Abstract
Objective
Despite the many medical benefits, cupping therapy can be difficult for some patients due to unpleasant marks on the skin. As patients are afraid of the potential painful sensation from cupping therapy, the skin reactions might produce vigilance for treatment as pain-related information. We investigated whether individuals show negative emotions and attentional bias toward pain-related residual marks from cupping therapy on the body using an eye-tracking method.
Methods
Fifty pain-free volunteers were presented with four different kinds of visual stimulation, such as the back or face region and with or without cupping marks on the skin. A cupping and a control image were presented on one screen with one image on the left side of the screen and the other on the right (locations of the images were counterbalanced across participants). The eye movements of the participants were measured while they viewed the pictures. They completed the Empathy Quotient questionnaire before the experiment and evaluated the unpleasantness level to each image during the task.
Results
Images of the back and face with cupping marks were rated significantly more unpleasant and showed a significant attentional bias (significantly longer percentage fixation time) than the control images (attentional bias score: Back + cupping: 48.1 ± 2.8%; Back: −0.7 ± 3.4%; Face + cupping: 34.5 ± 2.5%; Face: −2.2 ± 2.9%). Individuals with greater empathy exhibited significantly higher unpleasantness (r = 0.323, p < 0.05) and less attentional bias (r = −0.279, p < 0.05) to the images with cupping marks.
Conclusion
The skin reactions caused by cupping therapy evoked negative emotional responses as well as attentional bias to the reaction sites. Our findings suggest that the emotional and attentional responses to cupping therapy might reflect potential reluctance to this therapy.
Keywords: attentional bias, cupping, emotion, empathy, eye-tracking
Introduction
Cupping therapy, one of many complementary and alternative therapies, has gained popularity and has attracted much attention on social media.1 This treatment is widely used for pain relief by enhancing microcirculation of the skin.2–4 Cupping therapy causes negative pressure to stretch the skin and underlying tissue, dilate the capillaries, and enhance blood volume and tissue oxygenation at the treatment site.5–7 During this process, patients inevitably experience circular red marks on the treated sites, which usually elicit negative emotional responses. Although these marks are not harmful to the body and disappear in a few days, some patients are still afraid of being treated with cupping therapy.8,9 Our previous study showed that individuals experienced unpleasant and aroused emotional states to the visual images of cupping marks on the skin.10 In addition to residual skin discoloration, cupping therapy evokes a painful sensation during treatment, which is followed by a negative emotional response. Understanding the negative emotional responses to cupping therapy is a core component of dealing with aversion to this treatment.
There is large and growing evidence on attentional bias to negative information, i.e., the preferential allocation of attention toward pain-related information.11,12 Meta-analyses have shown that chronic pain patients have greater bias to pain-associated information.11,12 Eye-tracking methodology continuously measures visual-spatial attention and overcomes the shortcomings of a brief snapshot of attentional bias inferred from the visual probe task.13 Recent eye-tracking studies have shown that individuals with chronic pain exhibit greater vigilance to pain-related stimuli and earlier disengagement from pain words.14–17 The initial vigilance for pain-related stimuli reflects an adaptive reaction to detect subsequent pain.18 Furthermore, the attentional bias toward pain-related information is considered to be an important factor for the development and the maintenance of chronic pain.19,20
As cupping therapy might be painful and evoke negative emotional responses in patients, it is assumed that the cupping-associated negative response, skin discoloration, creates greater attentional bias to the therapy-related sites. The present study used eye-tracking to investigate whether pain-related stimuli (cupping therapy) on the back and the face induce negative emotions and attentional bias toward these stimuli.
Materials and Methods
Participants
Fifty healthy volunteers (under 40 age) were recruited via online advertisements directed at students attending Kyung Hee University and Korea University in Seoul, Republic of Korea. Participants had normal or corrected-to-normal vision, and none had any kind of psychiatric or neurological disorder. None of the participants suffered from acute or chronic pain. Participants working or majoring in the medical field, eg, medical or nursing students, were excluded. All participants received a detailed explanation of the experimental procedure, and provided written informed consent prior to the start of the study. This experiment was conducted in accordance with the Declaration of Helsinki and approved by the Institutional Review Board of Kyung Hee University (KHSIRB-18-075).
