Abstract
A novel Bacillus licheniformis strain (DM-1) was isolated from a mature reservoir in Dagang oilfield of China. DM-1 showed unique properties to utilize petroleum hydrocarbons and agroindustrial by-product (molasses) for exopolysaccharide (EPS) production under oil recovery conditions. The DM-1 EPS was proven to be a proteoglycan with a molecular weight of 568 kDa. The EPS showed shear thinning properties and had high viscosities at dilute concentrations (<1%, w/v), high salinities, and elevated temperatures. Strain DM-1 could degrade long-chain n-alkanes up to C36. Viscosity reduction test have shown that the viscosity of the crude oil was reduced by 40% compared with that before DM-1 treatment. Sand pack flooding test results under simulated reservoir conditions have shown that the enhanced oil recovery efficiency was 19.2% after 7 days of in-situ bioaugmentation with B. licheniformis DM-1. The obtained results indicate that strain DM-1 is a promising candidate for in situ microbial enhanced oil recovery (MEOR).
Subject terms: Environmental biotechnology, Industrial microbiology, Microbiology, Applied microbiology, Environmental microbiology
Introduction
Two-thirds of crude oil remains entrapped in oil reservoirs even when it is subjected to routine water and gas flooding1,2. Effective exploitation of these untouched crude oil is critical to meeting the world’s growing energy demands, and attention has been focused on tertiary oil recovery technology to enhance oil recovery (EOR) to produce more crude oil in the past decades3,4. Polymer flooding is one of the most widely used techniques in tertiary oil recovery. The mechanism of polymer flooding is to add a high-viscosity water-soluble polymer into the injected water to expand the swept volume and improve mobility ratio, thereby enhancing the oil recovery5,6. Sheng et al.7 collected and surveyed 733 polymer-flooding projects in 24 countries worldwide and concluded that the median incremental oil recovery is approximately 6.7%.
Nowadays, partially hydrolyzed polyacrylamide and its derivatives are the most widely used substances in polymer flooding and have been used in a lot of oil fields for large-scale oil production8,9. However, these chemical synthetic polymers that are toxic to the environment and difficult to decompose6. Therefore, biologically produced polymers (biopolymers) have attracted increasing attention because they are eco-friendly and have superior tolerance to salts and temperature10.
More than 10 biopolymers, including schizophyllan, xanthan, guar gum, hydroxyethylcellulose, scleroglucan, carboxymethycellulose, and lignin, have been reported for EOR field studies. The field application of schizophyllan flooding in Bockstedt oil field (Germany) has shown that the oil production rate increases by more than 20% compared with that of waterflooding11.
Two main strategies are available for the use of biopolymer in MEOR: 1) ex-situ where biopolymer is directly injected into the oil reservoir with the injected water and 2) in-situ where suitable microbes are injected to produce the desired biopolymer in the oil reservoir. In most cases, biopolymer flooding is carried out via the first strategy. For example, xanthan gum (obtained from Xanthomonas campestris pv. campestris), the most widely known and used biopolymer, is normally produced ex-situ and injected into oil reservoirs with the injected water to EOR. However, the ex-situ process, for example, determines optimal fermentation conditions, purifies the EPS, transports and injects it into the well, and leads to high production costs, which limits its use in the oil field12. In contrast, the in-situ process that injects suitable microorganisms into the oil reservoir to produce the desired polymer seems to be cost-effective. For example, BNP29, a strain of B. licheniformis isolated from a German oil reservoir, can improve oil recovery efficiency by up to 22.1% after in-situ microbial treatment13, and ZR3, an engineered strain constructed from a EPS-producing strain and a thermophilic strain, can improve oil recovery by up to 11.3% of the OOIP over water flooding after 7 days in-situ bioaugmentation14.
Many depleted and mature reservoirs are available in China that need to be rejuvenated. However, few researches have investigated indigenous microorganisms in local oil fields for MEOR. In this study, an EPS-producing strain (DM-1) was isolated from oil field-produced water, and its EPS production capacity under extreme environmental conditions was studied. Sand pack flooding tests were performed to determine the effects of in-situ bioaugmentation with B. licheniformis DM-1.
Materials and Methods
Crude oil and reagents
Crude oil with a viscosity (50 °C) of 110 mPa·s and a density of 0.87 g/cm3 was obtained from Dagang Oilfield. Analytical-grade chemical reagents were used in the experiments.
Microorganism
Strain DM-1, which can degrade and emulsify crude oil (Fig. 1), was isolated from a mature reservoir in Dagang oilfield of China and deposited in China General Microbiological Culture Collection Center with the deposit number of CGMCC 1.6128. Pure culture of DM-1 was preserved in sterile glycerol suspensions (20%, v/v) at −80 °C; for routine experiments, DM-1 was maintained on LB agar slant at 4 °C and subcultured at an interval of 30 days.
Figure 1.
Ability of B. licheniformis DM-1 to degrade crude oil. (A) Crude oil in BH medium without inoculum of DM-1. (B) Crude oil in BH medium inoculated with DM-1. Results were obtained at 55 °C for 7 days.
Identification of strain DM-1
DM-1 was identified based on physiological and morphological characteristics and 16 S rRNA sequence analysis. Chromosomal DNA was extracted from B. licheniformis DM-1 by using a Bacteria DNA Kit (Takara Bio Inc.) according to the manufacturer’s instructions. The 16S rRNA gene sequence was amplified by using the universal primers 27 F (5'-GAGAGTTTGATCCTGGCTCAG-3') and 1541 R (5'-AAGGAGGTGATCCAGCCCGCA-3'). The purified amplified product was sent to Takara Bio Inc. (Dalian) for sequencing. The 16S rRNA gene sequence was used to search the GenBank database (https://blast.ncbi.nlm.nih.gov/Blast.cgi) with the BlastN algorithm and determine the relative phylogenetic position. Phylogenetic analysis based on neighbor-joining method15 was performed by using MEGA 6.016. The stability of relationships was assessed by performing bootstrap analyses of the neighbor-joining data based on 1,000 resamplings.
