Abstract
This study aimed to identify the independent and combined effects of age, BMI at first prenatal visit and GWG on the risk of GDM. A retrospective cohort study of 1,951 pregnant women in Seremban district, Negeri Sembilan, Malaysia. GDM was defined as fasting plasma glucose (FPG) ≥5.6 mmol/l and/or 2-hour postprandial plasma glucose (2hPPG) ≥7.8 mmol/l. A higher percentage of women with GDM had 2 risk factors (29.0%) or >2 risk factors (8.6%) compared to non-GDM women (2 risk factors: 25.5%; >2 risk factors: 5.0%). In general, women with ≥2 risk factors were respectively 1.36–2.06 times more likely to have GDM compared to those without risk factors. Older maternal age and being overweight/obese were significantly associated with risk of GDM. Overweight/obese women with age ≥35 years had 2.45 times higher risk of GDM and having excessive GWG at second trimester further increased the risk of GDM. Age and BMI are independent risk factors for GDM but not GWG in the first and second trimester. The findings emphasize the need to focus on a healthy BMI before pregnancy and optimal GWG during pregnancy to improve pregnancy outcomes.
Subject terms: Risk factors, Metabolic disorders
Introduction
In 2017, of the 16.2% (21.3 million) live births affected by hyperglycaemia in pregnancy, 86.4% (18.4 million) of these cases were due to gestational diabetes mellitus1. Globally, the prevalence of gestational diabetes mellitus (GDM) is estimated at 6–12% of all pregnancies2. The National Health and Morbidity Survey (2016) reported that the prevalence of GDM among Malaysian mothers aged 15–49 years old was 13.5%3. The GDM rate in Malaysian population (8.7–29.7%)4–6 was significantly higher than the reported rates in many Western (2.0–9.2%)7,8 and Asian countries (2.8–25.0%)9–12.
The known risk factors for GDM are advanced age, obesity, excessive gestational weight gain (GWG), ethnicity, family history of diabetes, history of GDM, high parity, short stature, polycystic ovary syndrome (PCOS) and previous large-for-gestational (LGA) births13–19. Advanced maternal age is a well-documented risk factor for GDM, as previous studies consistently reported that older pregnant women were more likely to develop GDM13,14,20–24. Although maternal age is an established risk factor, there is no consensus regarding a valid cut-off value. The American Diabetes Association recommended the use of ≥25 years as a risk factor for GDM25, but this recommendation is supported by very limited data26. A retrospective study of 138,530 Japanese women reported that the prevalence of GDM increased with advancing age, with women aged 40 years and above showing a relative risk of 15.1%20.
Studies on the associations between pre-pregnancy BMI and GWG with risk of GDM showed that pre-pregnancy obesity and excessive GWG are independent risk factors for the development of GDM. While overweight or obese women had an increased risk of GDM compared to lean or normal-weight women27, excessive GWG, particularly between early and mid-pregnancy, was postively associated with risk of GDM28,29. Studies have also reported that overweight or obese women were more likely to show excessive GWG30–32 and that pre-pregnancy overweight or obesity combined with excessive GWG confers a higher risk of complications in pregnancy, particularly GDM15,33.
The Malaysian National Health and Morbidity Survey (NHMS) showed that in the period between 1996 and 2015, there was a 26.6% increase in the prevalence of overweight and obesity (BMI ≥25.0 kg/m2) among females aged ≥18 years old. With the increasing average age of Malaysian women having their first child34,35 and the rising prevalence of overweight and obesity among women of childbearing age36, the risk of having higher pre-pregnancy BMI and excessive GWG are inevitable37,38. Although it is well established that increasing maternal age, overweight/obesity, and excessive GWG are independent determinants of GDM, no study has specifically evaluated the combined effects of maternal age, BMI and GWG on the risk of GDM. Thus, the aim of this study was to identify the independent and combined effects of age, BMI at first prenatal visit and GWG on the risk of GDM.
Results
Characteristics of women
The baseline characteristics of 1,951 pregnant women included in the analysis are presented in Table 1. The mean age of women was 29.08 ± 4.44, with about 88.3% of women under35 years. Most of the women were Malays (83.9%), had low-secondary education (59.8%) and were employed (61.0%). About 28.4% of women were nulliparous. The mean height and BMI at first prenatal visit were 1.56 ± 0.06 m, and 24.76 ± 5.49 kg/m2, respectively. More than half (54.75%) had a height over 1.56 m. According to the BMI calculated at the first prenatal visit, 556 (28.5%) were overweight, and 292 (15.0%) were obese. Two-thirds of women gained weight above the recommended range in the second trimester (42.9%). The mean fasting plasma glucose (FPG) and 2-hour plasma glucose (2hPG) were 4.37 ± 0.51 mmol/l and 6.10 ± 1.41 mmol/l. About 13.1% of the women were diagnosed with GDM.
