Abstract
To estimate the incidence of pulmonary tuberculosis (PTB) in Chinese diabetes patients and to evaluate the effect of blood glucose on PTB risk, a retrospective cohort study was built based on the diabetes management system in Shanghai and included 240,692 adults aged 35 or above. Incidences of PTB in all diabetes patients and by subgroups were calculated and compared. Multivariable Cox regression models with restricted cubic splines were used to evaluate the association of fasting plasma glucose (FPG) with the risk of PTB. A total of 439 incident PTB cases were identified in the cohort after an average of 3.83 years of follow-up. The overall PTB incidence rate was 51.3/100,000 in diabetes patients, and annual incidence remained higher than that in general population. The PTB incidence rate of diabetes patients was higher in men than in women (86.2 vs. 22.1 per 100,000) and was highest in patients with body mass index (BMI) < 18.5 kg/m2 (215.2/100,000) or FPG ≥ 10 mmol/L (143.2/100,000). Our results suggest that the risk of tuberculosis may be greater at higher levels of FPG in diabetes patients of normal weight. Specific tuberculosis screening strategies for different characteristic diabetes population should be provided to prevent and control tuberculosis in China.
Subject terms: Type 2 diabetes, Tuberculosis
Introduction
Tuberculosis (TB) remains a major global public health issue despite its slowly decreased incidence, particularly in developing countries. The World Health Organization (WHO) reported that an estimated 10 million people developed TB and 1.6 million people died of TB worldwide in 20171. At the same time, type 2 diabetes mellitus (T2DM) is a global epidemic. It is estimated that there were around 425 million prevalent diabetes patients worldwide in 2017, and the number is expected to be almost 700 million by 20452.
The associations of T2DM with the incidence, severity and clinical outcome of TB have been well documented3–7. The estimated number of incident TB attributable to T2DM increased from 10% in 2010 to 15% in 2013, globally, and almost 17% of diabetes-associated TB cases occurred in China8. A recent meta-analysis demonstrated that T2DM was associated with a two- to four-fold increased risk of active TB9. Shanghai, a typical megacity of China, experiencing rapid population aging, urbanization, industrialization and changes in lifestyle, is bearing heavy dual burden of prevention and control for diabetes and TB. Although TB incidence rate is relatively low in Shanghai, approximately 26.9 per 100,000 in 2015, the prevalence of diabetes is dramatically increasing. A cross-sectional investigation in 2013 reported that overall weighted prevalence of diabetes was 17.6% among Shanghai residents aged 35 or above, which was much higher than the nationwide average level10.
Therefore, to realize the WHO’s END TB Strategy, which was approved by the 67th World Health Assembly in 201411, the Shanghai Municipal Commission of Health and Family Planning launched “Plan for Hierarchical Diagnosis and Treatment and Comprehensive Prevention and Treatment of Tuberculosis” in 2017, particularly addressing the implementation of screening for TB in patients with T2DM. However, the validity and reliability of screening for TB among T2DM were not convincing due to the lack of well-determined screening criteria and methods. A prospective study assessing the screening for TB in T2DM patients in community health settings in Kunming, China, found only a small number of TB cases were diagnosed among the T2DM patients with a positive symptom screen12. Owing to limited medical resources and relatively low incidence rate in Shanghai, it is important to identify risk factors of TB in T2DM patients in order to obtain more beneficial effectiveness.
Additionally, several studies have suggested that adequate management of blood glucose would have a significant positive influence on the reduction of TB incidence and mortality13–15. However, on the one hand, published studies on comprehensive epidemics of TB among T2DM patients in China are considerably insufficient. On the other hand, evidence on the association between TB incidence risk and blood glucose is still not consistent worldwide16–18.
Given the above situation, our study was aimed to analyse epidemiological characteristics of pulmonary tuberculosis (PTB) in T2DM patients and its association with fasting plasma glucose (FPG), and eventually to optimize TB screening strategies among T2DM patients in Shanghai, China.
Methods
Data sources
The diabetes patient data used in the study were from Shanghai Standardized Diabetes Management System (SSDMS) operated by Shanghai Municipal Center for Disease Control and Prevention. The system was initiated in 2004 and well-established after the launch of the National Basic Public Health Service Program (NBPHSP) in 200919. According to the requirement of the NBPHSP, in Shanghai, community health centres (CHCs) take responsibility to provide management for T2DM patients aged 35 years or above and to upload the electronic records to SSDMS. Nearly half of all diagnosed T2DM cases in Shanghai were registered in the system10, including newly diagnosed cases through community-based screenings or physical examinations and previously diagnosed cases through routine outpatient visits. Baseline information for each T2DM case in SSDMS, including height, weight, waist and hip circumference, blood pressure and blood glucose, were collected through an initial assessment before being registered.
Incident PTB of managed T2DM patients was identified through the record linkage to the Shanghai TB surveillance system and mandatory reporting system using the unique identification card number for each individual. The Shanghai TB surveillance and mandatory reporting system has been established since the 1990s, covering all PTB patients in Shanghai. Each TB patient in the system was confirmed by physicians in designated TB hospitals/clinics through conducting a sputum smear test and bacterial culture.
The deaths of managed T2DM patients were identified by annual record linkage to the Shanghai Vital Statistics Registry during the whole study period.
This study was approved by the Ethical Review Committee of Shanghai Municipal Center for Disease Control and Prevention. Since this was a retrospective study and all patients’ information used was routinely collected through the SSDMS, the Shanghai TB surveillance and mandatory reporting system and the Shanghai Vital Statistics Registry, requirement for informed consent was exempted by the committee mentioned above.