Exopolysaccharide production
Active bacterial culture was prepared by transferring one loop of strain DM-1 from agar slant into 10 ml of LB medium and incubated at 45 °C at 180 rpm for 12 h. The bacterial cells in the culture broth were collected via centrifugation at 6,000 rpm for 10 min. Thereafter, bacterial cells were washed thrice with sterile water and were used to inoculate 100 ml sterilized Bushnell–Haas (BH) medium (KH2PO4 0.1%, CaCl2 0.002%, NH4NO3 0.1%, MgSO4·7H2O 0.02%, K2HPO4 0.1%, FeCl3 0.005%, pH 7.0) supplemented with petroleum or nonpetroleum carbon sources, such as crude oil, liquid paraffin, starch, glucose, sucrose, and molasses (2%, v/v) or (2%, w/v). Nitrogen was injected to replace the air in the flasks14, and the flasks were sealed with non-vented rubber stoppers after inoculation of strain DM-1 into the Erlenmeyer flasks to create anaerobic environmental conditions. The parameters studied for EPS production included salt concentration (0, 1, 3, 5, 7, 9, and 11%, w/v of NaCl) and varying temperatures (30 °C, 40 °C, 45 °C, 50 °C, and 55 °C). Strain DM-1 was routinely cultured in 300 ml flasks, and culture broth was taken at intervals to analyze EPS production and bacterial cell growth (OD600).
Preliminary chemical characterization of the exopolysaccharide
The strain DM was cultured under optimal conditions (BH medium supplemented with 2% sucrose, 5% (w/v) NaCl, 45 °C; 60–70 h of incubation), and then EPS was purified and analyzed to determine the nature of EPS. The method of polymer purification was according to the method of Ortega–Morales et al.17 with slight modifications. In brief, bacterial cells were removed through centrifugation at 10,000 × g and 4 °C for 20 min and filtered through HVLP filters with a pore diameter of 0.47 µm. The supernatant was kept at 4 °C overnight, and EPS was precipitated by adding two volumes of cold absolute ethanol. The precipitate was recovered through centrifugation at 120,000 × g and 4 °C for 20 min, dissolved in a small volume of distilled water, subsequently dialyzed against deionized water for 48 h, reprecipitated, and dried at 40 °C. The total carbohydrate content was evaluated by phenol–sulfuric acid method described by Dubois et al.18. Protein content was determined by using the Lowry method19. Bovine serum albumin (BSA) was used as the calibration standard. The lipid content was determined by using gravimetric estimation20. The sugar components and proportions of the purified EPS were investigated by using HPLC method21. The functional groups in the bioemulsifier were analyzed by Fourier transform-infrared spectroscope.
Physicochemical characterization of the DM-1 EPS
Rheological characterization
The rheological properties of the DM-1 EPS, at concentrations ranging from 0.25% to 0.75% (w/v), were measured at 25 °C at a shear rate of 20 s−1 using a digital rotational viscosimeter (BROOKFIELD, LVDV-II + Pro, USA). EPS solutions (1.0%, w/v) were incubated in a water bath at 100 °C for 10, 20, 30, 40, 50, and 60 min to investigate thermal stability, and the viscosity of the solution at the corresponding temperature was measured. Moreover, the viscosity of xanthan gum (1.0%, w/v) was tested under the same conditions. The effect of salinity on viscosity activity was determined by adjusting the concentration of added NaCl to 1%, 3%, 5%, 7%, 9%, 11%, 13%, and 15%, w/v of NaCl. The sample was allowed to equilibrate for 2 min before the viscosity was determined. Values presented are the mean of triplicate analyses.
Degradation of n-alkanes
The ability of DM-1 to degrade n-alkanes was tested according to a modified method described by Zhou et al.22. Briefly, inoculum (2%, v/v) of B. licheniformis DM-1 was added to 100 ml BH medium supplemented with 1% (w/v) individual n-alkanes (C8–C40) in 300 ml Erlenmeyer flasks. The uninoculated flasks were used as controls. The flasks were sealed with non-vented rubber stoppers and incubated for 10 d at 45 °C. Thereafter, the residual alkanes in the culture broth were extracted with 300 ml n-hexane. The alkane extracts were then analyzed via gas chromatography (GC) analysis, and their relative abundances were calculated23. GC analysis was performed on an Agilent 6820 machine by using an FID detector (Agilent 6820, United States) equipped with an HP-PONA column (50 m × 0.2 mm × 0.5 mm). The column temperature was kept at 120 °C for 2 min and then raised to 300 °C at a rate of 5 °C/min. Nitrogen was used as a carrier gas.
Reduction of oil viscosity
The oil degradation characteristics of DM-1 strain prompted us to study the potential role of DM-1 in the viscosity reduction of crude oil. The property of viscosity reduction of crude oil by B. licheniformis DM-1 was studied by the method of Zhou et al.22.
In brief, DM-1 strain was cultured in an LB medium and incubated at 45 °C and 180 rpm for 14 h. Then, 30 ml of the culture broth and 30 g of crude oil were added to 300 ml Erlenmeyer flasks. The flasks were sealed with rubber stoppers and incubated at 45 °C for 15 days. The sample was heated to 80 °C and then centrifuged at 12,000 × g for 10 min for dehydration. Oil viscosity was also measured at 50 °C by using a Brookfield viscometer (LVDV-II + Pro, USA).
