Graphical abstract
Keywords: Coronavirus, SARS-CoV-2, Immune escape, Inflammation, Cytokine storm
Abstract
Coronavirus Disease 2019 (COVID-19) has sparked a global pandemic, affecting more than 4 million people worldwide. The severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) can cause acute lung injury (ALI) and even acute respiratory distress syndrome (ARDS); with a fatality of 7.0 %. Accumulating evidence suggested that the progression of COVID-19 is associated with lymphopenia and excessive inflammation, and a subset of severe cases might exhibit cytokine storm triggered by secondary hemophagocytic lymphohistiocytosis (sHLH). Furthermore, secondary bacterial infection may contribute to the exacerbation of COVID-19. We recommend using both IL-10 and IL-6 as the indicators of cytokine storm, and monitoring the elevation of procalcitonin (PCT) as an alert for initiating antibacterial agents. Understanding the dynamic progression of SARS-CoV-2 infection is crucial to determine an effective treatment strategy to reduce the rising mortality of this global pandemic.
1. Introduction
Since its first emergence in December 2019, the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), a novel human coronavirus (HCoV), has spread across the world at an alarming rate. SARS-CoV-2 was named by the International Committee on Taxonomy of Viruses (ICTV) based on the phylogenetic similarity to SARS-CoV [1,2]. Coronavirus Disease 2019 (COVID-19), the diseases caused by SARS-CoV-2, is far more extensive than the previous HCoV-diseases, with the number of confirmed patients worldwide has currently exceeded 4.0 million [[3], [4], [5]].
After the outbreak of COVID-19, angiotensin-converting enzyme 2 (ACE2) was promptly been identified as the functional receptor of SARS-CoV-2 [2]. ACE2 is mainly distributed on the surface of well-differentiated airway epithelial cells (especially ciliated cells), and the respiratory tract has shown to be a predominant channel for HCoV to invade the human body [6]. SARS-CoV-2 can cause severe lower respiratory tract infection, resulting in acute lung injury (ALI), acute respiratory distressed syndrome (ARDS), and potentially death [7]. A retrospective study of 1099 COVID-19 patients from Zhong Nanshan's group showed, 91.1 % of the patients developed pneumonia and 3.4 % of the patients developed ARDS [8]. While Chen and colleagues reported that over 17 % of the patients with COVID-19 in Wuhan developed ARDS [9]. The fatality rate worldwide has currently reached 7.0 % [3], and respiratory failure has been considered to be the leading cause (69.5 %) of SARS-CoV-2 related death [10]. In order to provide some implications for clinical therapy and drug exploration, we reviewed COVID-19 related clinical studies by searching the keywords "SARS-CoV-2", "COVID-19" and "2019-nCoV" on Sinomed, Pubmed, Embase, WHO, and medRxiv, to explore the possible influencing factors behind COVID-19 exacerbation and related biochemical indicators.
2. Lymphopenia
Several clinical studies have shown that lymphopenia occurs frequently in COVID-19 patients, manifested as the decrease of total T cells, CD4+T cells and CD8+T cells in the peripheral blood; with the degree of decline related to the severity of the disease. Compared with patients with mild symptoms, a higher proportion of severe or deceased patients had lymphopenia [[10], [11], [12], [13], [14]], which is similar to the observation during SARS-CoV infection [15]. According to the Diagnosis and Treatment Protocol for Novel Coronavirus Pneumonia (Trial Version 7) published by National Health Commission of China [16], an autopsy report of a patient who died from COVID-19 indicated (1) the spleen atrophied significantly, with apparent focal hemorrhage and necrosis; (2) lymphocytes in the lymph nodes were depleted and necrotized; and (3) the number of CD4+T and CD8+T cells in spleen and lymph nodes decreased significantly. These pathological characteristics coincide with that of patients who died of SARS-CoV infection [17]. Furthermore, compared with SARS in 2003, severe COVID-19 tends to have a higher viral load and a longer virus-shedding period [18,19]. Since the RNA sequence of SARS-CoV was found in the macrophages and aggregated T cells of lymph nodes [20], the direct impairment from SARS-CoV-2 may be an important cause of lymphopenia in patients with COVID-19.
The severe damage in lymphoid organs by SARS-CoV-2 may relate to the ineffective control of the virus infection during the early stage, which has been seen in SARS-CoV infection [21]. Type Ⅰ interferon (IFN), including IFN-α and IFN-β, plays a critical role in prohibiting viral infections and initiating adaptive immune responses; including in SARS-CoV infection [21,22]. Type Ⅰ IFN can inhibit virus replication in vitro as well as in macaques and mice [[23], [24], [25], [26]]. However, SARS-CoV may delay or evade innate immune response through early antagonism on IFN response, in which IFN can only be detected after SARS-CoV reached a high titer [7,27,28] Present evidence implied that the viral load of SARS-CoV-2 peaks at the time of presentation, even earlier than the time to peak of SARS-CoV [29]. We suspected that similar to SARS-CoV, SARS-CoV-2 may escape from the immune response through the “physical” cover of double-membrane vesicles (DMV) and the “chemical” interference of the proteins encoded by itself [30,31],
The clearance of the virus mainly depends on cellular immunity [32]. Innate antiviral signaling is initiated upon the recognition of pathogen-associated molecular patterns (PAMPs) by specific pattern recognition receptor (PRR) molecules expressed on host cells [33,34]. This ultimately leads to the activation of transcriptional factors, primarily interferon regulatory factor (IRF) and nuclear factor-kappaB (NF-κB), for the induction of type Ⅰ IFN and other proinflammatory mediators [33,34]. RIG-Ⅰ like receptors (RLR) and Toll-like receptors (TLR) are two types of PRR that can recognize viral PAMP [34]. SARS-CoV encoded structural proteins (such as N protein and M protein), nonstructural proteins (nsp), and accessory proteins (like ORF3b and ORF6) to antagonize the recognition and signal transduction [31]. N protein inhibits INF-β synthesis by inhibiting the activation of IRF-3 and NF-kB [35]; M protein prevents gene transcription of INF-β by inhibiting the activation of IRF-3/IRF-7 transcription factors [36]; other proteins such as nsp1, nsp3, ORF3b and ORF6 et al. can also antagonize IFN responses [[37], [38], [39], [40], [41], [42], [43]]. Recently, a team from the University of Chicago identified that the protein nsp15 from SARS-CoV-2 is 89 % identical to the nsp15 found in SARS-CoV [44]. Since nsp15 acts as an endoribonuclease for the virus’s double-stranded RNA, inhibition of nsp15 could slow viral replication [45]. Most recently, Kim et al. reported SARS-CoV-2 can also express ORF7a, 3a, 8, 6, and 7b [46], indicating that targeting these proteins may help to recover the inhibition of SARS-CoV-2 infection at an early stage.
In addition to lymphopenia, SARS-CoV-2 may also induce T cell exhaustion [47]. Expression of immune-checkpoint molecules such as programmed cell death 1 (PD-1) and T cell immunoglobulin and mucin domain-3 (TIM-3), accompanied by the elevation of anti-inflammatory cytokines, is known to be the common indicators of T cell depletion [48]. Wang’s team has observed that up-regulated PD-1 and TIM-3 between COVID-19 patients and healthy individuals, and between prodromal stages and overtly symptomatic stages among COVID-19 patients; which was also associated with the increased levels of anti-inflammatory IL-10 [47]. The expression of inhibitory immune receptors T-cell immunoglobulin and ITIM domain (TIGIT) and the CD94/NK group 2 member A (NKG2A) on CD8 + T cells were increased significantly in COVID-19 patients [49,50]. However, our current knowledge about T cell (mainly CD8 + T cell) depletion gained from the research of tumors and chronic viral infection, such as HIV and HBV. It is generally believed that this phenomenon occurs when the body has been stimulated by antigens for a “long” period of time, which is not in line with acute viral infection [48,51]. Therefore, it is not clear that T cell exhaustion is actually accruing in SARS-CoV-2 infection.
Since SARS-CoV-2 can escape from the host immunity by inhibiting the production of type I IFN during the early stage of infection, an artificial supplement of IFN-α/β during the early stage of COVID-19 infection may help to relieve injuries caused by the virus. However, we do not recommend applying glucocorticoids (GSs) in the early stage, as early use of immunosuppressive medications may exacerbate the virus's damage to the body.