Table 1.
Characteristics of women (N = 1951).
| n (%) | Mean ± SD | |
|---|---|---|
| Age (years) | 29.08 ± 4.44 | |
| <35 | 1723 (88.3) | |
| ≥ 35 | 228 (11.7) | |
| Ethnicity | ||
| Malay | 1636 (83.9) | |
| Others | 315 (16.1) | |
| Education | ||
| Lower-secondary | 1166 (59.8) | |
| Others | 785 (40.2) | |
| Occupation | ||
| Housewife | 761 (39.0) | |
| Working | 1190 (61.0) | |
| Parity | 1.43 ± 1.31 | |
| 0 | 554 (28.4) | |
| 1 | 521 (26.7) | |
| 2 | 568 (29.1) | |
| ≥3 | 308 (15.8) | |
| Height (m) | 1.56 ± 0.06 | |
| <1.56 | 884 (45.3) | |
| ≥1.56 | 1067 (54.7) | |
| BMI at first prenatal visit (kg/m2) | 24.76 ± 5.49 | |
| Underweight (<18.50) | 207 (10.6) | |
| Normal (18.50–24.99) | 896 (45.9) | |
| Overweight/ (25.00–29.99) | 556 (28.5) | |
| Obese (≥30.00) | 292 (15.0) | |
| GWG at first trimester (kg) | 0.25 ± 0.06 | |
| Inadequate (<IOM) | 1166 (59.8) | |
| Adequate (= IOM) | 585 (30.0) | |
| Excessive (>IOM) | 200 (10.2) | |
| GWG at second trimester (kg) | 5.90 ± 3.41 | |
| Inadequate (<IOM) | 626 (32.1) | |
| Adequate (= IOM) | 505 (25.9) | |
| Excessive (>IOM) | 820 (42.0) | |
| Maternal glucose level | ||
| OGTT at 28th weeks of gestation (mmol/L) | ||
| Fasting plasma glucose (FPG) | 4.37 ± 0.51 | |
| 2-hours plasma glucose (2hPG) | 6.10 ± 1.41 | |
| GDM according to MOH criteria‡ | 255 (13.1) | |
Note. ‡GDM according to MOH criteria, either of both FPG ≥5.6 mmol/l or 2hPG ≥7.8 mmol/L.
Risk factors between non-GDM and GDM women
Table 2 shows the presence of risk factors among non-GDM and GDM women. There was a higher percentage of women with GDM having age ≥35 years (19.6%) and being overweight/obese (51.4%) as compared to non-GDM women (10.5% age ≥35 years; 42.3% overweight/ obese). However, no significant difference was observed for GWG in the first and second trimester between non-GDM and GDM women. There was a significant difference in the number of risk factors for GDM between non-GDM and GDM women, in that a higher proportion of GDM women having 2 (29.0%) or more than 2 risk factors (8.6%) compared to non-GDM women (25.5% 2 risk factors and 5.0% >2 risk factors). Only a small proportion of women (0.4–0.8%) had all 4 risk factors.
Table 2.
Proportion of risk factors between non-GDM and GDM.
| Factors | Total (n = 1951) | Non-GDM (n = 1696) | GDM(n = 255) | p-value |
|---|---|---|---|---|
| n (%) | ||||
| Age ≥35 years | 228 (11.7) | 178 (10.5) | 50 (19.6) | 0.001* |
| Overweight/Obese (≥25.00 kg/m2) | 848 (43.5) | 717 (42.3) | 131 (51.4) | 0.02* |
| Excessive GWG at the first trimester | 200 (10.3) | 177 (10.4) | 23 (9.0) | 0.49 |
| Excessive GWG at the second trimester | 820 (42.0) | 713 (42.0) | 107 (42.0) | 0.36 |
| Number of risk factors¥ | ||||
| None | 582 (29.8) | 518 (30.5) | 64 (25.1) | 0.02* |
| 1 risk factor | 756 (38.7) | 661 (39.0) | 95 (37.3) | |
| 2 risk factors | 507 (26.0) | 433 (25.5) | 74 (29.0) | |
| >2 risk factors | 106 (5.4) | 84 (5.0) | 22 (8.6) | |
| 3 risk factors | 98 (5.0) | 78 (4.6) | 20 (7.8) | |
| 4 risk factors | 8 (0.4) | 6 (0.4) | 2 (0.8) | |
¥Age ≥35 years, Overweight/Obese (≥25.00 kg/m2), excessive GWG at first trimester, excessive GWG at second trimester.
