantigens. Pathogens 2018, 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kwiecinski J, Rhost S, Lofbom L, Blomqvist M, Mansson JE, Cardell SL, Jin T: Sulfatide attenuates experimental Staphylococcus aureus sepsis through a CD1d-dependent pathway. Infect Immun 2013, 81:1114–1120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shaler CR, Choi J, Rudak PT, Memarnejadian A, Szabo PA, Tun-Abraham ME, Rossjohn J, Corbett AJ, McCluskey J, McCormick JK, et al. : MAIT cells launch a rapid, robust and distinct hyperinflammatory response to bacterial superantigens and quickly acquire an anergic phenotype that impedes their cognate antimicrobial function: Defining a novel mechanism of superantigen-induced immunopathology and immunosuppression. PLoS Biol 2017, 15:e2001930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Szabo PA, Rudak PT, Choi J, Xu SX, Schaub R, Singh B, McCormick JK, Haeryfar SMM: Invariant Natural Killer T Cells Are Pathogenic in the HLA-DR4-Transgenic Humanized Mouse Model of Toxic Shock Syndrome and Can Be Targeted to Reduce Morbidity. J Infect Dis 2017, 215:824–829. [DOI] [PubMed] [Google Scholar]
- 34.Klak M, Anakkala N, Wang W, Lange S, Jonsson IM, Tarkowski A, Jin T: Tranexamic acid, an inhibitor of plasminogen activation, aggravates staphylococcal septic arthritis and sepsis. Scand J Infect Dis 2010, 42:351–358. [DOI] [PubMed] [Google Scholar]
- 35.Peetermans M, Meyers S, Liesenborghs L, Vanhoorelbeke K, De Meyer SF, Vandenbriele C, Lox M, Hoylaerts MF, Martinod K, Jacquemin M, et al. : Von Willebrand factor and ADAMTS13 impact on the outcome of Staphylococcus aureus sepsis. J Thromb Haemost 2019. [DOI] [PubMed] [Google Scholar]
- 36.Soerensen KE, Olsen HG, Skovgaard K, Wiinberg B, Nielsen OL, Leifsson PS, Jensen HE, Kristensen AT, Iburg TM: Disseminated intravascular coagulation in a novel porcine model of severe Staphylococcus aureus sepsis fulfills human clinical criteria. J Comp Pathol 2013, 149:463–474. [DOI] [PubMed] [Google Scholar]
- 37.Silasi R, Keshari RS, Lupu C, Van Rensburg WJ, Chaaban H, Regmi G, Shamanaev A, Shatzel JJ, Puy C, Lorentz CU, et al. : Inhibition of contact-mediated activation of factor XI protects baboons against S aureus-induced organ damage and death. Blood Adv 2019, 3:658–669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Skjeflo EW, Christiansen D, Fure H, Ludviksen JK, Woodruff TM, Espevik T, Nielsen EW, Brekke OL, Mollnes TE: Staphylococcus aureus-induced complement activation promotes tissue factor-mediated coagulation. J Thromb Haemost 2018, 16:905–918. [DOI] [PubMed] [Google Scholar]
- 39.Surewaard BGJ, Thanabalasuriar A, Zeng Z, Tkaczyk C, Cohen TS, Bardoel BW, Jorch SK, Deppermann C, Bubeck Wardenburg J, Davis RP, et al. : alpha-Toxin Induces Platelet Aggregation and Liver Injury during Staphylococcus aureus Sepsis. Cell Host Microbe 2018, 24:271–284 e273.Through intravital microscopy and mouse models, demonstrates how poisoning of platelets by S. aureus alpha-toxin causes abbernat coagulation andf microthrombi formation in host vasculature.