3. Cytokine storm
Although the direct damage from the viruses contributes to the initiation of the disease, the cytokine storm caused by HCoV plays a vital role in the development of ALI and ARD [7]. Cytokines are a group of small molecular proteins secreted by immune and tissue cells that includes interleukin (IL), colony-stimulating factor (GSF), IFN, tumor necrosis factor (TNF), growth factor (GF) and chemokines. Cytokines participate in immune responses through the activation of multiple signaling pathways, such as JAK-STAT, TRAF-NF-κB, TRAF-AP-1, and IRAK-NF-κB [32]. The term “cytokine storm” appeared for the first time in an article relating to graft-versus-host disease in 1993 [52] as an interpretation for mechanisms of immune diseases or chronic inflammation. Gradually, scientists found that viruses, including cytomegalovirus, streptococci, influenza virus, and SARS-CoV, can also evoke cytokine storms [[53], [54], [55], [56]]. During viral infection, PRRs activate IRF and NF-κB pathways by recognizing PAMP, then promote the release of pro-inflammatory cytokines and chemokines from infected local epithelial cells or macrophages. These cytokine-activated macrophages and virus-infected dendritic cells (DC) lead to a broader immune response, attracting more inflammatory cells to inflammatory sites, releasing a large number of cytokines, which eventually, results in a cytokine storm. Excessive cytokines may spill into the circulatory system and cause systemic cytokine storms, resulting in multiple organ dysfunction [7,57].
Previously, it has been found that the serum levels of pro-inflammatory cytokines [IFN- γ, IL-1, IL-6, IL-12, and transforming growth factor-β (TGF-β)], and chemokines (CCL2, CXCL9, CXCL10, and IL-8) in SARS-CoV infected patients were higher than those in healthy individuals. While the level of anti-inflammatory cytokine IL-10 in severe patients was significantly lower than that in healthy controls [[58], [59], [60]]. At the beginning of the epidemic, Huang et al. reported that the plasma levels of IL2, IL7, GSCF, CXCL10, MCP1, MIP1A, and TNF-α were higher in COVID-19 patients in the intensive care unit (ICU) than those of COVID-19 patients outside the ICU [61]. Furthermore, the relatively higher level of serum ferritin was also seen in deceased patients comparing with discharged patients, and in severe patients comparing with mild patients [62,63]. Thus, the pattern of cytokine storm in COVID-19 patients was thought to be similar to secondary hemophagocytic lymphohistiocytosis (sHLH) [[64], [65], [66]]. Unlike the cytokines profile of SARS patients, COVID-19 patients have a remarkable increase of anti-inflammatory cytokines IL-10 (Table 1 ), which has been often seen in HLH patients in previous studies [61,67,68]. Compared with other increased cytokines, increased IL-10 was frequently associated with IL-6 in patients with severe disease(Table 1) [13,69,47,61], suggesting both IL-10 and IL-6 are the indicators of COVID-19 exacerbation.
Table 1.
Cytokines of COVID-19 patients that statistically elevated in different clinical studies.
| Studies and area | Number of patients | Type of patients* | Cytokines that statistically increased in all patients | Cytokines that statistically associated with disease severity |
|---|---|---|---|---|
| Zhang et al, Wuhan [10] | 82 | Dead | IL-6 | Made no comparison |
| Wang et al, Guangzhou [70] | 11 | Severe | IL-6 | Made no comparison |
| Huang et al, Wuhan [61] | 41 | Mixed | IL-1β, IL-1RA, IL-7, IL-8, IL-9, IL-10, FGF-2, G-CSF, GM-CSF, IFN-γ, CCL2, CXCL10, MIP1A, MIP1B, PDGF, TNF-α, VEGF | IL-2, IL-7, IL-10, G-SCF, CXCL10, CCL2, MIP1A, TNF-α |
| Chen et al, Wuhan [71] | 29 | Mixed | IL-2R, IL-6 | IL-2R, IL-6 |
| Liu et al, Wuhan [69] | 40 | Mixed | IL-2, IL-4, IL-6, IL-10, IFN-γ, TNF-α | IL-2, IL-6, IL-10, IFN-γ |
| Chen et al, Wuhan [14] | 21 | Mixed | IL-6 | IL-2R, IL-6, IL-10, TNF-α |
| Diao et al, Wuhan [47] | 522 | Mixed | IL-6, IL-10, TNF-α | IL-6, IL-10, TNF-α |
| Wen et al, Beijing [72] | 46 | Mixed | NA | IL-6 |
| Wan et al, Chongqing [13] | 123 | Mixed | NA | IL-6 |
| Wang et al, Wuhan [73] | 69 | Mixed | IL-6, IL-10 | IL-6, IL-10 |
| Qin et al, Wuhan [63] | 452 | Mixed | NA | TNF-α, IL-2R, IL-6, IL-8, IL-10 |
The Patients that included in these studies are in different conditions. Mixed: the study included both mild and severe cases. Severe: the study only included severe cases. Dead: the study only included dead patients.
IL-6, a kind of pleiotropic cytokine, is expressed by immune cells such as DC, monocytes, macrophages, B cells, and subsets of activated T cells, as well as by non-immune cells like fibroblasts, epithelial cells, and keratinocytes [[74], [75], [76], [77]]. It can activate JAK/STAT, Ras/ERK/C/EBP, and PⅠ3 K/Akt pathways through IL-6R and signal-transducing coreceptor gp130 [78,79]. IL-6 also regulates B cell proliferation and differentiation and induces T cell differentiation [[80], [81], [82]]. Although IL-6 has been proposed to be involved in the repair of lung injury in the mouse model of influenza A H1N1 virus [83], it contributes to the pathogenesis of inflammatory or autoimmunity diseases [84,85], and excessive production of IL-6 leads to serious disease progression in viral infection [86,87].
IL-10 is also a pleiotropic cytokine that limits and terminates inflammatory responses through inhibiting antigen-presenting cells (APC), pro-inflammatory cytokines production, and T cell response [88]. Various subpopulations of CD4+ helper T (Th) cells, including Th1, Th2, and Th17, produce IL-10, as an important self-regulatory function of Th cells during infections [[89], [90], [91], [92]]. Although reducing IL-10 levels could improve the resistance to infection in some animal models, the weakening control of inflammatory responses may cause significant damage to the hosts at the same time [93]. Thus, IL-10 plays a central role in maintaining a balance between damage and protection.
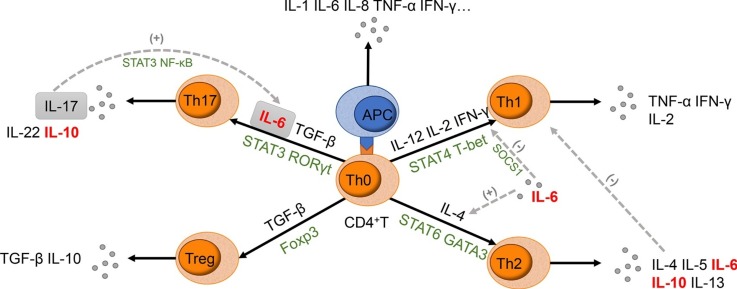
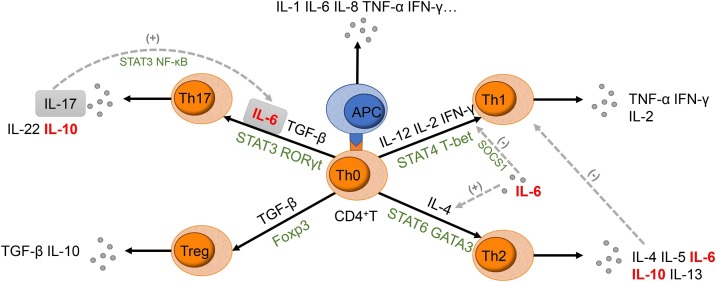
IL-6 induces näive T cells to differentiate into Th2 cells and Th17 cells, and the activated Th2 and Th17 cells produce IL-10 that regulates the immune response [[94], [95], [96], [97], [98]]. (Fig. 1 ). The similarity in the signaling network between IL-10 receptors and IL-6R suggests the common biological process among IL-10 and IL-6 [99]. It has been reported that IL-6 cooperated with IL-10 to eliminate pathogens and modulate the cellular immune response in the patient infected with the H1N1 influenza virus, but the produced immunosuppressive environment delayed virus clearance or increase secondary infection [86,100]. Pathological findings of a deceased COVID-19 patient showed elevated IL-6 and IL-10 with increased highly proinflammatory CCR4+CCR6+Th17 in peripheral CD4+T cells [101]. Rapid accumulation of proinflammatory cytokines stimulates the over-reaction of anti-inflammatory response, resulting in severe immune injury in patients. Chinese Diagnosis and Treatment Protocol for Novel Coronavirus Pneumonia (Trial Version 7) suggests the increase of IL-6 as an indicator for critical patients [16]. We recommend measuring both IL-10 and IL-6 as the signs of the cytokine storm intensification in COVID-19 patients.
