The paradigmatic notion of apoptosis as a non-cell autonomous inert process has been challenged since the original discovery that dying cells can produce and release ‘find-me’ signals that account for phagocytes recruitment, corpse engulfment and tissue remodeling in distinct physiological contexts [1]. In the time frame spanning from the initiation of the apoptotic cascade to immune clearance, it remains unclear how dying cells influence neighboring cells in a tissue.
In a recent Nature paper, Ravichandran and collaborators hypothesize that apoptotic cells may coordinate pathophysiologically relevant stress responses by communicating their demise to neighboring live cells through their metabolic secretome [2]. The authors used different cellular models (Jurkat T cells, bone marrow derived macrophages, and primary mouse thymocytes) exposed to different apoptotic cues, in experimental settings in which 80% of the cells became apoptotic (yet maintained plasma membrane integrity). In these models, the authors performed an untargeted mass spectrometric metabolomics analysis of the intracellular versus extracellular metabolomes. Five metabolites (adenosine monophosphate, guanosine 5′-monophosphate, creatine, spermidine, and glycerol-3-phosphate) were found to be differentially enriched in the supernatant of apoptotic cells, irrespective of the cell line tested. A sixth extracellular metabolite, adenosine triphosphate (previously known as a chemotactic factor for inflammatory immune cells recruitment) [3] was found commonly upregulated after luciferase-based assessment. Importantly, the secretion of these metabolites was significantly attenuated by pre-treatment of cells with the pan-caspase inhibitor Z-VAD-fmk [4], reinforcing the idea that the apoptotic process is associated to the active release of biologically meaningful metabolites [2].
Intriguingly, only a selective subset of metabolites (even within the same metabolic route) was secreted by dying cells, suggesting the existence of a maximum size limit for the release of metabolites during apoptosis. In line with this speculation, the authors found that the Pannexin 1 (PANX1) channel, which can be cleaved and activated by effector caspases (caspase-3 or -7) in the plasma membrane [5], was responsible for the apoptosis-associated release of these intracellular metabolites [2]. Accordingly, a series of experimental maneuvers including the genetic ablation of PANX1, the expression of a caspase-insensitive dominant PANX1 mutant or the pharmacological inhibition of PANX1, invariably abrogated metabolite secretion (without impairing the apoptotic program). Metabolomics analysis of the secretome of apoptotic cells performed with or without PANX1 inhibition allowed to define a PANX1-dependent extracellular metabolic signature that included, beyond the abovementioned metabolites, fructose-1,6-bisphosphate [FBP], dihydroxyacetone phosphate, UDP-glucose, and inosine-5′-monophosphate [IMP].
The authors then investigated whether metabolites secreted by apoptotic cells would represent an epiphenomenon or actually would mirror the pre-mortem metabolic activity of dying cells. Based on the observation that significant levels of spermidine were released across all the models assessed [2], and knowing that exogenous supplementation of spermidine to mice is able to exert a plethora of beneficial effects in different physiopathological conditions (including but not limited to lifespan extension, cardioprotection and anti-obesity effects) [6], the authors focused their attention on spermidine biosynthetic pathways in apoptotic cells. In contrast to the recent finding that a large subset of RNA species undergoes PNPT1-dependent decay upon apoptosis-associated MOMP [7], Ravichandran et al. found that mRNAs encoding enzymes involved in spermidine biosynthesis were retained during the apoptotic process. Accordingly, 13C-labeled arginine administered to dying cells was rapidly converted into spermidine, corroborating the hypothesis that polyamine biosynthesis is elevated in pre-mortem conditions [8]. Altogether, these results highlight an unexpected role for neo-synthesized extracellular spermidine in the regulation of tissue homeostasis and local inflammation and pave the way to new lines of research that aim at exploring the role of polyamines metabolism in the regulation of tissue regeneration and wound healing [6].
To obtain further insights on the paracrine effect of the apoptotic secretome, phagocytic LR73 Chinese hamster ovary cells were exposed to the supernatants of WT or PANX−/− Jurkat T cells. RNAseq analysis confirmed that metabolites secreted in a PANX1-dependent fashion caused extensive reprogramming of the transcriptome, including increase expression of genes involved in tissue repair and wound healing (AREG and PTGS2), immune tolerance (NR4A1 and PBX1) and metabolism (SLC14A1, SGK1, and UAP1).