Empathy Quotient Questionnaire
All participants completed the Empathy Quotient (EQ) questionnaire before the experiment, which measures empathic characteristics of adults with normal intelligence.21 This questionnaire consists of 40 empathy associated questions and 20 filler questions. We used the validated Korean version of the EQ questionnaire in this study.22 The internal consistency of the EQ in the current study was evaluated by the Cronbach’s alpha coefficient. The 40-items scale, excluding the 20 filler items, had a Cronbach’s alpha of 0.867, which represents a good internal consistency.
Pain-Related Visual Stimuli Caused by Cupping Therapy
To investigate whether pain-related visual stimuli related to cupping therapy on the back and the face induce negative emotions and attentional bias toward the stimuli, the participants were presented four different types of visual stimuli: images of a back with cupping marks (Back + cupping), images of a back without cupping marks (Back), images of a face with cupping marks (Face + cupping), and images of a face without cupping marks (Face). We used different back (n = 10) and facial (n = 10) images. The back images were taken with a digital camera, and the facial images were obtained from the Korea University Facial Expression Collection and have been well validated for research in the Korean population.23 All faces had a neutral expression. To avoid the effect of differences other than cupping therapy, we used Adobe Photoshop to create cupping marks on the original back and facial images.
We created 60 pairs of two horizontally aligned pictures: 20 pairs of one Back (control) and one Back + cupping (target) image, 20 pairs of one Face (control) and one Face + cupping (target) image, and 20 pairs of two control images (Back and Back; Face and Face). The positions of the two images in each pair, either left or right, were counterbalanced to eliminate the effect of image location. All pictures were approximately 10 cm wide and 12.5 cm high, and the distance between the inner edges of each picture in each pair was approximately 12 cm.
Eye Movement Measurements
The 60 sets of images used for the eye-tracking task were presented in random order across participants. The experimental stimuli were displayed on a 17-inch LCD-TFT monitor located approximately 70 cm from the participants’ eyes (with a visual angle of 10.2° horizontally and 25.6° vertically). The programs iView XTM RED and iView X Experiment Center 2.4 (SensoMotoric Instruments, Teltow, Germany) were used to present the visual stimuli and to calibrate and record eye movements.
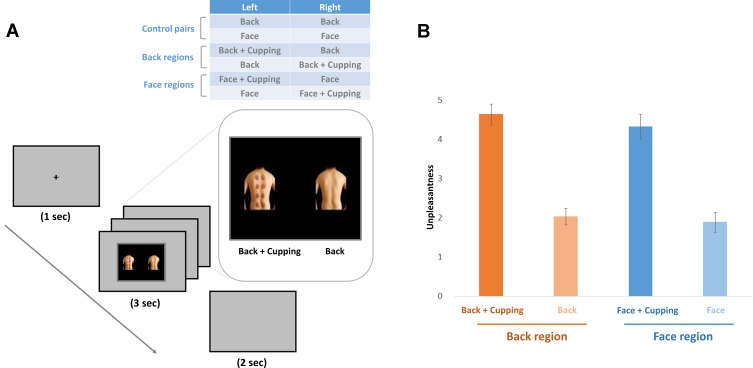
Each trial started with a fixation cross at the center of the screen for 1000 ms, which was replaced with a pair of pictures for 3000 ms. Then, a black screen appeared for 2000 ms (Figure 1A). Participants were asked to look at the fixation cross at the start of each trial and refrain from moving their head during the task.
Figure 1.
(A) Experimental design. We used four different kinds of visual stimuli: the back region with or without cupping and the facial region with or without cupping. Both the cupping and control images were presented on one screen at the same time, either on the left or right side of the screen. A fixation cross was displayed at the center of the screen for 1000 ms at the start of each trial. The fixation cross was followed by visual stimuli, which were displayed for 3000 ms. Then, a 2000 ms black screen was presented. (B) Unpleasantness ratings to the images. Both images with cupping marks on the back and on the face showed significantly more unpleasantness than the control images without cupping marks.