- 40.Liesenborghs L, Verhamme P, Vanassche T: Staphylococcus aureus, master manipulator of the human hemostatic system. J Thromb Haemost 2018, 16:441–454. [DOI] [PubMed] [Google Scholar]
- 41.Guo Y, Li J, Hagstrom E, Ny T: Beneficial and detrimental effects of plasmin(ogen) during infection and sepsis in mice. PLoS One 2011, 6:e24774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kerschen E, Hernandez I, Zogg M, Maas M, Weiler H: Survival advantage of heterozygous factor V Leiden carriers in murine sepsis. J Thromb Haemost 2015, 13:1073–1080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Schouten M, van ‘t Veer C, van der Poll T, Levi M: Effect of the factor V Leiden mutation on the incidence and outcome of severe infection and sepsis. Neth J Med 2012, 70:306–310. [PubMed] [Google Scholar]
- 44.Crosby HA, Kwiecinski J, Horswill AR: Staphylococcus aureus Aggregation and Coagulation Mechanisms, and Their Function in Host-Pathogen Interactions. Adv Appl Microbiol 2016, 96:1–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Prasad JM, Gorkun OV, Raghu H, Thornton S, Mullins ES, Palumbo JS, Ko YP, Hook M, David T, Coughlin SR, et al. : Mice expressing a mutant form of fibrinogen that cannot support fibrin formation exhibit compromised antimicrobial host defense. Blood 2015, 126:2047–2058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Crosby HA, Schlievert PM, Merriman JA, King JM, Salgado-Pabon W, Horswill AR: The Staphylococcus aureus Global Regulator MgrA Modulates Clumping and Virulence by Controlling Surface Protein Expression. PLoS Pathog 2016, 12:e1005604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.McAdow M, Kim HK, Dedent AC, Hendrickx AP, Schneewind O, Missiakas DM: Preventing Staphylococcus aureus sepsis through the inhibition of its agglutination in blood. PLoS Pathog 2011, 7:e1002307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Walker JN, Crosby HA, Spaulding AR, Salgado-Pabon W, Malone CL, Rosenthal CB, Schlievert PM, Boyd JM, Horswill AR: The Staphylococcus aureus ArlRS two-component system is a novel regulator of agglutination and pathogenesis. PLoS Pathog 2013, 9:e1003819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Yu W, Kim HK, Rauch S, Schneewind O, Missiakas D: Pathogenic conversion of coagulase-negative staphylococci. Microbes Infect 2017, 19:101–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Rothfork JM, Dessus-Babus S, Van Wamel WJ, Cheung AL, Gresham HD: Fibrinogen depletion attenuates Staphyloccocus aureus infection by preventing density-dependent virulence gene up-regulation. J Immunol 2003, 171:5389–5395. [DOI] [PubMed] [Google Scholar]
- 51.Bokarewa MI, Jin T, Tarkowski A: Staphylococcus aureus: Staphylokinase. Int J Biochem Cell Biol 2006, 38:504–509. [DOI] [PubMed] [Google Scholar]
- 52.Kwiecinski J, Josefsson E, Mitchell J, Higgins J, Magnusson M, Foster T, Jin T, Bokarewa M: Activation of plasminogen by staphylokinase reduces the severity of Staphylococcus aureus systemic infection. J Infect Dis 2010, 202:1041–1049. [DOI] [PubMed] [Google Scholar]
- 53.Liesenborghs L, Meyers S, Lox M, Criel M, Claes J, Peetermans M, Trenson S, Vande Velde G, Vanden Berghe P, Baatsen P, et al. : Staphylococcus aureus endocarditis: distinct mechanisms of bacterial adhesion to damaged and inflamed heart valves. Eur Heart J 2019, 40:3248–3259.With mouse endocarditis models demonstrates two distinct mechanisms of S. aureus adhesion to vessel wall, depending on whether endothelium is physically damaged or inflamed.