To extend the relevance of these finding to in vivo settings, Ravichandran et al. took advantage of a Panx1fl/fl; Cd4-Cre mouse in which (upon 4-hydroxytamoxifen administration) Panx1 was deleted only in thymocytes (yet remained functionally expressed in the thymic myeloid compartment). After dexamethasone-induced induction of apoptosis in Cd4+ thymocytes [9], the gene expression profile of CD11b+ and CD11c+ myeloid cells exposed to the apoptotic secretome presented a high degree of convergence with the in vitro signature. Consistently, the genetic ablation of Panx1 was able to abolish secretome-induced gene expression changes, further highlighting the importance of Panx1 for the paracrine effects of the apoptotic secretome [2].
Driven by the observation that apoptotic cells secretome elicited a prominent anti-inflammatory gene signature in target cells, the authors decided to directly assess the pre-clinical values of these metabolites in two different mouse models of exacerbated inflammation, namely rheumatoid arthritis and host-graft lung transplant rejection [10, 11]. In these models, administration of two different cocktails of three (Memix-3) or six (Memix-6) metabolites that both contained spermidine (but not the administration of single metabolites) alleviated signs of excessive myeloid cell activation and T-cell mediated lung rejection.
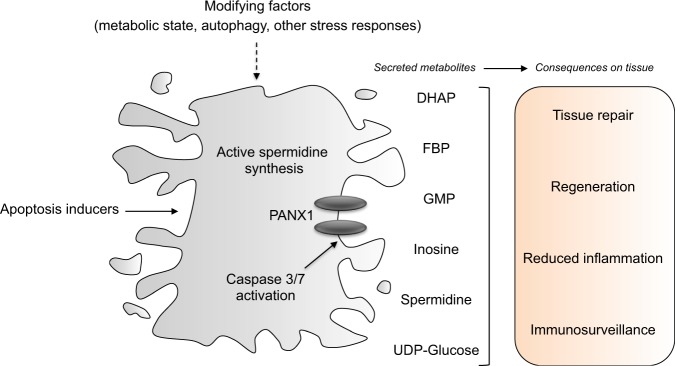
Overall, these data open the way to different therapeutic perspectives, depending on the pathological context (Fig. 1). Thus, apoptosis-inducing therapies might be combined with the administration of metabolic cocktails inspired by the premortem secretome to counteract tissue fibrosis/inflammation and to promote tissue repair/regeneration [12, 13]. Moreover, the activation of these pathways may be advantageous in a scenario of pro-apoptotic immunogenic chemotherapy, as PANX1 channels might enhance myeloid cell recruitment into tumors and foster the initiation of the cancer-immunity cycle [14].
Fig. 1. Pathophysiological implication of metabolite release by apoptotic cells on tissue dynamics.
Apopotic cells display active polyamine biogenesis and influence the behavior of neighbors live cells through the PANX1-dependent secretion of a combination of metabolites. The biological effects of the apoptotic secretome (that could be modulated upstream by strategies targeting metabolic circuitries or cellular responses to stress) may be harnessed to improve tissue response to damage and anti-cancer immunosruveillance.
Acknowledgements
GK is supported by the Ligue contre le Cancer (équipe labellisée); Agence National de la Recherche (ANR)—Projets blancs; ANR under the frame of E-Rare-2, the ERA-Net for Research on Rare Diseases; Association pour la recherche sur le cancer (ARC); Cancéropôle Ile-de-France; Chancellerie des universités de Paris (Legs Poix), Fondation pour la Recherche Médicale (FRM); a donation by Elior; European Research Area Network on Cardiovascular Diseases (ERA-CVD, MINOTAUR); Gustave Roussy Odyssea, the European Union Horizon 2020 Project Oncobiome; Fondation Carrefour; High-end Foreign Expert Program in China (GDW20171100085 and GDW20181100051), Institut National du Cancer (INCa); Inserm (HTE); Institut Universitaire de France; LeDucq Foundation; the LabEx Immuno-Oncology; the RHU Torino Lumière; the Seerave Foundation; the SIRIC Stratified Oncology Cell DNA Repair and Tumor Immune Elimination (SOCRATE); and the SIRIC Cancer Research and Personalized Medicine (CARPEM). FP is supported by a Karolinska Institute Starting Grant; Starting Grant from the Swedish Research Council (2019_02050_3).