Fig. 1.
Schematic diagram of the role IL-6 played in CD4+T cell differentiation.
IL-6 incites näive CD4+T cells to differentiate into both Th2 cells, instead of Th1 cells, and Th17 cells, instead of Treg. Together with TGF-β, IL-6 leads to the activation of STAT3; thus relieving the repression of RORγt and promoting the Th17 cells transcriptional program. It has also been established that IL-17 produced by activated Th17 cells are able to trigger a positive-feedback loop of IL-6 expression through NF-κB and STAT3 signaling. Additionally, IL-6 prevents T cells from differentiating into Th1 cells through the inhibition of TNF-γ signaling by the upregulation of suppressor of cytokine signaling (SOCS1). IL-6 can also induce the production of endogenous IL-4, consequently driving the T cells to differentiate into Th2 cells. This, in turn, inhibits the differentiation and function of Th1 cells due to the cytokines produced from Th2 cells.
Great attention has been paid to the cytokine storm in COVID-19 infection to address the immediate need for reducing the rising mortality. Anti-inflammatory medications are commonly used for the management of cytokine storm, which includes GCs, intravenous immune globulin (IVIG), and inflammatory cytokines antagonists (such as IL-1R antagonists, IL-6R antagonists, and JAK inhibitors). However, GCs may slow down the clearance of the coronavirus [102]. High-dose IVIG at the early stage of clinical deterioration was reported to be successful in three cases [103], and an ongoing randomized controlled trial (RCT) of IVIG in severe COVID-19 patients (NCT04261426) may provide more authoritative evidence of this treatment.
Since IL-1β and IL-6 are the major cytokines involved in the pathogenesis of sHLH [65], the IL-6R antagonist (tocilizumab and sarilumab) and IL-1R antagonists (anakinra) may be effective choices. A retrospective study of continuous intravenous infusion of anakinra in five patients established a rapid serologic and subsequent clinical improvement in adult patients with sHLH [104] and a randomized, placebo-controlled phase III trial indicated that anakinra reduced mortality in sepsis patients with features of sHLH [105]. Tocilizumab, which attracts more attention compared with anakinra, was found to improve fever, hypoxemia, lung lesions, and CRP levels in most of the severe COVID-19 patients with no obvious adverse reactions in retrospective studies, but several patients still aggravated [106,107]. Serum level of IL-6 after tocilizumab therapy tended to further spike initially, and then decrease, while the patients who failed treatment exhibited a persistent and dramatic increase of IL-6 [107,108]. Nevertheless, we cannot draw any firm conclusions on the efficacy and safety of anakinra or tocilizumab due to the limited sample size and retrospective method of the present evidence. Further studies are required to determine the probability of potential secondary infection, and to find an appropriate biochemical indicator for monitoring the therapeutic effect. Clinical trials evaluating anakinra (NCT04341584, NCT04324021, NCT04339712, and NCT04330638) and tocilizumab (ChiCTR2000029765, ChiCTR2000030796, ChiCTR2000030894, NCT04317092, NCT04335071, and NCT04320615 et al) in the treatment of COVID-19 are ongoing across the world.
4. Elevation of procalcitonin (PCT)
Secondary bacterial infection has been found to further exacerbate viral pneumonia, which is also related to the imbalance of T cells and plasma cytokines [109]. Bacterial coinfection is known to be associated with approximately 40 % of viral respiratory tract infections requiring hospitalization [110]. Early detection of bacterial infection and timely intervention will help to alleviate the deterioration of COVID-19. Procalcitonin (PCT), released by bacterial infectious tissues under the irritation of pro-inflammatory cytokines, is a more specific marker of serious bacterial infection compared to C-reactive protein (CRP) and IL-6 [111] PCT-based strategy has been applied to guide antibiotic use in ICU or emergency wards, since the serum PCT levels in patients with severe bacterial infections are much higher than those with simple viral infections or non-specific inflammatory diseases [[111], [112], [113]]. Antimicrobial interventions are encouraged in patients with lower respiratory tract infection who has the value of PCT > 0.25 ng/mL or > 0.5 ng/mL, for they are likely or highly-likely respectively, to undergo a bacterial infection [113,114].
Multiple studies have shown that PCT levels increased in critical or deceased COVID-19 patients. (Table 2 ) Li et al. [115] reported that 90.5 % of deceased patients had elevated PCT levels (0.11−75 ng/mL), including PCT > 0.25 ng/mL in 12 patients and PCT > 0.5 ng/mL in 9 patients, and the PCT value increased from the admission to death. In another retrospective study of 1099 COVID-19 patients, higher proportions of PCT > 0.5 ng/mL were found in severe cases than mild cases (13.7 % vs 3.7 %) [8]. Patients who reached primary composite endpoint (including admission to an ICU, the use of mechanical ventilation, or death) had a higher proportion of PCT > 0.5 ng/mL than patients without end-point events (24.0 % vs 3.9 %) [8]. The correlation between increased PCT and severe COVID-19 was also observed in pediatric patients [116]. These findings, although preliminary, suggested that bacterial infection may promote the aggravation of COVID-19. Timely measuring PCT as a sign of secondary bacterial infection for the decision of antibacterial agents use is critical for COVID-19 patients. The aggravation of COVID-19 ascribed to the secondary bacterial infection and the adverse events caused by unreasonable use of antibiotics could be reduced. The most effective selection of the antibiotic agents should be based on bacterial culture. In consideration of the dysregulation of the immune system in severe COVID-19 patients, antibiotic agents with immunomodulatory effects, such as macrolides (erythromycin and clarithromycin) might be a better option. Previous studies have shown that in addition to antibacterial effects, these drugs can also alleviate inflammatory reactions by reducing the accumulation of pro-inflammatory cytokines and regulating neutrophil function and apoptosis [[117], [118], [119], [120]].
Table 2.
PCT value of severe or deceased COVID-19 patients in different clinical studies.