Study design and population
A retrospective cohort was established based on the Shanghai Standardized Diabetes Management System. By the end of 2015, a total of 552,377 T2DM patients had been registered in the system. In this study, we excluded 311,685 patients diagnosed with T2DM before January 1, 2010. Ultimately, a total of 240,692 T2DM patients were included in the present analyses. The patients were censored as lost to follow-up if they died or moved out of Shanghai.
Related definitions
T2DM patients were defined as (1) FPG level of 7.0 mmol/L or higher, or (2) 2-h plasma glucose (venous plasma glucose 2 h after ingestion of 75 g oral glucose load) level of 11.1 mmol/L or higher according to the 1999 WHO criteria20. The subgroups of FPG level were defined for certain analysis: <4.4 mmol/L, 4.4–5.9 mmol/L, 6.0–7.9 mmol/L, 8.0–9.9 mmol/L and ≥10.0 mmol/L according to the Chinese expert consensus on management of diabetes in Chinese adults21.
PTB cases were defined as individuals who had positive sputum smear, positive sputum culture, or pulmonary lesions of tuberculosis that had been confirmed by pathological examination according to the Guideline for National TB Control Program22. Additionally, in our study, only individuals whose date of diagnosis with PTB was posterior to the date of diagnosis with T2DM could be confirmed as PTB cases.
Body mass index (BMI) was calculated as weight (kg) divided by the square of height (m). The diabetes patients were classified into three groups according to BMI level: underweight (<18.5 kg/m2), normal weight (18.5–24.9 kg/m2) or overweight/obese (≥25 kg/m2)23. Central obesity was defined as waist circumference over 90 cm for men or more than 85 cm for women. High blood pressure was defined as systolic blood pressure ≥140 mmHg or diastolic blood pressure ≥90 mmHg.
Statistical analysis
The annual incidence of PTB among T2DM patients in the cohort from 2010 to 2015 was calculated as the number of PTB cases divided by the average number of patients, which was estimated based upon the population numbers at the start and end of each year. The age-standardized rates per 100,000 for each year were calculated using the direct method, based on the 2010 Shanghai standardized population by 5-year age groups from the Shanghai Vital Statistics Registry and age-specific annual incidence. The annual incidence in the general population from 2010 to 2015 was calculated by the number of incident PTB divided by the number of adults aged 35 or above in the Shanghai population in a given year. Person-years (PYs) of follow-up were calculated from the date that T2DM was first diagnosed to the date of diagnosis with PTB, the date of loss to follow-up, or the end of the study, December 31, 2015, whichever occurred first. The overall or group-specific incidence rate of PTB was calculated by the number of incident PTB cases divided by person-years of follow-up.
Comparison of annual incidence of PTB between T2DM patients and the general population was conducted using a Z test. A log-rank test was used to examine the difference of PTB incidence rate across subgroups, and a Kaplan-Meier survival curve was used by FPG category. The Cox proportional hazards regression model was applied to access the association between FPG and risk of PTB incidence. FPG was used as a continuous variable as well as a categorical variable and confounding variables including age, sex and BMI were adjusted in the model. The potential non-linear relationship was also detected among all T2DM patients and by sex using restricted cubic splines (RCS) with the 5th, 50th and 95th percentiles as fixed knots24 and FPG was used as a continuous variable. Two statistical tests were performed through the RCS procedure. The first null hypothesis was that the regression coefficients of both linear and non-linear terms of the factor were equal to zero, and the result was presented as “P for overall association”. The other one was the test of the regression coefficient of nonlinear term, and the result of “P for non-linearity” <0.05 implied a non-linear association.
Statistical analyses were performed using SAS version 9.4 (SAS Institute, Inc., Cary, North Carolina). RCS was completed by SAS macro%RCS. P < 0.05 was considered statistically significant.
Results
As shown in Table 1, a total of 110,262 male (45.8%) and 130,430 female (54.2%) T2DM patients were included in this study. The proportion of T2DM patients aged 65 years or older increased continuously from 36.4% in 2010 to 49.9% in 2015. The proportion of women remained higher than men over the six calendar years.
Table 1.
Description of newly enrolled and eligible T2DM patients in the cohort by calendar year.
| N (%) | 2010 | 2011 | 2012 | 2013 | 2014 | 2015 | P value* |
|---|---|---|---|---|---|---|---|
| Overall | 48 289 (20.1) | 47 506 (19.7) | 49 125 (20.4) | 39 133 (16.3) | 32 836 (13.6) | 23 803 (9.9) | |
| Age group (years) | <0.001 | ||||||
| 35–44 | 1801 (3.7) | 1650 (3.5) | 1606 (3.3) | 1197 (3.1) | 915 (2.8) | 610 (2.6) | |
| 45–54 | 10 699 (22.2) | 9427 (19.8) | 8846 (18.0) | 5862 (15.0) | 4543 (13.8) | 2873 (12.1) | |
| 55–64 | 18 197 (37.7) | 17 920 (37.7) | 18 263 (37.2) | 14 857 (38.0) | 12 245 (37.3) | 8452 (35.5) | |
| 65–74 | 10 953 (22.7) | 11 073 (23.3) | 12 347 (25.1) | 10 152 (25.9) | 8881 (27.1) | 6903 (29.0) | |
| 75– | 6639 (13.8) | 7436 (15.7) | 8063 (16.4) | 7065 (18.1) | 6252 (19.0) | 4965 (20.9) | |
| Sex | 0.001 | ||||||
| Men | 21 843 (45.2) | 21 557 (45.4) | 22 801 (46.4) | 17 929 (45.8) | 15 110 (46.0) | 11 022 (46.3) | |
| Women | 26 446 (54.8) | 25 949 (54.6) | 26 324 (53.6) | 21 204 (54.2) | 17 726 (54.0) | 12 781 (53.7) |
*P values were calculated using a Chi-square test.