In-situ microbial oil recovery
The potential application of strain DM-1 for in-situ MEOR was investigated by using the sand pack flooding method23. Sand samples (40–60 mesh), obtained from Dagang Oilfield, were washed initially with chloroform to remove crude oil and then with deionized water. They were dried at 90 °C for 24 h and filled into stainless steel columns to prepare sand pack models. The columns was 36 mm in diameter and 600 mm in length. The column permeability and porosity were 3.22 μm2 and 23.2%, respectively. All the sandpacks in the setups were placed horizontally. The column was saturated by injecting formation brine from the specified reservoir. The physical and chemical parameters of formation brine are listed in Table S1. The flow rate of flooding was set at 2 ml/min. Crude oil was filled in a tank and injected into the sand pack to replace the brine. The initial oil saturation was judged by measuring the volume of brine replaced by oil saturation, which is also called OOIP. After aging for 24 h at 45 °C, brine was injected (first water flooding) until no oil was monitored in the other end of the cores which means that the core reached its residual oil saturation. Thereafter, 0.35 pore volume (PV) of nutrient (BH medium supplemented with 2.0% molasses) mixed with 0.15 PV of microbes (109 cells/ml) were injected into the water-flooded core, and shut-in at 45 °C for 7 days. Finally, brine was injected again until no more oil was observed in the outlet of the cores, and the amount of oil released was carefully recorded. The blank experiments were performed in the same conditions, but no bacterial cells were injected into the cores. The controls with only brine nutrients or DM-1 were performed under the same conditions to background information.
Data analysis
All analyses in this article were carried out in triplicate, and results were statistically analyzed by using SPSS 16.0.
Results and Discussion
In case of in-situ MEOR, the severe reservoir conditions (high temperature, anoxic, and hydrocarbon toxicity) affected the growth of microbes, thus influencing the efficiency of oil recovery24. Therefore, searching suitable microbes that can grow in oil reservoir and produce the products needed for oil recovery are necessary25,26. Related studies have shown that the isolation of indigenous bacteria from the target oil fields is a good method because they have adapted to the conditions of that reservoir3. A total of six hydrocarbon-degrading strains were isolated from a deep oil reservoir in northern China. Out of these isolates, strain DM-1 was selected for further investigation because it showed unique superiority to utilize crude oil for production of EPS under extreme environmental conditions.
Characterization of strain DM-1
Strain DM-1 was able to grow at 15 °C to 55 °C (optimum 45 °C), pH 6–10 (optimum 6.0–7.5), and NaCl concentration 0%–11% (w/v) (optimum 4%–5%). The ability to produce EPS was suggested by its sticky mucoid colony morphology (Fig. 2) Strain DM-1 was rod-like, facultative anaerobic, Gram-positive, and spore-forming, similar to the Bacillus species. The 16S rRNA gene sequence (1,541 nucleotides) was deposited in GenBank (accession number DQ539620) and compared with its database. Alignment of the 16S rRNA gene with the closest type strains showed 99.9% similarity to B. licheniformis strain NCTC8721 (accession number LR134165). Strain DM-1 was identified as a type of B. licheniformis based on physiological characteristics and 16S rRNA gene analysis (Fig. S1).
Figure 2.
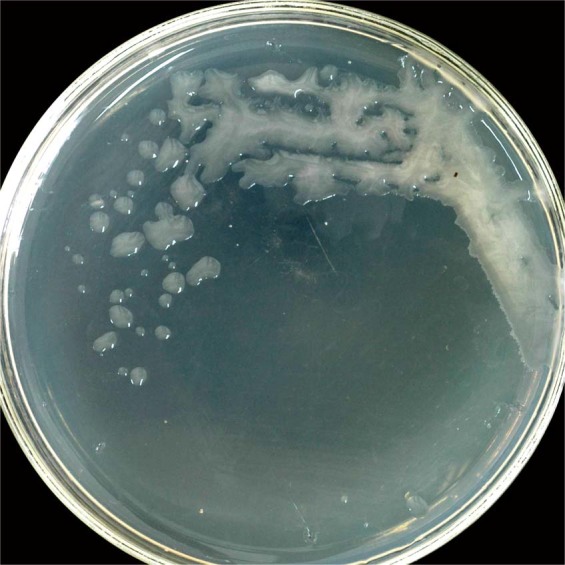
Mucoid colonies of B. licheniformis DM-1 on LB agar at 55 °C for 24 h.
Productivity studies
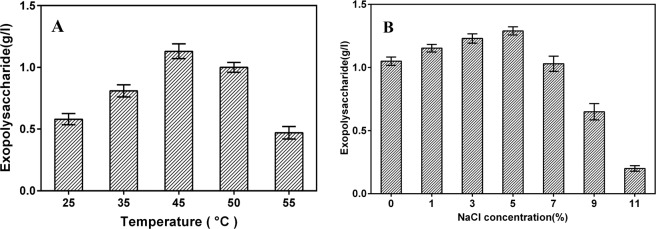
The nature and quantity of EPS synthesized by a microorganism are genetically determined, but they are greatly influenced by cultivation conditions and components of medium, particularly carbon sources27. The effects of the cultivation conditions (temperature and salinity) on the growth of strain DM-1 and EPS yield are shown in (Fig. 3). Maximum EPS yield was achieved by incubation at 45 °C for about 60 h. The salinity in the range of 0%–7% has minimal effect on the growth of DM-1 and the yield of EPS, but the EPS yield was significantly reduced (0.2 g/l) when the NaCl concentration reached 11%. This result was consistent with several previous reports24. Six different carbon sources were examined for their effectiveness on EPS cproduction of strain DM-1 in BH medium with C-sources (Fig. 4). The highest DM-1 EPS production was 1.29 g/l when sucrose was used as the source of carbon. In EPS production, sugars are often the most common and effective carbon sources28. However, cheaper substrates (molasses, cheese whey, and glycerol byproduct) were sufficient to produce several bacterial EPSs29–31. The choice of cheap raw materials is an important way to reduce the production cost as they account for 50% of the final production expenses and also reduce the cost of waste disposal28,32. The ability of using agroindustrial by-product (molasses) to produce EPS makes DM-1 economic valuable in oil fields that require large amounts of EPS for flooding12.
Figure 3.
Effect of (A) temperature and (B) salinity on EPS production of B. licheniformis DM-1. Cultivation was carried out under optimal conditions (BH medium supplemented with 2% sucrose and 5% w/v NaCl; 45 °C) unless stated otherwise.
Figure 4.
