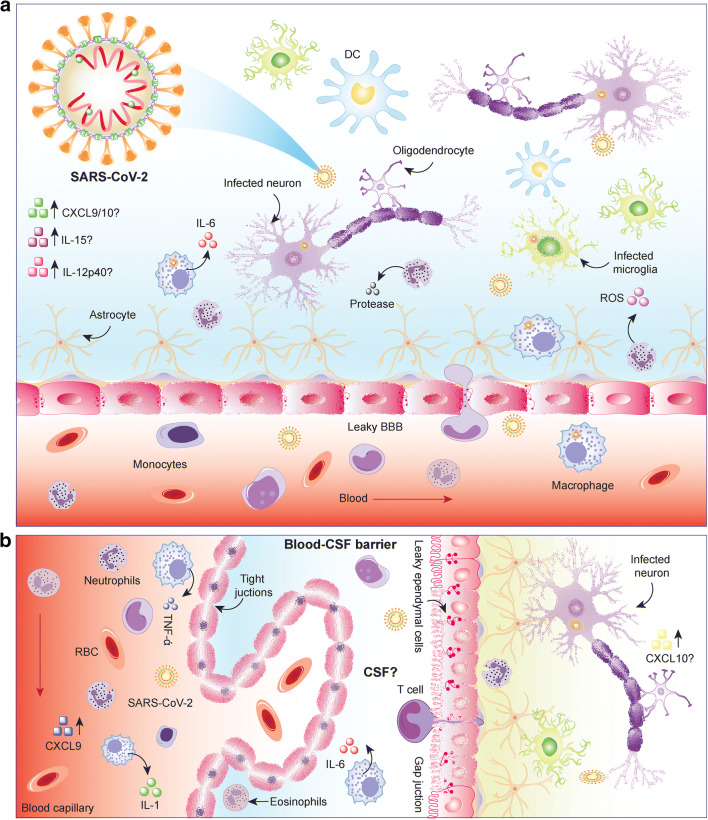
Abstract
A number of neurological disease complications have been seen following infection with the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). While most person with COVID-19 respiratory disease demonstrate headache, nausea and vomiting, up to 40% present also experience dizziness, confusion, cerebrovascular disease, muscle pain, ataxia and seizures. Loss of taste and smell, defects in visual acuity and pain occur in parallel. Such central nervous system (CNS) signs and symptoms linked to laboratory-confirmed SARS-CoV-2 infection is often life threatening. Health care providers currently evaluating patients with neurologic symptoms need consider COVID-19 in any differential diagnosis. These considerations will facilitate prompt testing, isolation and prevention of viral transmission speeding best clinical outcomes.
Graphical Abstract
Keywords: COVID-19, SARS-CoV-2, CNS, Neurologic manifestations, Animal models, Guillain-Barré syndrome, Therapeutics
The Coronavirus (CoV)
CoVs are enveloped positive sense RNA viruses that have four genera: alphacoronavirus, betacoronavirus, gammacoronavirus and deltacoronavirus (Fehr and Perlman 2015). CoV are spherical with a diameter of 125 nm (Zhao et al. 2012). The viral genome is 30 kb long with a 5′ cap and 3′ poly-A tail. Up to 20 kb of the viral genome codes non-structural proteins, whereas 10 kb codes for structural proteins. The latter include the viral spike (S), envelop (E), membrane (M) and nucleocapsid (N) (Brian and Baric 2005). The spike proteins project form the envelop surface of the virus giving the virus a solar corona-like appearance, and hence the name corona (Beniac et al. 2006). Spikes are composed of two proteins, termed S1 and S2, and these S1/S2 form homotrimers that are heavily N-linked glycosylated; specific regions of the S1 protein (termed Receptor Binding Domains, RBD). These engage the ACE2 receptors expressed on cell surface receptors to enter host cells (Delmas and Laude 1990). After binding with the host cell surface receptor, the S1/S2 containing spike is cleaved by a furin-like protease to generate two polypeptides. S1 contains the receptor-binding domain (RDB) and S2 forms the spike protein stalk (Luytjes et al. 1987; Abraham et al. 1990). Human CoV belonging to the betacoronavirus family cause respiratory and enteric diseases and include human CoV 229E, OC43, NL63, HKU1, severe acute respiratory syndrome (SARS) and the Middle East Respiratory Syndrome (MERS). All can lead to pulmonary disease with significant associated morbidities and mortality rates. The present outbreak of the CoV referenced as SARS-CoV-2 was reported from Wuhan, China in late December 2019. Later, on February 11, the World Health Organization (WHO) named the viral strain SARS-CoV-2 and declared its spread a pandemic. The disease that results from SARS-CoV-2 infection is called COVID-19 (reviewed recently by Rothan and Byrareddy 2020). SARS-CoV-2 belongs to the clade of SARS-CoVs that include bat-derived viruses (Hu et al. 2017; Luk et al. 2019). Like SARS-CoV, SARS-CoV-2 uses the angiotensin-converting enzyme 2 (ACE-2) receptor to infect host cells that includes, but are not limited to, airway epithelium and parenchyma cells, vascular endothelial cells, kidney and small intestinal cells (Baig et al. 2020). The S1-S2 cleavage site of the spike of SARS-CoV-2 has a unique 12 nucleotide insertion sequence with three predicted O-linked glycans; whereas most spikes of other HCoV have N-linked glycans (Andersen et al. 2020). The function of the O-linked glycan contains a predicted ‘mucin-like domain’ with a glycan shield that can promote immune escape (Bagdonaite and Wandall 2018). While awaiting further confirmation, SARS-CoV-2 has up to 96% similarity in genomic sequence with CoVs isolated from horseshoe bats found in caves within the Hunnan China. The bat SARS-CoV is named RaTG13 which was isolated from the Chinese rufous horseshoe bat (Rhinolophus sinicus). All support the idea that the bat is the natural viral reservoir (Zhou et al. 2020).