- 54.Claes J, Liesenborghs L, Peetermans M, Veloso TR, Missiakas D, Schneewind O, Mancini S, Entenza JM, Hoylaerts MF, Heying R, et al. : Clumping factor A, von Willebrand factor-binding protein and von Willebrand factor anchor Staphylococcus aureus to the vessel wall. J Thromb Haemost 2017, 15:1009–1019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hienz SA, Schennings T, Heimdahl A, Flock JI: Collagen binding of Staphylococcus aureus is a virulence factor in experimental endocarditis. J Infect Dis 1996, 174:83–88. [DOI] [PubMed] [Google Scholar]
- 56.Kulhankova K, Kinney KJ, Stach JM, Gourronc FA, Grumbach IM, Klingelhutz AJ, Salgado-Pabon W: The Superantigen Toxic Shock Syndrome Toxin 1 Alters Human Aortic Endothelial Cell Function. Infect Immun 2018, 86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Powers ME, Becker RE, Sailer A, Turner JR, Bubeck Wardenburg J: Synergistic Action of Staphylococcus aureus alpha-Toxin on Platelets and Myeloid Lineage Cells Contributes to Lethal Sepsis. Cell Host Microbe 2015, 17:775–787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Powers ME, Kim HK, Wang Y, Bubeck Wardenburg J: ADAM10 mediates vascular injury induced by Staphylococcus aureus alpha-hemolysin. J Infect Dis 2012, 206:352–356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Casadevall A, Fang FC, Pirofski LA: Microbial virulence as an emergent property: consequences and opportunities. PLoS Pathog 2011, 7:e1002136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Pirofski LA, Casadevall A: Q and A: What is a pathogen? A question that begs the point. BMC Biol 2012, 10:6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Recker M, Laabei M, Toleman MS, Reuter S, Saunderson RB, Blane B, Torok ME, Ouadi K, Stevens E, Yokoyama M, et al. : Clonal differences in Staphylococcus aureus bacteraemia-associated mortality. Nat Microbiol 2017, 2:1381–1388.Through phenotyping and genotyping a large collection of sepsis S. aureus isolates and combining it with clinical data and machine learning, demonstrates that factors predicting disease outcome differ between clonal lineages of S. aureus.
- 62.Nilsson IM, Lee JC, Bremell T, Ryden C, Tarkowski A: The role of staphylococcal polysaccharide microcapsule expression in septicemia and septic arthritis. Infect Immun 1997, 65:4216–4221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Boyle-Vavra S, Li X, Alam MT, Read TD, Sieth J, Cywes-Bentley C, Dobbins G, David MZ, Kumar N, Eells SJ, et al. : USA300 and USA500 clonal lineages of Staphylococcus aureus do not produce a capsular polysaccharide due to conserved mutations in the cap5 locus. mBio 2015, 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Rauch S, Gough P, Kim HK, Schneewind O, Missiakas D: Vaccine protection of leukopenic mice against Staphylococcus aureus bloodstream infection. Infect Immun 2014, 82:4889–4898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.DeLeo FR, Kennedy AD, Chen L, Bubeck Wardenburg J, Kobayashi SD, Mathema B, Braughton KR, Whitney AR, Villaruz AE, Martens CA, et al. : Molecular differentiation of historic phage-type 80/81 and contemporary epidemic Staphylococcus aureus. Proc Natl Acad Sci U S A 2011, 108:18091–18096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Sharma-Kuinkel BK, Mongodin EF, Myers JR, Vore KL, Canfield GS, Fraser CM, Rude TH, Fowler VG, Jr., Gill SR: Potential Influence of Staphylococcus aureus Clonal Complex 30 Genotype and Transcriptome on Hematogenous Infections. Open Forum Infect Dis 2015, 2:ofv093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Fowler VG Jr., Nelson CL, McIntyre LM, Kreiswirth BN, Monk A, Archer GL, Federspiel J, Naidich S, Remortel B, Rude T, et al. : Potential associations between hematogenous complications and bacterial genotype in Staphylococcus aureus infection. J Infect Dis 2007, 196:738–747. [DOI] [PubMed] [Google Scholar]