Unpleasantness Rating
To minimize cognitive load, the unpleasantness ratings were completed separately from the eye movement measurements. The participants were given 6000 ms to evaluate the unpleasantness of each image. Participants rated the perceived unpleasantness of the image using a 5-point Likert-type rating scale (1 = not unpleasant, 5 = very unpleasant). A 2000 ms rest period was provided after the unpleasantness ratings. All experiments were conducted in an air-conditioned (24 ± 2°C) soundproofed room.
Data Analysis
The eye-tracking data were analyzed using the eye-tracking data analysis program BeGaze 2.4 (SensoMotoric Instruments). Regions of interest (ROIs) were defined for all sets of images; square-shaped ROIs were drawn to cover the back, and round-shaped ROIs were drawn to cover the face. The fixation time percentage on the ROIs was measured by BeGaze, and the attentional bias score was calculated for each participant by subtracting the mean fixation percentage (the mean percentage of fixations made on each ROI) of the control images (images without cupping marks) from the mean fixation percentage of the target images (images with cupping marks). The scores for the control pairs (pairs of back/back and face/face without cupping marks) were calculated using the mean fixation percentage of the left and right images, and the attentional bias scores of the control pairs were used as a control.
Data were analyzed using a 2 × 2 analysis of variance (ANOVA), with two within factors for the attentional bias score; one for the presence of cupping marks (with marks and without marks) and the other for the body parts where the cupping marks were drawn (back and face). The results are expressed as mean ± standard error. Pearson’s correlation analysis was also performed to determine the relationship between subjective unpleasantness ratings and attentional bias with the EQ score. Statistical analyses were performed using Jamovi software (version 0.9; http://www.jamovi.org), and a p-value < 0.05 was considered significant.
Results
Participants
Fifty participants included 24 females (22.3 ± 0.6 years) and 26 males (24.2 ± 0.5 years) in this study. Among fifty participants, 24 participants had prior experience with cupping therapy, but 26 participants had no prior experiences.
Psychophysical Response to Pain-Related Visual Stimuli Caused by Cupping Therapy
We conducted a 2 × 2 ANOVA with two within factors for the unpleasantness ratings; one for the presence of cupping marks (with marks and without marks) and the other for the body parts where the cupping marks were drawn (back and face). ANOVA revealed a significant main effect of cupping marks (F = 184.4, p < 0.001) while the effect of location (back and face) was not significant (F = 0.495, p = 0.483). The interaction (cupping effect × location effect) was also not significant (F = 0.181, p = 0.672). Participants reported significantly higher unpleasant ratings to the images with cupping skin marks than to the control images (Back + cupping: 4.64 ± 0.26; Back: 2.03 ± 0.21; Face + cupping: 4.34 ± 0.31; Face: 1.89 ± 0.26) (Figure 1B).
Attentional Bias Towards Cupping Therapy
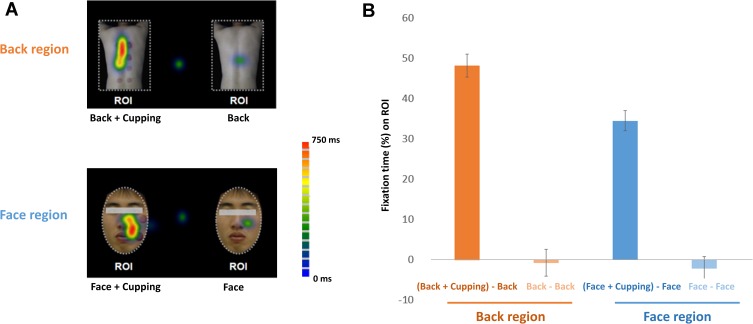
A 2 × 2 ANOVA was conducted for the attentional bias score. ANOVA showed a significant main effect of cupping (F = 241.4, p < 0.001) and location (F = 5.92, p < 0.05), and a significant interaction of the effects of cupping and location (F = 4.89, p < 0.05) (Figure 2A). Attentional bias to cupping therapy on the back was longer than that on the facial region (back, 48.1 ± 2.8% vs face, 34.5 ± 2.5%). Eye fixation time percentage on the ROIs of the target images was significantly longer than the ROIs on the control images (Back + cupping: 48.1 ± 2.8%; Back: −0.7 ± 3.4%; Face + cupping: 34.5 ± 2.5%; Face: −2.2 ± 2.9%) (Figure 2B).