The effects of age, BMI and GWG to the GDM risk
Table 3 shows the independent and combined effects of age, BMI at first prenatal visit and GWG at the first and second trimester to the risk of GDM, adjusted for covariates. The findings show that maternal age and BMI at first prenatal visit had significant independent associations with the risk of GDM for both categorical and continuous variable. Women with older age (≥35 years) (aOR = 2.08, 95% CI = 1.42–3.07) were significantly at higher risk for GDM than women aged <35 years. Overweight/obese women (aOR = 1.44, 95% CI = 1.04–1.81) had significantly higher risk for GDM compared to underweight/normal weight women. No significant independent effects were observed between GWG in the first and second trimester with the risk of GDM. Women with two risk factors and >2 risk factors had 1.36 times and 2.06 times higher risk for GDM compared to those women with no risk factor.
Table 3.
Independent and combine factors associated with the risk of gestational diabetes mellitus (GDM).
| GDM | p-value | |
|---|---|---|
| Adjusted OR [95% CI] | ||
| Factors (continuous) | ||
| Age (years) | 1.06 [1.04–1.10] | 0.001* |
| BMI at first prenatal visit (kg/m2) | 1.03 [1.01–1.06] | 0.01* |
| GWG at the first trimester (kg) | 0.92 [0.85–1.01] | 0.05 |
| GWG at second trimester (kg) | 0.97 [0.93–1.01] | 0.11 |
| Independent factors (categorical) | ||
| Age (years) | ||
| <35 | 1.00 | |
| ≥35 | 2.08 [1.42–3.07] | 0.001** |
| BMI at first prenatal visit (kg/m2) | ||
| Underweight (<18.50) | 0.78 [0.47–1.31] | 0.35 |
| Normal (18.50–24.99) | 1.00 | |
| Overweight/obese (≥25.00) | 1.44 [1.04–1.81] | 0.02* |
| GWG at the first trimester (kg) | ||
| Inadequate (<IOM) | 1.25 [0.92–1.70] | 0.15 |
| Adequate (= IOM) | 1.00 | |
| Excessive (>IOM) | 1.01 [0.61–1.66] | 0.97 |
| GWG at the second trimester (kg) | ||
| Inadequate (<IOM) | 1.29 [0.91–1.84] | 0.16 |
| Adequate (= IOM) | 1.00 | |
| Excessive (>IOM) | 1.17 [0.83–1.65] | 0.37 |
| Combined factors | ||
| Number of risk factorsa | ||
| No risk factor | 1.00 | |
| 1 risk factor | 1.15 [0.82–1.62] | 0.41 |
| 2 risk factors | 1.36 [1.01–1.96] | 0.04* |
| >2 risk factors | 2.06 [1.18–3.58] | 0.01* |
Note. Non-GDM as the reference group (GDM according to MOH criteria, either of both FPG ≥5.6 mmol/l or 2hPG ≥7.8 mmol/L).
a1 risk factor was defined as having any 1 of the 4-risk factors; 2 risk factors was defined as having any 2 of the 4 risk factors; >2 risk factors were defined as having more than 2 risk factors.
Adjusted for ethnicity, parity, gestational weeks at OGTT performed.
*p < 0.05.
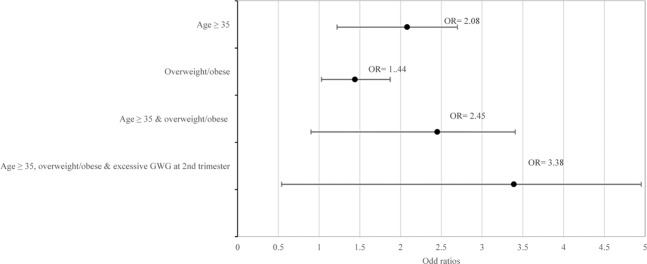
Figure 2 provides further insights into the independent and combined effects of significant factors on the risk of GDM. Overweight/obese women had 1.44 times higher risk for GDM while women aged 35 years and above had 2 times higher risk for GDM. Women with both aged 35 years and above and overweight/obese had a higher risk of GDM (aOR = 2.45, 95% CI = 1.50–4.00). The risk for GDM was further increased if the women were aged 35 years and above, overweight/obese and had excessive GWG in the second trimester (aOR = 3.38, 95% CI = 1.83–6.24) compared to women without these risk factors.