A total of 439 incident PTB cases were identified during the study period. As presented in Table 2, the annual incidence of PTB in T2DM patients did not change substantially from 2010 to 2015 and was significantly higher than in the general population except in 2010. While the annual incidence and age-standardized rate (ASR) of PTB in T2DM patients ranged from 76.4 and 99.9/100,000 in 2011 to 44.5 and 50.8/100,000 in 2015, respectively, the incidence in the general population was around 30.0/100,000 during the six years. The incidence was much higher in men than in women in both T2DM patients and general population.
Table 2.
Comparison of annual incidence of PTB/100000 population in T2DM patients and the general population in Shanghai by calendar year.
| Population | Calendar year | Population at risk | No. of PTB cases | Annual incidence (1/100000) | ASR (1/100000) | Annual incidence in general population (1/100000) | P value* | ||
|---|---|---|---|---|---|---|---|---|---|
| Estimate | 95% CI | ||||||||
| All subjects | |||||||||
| 2010 | 36 777 | 17 | 46.2 | 67.0 | 16.9 | 117.2 | 29.3 | 0.058 | |
| 2011 | 72 031 | 55 | 76.4 | 99.9 | 63.3 | 136.5 | 28.9 | <0.001 | |
| 2012 | 120 266 | 63 | 52.4 | 73.0 | 46.1 | 100.0 | 28.1 | <0.001 | |
| 2013 | 164 145 | 91 | 55.4 | 64.7 | 42.5 | 86.9 | 29.1 | <0.001 | |
| 2014 | 199 592 | 112 | 56.1 | 53.6 | 41.3 | 65.9 | 31.0 | <0.001 | |
| 2015 | 226 920 | 101 | 44.5 | 50.8 | 34.4 | 67.2 | 29.4 | <0.001 | |
| Men | |||||||||
| 2010 | 16 530 | 10 | 60.5 | 88.6 | 8.1 | 169.2 | 46.0 | 0.383 | |
| 2011 | 32 616 | 43 | 131.8 | 156.3 | 96.6 | 215.9 | 44.6 | <0.001 | |
| 2012 | 54 753 | 47 | 85.8 | 105.6 | 66.3 | 144.9 | 43.3 | <0.001 | |
| 2013 | 74 986 | 72 | 96.0 | 105.4 | 68.8 | 142.0 | 44.7 | <0.001 | |
| 2014 | 91 237 | 86 | 94.3 | 87.1 | 65.7 | 108.4 | 47.3 | <0.001 | |
| 2015 | 103 798 | 78 | 75.2 | 79.1 | 54.2 | 104.0 | 44.5 | <0.001 | |
| Women | |||||||||
| 2010 | 20 247 | 7 | 34.6 | 39.5 | 7.1 | 71.9 | 13.2 | 0.008 | |
| 2011 | 39 415 | 12 | 30.5 | 49.1 | 10.5 | 87.8 | 13.8 | 0.005 | |
| 2012 | 65 513 | 16 | 24.4 | 44.0 | 3.5 | 84.5 | 13.3 | 0.014 | |
| 2013 | 89 159 | 19 | 21.3 | 22.8 | 7.8 | 37.7 | 14.0 | 0.065 | |
| 2014 | 108 356 | 26 | 24.0 | 24.9 | 11.4 | 38.4 | 15.3 | 0.020 | |
| 2015 | 123 123 | 23 | 18.7 | 25.1 | 3.2 | 46.9 | 14.8 | 0.259 | |
Abbreviation: T2DM, type 2 diabetes; PTB, pulmonary tuberculosis; ASR: age-standardized rate.
*P values were calculated using a Z test.
The patients were followed up for 855,782 person-years. As shown in Table 3, the incidence rate of PTB in T2DM patients was 51.3 per 100,000 person-years in all patients, 86.2 per 100,000 person-years in men and 22.1 per 100,000 person-years in women. A significant difference was observed in incidence rate of PTB by age groups, BMI, status of central obesity and FPG level. In particular, incidence of PTB reached 215.2 per 100,000 person-years in T2DM patients with BMI < 18.5 kg/m2 (442.0 per 100,000 person-years in men and 77.0 per 100,000 person-years in women), much higher than those in T2DM patients with BMI ≥ 18.5 kg/m2. A significantly higher incidence of PTB was also observed in T2DM patients with FPG ≥ 10.0 mmol/L, which was as high as 143.2 per 100,000 person-years in all patients. Moreover, in the Kaplan-Meier plot, there was a significant separation between the subgroup of FPG ≥ 10.0 mmol/L and the other four subgroups (Fig. 1). Interestingly, we did not find a significant difference in the incidence rate of PTB by FPG levels in women.
Table 3.
Incidence rate of PTB per 100000 person-years among T2DM patients by subgroups.