Clinical Manifestations and Laboratory Diagnosis of SARS-CoV-2 Infection
SARS CoV-2 infection leads to significant disease now termed COVID-19. Symptoms of COVID-19 include fever, cough, diarrhea, and fatigue progressing to severe respiratory impairments and the acute respiratory distress syndrome (ARDS). A diagnosis of COVID-19 disease is made by molecular-based (real-time reverse transcriptase-polymerase chain reaction) viral nucleic acid detection and is supported by panels of hematological (reduced numbers of CD45+, CD3+, CD4+, CD8+, CD19+ and CD16/56+ cells, abnormal liver and metabolic functions. These are coincident with upper and lower respiratory tract disease sometimes progressing to ARDS (Fu et al. 2020; Pan et al. 2020). However, such signs and symptoms now appear to be simply a part of the constellation of presenting clinical features for COVID-19. Indeed, recent studies affirm that neurologic disease manifestations are a significant part of an already large number of disease manifestations (Asadi-Pooya and Simani 2020). It is known that invasion of the central nervous system (CNS by the human CoV (HCoV) occurs shortly after infection and through immune escape. The virus enters the CNS and infects neurons creating a range of disease-related consequences (McGavern and Kang 2011; Desforges et al. 2014a). This includes more widespread neurodegeneration (Li et al. 2016b; Desforges et al. 2019; Wu et al. 2020). CNS disease manifestations commonly appears directly after infection, late in the disease course or following recovery (Conde Cardona et al. 2020). These observations are not only seen for SARS-CoV-2 but are mirrored by all betacoronaviruses (Desforges et al. 2019). Such findings were affirmed during the SARS-CoV pandemic of 2002–2003. During this time autopsy examinations demonstrated HCoV particles in infected human brain tissues. CNS invasion of zoonotic viruses offers no potential evolutionary advantage. This is underscored by the fact that immune viral clearance is limited for neuronal viral infections. Indeed, antiviral cytolytic T cells demonstrate reduced surveillance activities in the brain (Koyuncu et al. 2013). In a recent case study, meningoencephalitis was seen as a major clinical manifestation of COVID-19. Notably this was recorded without respiratory symptoms (Duong et al. 2020). In this report, a person with a history of type-2 diabetes presented with a low-grade fever of 100.5 ° F, headache, stiff neck, photophobia, seizure without respiratory distress. Anti-epileptic therapy was started for the management of viral meningitis. The clinical progression demonstrated rapid clinical deterioration with disorientation and hallucinations. SARS-CoV-2 detection tests were positive, and she was treated with hydroxychloroquine with improvement in symptoms during a clinical course of 5 days following admission to hospital. In a second case, a young man in his twenties presented total anosmia and ageusia with high suspicion of SARS-CoV-2 infection (Stripp and Sondergaard 2020).
CoV and Animal Models of CNS Infection
The localization of the virus in brain tissues was studied in mice infected with SARS-CoV34 and MERS-CoV13 (Netland et al. 2008). The brainstem was the target source of viral infection (McCray et al. 2007; Netland et al. 2008; Li et al. 2016a). As cardio-respiratory impairments could reflect brainstem dysfunction it may, in part, be responsible for ARDS as frequently occurs as a cause of COVID-19 mortality among SARS-CoV-2 infected patients (Netland et al. 2008; Dube et al. 2018; Li et al. 2020). This reflects findings in other neurotropic CoVs that included mouse hepatitis virus, porcine hemagglutinating encephalitis virus, and feline CoV (Greig et al. 1962; Arbour et al. 2000; Foley et al. 2003). While HCoV infections are restricted to the upper and lower respiratory systems, it is clear that virus can cross epithelial barriers to reach the periphery and then be transmitted to other organs including the CNS by hematogenous spread (Gu et al. 2005; Nicholls et al. 2006; Desforges et al. 2014a, b). Most of the human CoV strains including 229E, OC43 and SARS productively infect mononuclear phagocytes (MP). It has been suggested that the virus manipulates innate immune responses intrinsic to this cell lineage and subsequently serves as a reservoir for the virus. MP infection of alveolar macrophages can trigger innate immune activation and release of pro-inflammatory secretory factors that may also, in part, by responsible for lung tissue injury and ARDS.