Figure 2.
Odd ratios for independent and combined effects of significant factors associated with the risk of GDM. Note. No risk factor as the reference group.
Discussion
The present study showed that within our study population more than two-thirds of women had at least one risk factor, with 38.7% and 31.4% had one risk factor and two or more risk factors. Women aged 35 years and above with BMI of overweight/obese had 2.45 times higher risk for GDM, while women with a combination of three risk factors (aged 35 years and above, overweight/obese and with excessive GWG in the second trimester) had 3.38 times higher risk of GDM compared to women with no risk factors. The clustering of these risk factors might confer a greater risk for adverse outcomes, such as large-for-gestational-age (LGA) infants, caesarean delivery. These findings emphasize the importance of addressing not only the individual established risk factors but also the combined effect of these biological and behavioural factors on risk of GDM.
In the present study, age 35 years and above and overweight/obese were independently associated with the risk of GDM. This finding was consistent with previous studies that reported older pregnant women were more likely to develop GDM13,14,39. To date, there has been an increasing trend among women in developing countries to delay their first pregnancy. This trend to postpone childbearing is driven largely by the desire to complete education or professional career development40. Thus, the number of women giving birth for the first time at the age of 35 years and above has increased steadily34. Similarly, in Malaysia, maternal age for having the first baby is increasing35. Older maternal age also increases the risk that women might encounter a second pregnancy soon after the previous delivery, carrying along some additional weight and abdominal fat mass due to a short interpregnancy interval, especially in the case of high GWG in the previous pregnancy and postpartum weight retention. The Malaysian Adult Nutrition Survey (MANS) 2016 showed that about 40–55% of women of reproductive age (20–39 years old) were overweight and obese36. As the prevalence of overweight/obesity among women entering pregnancy is likely to increase in Malaysia, this could further contribute to an increase in obesity-related adverse pregnancy outcomes.
Normal ageing is associated with deterioration of endocrine functions, such as the decline in β-cell function and insulin sensitivity41. The development of GDM is strongly influenced by an individuals’ reduced ability to secrete insulin. In addition, older women are also at an increased risk for both acute and chronic cardiovascular complications, such as coronary artery disease, atherosclerosis, heart failure, and stroke42. The underlying mechanism of how maternal age acts as a risk factor for GDM is still unclear but age related alterations in glucose-insulin regulation as well as vascular aging may contribute to poor pregnancy outcomes in older women.
Overweight/obese women in this study had a significantly higher risk of GDM compared to normal weight women. The findings of the present study are similar to those of previous studies in Western populations15,17,28,33,43. Several factors may explain the association between pre-pregnancy BMI and GDM. As metabolic changes occur during pregnancy, particularly declining insulin sensitivity in late pregnancy, obese pregnant women may experience a higher risk of homeostatic dysregulation during pregnancy44. Obese women have approximately 40% higher in insulin resistance compared to normal weight women45. Inflammation is another possible explanation for the association between obesity and GDM46, in that overweight and obesity are associated with increased levels of inflammation. Bastard et al. (2000) found that an increase in inflammation, especially interleukin-6 (IL-6), among obese individuals was associated with insulin resistance47.
The present study showed that neither GWG in the first nor the second trimester was independently associated with the risk of GDM. This finding was inconsistent with a previous study in China39, which reported that GWG in the first and second trimester were associated with the risk of GDM only among obese and aged >35 years women. The inconsistent findings could be due to different methodological differences used, such as study design and timing of measurement. The study in China was a prospective study and weight gain in the first trimester was defined as the difference between weight in the early second trimester and self-reported pre-pregnancy weight. The present study in Malaysia was a retrospective study and the GWG in the first trimester was defined as the difference in body weight between early second trimester and first prenatal visit, likely resulting in a more reliable estimate.
This study also showed that women with a combination of two risk factors (aged 35 years and above & BMI overweight/obese) had a higher risk of GDM as compared to women with individual risk factor of either being 35 years and above or overweight / obese. Although excessive GWG in the second trimester was not a significant risk factor of GDM, the combination of three risk factors (aged 35 years and above, overweight/obese and had an excessive GWG in the second trimester) significantly increased the risk of GDM. This finding indicates that maternal age and BMI are more important risk factors than GWG, and this notion is supported by a recent meta-analysis that found the association between maternal pre-pregnancy BMI with the risk for any adverse outcome, including GDM was stronger than the association of GWG48. The absolute risk of overweight women for any adverse outcomes increased from 37.3% for GWG of 2.0–3.9 kg to 56.4% for GWG of 28.0 kg or greater48. Since age is a non-modifiable risk factor for GDM, the focus on an effective intervention to prevent the risk of developing of GDM should be aimed at helping overweight or obese women to lose weight before pregnancy and to limit weight gain in pregnancy.