Compliance with ethical standards
Conflict of interest
GK is cofounder of Samsara Therapeutics, everImmune and Therafast Bio.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Guido Kroemer, Email: kroemer@orange.fr.
Federico Pietrocola, Email: federico.pietrocola@ki.se.
References
- 1.Nagata S. Apoptosis and clearance of apoptotic cells. Annu Rev Immunol. 2018;36:489–517. doi: 10.1146/annurev-immunol-042617-053010. [DOI] [PubMed] [Google Scholar]
- 2.Medina CB, Mehrotra P, Arandjelovic S, Perry JSA, Guo Y, Morioka S. Metabolites released from apoptotic cells act as tissue messengers. Nature. 2020;580:130–5. doi: 10.1038/s41586-020-2121-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wang Y, Martins I, Ma Y, Kepp O, Galluzzi L, Kroemer G. Autophagy-dependent ATP release from dying cells via lysosomal exocytosis. Autophagy. 2013;9:1624–5. doi: 10.4161/auto.25873. [DOI] [PubMed] [Google Scholar]
- 4.Galluzzi L, Lopez-Soto A, Kumar S, Kroemer G. Caspases connect cell-death signaling to organismal homeostasis. Immunity. 2016;44:221–31. doi: 10.1016/j.immuni.2016.01.020. [DOI] [PubMed] [Google Scholar]
- 5.Chekeni FB, Elliott MR, Sandilos JK, Walk SF, Kinchen JM, Lazarowski ER, et al. Pannexin 1 channels mediate ‘find-me’ signal release and membrane permeability during apoptosis. Nature. 2010;467:863–7. doi: 10.1038/nature09413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Madeo Frank, Eisenberg Tobias, Pietrocola Federico, Kroemer Guido. Spermidine in health and disease. Science. 2018;359(6374):eaan2788. doi: 10.1126/science.aan2788. [DOI] [PubMed] [Google Scholar]
- 7.Liu X, Fu R, Pan Y, Meza-Sosa KF, Zhang Z, Lieberman J. PNPT1 release from mitochondria during apoptosis triggers decay of Poly(A) RNAs. Cell. 2018;174:187–201. e112. doi: 10.1016/j.cell.2018.04.017. [DOI] [PubMed] [Google Scholar]
- 8.Pegg AE. Mammalian polyamine metabolism and function. IUBMB Life. 2009;61:880–94. doi: 10.1002/iub.230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tonomura N, McLaughlin K, Grimm L, Goldsby RA, Osborne BA. Glucocorticoid-induced apoptosis of thymocytes: requirement of proteasome-dependent mitochondrial activity. J Immunol. 2003;170:2469–78. doi: 10.4049/jimmunol.170.5.2469. [DOI] [PubMed] [Google Scholar]
- 10.Smolen JS, Aletaha D, Barton A, Burmester GR, Emery P, Firestein GS, et al. Rheumatoid arthritis. Nat Rev Dis Prim. 2018;4:18001. doi: 10.1038/nrdp.2018.1. [DOI] [PubMed] [Google Scholar]
- 11.Mori DN, Kreisel D, Fullerton JN, Gilroy DW, Goldstein DR. Inflammatory triggers of acute rejection of organ allografts. Immunol Rev. 2014;258:132–44. doi: 10.1111/imr.12146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhao X, Kwan JYY, Yip K, Liu PP, Liu FF. Targeting metabolic dysregulation for fibrosis therapy. Nat Rev Drug Discov. 2020;19:57–75. doi: 10.1038/s41573-019-0040-5. [DOI] [PubMed] [Google Scholar]
- 13.Eming SA, Wynn TA, Martin P. Inflammation and metabolism in tissue repair and regeneration. Science. 2017;356:1026–30. doi: 10.1126/science.aam7928. [DOI] [PubMed] [Google Scholar]
- 14.Kroemer G, Galluzzi L, Kepp O, Zitvogel L. Immunogenic cell death in cancer therapy. Annu Rev Immunol. 2013;31:51–72. doi: 10.1146/annurev-immunol-032712-100008. [DOI] [PubMed] [Google Scholar]