CNS Viral Entry and Neurological Disease Manifestations
HCoV enters the CNS from blood and peripheral organs then spreads to the brain (Collins 2002; Nicholls et al. 2006; Desforges et al. 2007). HCoV may also enter CNS through neuronal dissemination, in which the virus initially infects peripheral neurons and from there, using host cell machinery, infects CNS neurons (Koyuncu et al. 2013) (Fig. 1). Using the murine model of HCoV infection, it was shown that SARS and OC43 were able to enter the olfactory bulb after exposure by the nasal route; then using olfactory pathways invades the CNS then specifically the brain stem (Netland et al. 2008; Dube et al. 2018). The fact that COVID-19 patients have impaired olfactory function supports this view (Eliezer et al. 2020). Furthermore, the CT scan and MRI analysis of this patient revealed ‘bilateral inflammatory obstruction of the olfactory clefts’ (Eliezer et al. 2020). SARS-CoV-2 may also play a role in impaired olfactory function due to its structural and genomic similarities to SARS-CoV, as well as its shared mechanism of entrance into host cells by use of the ACE-2 receptor. Xydakis et al., reported anosmia alone or in combination of parageusia is frequently encountered in COVID-19 patients (Xydakis et al. 2020). “The American Academy of Otolaryngology—Head and Neck Surgery” and “The British Association of Otorhinolaryngology” recommends adding these symptoms in the list of primary screening for COVID-19 (Xydakis et al. 2020). As the impaired ability to smell and test are a common manifestation of respiratory neurotropic viral invasion of the olfactory system, we suspect there is a possibility that SARS-CoV-2 can infect the olfactory system and may enter the CNS using the olfactory pathway. A retrospective study substantiates this, wherein, 36.4% of patients out of 214 confirmed cases of SARS-CoV-2 have been documented to present with varying degree of neurological manifestations that include skeletal muscle injury, delirium and acute cerebrovascular disease (Mao et al. 2020) (Fig. 2). Another study from China during the early days of the pandemic, reported neurological manifestations including confusion and headache (Chen et al. 2020b). This report supports results of the previous studies that showed the presence of high titers of HCoV antibodies found in the cerebrospinal fluid of multiple sclerosis (MS) patients as compared with controls, although both groups had similar levels of serum antibody (Salmi et al. 1982). This is further supported by the finding of the presence of HCoV RNA in post-mortem brain tissues from MS patients (Stewart et al. 1992). The findings suggest that MS patients are at higher risk of HCoV neuroinvasion.
Fig. 1.
The neurovirulence of SARS-CoV-2. Increasing data has now provided evidence that SARS-C0V-2 is both neurotropic and neurovirulent. The virus invades the CNS soon after infection and gains access into the CSF and to brain subregions that include the brain stem and cortex. After crossing the blood-brain barrier (BBB) virus can replicate in microglia and neurons with collateral damage to the barrier. An inflammatory cascade is set into motion that includes ongoing collateral damage and secondary seizures, delirium and stroke. A key finding that is the requirements of the ACE2 cell entry receptor. The CoV spike glycoprotein, by which SARS-CoV-2 binds to cell membranes. The expression of this receptor in neurons and endothelial cells outlines the virus neuroinvasive potential. It is possible that both respiratory and neural failures are linked to brain stem damage and as such both direct infection and indirect inflammatory mechanisms are likely both operative. Differential host immune-mediated responses may determine outcomes. Disease models are surely needed to investigate potential neurological complications and to explore mechanisms of alternative immune-mediated pathogenicity and developmental therapies
Fig. 2.
Neurological signs and symptoms occur as a result of SARS-COV-2 infection. These are in addition to life threatening ARDS. These include, but are not limited to, headache, dizziness, myalgia and fatigue, ARDS (a primary part of the disease complex, brain stem (respiratory and cardiac) impairments, primary cardiac disease, anosmia, an inflammatory encephalitis, delirium and cognitive impairments, stroke, seizures, spinal cord injuries and the Guillain-Barre syndrome. All are associated with SARS-CoV-2 infections
It is of interest to examine the data on studies performed using the mouse hepatitis virus (MHV), an enveloped RNA virus that belongs to the coronaviridae family. The MHV produces acute infection of the CNS that gradually gets controlled by cytolytic CD8+ T cells. However, MHV has the capacity to immune escape leading to the establishment of a chronic CNS infection and progressive demyelination in the brain, which is a hallmark of MS (Wu et al. 2000; Bergmann et al. 2006). As SARS-CoV-2, belongs to same family as MHV, it may also be responsible for demyelination in the brain and lead to conditions similar to that noted in MS patients. It is important to remember that MS patients are treated with corticosteroids. They thus remain in an immune-compromised state and fall in a high-risk group of getting infected with SARS-CoV-2, which warrants extra careful surveillance of MS patients during this ongoing global pandemic.