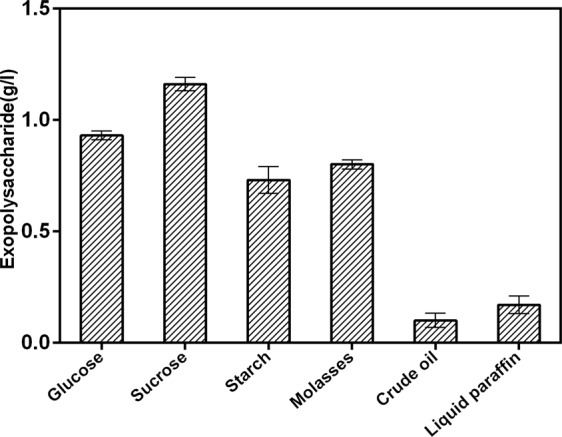
Effect of carbon sources on EPS production of B. licheniformis DM-1. Cultivation was carried out at 45 °C with a salinity of 5%.
Strain DM-1 showed unique properties to utilize hydrocarbons for the production of EPS, although the yield was not very high. This property is beneficial to the survival of DM-1 in the reservoir because crude oil is the most available and abundant carbon and energy sources in oil reservoirs33. The ability to utilize crude oil also makes DM-1 a potential agent for bioremediation of hydrocarbon contaminated sites, especially under in-situ conditions and extreme environments34. Accounting to relevant literature, many strains of B. licheniformis are able to grow and produce exopolymers, especially biosurfactants, at elevated temperature and high salt concentrations35,36. However, their ability to degrade long-chain alkanes and enhance oil recovery has rarely been reported37.
The production of EPS is often associated with the growth phase of bacteria. Some EPSs are only synthesized in the late logarithmic or stationary growth phase, while others are synthesized throughout the microbial growth process38. Figure 5 showed the dynamic relationships between bacterial cell growth (OD600), incubation time, and the EPS yield under optimal conditions (BH medium supplemented with 2% sucrose, 5% w/v NaCl, 45 °C, pH 7.0). The DM-1 EPS began to produce in the early growth stage, increased with the rise of bacterial cells and reached the highest value after about 60 h of incubation at the beginning of the stationary growth stage. Afterwards, EPS yield began to decline due to enzyme activity and cell lysis39. The maximum polysaccharide production was 1.29 g/l after 60 h of incubation under the culture conditions described above (Fig. 5).
Figure 5.
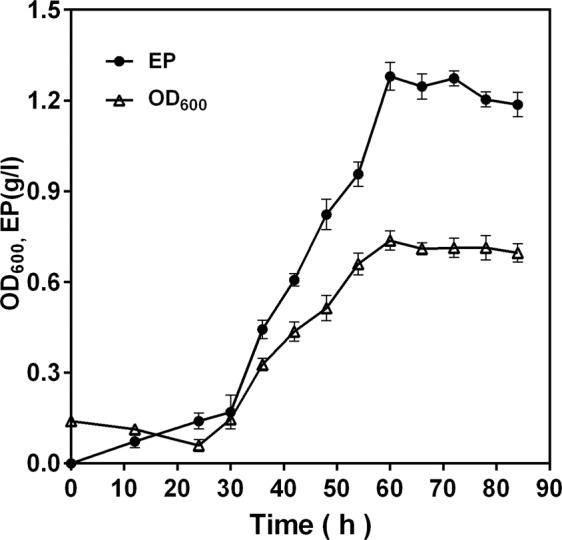
Time-course curve profile of EPS production (■) and bacterial growth (△) of B. licheniformis DM-1 on BH medium supplemented with 2% sucrose as carbon source at 45 °C.
Characterization of the EPS from strain DM-1
Results of FT-IR spectra analysis of the DM-1 polymer are shown in Fig. S2. The absorption peaks at 3422.41 and 1406.17 cm−1 were characteristic absorption peaks of O-H and C-O, which were caused by C-H and C-O stretching vibration, respectively. The peak at 2933.13 cm−1 was related to C-H stretching vibration. The absorption near 1600 cm−1 was the C-O-C stretching vibration. Furthermore, the weak peak at 780.42 cm−1 indicated that the sample contains β-D-type pyranose.
Compositional analyses revealed that the polysaccharide produced by DM-1 primarily consist of carbohydrate with relative percent of 67.4% sugar (w/w) and 27.6% (w/w) protein. HPLC analysis showed that the sugar fraction of the purified EPS contained an unusually large amount of mannose which accounted for approximately 91.87% of the total sugar weight (Fig. S3). Additionally, the other two sugars are glucose and galactose, which account for 7.95% and 0.18% of the total sugar weight, respectively. The protein of fraction of DM-1 EPS is mainly consist of Arg, Met, Ile, His and Lys identified by the paper chromatography method (Fig. S4).
Rheological properties of DM-1 polymer
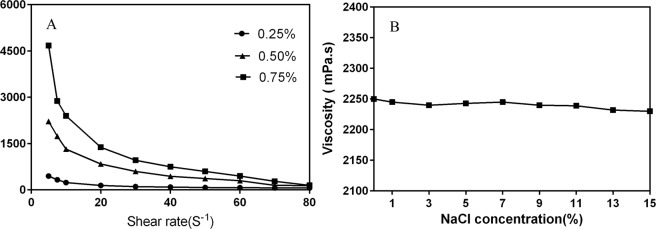
The viscosity of EPS at concentrations of 0.25%, 0.5%, and 0.75% at different shear rates were shown in Fig. 6. The DM-1 EPS showed a comparatively high viscosity at dilute concentrations. The viscosity value of 0.75% (w/v) EPS solution was 4672 mPa·s at 25 °C at a shear rate of 7.5·s−1. The viscosity of the EPS solution dropped sharply when the shear rate increased from 5.0 s to 80 s−1, indicating that the DM-1 EPS solution belonged to non-Newtonian behavior fluid. This shear thinning behavior of the EPS solution is mainly caused by its low relaxation state and destruction of the EPS structure40. The effects of concentration salinity on the viscosity of DM-1 polysaccharide showed that the DM-1 EPS solutions have high viscosities at each concentration tested, and increasing salt concentration scarcely reduced the viscosities of DM-1 EPS solutions.