| Studies | Patients’ categories | Number of patientsa | PCT value ng/mL (n, %) |
P value | ||||
|---|---|---|---|---|---|---|---|---|
| Rangeb | >0.05 | >0.1 | >0.25 | >0.50 | ||||
| Li et al [115] | Dead | 21 | 0.36 (0.13, 1.91) | NA | 19, 90.5 | 12, 57 | 9, 42 | – |
| Zhang et al [10] | Dead | 69 | 0.3 (0.1−1.1) | NA | 56, 81.2 | NA | NA | – |
| Chen et al [121] | Dead | 96 | 0.33 (0.14−0.65) | NA | NA | NA | 27, 28.1 | NA |
| Recovered | 140 | 0.05 (0.03−0.08) | NA | NA | NA | 3, 2.1 | ||
| Guan et al [8] | Severe | 173 | NA | NA | NA | NA | 12, 24.0 | NA |
| Non-severe | 926 | NA | NA | NA | NA | 19, 3.7 | ||
| Xiong et al [122] | Severe | 21 | 0.33 ± 0.27 | NA | NA | NA | NA | <.05d |
| Mild | 18 | 0.13 ± 0.11 | NA | NA | NA | NA | ||
| Yuan et al [123] | Severe | 31 | 0.05 (0.01−2.1) | NA | NA | NA | NA | .000 |
| Non-severe | 192 | 0.01 (0.01−0.4) | NA | NA | NA | NA | ||
| Zhang et al [124] | Severe | 50 | 0.1 (0.06−0.3) | NA | 25, 50.0 | NA | NA | <.001d |
| Non-severe | 68 | 0.05 (0.03−0.1) | NA | 16, 23.5 | NA | NA | ||
| Huang et al [61] | ICU | 12 | 0.1 (0.1−0.4) | NA | 6, 50.0 | 3, 25.0 | 3, 25.0 | .031d |
| Non-ICU | 27 | 0.1 (0.1−0.1) | NA | 6, 22.2 | 2, 7.4 | 0 | ||
| Wang et al [125] | ICU | 36 | NA | 27, 75.0 | NA | NA | NA | <.001e |
| Non-ICU | 102 | NA | 22, 21.6 | NA | NA | NA | ||
| Wan et al [126] | Severe | 40 | 0.11 (0.08−0.16) | NA | 19, 47.5 | 4, 10.0 | 1, 2.5 | <.0001d |
| Mild | 95 | 0.04 (0.03−0.06) | NA | 6, 6 | 0 | 0 | ||
| Peng et al [127] | Severe | 16 | 0.20 (0.15, 0.48) | NA | NA | NA | NA | <.001d |
| General | 96 | 0.11 (0.06, 0.20) | NA | NA | NA | NA | ||
| Qin et al [63] | Severe | 286 | 0.1 (0.0−0.2) | NA | NA | NA | NA | <.001d |
| Non-severe | 166 | 0.05 (0.03−0.09) | NA | NA | NA | NA | ||
| Li et al [128] | Critically severe | 16 | 0.44 ± 0.512 | NA | NA | NA | NA | .008d |
| Severe | 56 | 0.14 ± 0.353 | NA | NA | NA | NA | ||
| Moderate | 60 | 0.08 ± 0.279 | NA | NA | NA | NA | ||
| Wang et al [73] | SpO2 < 90 % | 14 | 0.13 (0.13−0.15) | NA | NA | NA | 0, 0 | .78d |
| SpO2 ≥ 90 % | 55 | 0.13 (0.13−0.15) | NA | NA | NA | 4, 8 | ||
| Shi et al [129] | With cardiac injury | 82 | 0.27 (0.10−1.22) | NA | NA | NA | NA | <.001d |
| Without | 334 | 0.06 (0.03−0.10) | NA | NA | NA | NA | ||
| Wu et al [130] | With ocular symptoms | 12 | NA | 8, 66.7 | NA | NA | NA | .03e |
| Without | 25 | NA | 7, 28.0 | NA | NA | NA | ||
| Qiu et al [116] | Moderate pediatric | 19 | 0.32 ± 0.19 | 5, 26.3 | NA | NA | NA | .0039d |
| Mild pediatric | 17 | 0.15 ± 0.13 | 1, 5.9 | NA | NA | NA | ||
| Sun et al [131] | Pediatric | 8 | 0.085 (0.05, 0.128) | 5, 62.5 | 3, 37.5 | 1, 12.5 | 1, 12.5 | – |
| Xia et al [132] | Pediatric | 20 | NA | 16, 80 | NA | NA | NA | – |
aNumber of patients with the record of PCT. b PCT values in different studies were expressed as different forms, including mean ± SD, median (IQR), and median (mix-max). c PCT value≥0.05. d P-value of the comparison between the PCT value of two groups. e P-value of the comparison between the proportion of the patients with increased PCT value in two groups. The tests with a P-value of <0.05 are considered statistically significant.
5. Conclusions
The ongoing COVID-19 pandemic is threatening the world with substantial mortality. SARS-CoV-2 could cause lymphopenia and cytokine storm, together with secondary bacterial infections, adding to the deterioration of the disease in severe cases of COVID-19. Understandings of the dynamic process of SARS-CoV-2 infection would help to make an appropriate decision on treatment strategies. We recommend measuring both IL-10 and IL-6 as a sign of intensification of cytokine storm in severe COVID-19 cases, though test IL-10 is not routinely carried out in the clinic setting. We also recommend using serum PCT level to guild the initiation of antibacterial agents in patient with COVID-19. Although the selection of immunomodulators is challenging, previous retrospective studies have revealed distinct immune regulators between the early and late stages of SARS-CoV-2 infection. For instance, supplementation of type Ⅰ IFN, instead of GCs, at the early stage of infection may help to control the virus and relieve injury. IL-1R and IL-6R antagonists could potentially be the effective choices for managing cytokine storm. However, these preliminary results would need to be validated from multiple ongoing RCTs. Due to the rapid nature of COVID-19 pandemic, global collaboration in research and information sharing is ultimately needed.
Declaration of Competing Interest
None.
References
- 1.Gorbalenya A.E., Baker S.C., Baric R.S., de Groot R.J., Drosten C., Gulyaeva A.A. The species Severe acute respiratory syndrome-related coronavirus: classifying 2019-nCoV and naming it SARS-CoV-2. Nat. Microbiol. 2020;5(4):536–544. doi: 10.1038/s41564-020-0695-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zhou P., Yang X.L., Wang X.G., Hu B., Zhang L., Zhang W. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature. 2020;579:270–273. doi: 10.1038/s41586-020-2012-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.World Health Organization (WHO); 2020. Coronavirus Disease 2019 (COVID-19) Situation Report – 112.https://www.who.int/docs/default-source/coronaviruse/situation-reports/20200511-covid-19-sitrep-112.pdf?sfvrsn=813f2669_2 Accessed 2020-05-12. [Google Scholar]
- 4.World Health Organization; 2003. Summary of Probable SARS Cases with Onset of Illness from 1 November 2002 to 31 July 2003.https://www.who.int/csr/sars/country/table2004_04_21/en/ Accessed 2003-12-31. [Google Scholar]
- 5.Eastern Mediterranean Regional Office (EMRO); Cairo, Egypt: 2020. MERS Situation Update, January 2020 World Health Organization (WHO)http://www.emro.who.int/pandemic-epidemic-diseases/mers-cov/mers-situation-update-january-2020.html Accessed 2020-1. [Google Scholar]
- 6.Jia H.P., Look D.C., Shi L., Hickey M., Pewe L., Netland J. ACE2 receptor expression and severe acute respiratory syndrome coronavirus infection depend on differentiation of human airway epithelia. J. Virol. 2005;79(23):14614–14621. doi: 10.1128/jvi.79.23.14614-14621.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Channappanavar R., Perlman S. Pathogenic human coronavirus infections: causes and consequences of cytokine storm and immunopathology. Semin. Immunopathol. 2017;39(5):529–539. doi: 10.1007/s00281-017-0629-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.W-j Guan, Z-y Ni, Hu Y., W-h Liang, C-q Ou, J-x He. Clinical Characteristics of Coronavirus Disease 2019 in China. N. Engl. J. Med. 2020;382:1708–1720. doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen N., Zhou M., Dong X., Qu J., Gong F., Han Y. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet (London, England). 2020;395(10223):507–513. doi: 10.1016/s0140-6736(20)30211-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhang B., Zhou X., Qiu Y., Feng F., Feng J., Jia Y. Clinical characteristics of 82 death cases with COVID-19. medRxiv. 2020 doi: 10.1101/2020.02.26.20028191. 2020.02.26.20028191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Qian K., Deng Y., Tai Y., Peng J., Peng H., Jiang L. Clinical characteristics of 2019 novel infected coronavirus pneumonia:A systemic review and meta-analysis. medRxiv. 2020 doi: 10.1101/2020.02.14.20021535. 2020.02.14.20021535. [DOI] [Google Scholar]
- 12.Lei L., Jian-ya G., Wan-mei H., Xian-xiang Z., Guo L. Clinical characteristics of 51 patients discharged from hospital with COVID-19 in Chongqing. China. medRxiv. 2020 doi: 10.1101/2020.02.20.20025536. 2020.02.20.20025536. [DOI] [Google Scholar]
- 13.Wan S., Yi Q., Fan S., Lv J., Zhang X., Guo L. Characteristics of lymphocyte subsets and cytokines in peripheral blood of 123 hospitalized patients with 2019 novel coronavirus pneumonia (NCP) medRxiv. 2020 doi: 10.1101/2020.02.10.20021832. 2020.02.10.20021832. [DOI] [Google Scholar]