Guillain-Barré Syndrome
Guillain-Barré Syndrome (GBS) is a rare autoimmune disorder that presents with bilateral weaknesses and neuromuscular paralysis (Willison et al. 2016). Among the different phenotypes of GBS “acute inflammatory demyelinating polyradiculoneuropathy” is the most common in which auto-antibodies attack the myelin membranes, whereas in “acute motor axonal neuropathy, auto-antibodies attack the axonal membranes of the peripheral nerves (Leonhard et al. 2019). Most of the patients have a history of suffering from a variety of infectious diseases within 4–6 weeks prior to the development of symptoms with GBS. The infectious agents that have so far been reported include Campylobacter jejuni, Mycoplasma pneumoniae, cytomegalovirus, herpes simplex virus, varicella zoster virus, Epstein-Barr virus, hepatitis A virus, hepatitis B and E, the human immunodeficiency virus (HIV), dengue, chikungunya and ZIKA viruses (Rodriguez et al. 2018). During the ZIKA virus epidemic (2016–2018), several studies reported the development of GBS among ZIKA infected patients, including children. The individuals who developed GBS after ZIKA virus infection had poor prognoses compared to the general population (Araujo et al. 2016; Arias et al. 2017; Barbi et al. 2018; Dirlikov et al. 2018; Major et al. 2018). A recent case report described the potential of SARS-CoV-2 infection which resulted in the detection of reversible GBS (Zhao et al. 2020). A 61-year-old woman with a travel history to Wuhan, China during the 3rd week of January 2020 was first asymptomatic for COVID-19 infection, but developed symptoms of GBS. Subsequently, during follow up treatment, she developed dry cough and fever when SARS-CoV-2 infection was confirmed. The patient was treated with Arbidol and boosted Lopinavir with Ritonavir. With treatment the patient clinical status returned to normal (Zhao et al. 2020). In prior reports, GBS was observed in four patients within a cohort of 23 with confirmed MERS CoV (Kim et al. 2017). Strikingly, the peripheral neurological complications develop after 2 to 3 weeks of recovery from respiratory illness (Kim et al. 2017). Although no direct correlation was established between the development of GBS and COVID-19 for this particular case, the incidence warrants follow up by performing an epidemiological study to monitor development of GBS and or associated neurological disorders among acute/chronic COVID-19 patients as well as in patients who successfully recover from the disease.
Therapeutics for COVID-19 Neurological Manifestations
The COVID-19 patients, who are above 60 years of age with comorbid conditions that include type 2 diabetes, hypertension, hyperlipidemia and chronic obstructive pulmonary disease may have elevated levels of serum D-dimer, that may lead to embolic vascular events (Chen et al. 2020a; Mao et al. 2020). This includes ischemic stroke necessitating treatment with anti-coagulants (Jin et al. 2020; Khosravani et al. 2020). Hypertensive patients with SARS-CoV-2 infection may have fluctuations in blood pressure that may lead to intracranial hemorrhage. In these patients, to control hypertension, ACE inhibitors should be replaced with calcium channel blockers or diuretics. Cerebrospinal fluid (CSF) should be tested by quantitative polymerase chain reaction tests to detect SARS-CoV-2 in patients with suspected intra-cranial infection. During this COVID-19 pandemic period, all patients coming to the emergency room should be evaluated for symptoms that overlap with neurological manifestations. Individuals suffering from an opioid use disorder are likely at higher risk of developing severe disease. All together the significant neurological disease manifestations now known warrant additional detailed study to explore the diagnostics, disease mechanisms and therapeutic options of COVID-19 disease.
Acknowledgements
We thank Michellie Thurman and Robin Taylor for editorial help. The work contained in this study is dedicated to the life and legacy of Ms. Harriet Singer whose philanthropy and dogged spirit enabled this and countless works possible for studies of human brain disease. This work was supported, in part, by R21MH113455, Frances E. Lageschulte and Evelyn B. Weese New Frontiers in Medical Research Fund to SNB and the Carol Swarts, M.D. Emerging Neuroscience Research Laboratory, the Margaret R. Larson Professorship, and the Frances and Louie Blumkin and Harriet Singer Research Foundations. P30MH062261 to HEG and SNB and 1R01AI145542-01A1, P01 DA028555, R01 NS36126, P01 NS31492, 2R01 NS034239, P01 MH64570, P01 NS43985, R01 AG043540, and 1 R56 AI138613-01A1 to HEG.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Arpan Acharya and Bhavesh D. Kevadiya contributed equally to this work.