Figure 6.
Effect of concentration, shear rate and salinity on the viscosities of DM-1 EPS.
The rates of viscosity decrease at 100 °C of xanthan gum and DM-1 EPS dissolved in distilled water was compared. The viscosity of the xanthan gum solution decreased more rapidly than did the viscosity of the DM-1 polymer solution. After 30 min at 100 °C, about a 50% decrease in viscosity (from 11506 to 5712 mPa·s) was observed for xanthan gum compared with about a 30% decrease in viscosity (from 6862 to 4795 mPa·s) for DM-1 EPS (Fig. 7). Thermal degradation of xanthan gum at 60 °C and 75 °C in distilled water has been previously observed35,41.
Figure 7.
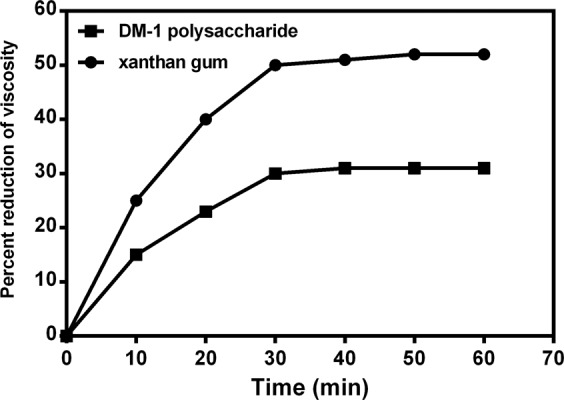
Effect of heating on the viscosities of xanthan gum and DM-1 EPS. The percent reduction in viscosity at 100 °C of a 1% (w/v) xanthan gum (●) or DM-1 EPS (■) was measured at 10-min intervals.
Utilization of n-alkanes (C12–C36) by B. licheniformis DM-1
Saturated alkanes (n-alkanes) are the main constituents of crude oil and are potentially the most available and abundant carbon and energy sources in oil reservoirs. Degradation of n-alkanes, especially long-chain alkanes, is of crucial importance for in-situ MEOR42. GC analysis results showed that strain DM-1 degraded a wide range of n-alkanes (C12–C36+), and preferentially degraded n-alkanes (C16–C22) with the degradation of higher than 70% after 10 d incubation (Fig. 8). Among them, the degradation of C18 was the highest, which was 81.33%, and the degradation rate of C36 was the lowest, which was 7.65%. To date, several thermophilic hydrocarbon-degrading microorganisms were isolated from oil wells, as follows: a strain of Acinetobacter sp., which is capable of degrading long-chain n-alkanes (C13-C44)43; Dietzia sp. DQ12-45-1b, which can utilize hydrocarbons with chain-lengths from C6 to C4023; and a strain of Geobacillus sp. and Rhodococcus sp., which are able to degrade n-alkanes up to C3622,44. To our knowledge, our study is the first report on a B. licheniformis strain that can degrade long-chain alkanes (C36+) at high temperature.
Figure 8.
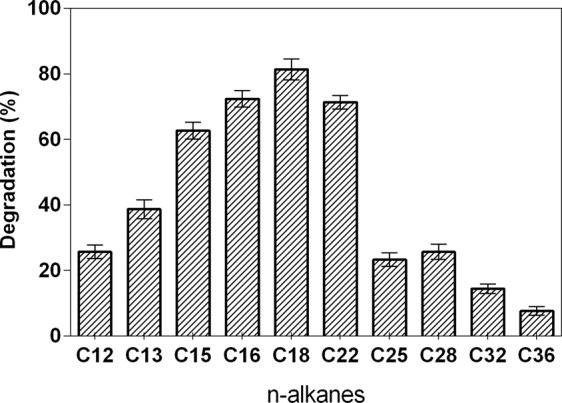
Degrading rate of the individual n-alkanes by B. licheniformis DM-1 over a period of 10 d (mean of three replicates; error bars represent s.d.).
Viscosity reduction of crude oil
The high viscosity of crude oil, especially in heavy oil reservoirs, is one of the major reasons that lead to the low oil recovery efficiency2. After treatment with DM-1 strain for 15 days, the crude oil viscosity decreased by 40%, from 110 mPa·s to 66 mPa·s. This indicates that strain DM-1 has potential for enhanced oil recovery by reducing the oil viscosity.
Oil recovery in the sandpack model
Sand pack flooding tests were carried out at simulated oil reservoir conditions to determine the in-situ MEOR efficiency of DM-1. The injected water and crude oil used in the tests were collected from the oil reservoir from which strain DM-1 was isolated. Moreover, a nutrient solution contained molasses (2%, w/v) was injected into the cores as the cosubstrate to improve EPS production and increase oil recovery. Results of the oil recovery were shown in Table 1. The oil recovery efficiency after the first water flooding was approximately 43%, which was attributed to volumetric sweep efficiency that occurred in the process of water injection14. After the first water flooding, bacterial culture and nutrient solution were injected to the cores, and the oil recovery efficiency increased by in-situ bioaugmentation with DM-1 was examined.The results revealed that for the nutrients flooding, 6.9% oil recovery efficiency was achieved, while for the in-situ test with strain DM-1 and nutrients (BH medium and 2% molasses), the recovery was 19.2% of OOIP. In contrast, it only increased by 3.8% with DM-1 injection only.
Table 1.
The parameters and results of the core-flooding test.
| Tested project | Porosity (%) | Permeability (μm2) | Oil recovery efficiency (%OOIP) | ||
|---|---|---|---|---|---|
| First water flooding | Subsequent water flooding | Recovery efficiency | |||
| Brine | 23.1 | 2.98 | 42.6 | 42.8 | 0.2 |
| DM-1+ Nutrients | 23.1 | 2.96 | 42.6 | 61.8 | 19.2 |
| DM-1 | 23.2 | 3.03 | 42.8 | 46.6 | 3.8 |
| Nutrients | 23.1 | 2.98 | 43.2 | 50.1 | 6.9 |
The oil recovery efficiency was within the range described in previous studies (Table 2).