Figure 2.
Attentional bias toward cupping therapy. (A) An example of the mean eye gaze fixation time percentage of a subject on the visual stimuli is represented as a heat map. The green-to-red color map indicates the average fixation time spent on each pixel. (B) The fixation time percentage was significantly higher in response to images with cupping marks on the body (both back and face) than control images. Attentional bias to cupping images was significantly greater for the images of the back compared to the images of the face.
Abbreviation: ROI, region of interest.
Furthermore, we compared attentional bias toward cupping marks based on experience with cupping therapy. No significant differences in attentional bias were observed toward cupping marks between experienced and unexperienced participants (Back + cupping: experienced, 48.9 ± 6.9% and unexperienced, 50.5 ± 5.8%; Face + cupping: experienced, 37.9 ± 4.4% and unexperienced, 36.4 ± 5.6%).
Correlations Between the EQ Score, Attentional Bias, and Unpleasantness
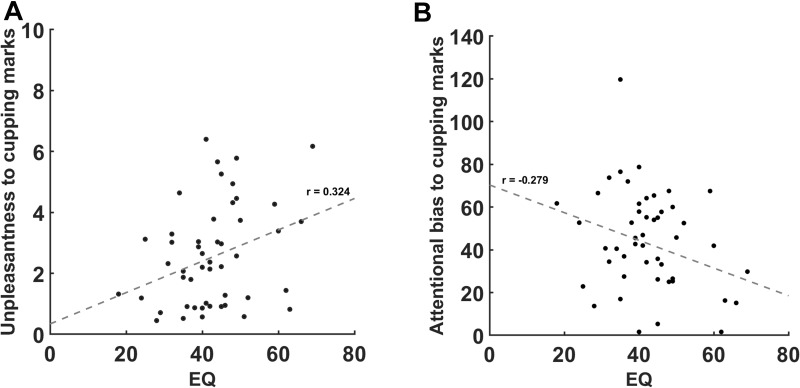
We found a significant positive correlation between the unpleasantness ratings for cupping therapy images and the EQ score (r = 0.323, p < 0.05) (Figure 3A), and a significant negative correlation between attentional bias to the ROIs of the target images and the EQ score (r = −0.279, p < 0.05) (Figure 3B). However, the magnitude of correlation coefficients was weak.
Figure 3.
Correlation between emotional response and attentional bias to cupping therapy and the Empathy Quotient scores. (A) Individuals with greater empathy showed significantly more unpleasantness to the cupping therapy images (r = 0.323, p < 0.05). (B) Individuals with greater empathy showed significantly less attentional bias to the cupping therapy images (r = −0.279, p < 0.05).
Abbreviation: EQ, Empathy Quotient questionnaire.
Discussion
The purpose of this study was to investigate attentional bias to cupping therapy-induced pain-related information using eye-tracking methodology. We demonstrated that the images of skin reactions caused by cupping therapy on the body evoked significant unpleasant responses compared to the control images. Similarly, the eye-tracking measures showed that participants exhibited greater attentional bias to the images with cupping marks on the body compared to the control images. Individuals with greater empathy showed more unpleasantness and less attentional bias to the cupping therapy images. These findings suggest that emotional and attentional responses to cupping therapy might reflect the potential reluctance to these marks.
In the current study, the eye-tracking measures showed that participants exhibited greater attentional bias toward cupping marks on the back and facial regions. These data are consistent with recent studies showing initial bias toward painful stimuli and attentional maintenance to the stimuli.14,18 The vigilance-avoidance hypothesis proposes an early attentional bias toward fearful stimuli followed by attentional avoidance of the stimulus.24 Initial vigilance is an orienting response, which induces rapid detection of potentially harmful stimuli.18 It is assumed that participants had greater initial vigilance to cupping therapy as an upcoming painful stimulus in the present study. It is well known that negative expectations of a treatment can reduce its clinical outcome.25,26 For example, nocebo effects can influence therapeutic efficacy and adherence to the treatment.27 To avoid an unnecessary nocebo effect of cupping therapy in clinical practice, it is necessary to minimize excessive vigilance to the stimuli and a potentially negative response, especially when it is used for medical treatment.