- 14.Chen G., Wu D., Guo W., Cao Y., Huang D., Wang H. Clinical and immunologic features in severe and moderate Coronavirus Disease 2019. J. Clin. Invest. 2020;130(5):2620–2629. doi: 10.1172/jci137244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yaqian M., Wei L., Junping W., Gang C. Epidemiological and clinical characteristics of SARS-CoV-2 and SARS-CoV: a system review. medRxiv. 2020 doi: 10.1101/2020.02.20.20025601. 2020.02.20.20025601. [DOI] [Google Scholar]
- 16.National health commission & national administration of traditional chinese medicine of P.R.CHina. Diagnosis and treatment protocol for novel coronavirus pneumonia (Trial version 7) Chin. Med. J. 2020;133 doi: 10.3760/cma.j.issn.0366-6999.2020.0027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ding Y., Wang H., Shen H., Li Z., Geng J., Han H. The clinical pathology of severe acute respiratory syndrome (SARS): a report from China. J. Pathol. 2003;200(3):282–289. doi: 10.1002/path.1440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liu J.-M.S.Y., Yan L.-M.-M., Wan L., Xiang T.-X.-X., Le A., Liu J.-M.-M. Viral dynamics in mild and severe cases of COVID-19. Lancet Infect. Dis. 2020;20(6):656–657. doi: 10.1016/S1473-3099(20)30232-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yang Jr, Deng Dt, Wu N., Yang B., Li H.J., Pan X.B. Persistent viral RNA positivity during recovery period of a patient with SARS-CoV-2 infection. J. Med. Virol. 2020 doi: 10.1002/jmv.25940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gu J., Gong E., Zhang B., Zheng J., Gao Z., Zhong Y. Multiple organ infection and the pathogenesis of SARS. J. Exp. Med. 2005;202(3):415–424. doi: 10.1084/jem.20050828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cervantes-Barragan L., Zust R., Weber F., Spiegel M., Lang K.S., Akira S. Control of coronavirus infection through plasmacytoid dendritic-cell-derived type I interferon. Blood. 2007;109(3):1131–1137. doi: 10.1182/blood-2006-05-023770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Le Bon A., Tough D.F. Links between innate and adaptive immunity via type I interferon. Curr. Opin. Immunol. 2002;14(4):432–436. doi: 10.1016/s0952-7915(02)00354-0. [DOI] [PubMed] [Google Scholar]
- 23.Haagmans B.L., Kuiken T., Martina B.E., Fouchier R.A., Rimmelzwaan G.F., van Amerongen G. Pegylated interferon-alpha protects type 1 pneumocytes against SARS coronavirus infection in macaques. Nat. Med. 2004;10(3):290–293. doi: 10.1038/nm1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Smits S.L., de Lang A., van den Brand J.M., Leijten L.M., van I.W.F., Eijkemans M.J. Exacerbated innate host response to SARS-CoV in aged non-human primates. PLoS Pathog. 2010;6(2) doi: 10.1371/journal.ppat.1000756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dahl H., Linde A., Strannegard O. In vitro inhibition of SARS virus replication by human interferons. Scand. J. Infect. Dis. 2004;36(11–12):829–831. doi: 10.1080/00365540410021144. [DOI] [PubMed] [Google Scholar]
- 26.Barnard D.L., Day C.W., Bailey K., Heiner M., Montgomery R., Lauridsen L. Evaluation of immunomodulators, interferons and known in vitro SARS-coV inhibitors for inhibition of SARS-coV replication in BALB/c mice. Antivir. Chem. Chemother. 2006;17(5):275–284. doi: 10.1177/095632020601700505. [DOI] [PubMed] [Google Scholar]
- 27.Channappanavar R., Fehr A.R., Vijay R., Mack M., Zhao J., Meyerholz D.K. Dysregulated Type I Interferon and Inflammatory Monocyte-Macrophage Responses Cause Lethal Pneumonia in SARS-CoV-Infected Mice. Cell Host Microbe. 2016;19(2):181–193. doi: 10.1016/j.chom.2016.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yoshikawa T., Hill T.E., Yoshikawa N., Popov V.L., Galindo C.L., Garner H.R. Dynamic innate immune responses of human bronchial epithelial cells to severe acute respiratory syndrome-associated coronavirus infection. PLoS One. 2010;5(1):e8729. doi: 10.1371/journal.pone.0008729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.To K.K., Tsang O.T., Leung W.S., Tam A.R., Wu T.C., Lung D.C. Temporal profiles of viral load in posterior oropharyngeal saliva samples and serum antibody responses during infection by SARS-CoV-2: an observational cohort study. Lancet Infect. Dis. 2020;20(5):565–574. doi: 10.1016/s1473-3099(20)30196-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Versteeg G.A., Bredenbeek P.J., van den Worm S.H., Spaan W.J. Group 2 coronaviruses prevent immediate early interferon induction by protection of viral RNA from host cell recognition. Virology. 2007;361(1):18–26. doi: 10.1016/j.virol.2007.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Totura A.L., Baric R.S. SARS coronavirus pathogenesis: host innate immune responses and viral antagonism of interferon. Curr. Opin. Virol. 2012;2(3):264–275. doi: 10.1016/j.coviro.2012.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cao X., Yao Z., Xiong S., Si C., YU Y. Medical Immunology. 7th edition. People’s Medical Publishing House Co., LTD; Beijing: 2018. [Google Scholar]
- 33.Liu S., Yan R., Chen B., Pan Q., Chen Y., Hong J. Influenza virus-induced robust expression of SOCS3 contributes to excessive production of IL-6. Front. Immunol. 2019;10:1843. doi: 10.3389/fimmu.2019.01843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhu G., Xu Y., Cen X., Nandakumar K.S., Liu S., Cheng K. Targeting pattern-recognition receptors to discover new small molecule immune modulators. Eur. J. Med. Chem. 2018;144:82–92. doi: 10.1016/j.ejmech.2017.12.026. [DOI] [PubMed] [Google Scholar]
- 35.Kopecky-Bromberg S.A., Martínez-Sobrido L., Frieman M., Baric R.A., Palese P. Severe acute respiratory syndrome coronavirus open reading frame (ORF) 3b, ORF 6, and nucleocapsid proteins function as interferon antagonists. J. Virol. 2007;81(2):548–557. doi: 10.1128/JVI.01782-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Siu K.L., Kok K.H., Ng M.H., Poon V.K., Yuen K.Y., Zheng B.J. Severe acute respiratory syndrome coronavirus M protein inhibits type I interferon production by impeding the formation of TRAF3.TANK.TBK1/IKKepsilon complex. J. Biol. Chem. 2009;284(24):16202–16209. doi: 10.1074/jbc.M109.008227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Frieman M., Ratia K., Johnston R.E., Mesecar A.D., Baric R.S. Severe acute respiratory syndrome coronavirus papain-like protease ubiquitin-like domain and catalytic domain regulate antagonism of IRF3 and NF-kappaB signaling. J. Virol. 2009;83(13):6689–6705. doi: 10.1128/jvi.02220-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chen X., Yang X., Zheng Y., Yang Y., Xing Y., Chen Z. SARS coronavirus papain-like protease inhibits the type I interferon signaling pathway through interaction with the STING-TRAF3-TBK1 complex. Protein Cell. 2014;5(5):369–381. doi: 10.1007/s13238-014-0026-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Huang C., Lokugamage K.G., Rozovics J.M., Narayanan K., Semler B.L., Makino S. SARS coronavirus nsp1 protein induces template-dependent endonucleolytic cleavage of mRNAs: viral mRNAs are resistant to nsp1-induced RNA cleavage. PLoS Pathog. 2011;7(12) doi: 10.1371/journal.ppat.1002433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wathelet M.G., Orr M., Frieman M.B., Baric R.S. Severe acute respiratory syndrome coronavirus evades antiviral signaling: role of nsp1 and rational design of an attenuated strain. J. Virol. 2007;81(21):11620–11633. doi: 10.1128/jvi.00702-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Frieman M., Yount B., Heise M., Kopecky-Bromberg S.A., Palese P., Baric R.S. Severe acute respiratory syndrome coronavirus ORF6 antagonizes STAT1 function by sequestering nuclear import factors on the rough endoplasmic reticulum/Golgi membrane. J. Virol. 2007;81(18):9812–9824. doi: 10.1128/jvi.01012-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Freundt E.C., Yu L., Park E., Lenardo M.J., Xu X.N. Molecular determinants for subcellular localization of the severe acute respiratory syndrome coronavirus open reading frame 3b protein. J. Virol. 2009;83(13):6631–6640. doi: 10.1128/jvi.00367-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Menachery V.D., Yount B.L., Jr., Josset L., Gralinski L.E., Scobey T., Agnihothram S. Attenuation and restoration of severe acute respiratory syndrome coronavirus mutant lacking 2’-o-methyltransferase activity. J. Virol. 2014;88(8):4251–4264. doi: 10.1128/jvi.03571-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Paul M. 2020. Drugs Previously in Development for SARS Could Be Effective for COVID-19. news.northwestern.edu, news.northwestern.eDu.https://news.northwestern.edu/stories/2020/03/new-coronavirus-protein-reveals-drug-target/ [Google Scholar]