Table 2.
Comparison of in-situ MEOR of different strains.
| Strain | Origin | Hydrocarbon utilization | Product | Test | Conditions | Oil recovery efficiency | Reference |
|---|---|---|---|---|---|---|---|
| Bacillus licheniformis DM-1 | A deep subterranean oil reservoir | Crude oil (C12-C36) | EPS | Sand pack flooding | 45 °C, 7 days | 3.8%–19.2% | This study |
| An engineered strain constructed from an EPS-producing strain and a thermophilic strain | Oil field | ND | EPS | Sand pack flooding | 40 °C, 7 days | 11.3% | 16 |
| Bacillus licheniformis BNP29 | Northern German oil reservoir | ND | EPS | Core flooding | 47 °C, 20 days | 9.3%–22.1% | 15 |
| Consortium of Clostridium spp. | Formation water from oil wells | ND | EPSs, acids, surfactants | Sand pack flooding, core flooding | 40 °C, 14 days | 26.7%, 10.1% | 46 |
| Consortium of Brevibacillus spp. | Brine samples from a high salinity petroleum reservoir | Crude oil | Lipopeptide | Sand pack flooding | 45 °C, 7 days | 7.03%–10.15% | 47 |
| Consortium of Enterobacter cloacae and Pseudomonas sp. | Crude oil-contaminated soil | Crude oil | Biosurfactant | Core flooding | 40 °C, 7 days | 19.1%–48.8% | 24 |
| Geobacillus stearothermophilus A-2 | Produced water of Dagang petroleum reservoir | Crude oil (C12-C36) | Bioemulsifier | Core flooding | 69 °C, 15 days | 6.8% | 22,48 |
| Consortium of Thermoanaerobacter spp. | A Mexican oil reservoir | Heavy hydrocarbon fractions | Biosurfactant, acids, gases | Core flooding | 70 °C, 20 days | 19.48% | 49 |
| Consortium of Thermoanaerobacter spp. | Formation fluids from different oil wells | ND | Biosurfactant, volatile fatty acids | Core flooding | 70 °C, 10 days | 19.2% | 50 |
| Consortium of Methanothermobacter sp. and Thermoanaerobacter sp. | Formation fluids from different oil wells | Crude oil | Volatile fatty acid, gases | Core flooding | 70 °C, 10 days | 15.49% | 51 |
| Bacillus subtilis M15-10-1 | Produced water of a water-flooded petroleum reservoir | — | Lipopeptide | Core flooding | 40 °C, 7 days | 16.71% | 52 |
ND, not determined; (−), Negative.
Our results indicated that the in-situ bioaugmentation with DM-1 can efficiently recover the trapped oil under harsh conditions similar to oil reservoir.
The improvement of oil recovery efficiency may be associated with the following oil displacement mechanisms of strain DM-1. First, strain DM-1 can produce an EPS with high viscosity, which can lower water-to-oil mobility, thus improving the sweep volume of the injected water. Second, strain DM-1 could degrade heavy oil fractions and decrease the viscosity of the crude oil. Upon exhaustion of the injected carbon source (molasses), DM-1 can degrade and utilize crude oil, thereby increasing the quantity of light crude oil. This lighter oil has low viscosity and better fluidity and is therefore easy to be recovered from producing well45.
Conclusion
An indigenous strain (B. licheniformis DM-1) showing superior properties required for in-situ MEOR was isolated from a mature oil reservoir in Dagang oilfield of China. B. Licheniformis DM-1 was able to utilize petroleum hydrocarbons and molasses for production of EPS under oil recovery conditions. The EPS from B. Licheniformis DM-1 was a glycoprotein with viscosifying and shear thinning properties. B. licheniformis DM-1 could degrade a wide range of n-alkanes with chain-lengths of C12–C36. Viscosity measurement shows that strain DM-1 reduced the viscosity of the crude oil by 40%. The results of sand pack flooding experiments showed that the in-situ bioaugmentation with strain DM-1 enhanced oil recovery by 19.2%, which highlighted its potential application in oil exploration. However, additional experimentations, such as further checking the EOR potential by using core plugs from a given oil field, monitoring the change in microbial communities during EOR via high-throughput sequencing, and evaluating problems caused by the growth of notorious sulfate-reducing bacteria, are necessary to support field trials.
Supplementary information
Acknowledgements
This study was supported by the Natural Science Foundation of Shandong Province of China (ZR2018LD005 and ZR2017PC010) and The key research and development program of Shandong Province (2019GNC106111). We are also thankful to ShineWrite. com (www.shinewrite.com) for English language editing.
Author contributions
Yanhui fan wrote the main manuscript text and analyzed the data; Jun Wang designed the idea of the manuscript; Chunming Gao and Wen Du designed the idea of the manuscript and checked the language of the manuscript; Yumiao Zhang analyzed the data and prepared Table 1.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
is available for this paper at 10.1038/s41598-020-65432-z.