| All subjects | Men | Women | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| No. of T2DM patients | Person years | No. of PTB cases | Incidence rate (1/100000) | P value* | No. of T2DM patients | Person years | No. of PTB cases | Incidence rate (1/100000) | P value* | No. of T2DM patients | Person years | No. of PTB cases | Incidence rate (1/100000) | P value* | |
| Overall | 240 692 | 855782 | 439 | 51.3 | 110262 | 389713 | 336 | 86.2 | 130 430 | 466069 | 103 | 22.1 | |||
| Age group (years) | <0.001 | 0.027 | 0.121 | ||||||||||||
| 35–44 | 7779 | 29150 | 23 | 78.9 | 4841 | 17937 | 17 | 94.8 | 2938 | 11213 | 6 | 53.5 | |||
| 45–54 | 42 250 | 163970 | 112 | 68.3 | 20 571 | 78761 | 90 | 114.3 | 21 679 | 85209 | 22 | 25.8 | |||
| 55–64 | 89 934 | 322037 | 157 | 48.8 | 40 610 | 144765 | 117 | 80.8 | 49 324 | 177272 | 40 | 22.6 | |||
| 65–74 | 60 309 | 207232 | 93 | 44.9 | 27 700 | 94633 | 74 | 78.2 | 32 609 | 112599 | 19 | 16.9 | |||
| 75– | 40 420 | 133393 | 54 | 40.5 | 16 540 | 53617 | 38 | 70.9 | 23 880 | 79776 | 16 | 20.1 | |||
| BMI (kg/m2) a | <0.001 | <0.001 | <0.001 | ||||||||||||
| <18.5 | 3491 | 12548 | 27 | 215.2 | 2140 | 4752 | 21 | 442.0 | 1351 | 7796 | 6 | 77.0 | |||
| 18.5–24.9 | 139 819 | 496125 | 321 | 64.7 | 74 468 | 230843 | 252 | 109.2 | 65 351 | 265281 | 69 | 26.0 | |||
| ≥25.0 | 91 108 | 329073 | 85 | 25.8 | 50 530 | 145608 | 58 | 39.8 | 40 578 | 183465 | 27 | 14.7 | |||
| Central obesity b | <0.001 | <0.001 | 0.010 | ||||||||||||
| Yes | 100 940 | 367961 | 132 | 35.9 | 42 155 | 154020 | 97 | 63.0 | 58 785 | 213941 | 35 | 16.4 | |||
| No | 128 844 | 458011 | 294 | 64.2 | 62 730 | 220968 | 228 | 103.2 | 66 114 | 237042 | 66 | 27.8 | |||
| High blood pressure c | 0.573 | 0.841 | 0.106 | ||||||||||||
| Yes | 45 833 | 159765 | 78 | 48.8 | 22 311 | 77590 | 66 | 85.1 | 23 522 | 82175 | 12 | 14.6 | |||
| No | 191 071 | 683430 | 357 | 52.2 | 86 145 | 306171 | 267 | 87.2 | 104 926 | 377259 | 90 | 23.9 | |||
| FPG (mmol/L) d | <0.001 | <0.001 | 0.245 | ||||||||||||
| <4.4 | 554 | 2017 | 1 | 49.6 | 271 | 1000 | 1 | 100.0 | 283 | 1018 | 0 | 0 | |||
| 4.4–5.9 | 29 235 | 113187 | 47 | 41.5 | 12 652 | 48308 | 33 | 68.3 | 16 583 | 64879 | 14 | 21.6 | |||
| 6.0–7.9 | 152 066 | 541679 | 260 | 48.0 | 68 481 | 243693 | 200 | 82.1 | 83 585 | 297985 | 60 | 20.1 | |||
| 8–9.9 | 26 188 | 87119 | 51 | 58.5 | 12 744 | 42131 | 39 | 92.6 | 13 444 | 44988 | 12 | 26.7 | |||
| ≥10 | 9956 | 30031 | 43 | 143.2 | 5307 | 15753 | 36 | 228.5 | 4649 | 14277 | 7 | 49.0 | |||
| Interval between diagnosis and registry (years) † | 0.378 | 0.569 | 0.353 | ||||||||||||
| <0.5 | 92 954 | 231161 | 115 | 49.8 | 42 660 | 104721 | 88 | 84.0 | 50 294 | 126440 | 27 | 21.4 | |||
| 0.5–0.9 | 34 267 | 119121 | 65 | 54.6 | 14 973 | 51336 | 51 | 99.4 | 19 294 | 67786 | 14 | 20.7 | |||
| 1–1.4 | 27 901 | 100759 | 50 | 49.6 | 12 817 | 45836 | 41 | 89.5 | 15 084 | 54922 | 9 | 16.4 | |||
| 1.5–1.9 | 19 589 | 85666 | 54 | 63.0 | 8876 | 38509 | 38 | 98.7 | 10 713 | 47158 | 16 | 33.9 | |||
| ≥2 | 65 981 | 319074 | 155 | 48.6 | 30 936 | 149311 | 118 | 79.0 | 35 045 | 169763 | 37 | 21.8 | |||
Abbreviation: T2DM, type 2 diabetes; PTB, pulmonary tuberculosis; BMI, body mass index; FPG, fasting plasma glucose.
*P values were calculated using a Log-rank test.
†Referring to interval between date of diagnosis of T2DM and date of registration in Shanghai Standardized Diabetes Management System.
a6274 missing values excluded from analysis; b10908 missing values excluded from analysis; c3788 missing values excluded from analysis; d22693 missing values excluded from analysis.
Figure 1.
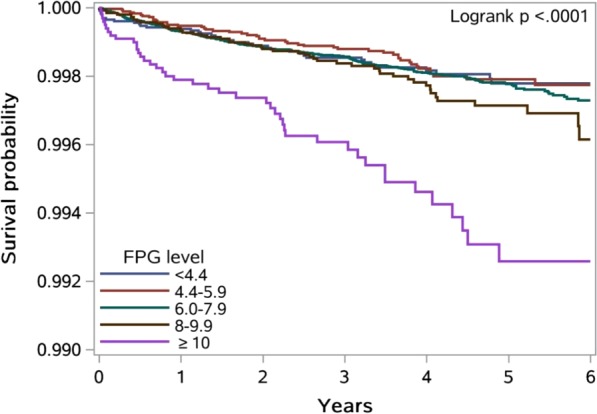
Kaplan-Meier curves of PTB-free survival in T2DM patients by FPG level. Abbreviation: FPG, fasting plasma glucose.