Contributor Information
Howard E. Gendelman, Email: hegendel@unmc.edu
Siddappa N. Byrareddy, Email: sid.byrareddy@unmc.edu
References
- Abraham S, Kienzle TE, Lapps W, Brian DA. Deduced sequence of the bovine coronavirus spike protein and identification of the internal proteolytic cleavage site. Virology. 1990;176:296–301. doi: 10.1016/0042-6822(90)90257-R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersen KG, Rambaut A, Lipkin WI, Holmes EC, Garry RF. The proximal origin of SARS-CoV-2. Nat Med. 2020;26:450–452. doi: 10.1038/s41591-020-0820-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Araujo LM, Ferreira ML, Nascimento OJ. Guillain-Barre syndrome associated with the Zika virus outbreak in Brazil. Arq Neuropsiquiatr. 2016;74:253–255. doi: 10.1590/0004-282X20160035. [DOI] [PubMed] [Google Scholar]
- Arbour N, Day R, Newcombe J, Talbot PJ. Neuroinvasion by human respiratory coronaviruses. J Virol. 2000;74:8913–8921. doi: 10.1128/JVI.74.19.8913-8921.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arias A, Torres-Tobar L, Hernandez G, Paipilla D, Palacios E, Torres Y, Duran J, Ugarte US, Ardila-Sierra A, Castellanos G. Guillain-Barre syndrome in patients with a recent history of Zika in Cucuta, Colombia: a descriptive case series of 19 patients from December 2015 to march 2016. J Crit Care. 2017;37:19–23. doi: 10.1016/j.jcrc.2016.08.016. [DOI] [PubMed] [Google Scholar]
- Asadi-Pooya AA, Simani L. Central nervous system manifestations of COVID-19: a systematic review. J Neurol Sci. 2020;413:116832. doi: 10.1016/j.jns.2020.116832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bagdonaite I, Wandall HH. Global aspects of viral glycosylation. Glycobiology. 2018;28:443–467. doi: 10.1093/glycob/cwy021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baig AM, Khaleeq A, Ali U, Syeda H. Evidence of the COVID-19 virus targeting the CNS: tissue distribution, host-virus interaction, and proposed neurotropic mechanisms. ACS Chem Neurosci. 2020;11:995–998. doi: 10.1021/acschemneuro.0c00122. [DOI] [PubMed] [Google Scholar]
- Barbi L, Coelho AVC, Alencar LCA, Crovella S. Prevalence of Guillain-Barre syndrome among Zika virus infected cases: a systematic review and meta-analysis. Braz J Infect Dis. 2018;22:137–141. doi: 10.1016/j.bjid.2018.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beniac DR, Andonov A, Grudeski E, Booth TF. Architecture of the SARS coronavirus prefusion spike. Nat Struct Mol Biol. 2006;13:751–752. doi: 10.1038/nsmb1123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergmann CC, Lane TE, Stohlman SA. Coronavirus infection of the central nervous system: host-virus stand-off. Nat Rev Microbiol. 2006;4:121–132. doi: 10.1038/nrmicro1343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brian DA, Baric RS. Coronavirus genome structure and replication. Curr Top Microbiol Immunol. 2005;287:1–30. doi: 10.1007/3-540-26765-4_1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen L, Li Q, Zheng D, Jiang H, Wei Y, Zou L, Feng L, Xiong G, Sun G, Wang H, Zhao Y, Qiao J (2020a) Clinical characteristics of pregnant women with Covid-19 in Wuhan, China. N Engl J Med [DOI] [PMC free article] [PubMed]
- Chen N, Zhou M, Dong X, Qu J, Gong F, Han Y, Qiu Y, Wang J, Liu Y, Wei Y, Xia J, Yu T, Zhang X, Zhang L. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020;395:507–513. doi: 10.1016/S0140-6736(20)30211-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins AR. In vitro detection of apoptosis in monocytes/macrophages infected with human coronavirus. Clin Diagn Lab Immunol. 2002;9:1392–1395. doi: 10.1128/CDLI.9.6.1392-1395.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conde Cardona G, Quintana Pajaro LD, Quintero Marzola ID, Ramos Villegas Y, Moscote Salazar LR. Neurotropism of SARS-CoV 2: mechanisms and manifestations. J Neurol Sci. 2020;412:116824. doi: 10.1016/j.jns.2020.116824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delmas B, Laude H. Assembly of coronavirus spike protein into trimers and its role in epitope expression. J Virol. 