Because of the biological relevance of the head and facial areas for vital functions, trigeminal pain on the face is more prone to sensitization than other sites of stimulation.28,29 Thus, it can be expected that attentional bias to a cupping image might be greater in the facial region than in the back region. However, the present study found that attentional bias to cupping images was greater in the back region than the facial region. Considering the size of the body area (back or face) presented in this study, we used a larger number of cupping marks on the back (n = 6–11) than on the face (n = 3). As we used a different number of cupping marks on the back and face, we were unable to confirm the body-specific effect of attentional bias to cupping therapy in the current study. It will be interesting to further investigate attentional bias to potentially painful stimuli on various body sites in a future study.
In our study, participants viewed the pictures of another’s body treated with cupping therapy. We found that individuals with higher EQ scores showed more unpleasantness and less attentional bias to another’s body part where they received cupping therapy. A vicarious response to another’s pain mediates the avoidance response to aversive stimuli,30 and empathy might be one of the important factors influencing vicarious pain.31 For example, individuals with a higher empathy score have greater brain responses in the insula while they are viewing painful stimuli given to others.32 Encoding of another person’s pain comes from shared sensory mirroring systems for pain.33 As more empathetic participants share the sensory mirroring system for pain, it is assumed that more empathetic individuals had more unpleasantness and more avoidance behavior (less attentional bias) to cupping therapy in the present study.
Notably, the level of fear of an upcoming painful stimulus affects the attentional bias to cupping therapy-associated painful information, and participants with previous cupping therapy experience might have less fear for the treatment than inexperienced participants, although we did not measure fear for the treatment. Individuals with a higher fear of pain display a longer initial gaze toward health catastrophe words during the visual probe task,16 and the fear level is associated with the maintenance of attention on painful depictions under higher threat conditions implying pain.34 In our study, no significant difference in attentional bias was observed toward cupping marks between experienced and unexperienced participants. Therefore, the enhanced attentional bias toward the cupping marks in the current study was not derived from a difference in fear originating from a difference in treatment experience.
There are several limitations that should be discussed. First, as our findings were based on relatively young participants, it is necessary to include more varied populations to generalize the results in a future study. Second, although eye-tracking measures are considered a strong indicator of visual attention, visual attention can occur in the absence of eye movement. To capture visual attention to cupping therapy, it is necessary to measure visual attention with other experimental methods, such as the dot-probe task. Third, the different types of the cupping marks might be one of the potential factors to influence attentional bias to cupping therapy marks. It will be interesting to examine different types of cupping therapy marks including shape or color in the future study. Last, we only included pain-free healthy participants in this study. It would be interesting to compare the attentional bias of pain-free participants to patients with chronic pain who want to be treated with cupping therapy in a future study.
In summary, eye-tracking results and psychophysical measurements revealed a negative emotional response and attentional bias to cupping therapy-related marks, indicating pain and fear, on the back and the face. Our findings suggest that vigilance for pain-associated information might reflect an adaptive reaction to potential harmful stimuli. Practitioner’s in-depth understanding of the negative responses of patients to cupping therapy will be necessary to deal with patients’ aversion and reluctance to cupping therapy in clinics.
Acknowledgments
This research was supported by Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Science, ICT & Future Planning (No. 2018R1D1A1B07042313).
Disclosure
The authors declare that there are no conflicts of interest regarding the publication of this paper.