- 45.Deng X., Hackbart M., Mettelman R.C., O’Brien A., Mielech A.M., Yi G. Coronavirus nonstructural protein 15 mediates evasion of dsRNA sensors and limits apoptosis in macrophages. Proc. Natl. Acad. Sci. U.S.A. 2017;114(21):E4251–e60. doi: 10.1073/pnas.1618310114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kim D., Lee J.-Y.-Y., Yang J.-S.-S., Kim J.W., Kim V.N., Chang H. The architecture of SARS-CoV-2 transcriptome. Cell. 2020;181(4):914–921. doi: 10.1016/j.cell.2020.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Diao B., Wang C., Tan Y., Chen X., Liu Y., Ning L. Reduction and Functional Exhaustion of T Cells in Patients with Coronavirus Disease 2019 (COVID-19) Front. Immunol. 2020;11(827):1–7. doi: 10.3389/fimmu.2020.00827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hashimoto M., Kamphorst A.O., Im S.J., Kissick H.T., Pillai R.N., Ramalingam S.S. CD8 t cell exhaustion in chronic infection and Cancer: opportunities for interventions. Annu. Rev. Med. 2018;69:301–318. doi: 10.1146/annurev-med-012017-043208. [DOI] [PubMed] [Google Scholar]
- 49.Zheng M., Gao Y., Wang G., Song G., Liu S., Sun D. Functional exhaustion of antiviral lymphocytes in COVID-19 patients. Cell. Mol. Immunol. 2020;17(5):533–535. doi: 10.1038/s41423-020-0402-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zheng H.Y., Zhang M., Yang C.X., Zhang N., Wang X.C., Yang X.P. Elevated exhaustion levels and reduced functional diversity of T cells in peripheral blood may predict severe progression in COVID-19 patients. Cell. Mol. Immunol. 2020;17(5):541–543. doi: 10.1038/s41423-020-0401-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lu F., Nong G. The role of programmed death factor 1 in viral infectious diseases. Chin J Contemp Pediatr. 2018;20(1):77–82. doi: 10.7499/j.issn.1008-8830.2018.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ferrara J.L., Abhyankar S., Gilliland D.G. Cytokine storm of graft-versus-host disease: a critical effector role for interleukin-1. Transplant. Proc. 1993;25(1 Pt 2):1216–1217. [PubMed] [Google Scholar]
- 53.Barry S.M., Johnson M.A., Janossy G. Cytopathology or immunopathology? The puzzle of cytomegalovirus pneumonitis revisited. Bone Marrow Transplant. 2000;26(6):591–597. doi: 10.1038/sj.bmt.1702562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Huang K.J., Su I.J., Theron M., Wu Y.C., Lai S.K., Liu C.C. An interferon-gamma-related cytokine storm in SARS patients. J. Med. Virol. 2005;75(2):185–194. doi: 10.1002/jmv.20255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Bisno A.L., Brito M.O., Collins C.M. Molecular basis of group A streptococcal virulence. Lancet Infect. Dis. 2003;3(4):191–200. doi: 10.1016/s1473-3099(03)00576-0. [DOI] [PubMed] [Google Scholar]
- 56.Short K.R., Kroeze E., Fouchier R.A.M., Kuiken T. Pathogenesis of influenza-induced acute respiratory distress syndrome. Lancet Infect. Dis. 2014;14(1):57–69. doi: 10.1016/s1473-3099(13)70286-x. [DOI] [PubMed] [Google Scholar]
- 57.Liu Q., Zhou Y.H., Yang Z.Q. The cytokine storm of severe influenza and development of immunomodulatory therapy. Cell. Mol. Immunol. 2016;13(1):3–10. doi: 10.1038/cmi.2015.74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Chien J.Y., Hsueh P.R., Cheng W.C., Yu C.J., Yang P.C. Temporal changes in cytokine/chemokine profiles and pulmonary involvement in severe acute respiratory syndrome. Respirology. 2006;11(6):715–722. doi: 10.1111/j.1440-1843.2006.00942.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Zhang Y., Li J., Zhan Y., Wu L., Yu X., Zhang W. Analysis of serum cytokines in patients with severe acute respiratory syndrome. Infect. Immun. 2004;72(8):4410–4415. doi: 10.1128/iai.72.8.4410-4415.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wong C.K., Lam C.W., Wu A.K., Ip W.K., Lee N.L., Chan I.H. Plasma inflammatory cytokines and chemokines in severe acute respiratory syndrome. Clin. Exp. Immunol. 2004;136(1):95–103. doi: 10.1111/j.1365-2249.2004.02415.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Huang C., Wang Y., Li X., Ren L., Zhao J., Hu Y. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet (London, England). 2020;395(10223):497–506. doi: 10.1016/s0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ruan Q., Yang K., Wang W., Jiang L., Song J. Clinical predictors of mortality due to COVID-19 based on an analysis of data of 150 patients from Wuhan, China. Intensive Care Med. 2020:1–3. doi: 10.1007/s00134-020-05991-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Qin C., Zhou L., Hu Z., Zhang S., Yang S., Tao Y. an official publication of the Infectious Diseases Society of America; 2020. Dysregulation of Immune Response in Patients with COVID-19 in Wuhan, China. Clinical Infectious Diseases. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Mehta P., McAuley D.F., Brown M., Sanchez E., Tattersall R.S., Manson J.J. COVID-19: consider cytokine storm syndromes and immunosuppression. Lancet (London, England) 2020;395(10229):1033–1034. doi: 10.1016/s0140-6736(20)30628-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Karakike E., Giamarellos-Bourboulis E.J. Macrophage activation-like syndrome: a distinct entity leading to early death in Sepsis. Front. Immunol. 2019;10:55. doi: 10.3389/fimmu.2019.00055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Crayne C.B., Albeituni S., Nichols K.E., Cron R.Q. The immunology of macrophage activation syndrome. Front. Immunol. 2019;10:119. doi: 10.3389/fimmu.2019.00119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Yang S.L., Xu X.J., Tang Y.M., Song H., Xu W.Q., Zhao F.Y. Associations between inflammatory cytokines and organ damage in pediatric patients with hemophagocytic lymphohistiocytosis. Cytokine. 2016;85:14–17. doi: 10.1016/j.cyto.2016.05.022. [DOI] [PubMed] [Google Scholar]
- 68.Osugi Y., Hara J., Tagawa S., Takai K., Hosoi G., Matsuda Y. Cytokine production regulating Th1 and Th2 cytokines in hemophagocytic lymphohistiocytosis. Blood. 1997;89(11):4100–4103. [PubMed] [Google Scholar]
- 69.Liu J., Li S., Liu J., Liang B., Wang X., Wang H. Longitudinal characteristics of lymphocyte responses and cytokine profiles in the peripheral blood of SARS-CoV-2 infected patients. EBioMedicine. 2020;55 doi: 10.1016/j.ebiom.2020.102763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Wang W., He J., Lie P., Huang L., Wu S., Lin Y. The definition and risks of Cytokine Release Syndrome-Like in 11 COVID-19-Infected Pneumonia critically ill patients: Disease Characteristics and Retrospective Analysis. medRxiv. 2020 doi: 10.1101/2020.02.26.20026989. 2020.02.26.20026989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Chen L., Liu H., Liu W., Liu J., Liu K., Shang J. Clinical characteristics of 29 cases of COVID-19. Chin J Tubere Respir Dis. 2020;(00):E005. [Google Scholar]
- 72.Wen K., Li W., Zhang D., Zhang A., Zhang B., Zhang T. Epidemiological and clinical characteristics of 46 patients with COVID-19 in Beijing. Chin J Infect. 2020;38(00):E011. doi: 10.3760/cma.j.cn311365-20200219-00086. [DOI] [Google Scholar]