References
- 1.Youssef N, Elshahed MS, Mclnerney MJ. Microbial processes in oil fields: culprits, problems, and opportunities. Adv. Appl. Microbiol. 2009;66:141–251. doi: 10.1016/S0065-2164(08)00806-X. [DOI] [PubMed] [Google Scholar]
- 2.Brown LR. Microbial enhanced oil recovery (MEOR) Curr. Opin. Microbiol. 2010;13:316–320. doi: 10.1016/j.mib.2010.01.011. [DOI] [PubMed] [Google Scholar]
- 3.Anbalagan S, et al. A review on systematic approach for microbial enhanced oil recovery technologies: opportunities and challenges. J. Cleaner Prod. 2020;258:120777. [Google Scholar]
- 4.Cui K, et al. Stimulation of indigenous microbes by optimizing the water cut in low permeability reservoirs for green and enhanced oil recovery. Sci. Rep. 2019;9:15772. doi: 10.1038/s41598-019-52330-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Taylor KC, Nasr-El-Din HA. Water-soluble hydrophobically associating polymers for improved oil recovery, a literature review. J. Pet. Sci. Eng. 1998;19:265–280. [Google Scholar]
- 6.Li YJ, et al. A microbial exopolysaccharide produced by sphingomonas species for enhanced heavy oil recovery at high temperature and high salinity. Energy Fuels. 2017;31:3960–3969. [Google Scholar]
- 7.Sheng J, Leonhardt B, Azri N. Status of polymer-flooding Technology. J. Can Petrol. Technol. 2015;54:116–126. [Google Scholar]
- 8.Jones D, Walters K. The behaviour of polymer solutions in extension dominated flows, with applications to enhanced oil recovery. Rheol. Acta. 1989;28:482–498. [Google Scholar]
- 9.Han DK, Yang CZ, Zhang ZQ, Lou ZH, Chang YI. Recent development of enhanced oil recovery in China. J. Petrol. Sci. Eng. 1999;22:181–188. [Google Scholar]
- 10.Scott AJ, Romero-Zerón L, Penlidis A. Evaluation of polymeric materials for chemical enhanced oil recovery. Processes. 2020;8:361. [Google Scholar]
- 11.Pu WF, Shen C, Wei B, Yang Y, Li YB. A comprehensive review of polysaccharide biopolymers for enhanced oil recovery (EOR) from flask to field. J. Ind. Eng. Chem. 2018;61:1–11. [Google Scholar]
- 12.Rellegadla S, Prajapat G, Agrawal A. Polymers for enhanced oil recovery, fundamentals and selection criteria. Biochem. Biotechnol. 2017;101:4387–4402. doi: 10.1007/s00253-017-8307-4. [DOI] [PubMed] [Google Scholar]
- 13.Yakimov MM, et al. The potential of Bacillus licheniformis, strains for in situ enhanced oil recovery. J. Petrol. Sci. Eng. 1997;18:147–160. [Google Scholar]
- 14.Sun S, et al. Construction and evaluation of an exopolysaccharide-producing engineered bacterial strain by protoplast fusion for microbial enhanced oil recovery. Technol. 2013;144:44–49. doi: 10.1016/j.biortech.2013.06.098. [DOI] [PubMed] [Google Scholar]
- 15.Saitou N, Nei M. The neighbor-joining method, a new method for reconstructing phylogenetic trees. Mol. Biol. Evol. 1987;4:406–425. doi: 10.1093/oxfordjournals.molbev.a040454. [DOI] [PubMed] [Google Scholar]
- 16.Tamura K, et al. MEGA6: Molecular evolutionary genetics analysis version 6.0. Mol. Biol. Evol. 2013;30:2725–2729. doi: 10.1093/molbev/mst197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ortega-Morales BO, et al. Characterization of extracellular polymers synthesized by tropical intertidal biofilm bacteria. J. Appl. Microbiol. 2007;102:254–264. doi: 10.1111/j.1365-2672.2006.03085.x. [DOI] [PubMed] [Google Scholar]
- 18.Dubois M, Gilles KA, Hamilton JK, Rebers PA, Smith F. Colorimetric method for determination of sugars and related substances. Anal. Chem. 1956;28:350–356. [Google Scholar]
- 19.Lowry OH, Rosebrough NJ, Farr AL, Farr AL. Protein measurement withthe folin phenol reagent. J. Biol. Chem. 1951;193:265–275. [PubMed] [Google Scholar]
- 20.Manocha MS, San-Blas G, Centeno S. Lipid composition of brasiliensis, possible correlation with virulence of different strains. Microbiology. 1980;117:147–154. doi: 10.1099/00221287-117-1-147. [DOI] [PubMed] [Google Scholar]
- 21.Squillaci G, et al. Production and properties of an exopolysaccharide synthesized by the extreme halophilic archaeon Haloterrigena turkmenica. Appl. Microbiol. Biotechnol. 2016;100:613–623. doi: 10.1007/s00253-015-6991-5. [DOI] [PubMed] [Google Scholar]
- 22.Zhou JF, et al. Heavy hydrocarbon degradation of crude oil by a novel thermophilic Geobacillus stearothermophilus strain A-2. Int. Biodeterior. Biodegrad. 2018;126:224–230. [Google Scholar]
- 23.Wang XB, et al. Degradation of petroleum hydrocarbons (C6–C40) and crude oil by a novel Dietzia strain. Bioresour. Technol. 2011;102:7755–7761. doi: 10.1016/j.biortech.2011.06.009. [DOI] [PubMed] [Google Scholar]
- 24.Darvishi P, Ayatollahi S, Mowla D, Niazi A. Biosurfactant production under extreme environmental conditions by an efficient microbial consortium, ERCPPI-2. Colloids Surf.,B. 2011;84:292–300. doi: 10.1016/j.colsurfb.2011.01.011. [DOI] [PubMed] [Google Scholar]
- 25.Makkar RS, Cameotra SS. Production of biosurfactant at mesophilic and thermophilic conditions by a strain of Bacillus subtilis. J. Ind. Microbiol. Biotechnol. 1998;20:48–52. [Google Scholar]
- 26.Joshi S, et al. Biosurfactant production using molasses and whey under thermophilic conditions. Bioresour. Technol. 2008;99:195–199. doi: 10.1016/j.biortech.2006.12.010. [DOI] [PubMed] [Google Scholar]
- 27.Freitas F, Alves VD, Reis MA. Advances in bacterial exopolysaccharides: from production to biotechnological applications. Trends Biotechnol. 2011;29:388–398. doi: 10.1016/j.tibtech.2011.03.008. [DOI] [PubMed] [Google Scholar]