1990;64:5367–5375. doi: 10.1128/JVI.64.11.5367-5375.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desforges M, Miletti TC, Gagnon M, Talbot PJ. Activation of human monocytes after infection by human coronavirus 229E. Virus Res. 2007;130:228–240. doi: 10.1016/j.virusres.2007.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desforges M, Le Coupanec A, Brison E, Meessen-Pinard M, Talbot PJ. Neuroinvasive and neurotropic human respiratory coronaviruses: potential neurovirulent agents in humans. Adv Exp Med Biol. 2014;807:75–96. doi: 10.1007/978-81-322-1777-0_6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desforges M, Le Coupanec A, Stodola JK, Meessen-Pinard M, Talbot PJ. Human coronaviruses: viral and cellular factors involved in neuroinvasiveness and neuropathogenesis. Virus Res. 2014;194:145–158. doi: 10.1016/j.virusres.2014.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desforges M, Le Coupanec A, Dubeau P, Bourgouin A, Lajoie L, Dube M, Talbot PJ (2019) Human coronaviruses and other respiratory viruses: underestimated opportunistic pathogens of the central nervous system? Viruses 12(1), pii: E14. 10.3390/v12010014 [DOI] [PMC free article] [PubMed]
- Dirlikov E, Major CG, Medina NA, Lugo-Robles R, Matos D, Munoz-Jordan JL, Colon-Sanchez C, Garcia M, Olivero-Segarra M, Malave G, Rodriguez-Vega GM, Thomas DL, Waterman SH, Sejvar JJ, Luciano CA, Sharp TM, Rivera-Garcia B. Clinical features of Guillain-Barre syndrome with vs without Zika virus infection, Puerto Rico, 2016. JAMA Neurol. 2018;75:1089–1097. doi: 10.1001/jamaneurol.2018.1058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dube M, Le Coupanec A, Wong AHM, Rini JM, Desforges M, Talbot PJ (2018) Axonal transport enables neuron-to-neuron propagation of human coronavirus OC43. J Virol 92 [DOI] [PMC free article] [PubMed]
- Duong L, Xu P, Liu A (2020) Meningoencephalitis without respiratory failure in a young female patient with COVID-19 infection in downtown Los Angeles, early April 2020. Brain Behav Immun. pii: S0889-1591(20)30509-2. 10.1016/j.bbi.2020.04.024 [DOI] [PMC free article] [PubMed]
- Eliezer M, Hautefort C, Hamel AL, Verillaud B, Herman P, Houdart E, Eloit C (2020) Sudden and complete olfactory loss function as a possible symptom of COVID-19. JAMA Otolaryngol Head Neck Surg. 10.1001/jamaoto.2020.0832 [DOI] [PubMed]
- Fehr AR, Perlman S. Coronaviruses: an overview of their replication and pathogenesis. Methods Mol Biol. 2015;1282:1–23. doi: 10.1007/978-1-4939-2438-7_1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foley JE, Rand C, Leutenegger C. Inflammation and changes in cytokine levels in neurological feline infectious peritonitis. J Feline Med Surg. 2003;5:313–322. doi: 10.1016/S1098-612X(03)00048-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu L, Wang B, Yuan T, Chen X, Ao Y, Fitzpatrick T, Li P, Zhou Y, Lin YF, Duan Q, Luo G, Fan S, Lu Y, Feng A, Zhan Y, Liang B, Cai W, Zhang L, Du X, Li L, Shu Y, Zou H (2020) Clinical characteristics of coronavirus disease 2019 (COVID-19) in China: a systematic review and meta-analysis. J Inf Secur. pii: S0163-4453(20)30170-5. 10.1016/j.jinf.2020.03.041 [DOI] [PMC free article] [PubMed]
- Greig AS, Mitchell D, Corner AH, Bannister GL, Meads EB, Julian RJ. A Hemagglutinating virus producing encephalomyelitis in baby pigs. Can J Comp Med Vet Sci. 1962;26:49–56. [PMC free article] [PubMed] [Google Scholar]
- Gu J, Gong E, Zhang B, Zheng J, Gao Z, Zhong Y, Zou W, Zhan J, Wang S, Xie Z, Zhuang H, Wu B, Zhong H, Shao H, Fang W, Gao D, Pei F, Li X, He Z, Xu D, Shi X, Anderson VM, Leong ASY. Multiple organ infection and the pathogenesis of SARS. J Exp Med. 2005;202:415–424. doi: 10.1084/jem.20050828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu B, Zeng LP, Yang XL, Ge XY, Zhang W, Li B, Xie JZ, Shen XR, Zhang YZ, Wang N, Luo DS, Zheng XS, Wang MN, Daszak P, Wang LF, Cui J, Shi ZL. Discovery of a rich gene pool of bat SARS-related coronaviruses provides new insights into the origin of SARS coronavirus. PLoS Pathog. 2017;13:e1006698. doi: 10.1371/journal.ppat.1006698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin H, Hong C, Chen S, Zhou Y, Wang Y, Mao L, Li Y, He Q, Li M, Su Y, Wang D, Wang L, Hu B (2020) Consensus for prevention and management of coronavirus disease 2019 (COVID-19) for neurologists. Stroke Vasc Neurol. pii: svn-2020-000382. 10.1136/svn-2020-000382 [DOI] [PMC free article] [PubMed]