- 73.Wang Z., Yang B., Li Q., Wen L., Zhang R. an official publication of the Infectious Diseases Society of America; 2020. Clinical Features of 69 Cases with Coronavirus Disease 2019 in Wuhan, China. Clinical Infectious Diseases. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Tanaka T., Narazaki M., Masuda K., Kishimoto T. Regulation of IL-6 in immunity and diseases. Adv. Exp. Med. Biol. 2016;941:79–88. doi: 10.1007/978-94-024-0921-5_4. [DOI] [PubMed] [Google Scholar]
- 75.Rose-John S. Interleukin-6 family cytokines. Cold Spring Harb. Perspect. Biol. 2018;10(2) doi: 10.1101/cshperspect.a028415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Matsukura S., Kokubu F., Noda H., Tokunaga H., Adachi M. Expression of IL-6, IL-8, and RANTES on human bronchial epithelial cells, NCI-H292, induced by influenza virus A. J. Allergy Clin. Immunol. 1996;98(6 Pt 1):1080–1087. doi: 10.1016/s0091-6749(96)80195-3. [DOI] [PubMed] [Google Scholar]
- 77.Stadnyk A.W. Cytokine production by epithelial cells. FASEB J. Off. Pub. Federation Am. Soc. Exp. Biology. 1994;8(13):1041–1047. doi: 10.1096/fasebj.8.13.7926369. [DOI] [PubMed] [Google Scholar]
- 78.Heinrich P.C., Behrmann I., Haan S., Hermanns H.M., Muller-Newen G., Schaper F. Principles of interleukin (IL)-6-type cytokine signalling and its regulation. Biochem. J. 2003;374(Pt 1):1–20. doi: 10.1042/bj20030407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Zhong Z., Wen Z., Darnell J.E., Jr. Stat3: a STAT family member activated by tyrosine phosphorylation in response to epidermal growth factor and interleukin-6. Science (New York, NY). 1994;264(5155):95–98. doi: 10.1126/science.8140422. [DOI] [PubMed] [Google Scholar]
- 80.Dienz O., Rud J.G., Eaton S.M., Lanthier P.A., Burg E., Drew A. Essential role of IL-6 in protection against H1N1 influenza virus by promoting neutrophil survival in the lung. Mucosal Immunol. 2012;5(3):258–266. doi: 10.1038/mi.2012.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Dienz O., Eaton S.M., Bond J.P., Neveu W., Moquin D., Noubade R. The induction of antibody production by IL-6 is indirectly mediated by IL-21 produced by CD4+ T cells. J. Exp. Med. 2009;206(1):69–78. doi: 10.1084/jem.20081571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Rose-John S., Winthrop K., Calabrese L. The role of IL-6 in host defence against infections: immunobiology and clinical implications. Nat. Rev. Rheumatol. 2017;13(7):399–409. doi: 10.1038/nrrheum.2017.83. [DOI] [PubMed] [Google Scholar]
- 83.Yang M.-L., Wang C.-T., Yang S.-J., Leu C.-H., Chen S.-H., Wu C.-L. IL-6 ameliorates acute lung injury in influenza virus infection. Sci. Rep. 2017;7:43829. doi: 10.1038/srep43829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Tisoncik J.R., Korth M.J., Simmons C.P., Farrar J., Martin T.R., Katze M.G. Into the eye of the cytokine storm. Microbiol. Mol. Boil. Rev. MMBR. 2012;76(1):16–32. doi: 10.1128/mmbr.05015-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Tanaka T., Narazaki M., Kishimoto T. IL-6 in inflammation, immunity, and disease. Cold Spring Harb. Perspect. Biol. 2014;6(10) doi: 10.1101/cshperspect.a016295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Yu X., Zhang X., Zhao B., Wang J., Zhu Z., Teng Z. Intensive cytokine induction in pandemic H1N1 influenza virus infection accompanied by robust production of IL-10 and IL-6. PLoS One. 2011;6(12) doi: 10.1371/journal.pone.0028680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Iwase S., Nakada T.A., Hattori N., Takahashi W., Takahashi N., Aizimu T. Interleukin-6 as a diagnostic marker for infection in critically ill patients: a systematic review and meta-analysis. Am. J. Emerg. Med. 2019;37(2):260–265. doi: 10.1016/j.ajem.2018.05.040. [DOI] [PubMed] [Google Scholar]
- 88.Moore K.W., de Waal Malefyt R., Coffman R.L., O’Garra A. Interleukin-10 and the interleukin-10 receptor. Annu. Rev. Immunol. 2001;19:683–765. doi: 10.1146/annurev.immunol.19.1.683. [DOI] [PubMed] [Google Scholar]
- 89.Rutz S., Ouyang W. Regulation of Interleukin-10 expression. Adv. Exp. Med. Biol. 2016;941:89–116. doi: 10.1007/978-94-024-0921-5_5. [DOI] [PubMed] [Google Scholar]
- 90.Trinchieri G. Interleukin-10 production by effector T cells: Th1 cells show self control. J. Exp. Med. 2007;204(2):239–243. doi: 10.1084/jem.20070104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Paul W.E., Seder R.A. Lymphocyte responses and cytokines. Cell. 1994;76(2):241–251. doi: 10.1016/0092-8674(94)90332-8. [DOI] [PubMed] [Google Scholar]
- 92.Jankovic D., Kugler D.G., Sher A. IL-10 production by CD4+ effector T cells: a mechanism for self-regulation. Mucosal Immunol. 2010;3(3):239–246. doi: 10.1038/mi.2010.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Stumhofer J.S., Silver J.S., Laurence A., Porrett P.M., Harris T.H., Turka L.A. Interleukins 27 and 6 induce STAT3-mediated T cell production of interleukin 10. Nat. Immunol. 2007;8(12):1363–1371. doi: 10.1038/ni1537. [DOI] [PubMed] [Google Scholar]
- 94.Diehl S., Rincon M. The two faces of IL-6 on Th1/Th2 differentiation. Mol. Immunol. 2002;39(9):531–536. doi: 10.1016/s0161-5890(02)00210-9. [DOI] [PubMed] [Google Scholar]
- 95.Korn T., Bettelli E., Oukka M., Kuchroo V.K. IL-17 and Th17 Cells. Annu. Rev. Immunol. 2009;27:485–517. doi: 10.1146/annurev.immunol.021908.132710. [DOI] [PubMed] [Google Scholar]
- 96.McGeachy M.J., Bak-Jensen K.S., Chen Y., Tato C.M., Blumenschein W., McClanahan T. TGF-beta and IL-6 drive the production of IL-17 and IL-10 by T cells and restrain T(H)-17 cell-mediated pathology. Nat. Immunol. 2007;8(12):1390–1397. doi: 10.1038/ni1539. [DOI] [PubMed] [Google Scholar]
- 97.Ogura H., Murakami M., Okuyama Y., Tsuruoka M., Kitabayashi C., Kanamoto M. Interleukin-17 promotes autoimmunity by triggering a positive-feedback loop via Interleukin-6 induction. Immunity. 2008;29(4):628–636. doi: 10.1016/j.immuni.2008.07.018. [DOI] [PubMed] [Google Scholar]
- 98.Rincon M., Anguita J., Nakamura T., Fikrig E., Flavell R.A. Interleukin (IL)-6 directs the differentiation of IL-4-producing CD4+ T cells. J. Exp. Med. 1997;185(3):461–469. doi: 10.1084/jem.185.3.461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Lai C.F., Ripperger J., Morella K.K., Jurlander J., Hawley T.S., Carson W.E. Receptors for interleukin (IL)-10 and IL-6-type cytokines use similar signaling mechanisms for inducing transcription through IL-6 response elements. J. Biol. Chem. 1996;271(24):13968–13975. doi: 10.1074/jbc.271.24.13968. [DOI] [PubMed] [Google Scholar]
- 100.La Gruta N.L., Kedzierska K., Stambas J., Doherty P.C. A question of self-preservation: immunopathology in influenza virus infection. Immunol. Cell Biol. 2007;85(2):85–92. doi: 10.1038/sj.icb.7100026. [DOI] [PubMed] [Google Scholar]