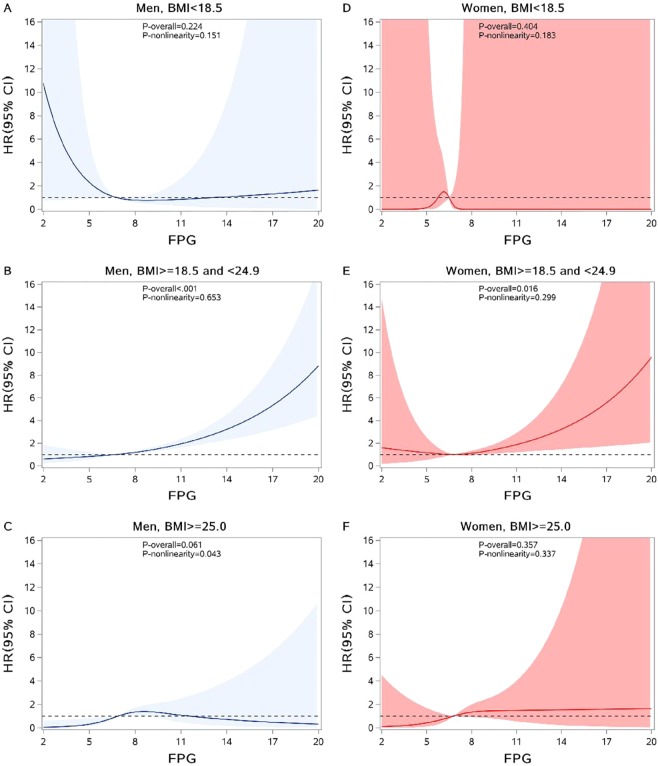
Further analysis was conducted to evaluate the association of FPG level with the risk of PTB in T2DM patients. After adjustment for sex, age and BMI, a significantly linear relationship between FPG and the risk of PTB incidence was observed in all subjects (Table 4; Fig. 2). When FPG was used as a categorical variable, the risk of PTB incidence in patients with high FPG level was 2.70 (95% CI: 1.95, 3.73) times higher than those with relatively normal FPG level (Table 4). The similar association pattern still existed in men and women separately. Since we found the interaction between BMI level and FPG level (P = 0.029), a stratified analysis by BMI level was performed. The significantly linear association of FPG and risk of PTB incidence only persisted in normal-weight patients both in men and women (Fig. 3). For underweight and overweight or obese patients, either significantly non-linear relationship or linear relationship was not observed.
Table 4.
Multivariable-adjusted hazard ratios and 95% confidence interval of PTB according to FPG.
| Categories of FPG | HR (95% CI) | ||
|---|---|---|---|
| All subjects* | Men† | Women† | |
| FPG as a continuous variable | 1.14 (1.09,1.19) | 1.15 (1.09, 1.20) | 1.12 (1.01, 1.24) |
| FPG level | |||
| <4.4 | 0.92 (0.64,1.31) | 0.87 (0.57,1.31) | 1.11 (0.55, 2.30) |
| 4.4–5.9 | 0.89 (0.65, 1.21) | 0.84 (0.58, 1.21) | 1.04 (0.58, 1.87) |
| 6.0–7.9 | 1.00 | 1.00 | 1.00 |
| 8–9.9 | 1.21 (0.90, 1.64) | 1.17 (0.83, 1.65) | 1.37 (0.74, 2.55) |
| ≥10 | 2.70 (1.95, 3.73) | 2.74 (1.92, 3.92) | 2.46 (1.12, 5.38) |
Abbreviation: FPG, fasting plasma glucose; PTB, pulmonary tuberculosis; HR, hazard ratio; CI, confidence interval.
*Adjusted for age, sex and BMI.
†Adjusted for age and BMI.
Figure 2.
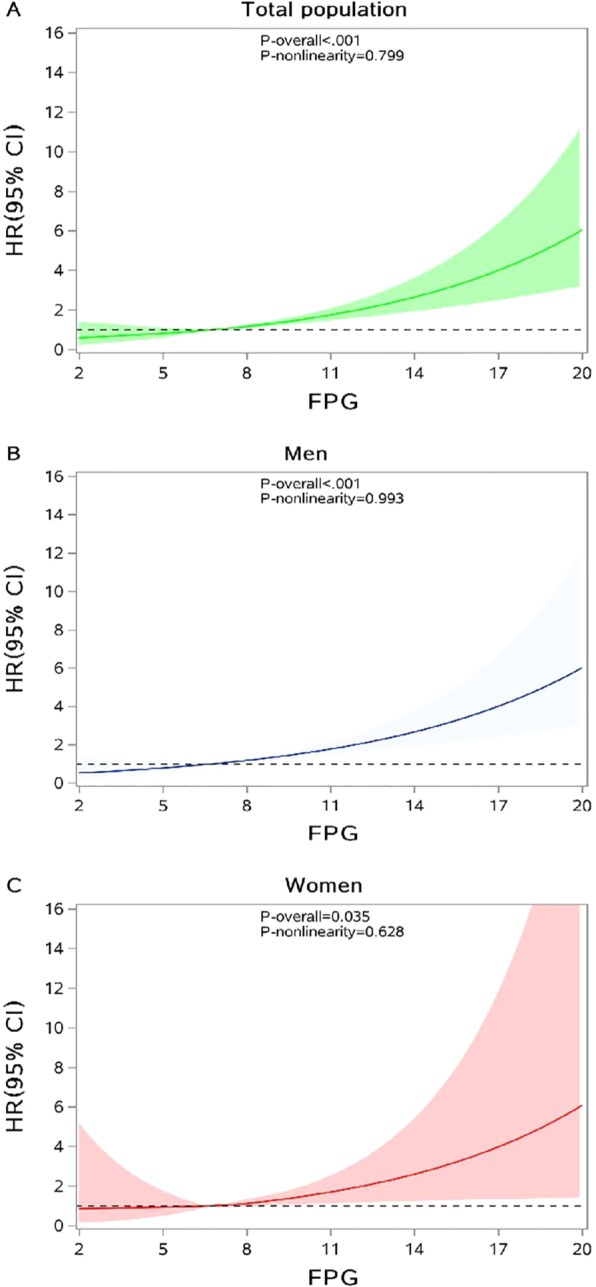
Association between FPG and risk of PTB among all T2DM patients(A), men (B) and women (C). The reference of FPG for these plots (with HR fixed as 1.0) was 6.8 mmol/L. HR was adjusted for age, sex, BMI. Abbreviations: FPG, fasting plasma glucose; HR, hazard ratio; CI: confidence interval.