- Khosravani H, Rajendram P, Notario L, Chapman MG, Menon BK (2020) Protected code stroke: Hyperacute stroke management during the coronavirus disease 2019 (COVID-19) pandemic. Stroke:STROKEAHA120029838. 10.1161/STROKEAHA.120.029838 [DOI] [PMC free article] [PubMed]
- Kim JE, Heo JH, Kim HO, Song SH, Park SS, Park TH, Ahn JY, Kim MK, Choi JP. Neurological complications during treatment of Middle East respiratory syndrome. J Clin Neurol. 2017;13:227–233. doi: 10.3988/jcn.2017.13.3.227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koyuncu OO, Hogue IB, Enquist LW. Virus infections in the nervous system. Cell Host Microbe. 2013;13:379–393. doi: 10.1016/j.chom.2013.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leonhard SE, Mandarakas MR, Gondim FAA, Bateman K, Ferreira MLB, Cornblath DR, van Doorn PA, Dourado ME, Hughes RAC, Islam B, Kusunoki S, Pardo CA, Reisin R, Sejvar JJ, Shahrizaila N, Soares C, Umapathi T, Wang Y, Yiu EM, Willison HJ, Jacobs BC. Diagnosis and management of Guillain-Barre syndrome in ten steps. Nat Rev Neurol. 2019;15:671–683. doi: 10.1038/s41582-019-0250-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li K, Wohlford-Lenane C, Perlman S, Zhao J, Jewell AK, Reznikov LR, Gibson-Corley KN, Meyerholz DK, McCray PB., Jr Middle East respiratory syndrome coronavirus causes multiple organ damage and lethal disease in mice transgenic for human Dipeptidyl peptidase 4. J Infect Dis. 2016;213:712–722. doi: 10.1093/infdis/jiv499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Li H, Fan R, Wen B, Zhang J, Cao X, Wang C, Song Z, Li S, Li X, Lv X, Qu X, Huang R, Liu W. Coronavirus infections in the central nervous system and respiratory tract show distinct features in hospitalized children. Intervirology. 2016;59:163–169. doi: 10.1159/000453066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li YC, Bai WZ, Hashikawa T (2020) The neuroinvasive potential of SARS-CoV2 may play a role in the respiratory failure of COVID-19 patients. J Med Virol. 10.1002/jmv.25728 [DOI] [PMC free article] [PubMed]
- Luk HKH, Li X, Fung J, Lau SKP, Woo PCY. Molecular epidemiology, evolution and phylogeny of SARS coronavirus. Infect Genet Evol. 2019;71:21–30. doi: 10.1016/j.meegid.2019.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luytjes W, Sturman LS, Bredenbeek PJ, Charite J, van der Zeijst BA, Horzinek MC, Spaan WJ. Primary structure of the glycoprotein E2 of coronavirus MHV-A59 and identification of the trypsin cleavage site. Virology. 1987;161:479–487. doi: 10.1016/0042-6822(87)90142-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Major CG, Dirlikov E, Medina NA, Lugo-Robles R, Matos D, Munoz-Jordan J, Colon-Sanchez C, Garcia-Negron M, Olivero-Segarra M, Malave-Gonzalez G, Thomas DL, Luciano CA, Waterman SH, Sejvar J, Sharp TM, Rivera-Garcia B. Implementation and evaluation of Guillain-Barre syndrome surveillance in Puerto Rico during the 2016 Zika virus epidemic. P R Health Sci J. 2018;37:S85–S92. [PMC free article] [PubMed] [Google Scholar]
- Mao L, Jin H, Wang M, Hu Y, Chen S, He Q, Chang J, Hong C, Zhou Y, Wang D, Miao X, Li Y, Hu B (2020) Neurologic manifestations of hospitalized patients with coronavirus disease 2019 in Wuhan, China. JAMA Neurol. 10.1001/jamaneurol.2020.1127 [DOI] [PMC free article] [PubMed]