References
- 1.Yin J, Basch CH, Adnan MM, Dottington Fullwood M, Menafro A, Fung IC. attributes of videos on youtube related to cupping therapy. Altern Ther Health Med. 2018;24(6):32–37. [PubMed] [Google Scholar]
- 2.Aboushanab TS, AlSanad S. Cupping therapy: an overview from a modern medicine perspective. J Acupunct Meridian Stud. 2018;11(3):83–87. doi: 10.1016/j.jams.2018.02.001 [DOI] [PubMed] [Google Scholar]
- 3.Al-Bedah AMN, Elsubai IS, Qureshi NA, et al. The medical perspective of cupping therapy: effects and mechanisms of action. J Tradit Complement Med. 2019;9(2):90–97. doi: 10.1016/j.jtcme.2018.03.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lauche R, Spitzer J, Schwahn B, et al. Efficacy of cupping therapy in patients with the fibromyalgia syndrome-a randomised placebo controlled trial. Sci Rep. 2016;6:37316. doi: 10.1038/srep37316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Huber R, Emerich M, Braeunig M. Cupping - is it reproducible? Experiments about factors determining the vacuum. Complement Ther Med. 2011;19(2):78–83. doi: 10.1016/j.ctim.2010.12.006 [DOI] [PubMed] [Google Scholar]
- 6.Kim S, Kim E, Jung G, Lee S, Kim JG. The hemodynamic changes during cupping therapy monitored by using an optical sensor embedded cup. J Biophotonics. 2019;12(5):e201800286. doi: 10.1002/jbio.201800286 [DOI] [PubMed] [Google Scholar]
- 7.Tham LM, Lee HP, Lu C. Cupping: from a biomechanical perspective. J Biomech. 2006;39(12):2183–2193. doi: 10.1016/j.jbiomech.2005.06.027 [DOI] [PubMed] [Google Scholar]
- 8.Kim SB, Lee YH. Numerical analysis of the change in skin color due to ecchymosis and petechiae generated by cupping: a pilot study. J Acupunct Meridian Stud. 2014;7(6):306–317. doi: 10.1016/j.jams.2013.09.004 [DOI] [PubMed] [Google Scholar]
- 9.Yoo SS, Tausk F. Cupping: east meets west. Int J Dermatol. 2004;43(9):664–665. doi: 10.1111/j.1365-4632.2004.02224.x [DOI] [PubMed] [Google Scholar]
- 10.Hong M, Lee IS, Ryu Y, Kim J, Chae Y. Cognitive and emotional aspect of cupping therapy. Brain Sci. 2020;10(3):144. doi: 10.3390/brainsci10030144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Crombez G, Van Ryckeghem DM, Eccleston C, Van Damme S. Attentional bias to pain-related information: a meta-analysis. Pain. 2013;154(4):497–510. doi: 10.1016/j.pain.2012.11.013 [DOI] [PubMed] [Google Scholar]
- 12.Schoth DE, Nunes VD, Liossi C. Attentional bias towards pain-related information in chronic pain; a meta-analysis of visual-probe investigations. Clin Psychol Rev. 2012;32(1):13–25. doi: 10.1016/j.cpr.2011.09.004 [DOI] [PubMed] [Google Scholar]
- 13.Schoth DE, Wu J, Zhang J, Guo X, Liossi C. Eye-movement behaviours when viewing real-world pain-related images. Eur J Pain. 2019;23(5):945–956. doi: 10.1002/ejp.1363 [DOI] [PubMed] [Google Scholar]
- 14.Fashler SR, Katz J. Keeping an eye on pain: investigating visual attention biases in individuals with chronic pain using eye-tracking methodology. J Pain Res. 2016;9:551–561. doi: 10.2147/JPR.S104268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Liossi C, Schoth DE, Godwin HJ, Liversedge SP. Using eye movements to investigate selective attention in chronic daily headache. Pain. 2014;155(3):503–510. doi: 10.1016/j.pain.2013.11.014 [DOI] [PubMed] [Google Scholar]
- 16.Yang Z, Jackson T, Chen H. Effects of chronic pain and pain-related fear on orienting and maintenance of attention: an eye movement study. J Pain. 2013;14(10):1148–1157. doi: 10.1016/j.jpain.2013.04.017 [DOI] [PubMed] [Google Scholar]
- 17.Yang Z, Jackson T, Gao X, Chen H. Identifying selective visual attention biases related to fear of pain by tracking eye movements within a dot-probe paradigm. Pain. 2012;153(8):1742–1748. doi: 10.1016/j.pain.2012.05.011 [DOI] [PubMed] [Google Scholar]