Figure 3.
Association between FPG and risk of PTB incidence among men with BMI < 18.5 kg/m2 (A), men with BMI between 18.5 and 24.9 kg/m2 (B), men with BMI ≥ 25.0 kg/m2 (C), women with BMI < 18.5 kg/m2 (D), women with BMI between 18.5 and 24.9 kg/m2 (E) and women with BMI ≥ 25.0 kg/m2 (F). The reference of FPG for these plots (with HR fixed as 1.0) was 6.8 mmol/L. HR was adjusted for age. Abbreviations: FPG, fasting plasma glucose; BMI, body mass index; HR, hazard ratio; CI, confidence interval.
Discussion
Although the association of TB and T2DM has been verified in several studies5,9,16,25, we noted that population-based cohort studies focusing on the risk of TB incidence among T2DM patients in Shanghai, China, were limited, where the burden of diabetes is soaring year by year. In our study, we found that the annual incidence of PTB remained higher than that in general population from 2010 to 2015, and the overall incidence rate was 51.3 per 100,000 person-years. There was a substantially higher incidence rate among patients with FPG ≥ 10 mmol/L (143.2 per 100,000 person-years). Especially in diabetes patients of normal weight, the risk of PTB incidence increased with increasing FPG.
The incidence of PTB was approximately 2–3 times higher in T2DM patients aged 35 or above than in the general population in Shanghai, China, from 2010 to 2015. Our results are consistent with a prospective study conducted in Dhaka, which reported that the incidence rate of confirmed PTB cases among DM patients was double than that observed in the general population26. Another prospective study, conducted in Yunnan province, China, demonstrated that the rate of TB incidence was almost 3 times higher than that found in the general population12. A retrospective cohort study in the English population also reported a similar result and found DM was associated with a more than twofold increased risk of PTB27. Additionally, compared to previous studies, overall incidence rate of TB in T2DM patients in Shanghai was much lower than other studies conducted in China but was higher than some studies in western countries. In a cohort study conducted in Taiwan, the cumulative incidence of TB was 1.92 cases per 1000 person-years for females and 3.25 cases per 1000 person-years for males28. A large UK population-based cohort study reported that the incidence of TB among patients with diabetes was 16.2 per 100,000 person-years16.
Our study found the incidence rate of PTB among T2DM patients was influenced by FPG level. The highest overall rate was 143.2 per 100,000 person-years in the subgroup of FPG ≥ 10.0 mmol/L. Furthermore, the risk of PTB incidence increased as FPG increased, presenting a linear relationship, which was consistent with a cohort study conducted in Taiwan18. However, not all the reported results were in line with ours. The aforementioned UK study reported that there was no statistically significant difference of incidence rate of TB among three subgroups of hemoglobin A1c16. Leegaard et al. conducted a population-based case-control study in Northern Denmark and found no statistically significant evidence for any association between TB and dysglycemia17. To explain the negative results, these two studies both mentioned that one of the limitations of their studies was inadequate adjustments for important individual level confounders from lifestyle and demographic risk factors such as BMI, which had a strong and consistent long-linear relationship with TB incidence23. Besides, in the UK cohort study, most of the patients had reasonably well-controlled diabetes, which may cause underestimation and could reflect that good glycaemic control could decrease the incidence risk of tuberculosis.
As mentioned above, a number of previous studies have verified BMI and TB incidence presented an inverse relationship23. Similarly, in our study, the incidence rates of PTB were 215.2, 64.7 and 25.8 per 100,000 person-years in underweight patients, normal-weight patients and overweight/obese patients, respectively, presenting a significantly decreasing tendency. When grouped by sex, this trend still existed. These results are consistent with two population-based cohort studies conducted in Taiwan29.
Therefore, on the basis of the aforementioned inverse relationship and the assumption that overweight and obesity are major drivers of T2DM, it is necessary to take BMI into consideration when it comes to the association between TB incidence risk and FPG. We found that a significantly linear relationship between risk of PTB incidence and FPG was only observed in normal-weight patients regardless of sex. In overweight patients, the FPG level did not have an impact on the incidence of TB, which means that the protective effect of high BMI may be stronger than the increase in risk due to diabetes. Lin et al. found that the overall association between BMI and TB risk was dominated by the direct protective effect of BMI that was not mediated through diabetes29. Nevertheless, the conclusion was not consistent in all studies. Kubiak et al. found that the burden of active TB associated with diabetes was similar for normal and overweight or obese adults in the Indian population30. However, they also stressed the finding may not be generalizable beyond the Indian population, who have a higher likelihood of developing diabetes at every BMI level. In our study, the significant association between FPG level and PTB risk was not observed among underweight T2DM patients either. Based on abundant epidemiological evidence that has ascertained the harmful effect of poor nutritional status (or low BMI) on TB incidence31,32, we may assume that such an effect also dominated in the progress of TB incidence in T2DM patients instead of FPG level, which is consistent with a prospective cohort study in Singapore reporting that underweight and T2DM were independent determinants for active TB33.