- McCray PB, Jr, Pewe L, Wohlford-Lenane C, Hickey M, Manzel L, Shi L, Netland J, Jia HP, Halabi C, Sigmund CD, Meyerholz DK, Kirby P, Look DC, Perlman S. Lethal infection of K18-hACE2 mice infected with severe acute respiratory syndrome coronavirus. J Virol. 2007;81:813–821. doi: 10.1128/JVI.02012-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGavern DB, Kang SS. Illuminating viral infections in the nervous system. Nat Rev Immunol. 2011;11:318–329. doi: 10.1038/nri2971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Netland J, Meyerholz DK, Moore S, Cassell M, Perlman S. Severe acute respiratory syndrome coronavirus infection causes neuronal death in the absence of encephalitis in mice transgenic for human ACE2. J Virol. 2008;82:7264–7275. doi: 10.1128/JVI.00737-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicholls JM, Butany J, Poon LL, Chan KH, Beh SL, Poutanen S, Peiris JS, Wong M. Time course and cellular localization of SARS-CoV nucleoprotein and RNA in lungs from fatal cases of SARS. PLoS Med. 2006;3:e27. doi: 10.1371/journal.pmed.0030027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan L, Mu M, Yang P, Sun Y, Wang R, Yan J, Li P, Hu B, Wang J, Hu C, Jin Y, Niu X, Ping R, Du Y, Li T, Xu G, Hu Q, Tu L (2020) Clinical characteristics of COVID-19 patients with digestive symptoms in Hubei, China: a descriptive, cross-sectional, multicenter study. Am J Gastroenterol 115(5):766–773 [DOI] [PMC free article] [PubMed]
- Rodriguez Y, Rojas M, Pacheco Y, Acosta-Ampudia Y, Ramirez-Santana C, Monsalve DM, Gershwin ME, Anaya JM. Guillain-Barre syndrome, transverse myelitis and infectious diseases. Cell Mol Immunol. 2018;15:547–562. doi: 10.1038/cmi.2017.142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothan HA, Byrareddy SN. The epidemiology and pathogenesis of coronavirus disease (COVID-19) outbreak. J Autoimmun. 2020;109:102433. doi: 10.1016/j.jaut.2020.102433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salmi A, Ziola B, Hovi T, Reunanen M. Antibodies to coronaviruses OC43 and 229E in multiple sclerosis patients. Neurology. 1982;32:292–295. doi: 10.1212/WNL.32.3.292. [DOI] [PubMed] [Google Scholar]
- Stewart JN, Mounir S, Talbot PJ. Human coronavirus gene expression in the brains of multiple sclerosis patients. Virology. 1992;191:502–505. doi: 10.1016/0042-6822(92)90220-J. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stripp TK, Sondergaard J (2020) [Possible unregistered SARS-CoV-2 infection in a young man with anosmia and ageusia]. Ugeskr Laeger 182(16), pii: V03200189 [PubMed]
- Willison HJ, Jacobs BC, van Doorn PA. Guillain-Barre syndrome. Lancet. 2016;388:717–727. doi: 10.1016/S0140-6736(16)00339-1. [DOI] [PubMed] [Google Scholar]
- Wu GF, Pewe L, Perlman S. Coronavirus-induced demyelination occurs in the absence of inducible nitric oxide synthase. J Virol. 2000;74:7683–7686. doi: 10.1128/JVI.74.16.7683-7686.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Y, Xu X, Chen Z, Duan J, Hashimoto K, Yang L, Liu C, Yang C (2020) Nervous system involvement after infection with COVID-19 and other coronaviruses. Brain Behav Immun [DOI] [PMC free article] [PubMed]
- Xydakis MS, Dehgani-Mobaraki P, Holbrook EH, Geisthoff UW, Bauer C, Hautefort C, Herman P, Manley GT, Lyon DM, Hopkins C (2020) Smell and taste dysfunction in patients with COVID-19. Lancet Infect Dis [DOI] [PMC free article] [PubMed]
- Zhao H, Shen D, Zhou H, Liu J, Chen S (2020) Guillain-Barre syndrome associated with SARS-CoV-2 infection: causality or coincidence? Lancet Neurol 19(5):383–384. 10.1016/S1474-4422(20)30109 [DOI] [PMC free article] [PubMed]
- Zhao L, Jha BK, Wu A, Elliott R, Ziebuhr J, Gorbalenya AE, Silverman RH, Weiss SR. Antagonism of the interferon-induced OAS-RNase L pathway by murine coronavirus ns2 protein is required for virus replication and liver pathology. Cell Host Microbe. 2012;11:607–616. doi: 10.1016/j.chom.2012.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou P, Yang XL, Wang XG, Hu B, Zhang L, Zhang W, Si HR, Zhu Y, Li B, Huang CL, Chen HD, Chen J, Luo Y, Guo H, Jiang RD, Liu MQ, Chen Y, Shen XR, Wang X, Zheng XS, Zhao K, Chen QJ, Deng F, Liu LL, Yan B, Zhan FX, Wang YY, Xiao GF, Shi ZL. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature. 2020;579:270–273. doi: 10.1038/s41586-020-2012-7. [DOI] [PMC free article] [PubMed] [Google Scholar]