- 18.Priebe JA, Messingschlager M, Lautenbacher S. Gaze behaviour when monitoring pain faces: an eye-tracking study. Eur J Pain. 2015;19(6):817–825. doi: 10.1002/ejp.608 [DOI] [PubMed] [Google Scholar]
- 19.Jackson T, Yang Z, Su L. Pain-related gaze biases and later functioning among adults with chronic pain: a longitudinal eye-tracking study. Pain. 2019;160(10):2221–2228. doi: 10.1097/j.pain.0000000000001614 [DOI] [PubMed] [Google Scholar]
- 20.Sharpe L, Haggman S, Nicholas M, Dear BF, Refshauge K. Avoidance of affective pain stimuli predicts chronicity in patients with acute low back pain. Pain. 2014;155(1):45–52. doi: 10.1016/j.pain.2013.09.004 [DOI] [PubMed] [Google Scholar]
- 21.Baron-Cohen S, Wheelwright S. The empathy quotient: an investigation of adults with asperger syndrome or high functioning autism, and normal sex differences. J Autism Dev Disord. 2004;34(2):163–175. doi: 10.1023/B:JADD.0000022607.19833.00 [DOI] [PubMed] [Google Scholar]
- 22.Kim J, Lee SJ. Reliability and validity of the korean version of the empathy quotient scale. Psychiatry Investig. 2010;7(1):24–30. doi: 10.4306/pi.2010.7.1.24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kim MW, Choi JS, Cho YS. The Korea university facial expression collection (KUFEC) and semantic differential rating of emotion. Kor J Psychol. 2011;30:1189–1211. [Google Scholar]
- 24.Mogg K, Bradley B, Milles F, Dixon R. Time course of attentional bias for threat scenes: testing the vigilance-avoidance hypothesis. Cogn Emot. 2004;18:689–700. doi: 10.1080/02699930341000158 [DOI] [Google Scholar]
- 25.Bingel U, Placebo Competence T. Avoiding nocebo effects to optimize treatment outcome. JAMA. 2014;312(7):693–694. doi: 10.1001/jama.2014.8342 [DOI] [PubMed] [Google Scholar]
- 26.Chae Y, Kim SY, Park HS, Lee H, Park HJ. Experimentally manipulating perceptions regarding acupuncture elicits different responses to the identical acupuncture stimulation. Physiol Behav. 2008;95(3):515–520. doi: 10.1016/j.physbeh.2008.07.027 [DOI] [PubMed] [Google Scholar]
- 27.Colloca L. Nocebo effects can make you feel pain. Science. 2017;358(6359):44. doi: 10.1126/science.aap8488 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schmidt K, Forkmann K, Sinke C, Gratz M, Bitz A, Bingel U. The differential effect of trigeminal vs. Peripheral pain stimulation on visual processing and memory encoding is influenced by pain-related fear. Neuroimage. 2016;134:386–395. [DOI] [PubMed] [Google Scholar]
- 29.Schmidt K, Schunke O, Forkmann K, Bingel U. Enhanced short-term sensitization of facial compared with limb heat pain. J Pain. 2015;16(8):781–790. doi: 10.1016/j.jpain.2015.05.003 [DOI] [PubMed] [Google Scholar]
- 30.Chang LJ, Gianaros PJ, Manuck SB, Krishnan A, Wager TD, Sensitive A. Specific neural signature for picture-induced negative affect. PLoS Biol. 2015;13(6):e1002180. doi: 10.1371/journal.pbio.1002180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Iacoboni M. Imitation, empathy, and mirror neurons. Annu Rev Psychol. 2009;60:653–670. doi: 10.1146/annurev.psych.60.110707.163604 [DOI] [PubMed] [Google Scholar]
- 32.Singer T, Seymour B, O’Doherty J, Kaube H, Dolan RJ, Frith CD. Empathy for pain involves the affective but not sensory components of pain. Science. 2004;303(5661):1157–1162. doi: 10.1126/science.1093535 [DOI] [PubMed] [Google Scholar]
- 33.Saarela MV, Hlushchuk Y, Williams AC, Schurmann M, Kalso E, Hari R. The compassionate brain: humans detect intensity of pain from another’s face. Cereb Cortex. 2007;17(1):230–237. doi: 10.1093/cercor/bhj141 [DOI] [PubMed] [Google Scholar]
- 34.Jackson T, Su L, Wang Y. Effects of higher versus lower threat contexts on pain-related visual attention biases: an eye-tracking study of chronic pain. J Pain. 2018;19(6):649–659. doi: 10.1016/j.jpain.2018.01.011 [DOI] [PubMed] [Google Scholar]