- 28.Suresh Kumar A, Mody K, Jha B. Bacterial exopolysaccharides–A perception. J. Basic Microbiol. 2007;47:103–117. doi: 10.1002/jobm.200610203. [DOI] [PubMed] [Google Scholar]
- 29.Li H, Xu H, Xu H, Li S, Ouyang PK. Biosynthetic pathway of sugar nucleotides essential for welan gum production in Alcaligenes sp. CGMCC 2428. Microbiol. Biotechnol. 2010;86:295–303. doi: 10.1007/s00253-009-2298-8. [DOI] [PubMed] [Google Scholar]
- 30.Banik RM, Santhiagu A, Upadhyay SN. Optimization of nutrients for gellan gum production by Sphingomonas paucimobilis ATCC-31461 in molasses based medium using response surface methodology. Bioresour. Technol. 2007;98:792–797. doi: 10.1016/j.biortech.2006.03.012. [DOI] [PubMed] [Google Scholar]
- 31.Reis EC, et al. Biopolymer synthesized by strains of Xanthomonas sp isolate from Brazil using biodiesel-waste. Macromol. Symp. 2010;296:347–353. [Google Scholar]
- 32.Makkar RS, Cameotra SC. Biosurfactant production by microorganisms on unconventional carbon sources. J. Surfactants Deterg. 1999;2:237–241. [Google Scholar]
- 33.Feng L, et al. Genome and proteome of long-chain alkane degrading Geobacillus thermodenitrificans NG80-2 isolated from a deep-subsurface oil reservoir. Proc. Natl. Acad. Sci. USA. 2007;104:5602–5607. doi: 10.1073/pnas.0609650104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wang L, et al. Isolation and characterization of a novel thermophilic Bacillus strain degrading long-chain n-alkanes. Extremophiles. 2006;10:347–356. doi: 10.1007/s00792-006-0505-4. [DOI] [PubMed] [Google Scholar]
- 35.Pfiffner SM, McInerney MJ, Jenneman GE, Knapp RM. Isolation of halotolerant, thermotolerant, facultative polymer-producing bacteria and characterization of the exopolymer. Appl. Environ. Microbiol. 1986;51:1224–1229. doi: 10.1128/aem.51.6.1224-1229.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Thaniyavarn J, et al. Production and characterization of biosurfactants from Bacillus licheniformis F2.2. Biosci., Biotechnol. 2003;67:1239–1244. doi: 10.1271/bbb.67.1239. [DOI] [PubMed] [Google Scholar]
- 37.Purwasena IA, Astuti DI, Syukron M, Amaniyah M, Sugai Y. Stability test of biosurfactant produced by Bacillus licheniformis DS1 using experimental design and its application for MEOR. J. Petrol. Sci. Eng. 2019;183:106383. [Google Scholar]
- 38.Sutherland IW. Microbial polysaccharides from gram-negative. Int. Dairy J. 2001;11:663–674. [Google Scholar]
- 39.Gong WX. Bioflocculant production by culture of Serratia ficaria and its application in wastewater treatment. Bioresour. Technol. 2008;99:4668–4674. doi: 10.1016/j.biortech.2007.09.077. [DOI] [PubMed] [Google Scholar]
- 40.Annapurna B, Meenakshi S, Bhat SD, Seshadri S. Microbial extracellular polysaccharide-based membrane in polymer electrolyte fuel cells. Chem. Eng. J. 2013;231:373–379. [Google Scholar]
- 41.Kennedy JF. Production, properties and applications of xanthan. Prog. Ind. Microbiol. 1984;19:319–371. [Google Scholar]
- 42.Atlas RM, Atlas MC. Biodegradation of oil and bioremediation of oil spills. Curr. Opin. Biotech. 1991;2:440–443. [Google Scholar]
- 43.Sakai Y, Maeng JH, Tani Y, Kato N. Use of long-chain n-alkanes (C13-C44) by an isolate, Acinetobacter sp. M-1. Biosci., Biotechnol., Biochem. 1994;58:2128–2130. [Google Scholar]
- 44.Harner NK, et al. Microbial processes in the Athabasca Oil Sands and their potential applications in microbial enhanced oil recovery. J. Ind. Microbiol. Biot. 2011;38:1761–1775. doi: 10.1007/s10295-011-1024-6. [DOI] [PubMed] [Google Scholar]
- 45.van Beilen J, et al. Cloning of Baeyer-Villiger monooxygenases from Comamonas, Xanthobacter and Rhodococcus using polymerase chain reaction with highly degenerate primers. Environ. Microbiol. 2003;5:174–182. doi: 10.1046/j.1462-2920.2003.00401.x. [DOI] [PubMed] [Google Scholar]
- 46.Arora P, Ranade DR, Dhakephalkar PK. Development of a microbial process for the recovery of petroleum oil from depleted reservoirs at 91–96 °C. Bioresour. Technol. 2014;165:274–278. doi: 10.1016/j.biortech.2014.03.109. [DOI] [PubMed] [Google Scholar]
- 47.She YH, Shu FC, Wang ZL, Yu LJ. Investigation of indigenous microbial enhanced oil recovery in a middle salinity petroleum reservoir. Advanced Materials Research. 2011;365:326–331. [Google Scholar]
- 48.Zhou JF, et al. A novel bioemulsifier from Geobacillus stearothermophilus A-2 and its potential application in microbial enhanced oil recovery. RSC Adv. 2016;6:96347–96354. [Google Scholar]
- 49.Castorena-Cortés G, et al. Coreflood assay using extremophile microorganisms for recovery of heavy oil in Mexican oil fields. J. Biosci. Bioeng. 2012;114:440–445. doi: 10.1016/j.jbiosc.2012.05.011. [DOI] [PubMed] [Google Scholar]
- 50.Sharma N, Lavania M, Kukreti V, Rana DP, Lal B. Laboratory investigation of indigenous consortia TERIJ-188 for incremental oil recovery. Front. Microbiol. 2018;9:2357. doi: 10.3389/fmicb.2018.02357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Rathi R, et al. Stimulation of an indigenous thermophillic anaerobic bacterial consortium for enhanced oil recovery. RSC Adv. 2015;5:88815. [Google Scholar]
- 52.Gao PK, et al. An exogenous surfactant-producing Bacillus subtilis facilitates indigenous microbial enhanced oil recovery. Front. Microbiol. 2016;7:186. doi: 10.3389/fmicb.2016.00186. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.