- 101.Xu Z., Shi L., Wang Y., Zhang J., Huang L., Zhang C. Pathological findings of COVID-19 associated with acute respiratory distress syndrome. Lancet Respir. Med. 2020;8(4):420–422. doi: 10.1016/s2213-2600(20)30076-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Russell C.D., Millar J.E., Baillie J.K. Clinical evidence does not support corticosteroid treatment for 2019-nCoV lung injury. Lancet (London, England). 2020;395(10223):473–475. doi: 10.1016/S0140-6736(20)30317-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Cao W., Liu X., Bai T., Fan H., Hong K., Song H. High-Dose Intravenous Immunoglobulin as a Therapeutic Option for Deteriorating Patients With Coronavirus Disease 2019. Open Forum Infect. Dis. 2020;7(3) doi: 10.1093/ofid/ofaa102. ofaa102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Adam Monteagudo L., Boothby A., Gertner E. Continuous intravenous anakinra infusion to calm the cytokine storm in macrophage activation syndrome. ACR Open Rheumatology. 2020;2(5):276–282. doi: 10.1002/acr2.11135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Shakoory B., Carcillo J.A., Chatham W.W., Amdur R.L., Zhao H., Dinarello C.A. Interleukin-1 receptor blockade is associated with reduced mortality in Sepsis patients with features of macrophage activation syndrome: reanalysis of a prior phase III trial. Crit. Care Med. 2016;44(2):275–281. doi: 10.1097/ccm.0000000000001402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Xu X., Han M., Li T., Sun W., Wang D., Fu B. Effective treatment of severe COVID-19 patients with tocilizumab. PNAS. 2020;117(20):10970–10975. doi: 10.1073/pnas.2005615117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Luo P., Liu Y., Qiu L., Liu X., Liu D., Li J. Tocilizumab treatment in COVID-19: a single center experience. J. Med. Virol. 2020 doi: 10.1002/jmv.25801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Zhang X., Song K., Tong F., Fei M., Guo H., Lu Z. First case of COVID-19 in a patient with multiple myeloma successfully treated with tocilizumab. Blood Adv. 2020;4(7):1307–1310. doi: 10.1182/bloodadvances.2020001907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Li W., Moltedo B., Moran T.M. Type I interferon induction during influenza virus infection increases susceptibility to secondary Streptococcus pneumoniae infection by negative regulation of gammadelta T cells. J. Virol. 2012;86(22):12304–12312. doi: 10.1128/jvi.01269-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Falsey A.R., Becker K.L., Swinburne A.J., Nylen E.S., Formica M.A., Hennessey P.A. Bacterial complications of respiratory tract viral illness: a comprehensive evaluation. J. Infect. Dis. 2013;208(3):432–441. doi: 10.1093/infdis/jit190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Muller B., Becker K.L., Schachinger H., Rickenbacher P.R., Huber P.R., Zimmerli W. Calcitonin precursors are reliable markers of sepsis in a medical intensive care unit. Crit. Care Med. 2000;28(4):977–983. doi: 10.1097/00003246-200004000-00011. [DOI] [PubMed] [Google Scholar]
- 112.Branche A.R., Walsh E.E., Vargas R., Hulbert B., Formica M.A., Baran A. Serum procalcitonin measurement and viral testing to guide antibiotic use for respiratory infections in hospitalized adults: a randomized controlled trial. J. Infect. Dis. 2015;212(11):1692–1700. doi: 10.1093/infdis/jiv252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Schuetz P., Christ-Crain M., Thomann R., Falconnier C., Wolbers M., Widmer I. Effect of procalcitonin-based guidelines vs standard guidelines on antibiotic use in lower respiratory tract infections: the ProHOSP randomized controlled trial. Jama. 2009;302(10):1059–1066. doi: 10.1001/jama.2009.1297. [DOI] [PubMed] [Google Scholar]
- 114.Schuetz P., Wirz Y., Sager R., Christ-Crain M., Stolz D., Tamm M. Procalcitonin to initiate or discontinue antibiotics in acute respiratory tract infections. Cochrane Database Syst. Rev. 2017;10(10) doi: 10.1002/14651858.CD007498.pub3. CD007498-CD. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Li X., Wang L., Yan S., Yang F., Xiang L., Zhu J. Clinical characteristics of 25 death cases with COVID-19: a retrospective review of medical records in a single medical center, Wuhan, China. Int. J. Infect. Dis. 2020;94:128–132. doi: 10.1016/j.ijid.2020.03.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Qiu H., Wu J., Hong L., Luo Y., Song Q., Chen D. Clinical and epidemiological features of 36 children with coronavirus disease 2019 (COVID-19) in Zhejiang, China: an observational cohort study. Lancet Infect. Dis. 2020;20(6):689–696. doi: 10.1016/s1473-3099(20)30198-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Buret A.G. Immuno-modulation and anti-inflammatory benefits of antibiotics: the example of tilmicosin. Can. J. Vet. Res. 2010;74(1):1–10. [PMC free article] [PubMed] [Google Scholar]
- 118.Ichikawa Y., Ninomiya H., Koga H., Tanaka M., Kinoshita M., Tokunaga N. Erythromycin reduces neutrophils and neutrophil-derived elastolytic-like activity in the lower respiratory tract of bronchiolitis patients. Am. Rev. Respir. Dis. 1992;146(1):196–203. doi: 10.1164/ajrccm/146.1.196. [DOI] [PubMed] [Google Scholar]
- 119.Takeshita K., Yamagishi I., Harada M., Otomo S., Nakagawa T., Mizushima Y. Immunological and anti-inflammatory effects of clarithromycin: inhibition of interleukin 1 production of murine peritoneal macrophages. Drugs Exp. Clin. Res. 1989;15(11–12):527–533. [PubMed] [Google Scholar]
- 120.Lee W.D., Flynn A.N., LeBlanc J.M., Merrill J.K., Dick P., Morck D.W. Tilmicosin-induced bovine neutrophil apoptosis is cell-specific and downregulates spontaneous LTB4 synthesis without increasing Fas expression. Vet. Res. 2004;35(2):213–224. doi: 10.1051/vetres:2004004. [DOI] [PubMed] [Google Scholar]
- 121.Chen T., Wu D., Chen H., Yan W., Yang D., Chen G. Clinical characteristics of 113 deceased patients with coronavirus disease 2019: retrospective study. BMJ. 2020;368:m1091. doi: 10.1136/bmj.m1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Xiong Juan, Jiang Wanli, Zhou Qian, Xiaoqing Hu, Liu Chunying. Clinical characteristics, treatment, and prognosis in 89 cases of COVID-2019. Med. J. Wuhan Univ. 2020;41(4):542–546. doi: 10.14188/j.1671-8852.2020.0103. [DOI] [Google Scholar]
- 123.Yuan J., Sun Y., Zuo Y., Chen T., Cao Q., Yuan G. Clinical characteristics of 223 patients with COVID-19 in Chongqing. Journal of Southwest University(Natural Science Edition) 2020;42(3):17–24. doi: 10.13718/j.cnki.xdzk.2020.03.003. [DOI] [Google Scholar]
- 124.Zhang J.J., Dong X., Cao Y.Y., Yuan Y.D., Yang Y.B., Yan Y.Q. Clinical characteristics of 140 patients infected with SARS-CoV-2 in Wuhan, China. Allergy. 2020;0:1–12. doi: 10.1111/all.14238. [DOI] [PubMed] [Google Scholar]
- 125.Wang D., Hu B., Hu C., Zhu F., Liu X., Zhang J. Clinical Characteristics of 138 Hospitalized Patients With 2019 Novel Coronavirus–Infected Pneumonia in Wuhan, China. JAMA. 2020;323(11):1061–1069. doi: 10.1001/jama.2020.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Wan S., Xiang Y., Fang W., Zheng Y., Li B., Hu Y. Clinical features and treatment of COVID-19 patients in northeast Chongqing. J. Med. Virol. 2020:1–10. doi: 10.1002/jmv.25783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Peng Y.D., Meng K., Guan H.Q., Leng L., Zhu R.R., Wang B.Y. [Clinical characteristics and outcomes of 112 cardiovascular disease patients infected by 2019-nCoV] Zhonghua Xin Xue Guan Bing Za Zhi. 2020;48(0):E004. doi: 10.3760/cma.j.cn112148-20200220-00105. [DOI] [PubMed] [Google Scholar]
- 128.Li H., Xiang X., Ren H., Xu L., Zhao L., Chen X. Serum Amyloid A Is a Biomarker of Severe Coronavirus Disease and Poor Prognosis. J. Infect. 2020;80(6):646–655. doi: 10.1016/j.jinf.2020.03.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Shi S., Qin M., Shen B., Cai Y., Liu T., Yang F. Association of cardiac injury with mortality in hospitalized patients with COVID-19 in Wuhan, China. JAMA Cardiol. 2020 doi: 10.1001/jamacardio.2020.0950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Wu P., Duan F., Luo C., Liu Q., Qu X., Liang L. Characteristics of Ocular Findings of Patients With Coronavirus Disease 2019 (COVID-19) in Hubei Province, China. JAMA Ophthalmol. 2020;138(5):575–578. doi: 10.1001/jamaophthalmol.2020.1291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Sun D., Li H., Lu X.X., Xiao H., Ren J., Zhang F.R. Clinical features of severe pediatric patients with coronavirus disease 2019 in Wuhan: a single center’s observational study. World J. Pediatr. 2020 doi: 10.1007/s12519-020-00354-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Xia W., Shao J., Guo Y., Peng X., Li Z., Hu D. Clinical and CT features in pediatric patients with COVID-19 infection: different points from adults. Pediatr. Pulmonol. 2020;55(5):1169–1174. doi: 10.1002/ppul.24718. [DOI] [PMC free article] [PubMed] [Google Scholar]