The major strengths of our study are as follows. To the best of our knowledge, it is the most representative and large-sample study in China to investigate the incidence rate of PTB by subgroups in T2DM patients and the association between PTB incidence and FPG grouped by BMI level. Furthermore, it is the first study to analyse the detailed incidence rate of PTB and association between PTB incidence and specific FPG level in diabetic patients in Shanghai. Moreover, making full use of information systems, including a diabetes management system and a TB surveillance and mandatory reporting system, ensured the authenticity and validity of the results.
Although we believe that our results shed light on the incidence of PTB in T2DM patients aged 35 or above in Shanghai, they still have several limitations. Since it is a retrospective study, reliable data of potential risk factors related to TB incidence, such as medication use, smoking history, alcohol consumption and physical activity34,35, were not available from the current system. Second, not all the T2DM patients were registered in the management system, which covered about half of all T2DM patients10. Selection bias existed because the registered DM cases were mainly identified through screenings and outpatient visits to community health centres. Elderly people tend to participate in screenings and visit community health centres, while young and middle-aged adults prefer the academic hospitals. It is possible that DM patients who were not registered were at greater risk of poor glycaemic status as well as greater risk of TB. Third, the information on glucose was based on a single FPG test at baseline, and whether it could reflect the individuals’ real glycaemic status when they were diagnosed with TB may be unclear. Once more accurate and reasonable data are available through future studies, we will conduct re-analysis of this issue. Fourth, the number of underweight T2DM patients was relatively rare in our study; thus, the curvilinear shape of the dose-response relationship in underweight patients was a little strange, though it was not significant, especially in women. Finally, T2DM patients enrolled near the end of the study would not have time to develop TB, which may underestimate the incidence of TB slightly. However, since it was a sustainable cohort and we will follow up those cases, complete analysis will be possible in future studies.
In summary, our study revealed that the increasing prevalence of diabetes has had a significant influence on the incidence rate of TB, which is higher in T2DM patients than in the general population. Our results also imply that the next step of screening for TB in diabetics should focus on determining and prioritizing the high-risk T2DM patients in order to improve cost-effectiveness. For normal-weight diabetics, those with very poor glycaemic control would be a targeted screening population, while it may not be cost-effective to provide extra screening for those with good glycaemic control. For underweight diabetics, improvement of nutritional status would be more vital instead of strict glycaemic control. For overweight or obese diabetics, finding the balance point of glycaemic control and appropriate BMI would be the key to preventing TB and complications of diabetes at the same time.
Acknowledgements
This study was supported by the Three-year Action Plan on Public Health, Phase IV, Shanghai, China (15GWZK0801) and the Study on Comprehensive Prevention and Treatment Model of Tuberculosis, Shanghai, China (2018ZX10715012).
Author contributions
Y.Y.L., J.T.G. and T.X. contributed equally to this paper. All authors contributed to the design of the study. Y.Y.L. and F.W. were mainly responsible for statistical analyses after discussion with all authors. All authors participated in data interpretation and writing of this article.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Yan Shi, Email: shiyan@scdc.sh.cn.
Fan Wu, Email: wufan_scdc@126.com.
References
- 1.World Health Organization. Global tuberculosis report 2018, http://apps.who.int/iris/bitstream/handle/10665/274453/9789241565646-eng.pdf?ua=1.
- 2.International Diabetes Federation. IDF diabetes atlas. http://diabetesatlas.org/IDF_Diabetes_Atlas_8e_interactive_EN/.
- 3.Marks SM. Diabetes and tuberculosis, US National Health Interview Survey, 2000-2005. Int. J. Tuberc. Lung Dis. 2011;15(7):982–4. doi: 10.5588/ijtld.10.0637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Baker MA, Lin HH, Chang HY, Murray MB. The risk of tuberculosis disease among persons with diabetes mellitus: a prospective cohort study. Clin. Infect. Dis. 2012;54(6):818–25.. doi: 10.1093/cid/cir939. [DOI] [PubMed] [Google Scholar]
- 5.Delgado-Sanchez G, et al. Association of Pulmonary Tuberculosis and Diabetes in Mexico: Analysis of the National Tuberculosis Registry 2000-2012. Plos One. 2015;10(6):e0129312. doi: 10.1371/journal.pone.0129312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hongguang C, et al. Impact of diabetes on clinical presentation and treatment outcome of pulmonary tuberculosis in Beijing. Epidemiol. Infect. 2015;143(1):150–6. doi: 10.1017/S095026881400079X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jimenez-Corona ME, et al. Association of diabetes and tuberculosis: impact on treatment and post-treatment outcomes. Thorax. 2013;68(3):214–20. doi: 10.1136/thoraxjnl-2012-201756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lonnroth K, Roglic G, Harries AD. Improving tuberculosis prevention and care through addressing the global diabetes epidemic: from evidence to policy and practice. lancet Diabetes Endocrinol. 2014;2(9):730–9. doi: 10.1016/S2213-8587(14)70109-3. [DOI] [PubMed] [Google Scholar]
- 9.Al-Rifai RH, Pearson F, Critchley JA, Abu-Raddad LJ. Association between diabetes mellitus and active tuberculosis: A systematic review and meta-analysis. Plos One. 2017;12(11):e0187967. doi: 10.1371/journal.pone.0187967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ruan Y, et al. Epidemiology of Diabetes in Adults Aged 35 and Older from Shanghai, China. Biomed. Env. Sci. 2016;29(6):408–16. doi: 10.3967/bes2016.053. [DOI] [PubMed] [Google Scholar]
- 11.World Health Organization. The End TB Strategy:Global strategy and targets for tuberculosis prevention, care and control after 2015[R]. Geneva:World Health Organization,2014.