- 68.King JM, Kulhankova K, Stach CS, Vu BG, Salgado-Pabon W: Phenotypes and Virulence among Staphylococcus aureus USA100, USA200, USA300, USA400, and USA600 Clonal Lineages. mSphere 2016, 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Monecke S, Coombs G, Shore AC, Coleman DC, Akpaka P, Borg M, Chow H, Ip M, Jatzwauk L, Jonas D, et al. : A field guide to pandemic, epidemic and sporadic clones of methicillin-resistant Staphylococcus aureus. PLoS One 2011, 6:e17936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Seymour CW, Kennedy JN, Wang S, Chang CH, Elliott CF, Xu Z, Berry S, Clermont G, Cooper G, Gomez H, et al. : Derivation, Validation, and Potential Treatment Implications of Novel Clinical Phenotypes for Sepsis. JAMA 2019, 321:2003–2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Jenul C, Horswill AR: Regulation of Staphylococcus aureus Virulence. Microbiol Spectr 2018, 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Cheung AL, Eberhardt KJ, Chung E, Yeaman MR, Sullam PM, Ramos M, Bayer AS: Diminished virulence of a sar-/agr- mutant of Staphylococcus aureus in the rabbit model of endocarditis. J Clin Invest 1994, 94:1815–1822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Painter KL, Krishna A, Wigneshweraraj S, Edwards AM: What role does the quorum-sensing accessory gene regulator system play during Staphylococcus aureus bacteremia? Trends Microbiol 2014, 22:676–685. [DOI] [PubMed] [Google Scholar]
- 74.Kang CK, Cho JE, Choi YJ, Jung Y, Kim NH, Kim CJ, Kim TS, Song KH, Choe PG, Park WB, et al. : agr dysfunction affects staphylococcal cassette chromosome mec type-dependent clinical outcomes in methicillin-resistant Staphylococcus aureus bacteremia. Antimicrob Agents Chemother 2015, 59:3125–3132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Kang CK, Kim YK, Jung SI, Park WB, Song KH, Park KH, Choe PG, Jang HC, Lee S, Kim YS, et al. : agr functionality affects clinical outcomes in patients with persistent methicillin-resistant Staphylococcus aureus bacteraemia. Eur J Clin Microbiol Infect Dis 2017, 36:2187–2191. [DOI] [PubMed] [Google Scholar]
- 76.Laabei M, Uhlemann AC, Lowy FD, Austin ED, Yokoyama M, Ouadi K, Feil E, Thorpe HA, Williams B, Perkins M, et al. : Evolutionary Trade-Offs Underlie the Multi-faceted Virulence of Staphylococcus aureus. PLoS Biol 2015, 13:e1002229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Yang CC, Sy CL, Huang YC, Shie SS, Shu JC, Hsieh PH, Hsiao CH, Chen CJ: Risk factors of treatment failure and 30-day mortality in patients with bacteremia due to MRSA with reduced vancomycin susceptibility. Sci Rep 2018, 8:7868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Hall PR, Elmore BO, Spang CH, Alexander SM, Manifold-Wheeler BC, Castleman MJ, Daly SM, Peterson MM, Sully EK, Femling JK, et al. : Nox2 modification of LDL is essential for optimal apolipoprotein B-mediated control of agr type III Staphylococcus aureus quorum-sensing. PLoS Pathog 2013, 9:e1003166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Altman DR, Sullivan MJ, Chacko KI, Balasubramanian D, Pak TR, Sause WE, Kumar K, Sebra R, Deikus G, Attie O, et al. : Genome Plasticity of agr-Defective Staphylococcus aureus during Clinical Infection. Infect Immun 2018, 86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Grundstad ML, Parlet CP, Kwiecinski JM, Kavanaugh JS, Crosby HA, Cho YS, Heilmann K, Diekema DJ, Horswill AR: Quorum Sensing, Virulence, and Antibiotic Resistance of USA100 Methicillin-Resistant Staphylococcus aureus Isolates. mSphere 2019, 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Horswill AR, Gordon CP: Structure-Activity-Relationship Studies of Small Molecule Modulators of the Staphylococcal Accessory Gene Regulator. J Med Chem 2019. [DOI] [PubMed] [Google Scholar]
- 82.Parlet CP, Kavanaugh JS, Crosby HA, Raja HA, El-Elimat T, Todd DA, Pearce CJ, Cech NB, Oberlies NH, Horswill AR: Apicidin Attenuates MRSA Virulence through Quorum-Sensing Inhibition and Enhanced Host Defense. Cell Rep 2019, 27:187–198 e186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Kwiecinski JM, Crosby HA, Valotteau C, Hippensteel JA, Nayak MK, Chauhan AK, Schmidt EP, Dufrene YF, Horswill AR: Staphylococcus aureus adhesion in endovascular infections is controlled by the ArlRS-MgrA signaling cascade. PLoS Pathog 2019, 15:e1007800.Through combination of in vitro experiments and mouse models, demonstrates that ArlRS and MgrA, by controlling surface protein expression, are master regulators of S. aureus adhesion to endothelium and vessel walls.