- 12.Lin Y, et al. Screening of patients with diabetes mellitus for tuberculosis in community health settings in China. Trop. Med. Int. Health. 2015;20(8):1073–80. doi: 10.1111/tmi.12519. [DOI] [PubMed] [Google Scholar]
- 13.Niazi AK, Kalra S. Diabetes and tuberculosis: a review of the role of optimal glycemic control. J. Diabetes Metab. Disord. 2012;11(1):28. doi: 10.1186/2251-6581-11-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Alisjahbana B, et al. The effect of type 2 diabetes mellitus on the presentation and treatment response of pulmonary tuberculosis. Clin. Infect. Dis. 2007;45(4):428–35. doi: 10.1086/519841. [DOI] [PubMed] [Google Scholar]
- 15.Dooley KE, Chaisson RE. Tuberculosis and diabetes mellitus: convergence of two epidemics. Lancet Infect. Dis. 2009;9(12):737–46. doi: 10.1016/S1473-3099(09)70282-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pealing L, et al. Risk of tuberculosis in patients with diabetes: population based cohort study using the UK Clinical Practice Research Datalink. BMC Med. 2015;13:135. doi: 10.1186/s12916-015-0381-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Leegaard A, et al. Diabetes, glycemic control, and risk of tuberculosis: a population-based case-control study. Diabetes care. 2011;34(12):2530–5. doi: 10.2337/dc11-0902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lee PH, et al. Glycemic Control and the Risk of Tuberculosis: A Cohort Study. PLoS Med. 2016;13(8):e1002072. doi: 10.1371/journal.pmed.1002072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Li X, et al. The primary health-care system in China. Lancet. 2017;390:2584–2594. doi: 10.1016/S0140-6736(17)33109-4. [DOI] [PubMed] [Google Scholar]
- 20.Chinese Diabetes Society China guideline for type 2 diabetes (2017) Chin. J. Diabetes. 2018;10(1):2–67. [Google Scholar]
- 21.Chinese Society of Endocrinology Chinese expert consensus on management of diabetes in Chinese adults. Chin. J. Endocrinol. Metab. 2013;29(3):189–95. [Google Scholar]
- 22.Chinese Center for Disease Control and Prevention. National Tuberculosis Control Programme Guideline in China (2008) Beijing, China: Peking Union Medical College Press; 2009. Disease Control Bureau and Medical Administrative Department of Ministry of Health of the People’s Republic of China.
- 23.Lönnroth K, Williams BG, Cegielski P, Dye C. A consistent log-linear relationship between tuberculosis incidence and body mass index. Int. J. Epidemiol. 2010;39(1):149–55. doi: 10.1093/ije/dyp308. [DOI] [PubMed] [Google Scholar]
- 24.Desquilbet L, Mariotti F. Dose-response analyses using restricted cubic spline functions in public health research. Stat. Med. 2010;29:1037–1057. doi: 10.1002/sim.3841. [DOI] [PubMed] [Google Scholar]
- 25.Martinez L, et al. Glycemic Control and the Prevalence of Tuberculosis Infection: A Population-based Observational Study. Clin. Infect. Dis. 2017;65(12):2060–8. doi: 10.1093/cid/cix632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rahim Z, et al. Pulmonary tuberculosis in patients with diabetes mellitus in Bangladesh. Int. J. Tuberc. Lung Dis. 2012;16(8):1132–3. doi: 10.5588/ijtld.11.0846. [DOI] [PubMed] [Google Scholar]
- 27.Young F, Wotton CJ, Critchley JA, Unwin NC, Goldacre MJ. Increased risk of tuberculosis disease in people with diabetes mellitus: record-linkage study in a UK population. J. Epidemiol. community health. 2012;66(6):519–23. doi: 10.1136/jech.2010.114595. [DOI] [PubMed] [Google Scholar]
- 28.Kuo M-C, et al. Type 2 Diabetes: An Independent Risk Factor for Tuberculosis: A Nationwide Population-Based Study. Plos One. 2013;8(11):e78924. doi: 10.1371/journal.pone.0078924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lin HH, et al. Association of Obesity, Diabetes, and Risk of Tuberculosis: Two Population-Based Cohorts. Clin. Infect. diseases: an. Off. Publ. Infect. Dis. Soc. Am. 2018;66(5):699–705. doi: 10.1093/cid/cix852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kubiak RW, et al. Interaction of nutritional status and diabetes on active and latent tuberculosis: a cross-sectional analysis. BMC Infect. Dis. 2019;19:627. doi: 10.1186/s12879-019-4244-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cegielski JP, McMurray DN. The relationship between malnutrition and tuberculosis: evidence from studies in humans and experimental animals. Int. J. tuberculosis lung disease: Off. J. Int. Union. Tuberculosis Lung Dis. 2004;8(3):286–98. [PubMed] [Google Scholar]
- 32.Odone A, Houben RM, White RG, Lonnroth K. The effect of diabetes and undernutrition trends on reaching 2035 global tuberculosis targets. lancet Diabetes endocrinology. 2014;2(9):754–64. doi: 10.1016/S2213-8587(14)70164-0. [DOI] [PubMed] [Google Scholar]
- 33.Soh AZ, et al. Diabetes and body mass index in relation to risk of active tuberculosis: a prospective population-based cohort. Int. J. Tuberc. Lung Dis. 2019;23(12):5. doi: 10.5588/ijtld.19.0094. [DOI] [PubMed] [Google Scholar]
- 34.Lin HH, Ezzati M, Murray M. Tobacco smoke, indoor air pollution and tuberculosis: a systematic review and meta-analysis. PLoS Med. 2007;4(1):e20. doi: 10.1371/journal.pmed.0040020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lönnroth K, Williams BG, Stadlin S, Jaramillo E, Dye C. Alcohol use as a risk factor for tuberculosis - a systematic review. BMC Public. Health. 2008;8:289. doi: 10.1186/1471-2458-8-289. [DOI] [PMC free article] [PubMed] [Google Scholar]