- 84.Seidl K, Leemann M, Zinkernagel AS: The ArlRS two-component system is a regulator of Staphylococcus aureus-induced endothelial cell damage. Eur J Clin Microbiol Infect Dis 2018, 37:289–292. [DOI] [PubMed] [Google Scholar]
- 85.Crosby HA, Tiwari N, Kwiecinski JM, Xu Z, Dykstra A, Jenul C, Fuentes EJ, Horswill AR: The Staphylococcus aureus ArlRS two-component system regulates virulence factor expression through MgrA. Mol Microbiol 2019.Through RNA-seq and series of in vitro experiments on purified ArlR and ArlS proteins, demonstrates that ArlRS and MgrA form a single regulatory cascade controlling expression of over 200 S. aureus genes.
- 86.Li L, Wang G, Cheung A, Abdelhady W, Seidl K, Xiong YQ: MgrA Governs Adherence, Host Cell Interaction, and Virulence in a Murine Model of Bacteremia Due to Staphylococcus aureus. J Infect Dis 2019, 220:1019–1028.Through mix of in vitro experiments and mouse models identifies MgrA as central for S. aureus sepsis outcome thanks to its regulation of S. aureus adhesion and antimicrobial peptide resistance.
- 87.Parraga Solorzano PK, Yao J, Rock CO, Kehl-Fie TE: Disruption of Glycolysis by Nutritional Immunity Activates a Two-Component System That Coordinates a Metabolic and Antihost Response by Staphylococcus aureus. mBio 2019, 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Radin JN, Kelliher JL, Parraga Solorzano PK, Kehl-Fie TE: The Two-Component System ArlRS and Alterations in Metabolism Enable Staphylococcus aureus to Resist Calprotectin-Induced Manganese Starvation. PLoS Pathog 2016, 12:e1006040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Liu CL, Chen ZJ, Wang F, Hou HY, Wang Y, Zhu XH, Jian C, Tian L, Yan SZ, Xu LQ, et al. : The impact of mgrA on progression of Staphylococcus aureus sepsis. Microb Pathog 2014, 71–72:56–61. [DOI] [PubMed] [Google Scholar]
- 90.Lei MG, Gudeta DD, Luong TT, Lee CY: MgrA Negatively Impacts Staphylococcus aureus Invasion by Regulating Capsule and FnbA. Infect Immun 2019, 87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Brothers KB, Rothstein MA: Ethical, legal and social implications of incorporating personalized medicine into healthcare. Per Med 2015, 12:43–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Drake TM, Knight SR, Harrison EM, Soreide K: Global Inequities in Precision Medicine and Molecular Cancer Research. Front Oncol 2018, 8:346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Ramaswami R, Bayer R, Galea S: Precision Medicine from a Public Health Perspective. Annual Review of Public Health, Vol 39 2018, 39:153–168. [DOI] [PubMed] [Google Scholar]
- 94.Parker D: Humanized Mouse Models of Staphylococcus aureus Infection. Front Immunol 2017, 8:512. [DOI] [PMC free article] [PubMed] [Google Scholar]