There are several limitations to the present study. As the study population comprised pregnant women from the Seremban district, Negeri Sembilan, the study findings may not be generalizable to all pregnant women in Malaysia. The anthropometric measurements were retrieved from antenatal clinic cards, which could potentially produce information bias and misclassification. This study used the recorded weight and height at first prenatal visit within the first 12 weeks of pregnancy to calculate the early pregnancy BMI which could under- or overestimate GWG. Besides, the IOM recommendations for GWG used for classification of GWG is based on the western population, which may not be appropriate for the current study population, albeit there is currently no recommendation for GWG for Asians available. There were methodological limitations for performing combined effects analysis, as some subgroups were relatively small. Despite these statistical limitations, the study findings may have important public health implications.
Conclusions
More than two-thirds of women in the present study had at least one risk factor of GDM, with 38.7% with one risk factor and 31.4% had two or more risk factors. Maternal age and early pregnancy BMI were stronger contributors to the risk of GDM than GWG. However, excessive GWG in the second trimester was associated with a further increased risk for GDM, yet only in overweight / obese women aged 35 years and above. With age of having children increasing and more women of reproductive age being overweight or obese, the risk of developing GDM is imminent in Malaysia. There is a need for public health awareness on the importance of having healthy pre-pregnancy BMI, achieving optimal weight gain during pregnancy and retaining minimal postpartum weight, particularly among older age women.
Methods
Study design and population
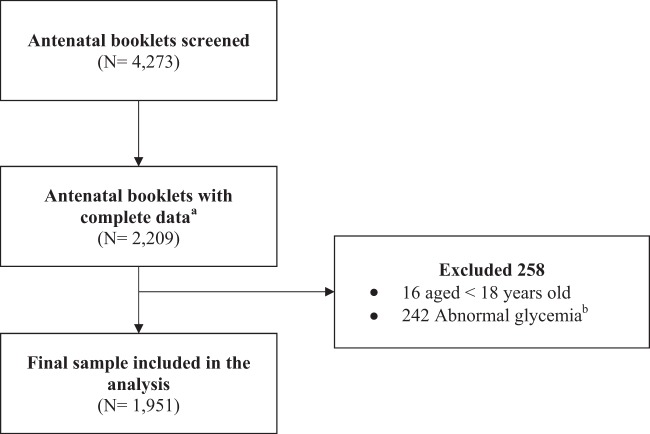
This was a retrospective cohort study of healthy, non-diabetic pregnant women having delivered at government hospitals between January 2010 and December 2012. A total of 4,273 antenatal booklets were screened, and 2,209 pregnant cases were initially identified for potential inclusion into this study. Two hundred and fifty-eight women were subsequently excluded from the analysis as follows: <18 years old (n = 16), and abnormal glycemia (n = 242). The final sample included in the analysis were 1951 pregnant women (Figs. 1 and 2).
Figure 1.
Sampling procedure. Note. aComplete data–complete all antenatal care visits. bNormal glycemia at first prenatal visit was defined as normal plasma glucose for the Ministry of Health Malaysia (MOH) criteria for the diagnosis of GDM49.
The study protocol was approved by the Medical Research Ethics Committee (MREC), Universiti Putra Malaysia (UPM/FPSK/100–9/2-MJKEtika) and the Medical Research Ethics Committee (MREC), Ministry of Health Malaysia (KKM/NIHSEC/08/0804/P12-613), which waived the requirement for informed consent as the data were analysed anonymously. This study was carried out in accordance with Good Clinical Practice (GCP) guidelines and the Declaration of Helsinki.
Measurements
Data sources
The source of data was antenatal clinic cards of pregnant women having delivered at government hospitals between January 2010 and December 2012. The clinic cards contained the patient’s background, antenatal care information, demographic characteristics, and obstetric history. Data were extracted from the antenatal clinic cards by trained enumerators.
Maternal glucose level
All pregnant women were required to take a standardized 2-hour 75 g OGTT in between 28th to 32nd week of gestation49. GDM was diagnosed if either or both FPG was ≥ 5.6 mmol/l or 2hPG is ≥ 7.8 mmol/l according to the Ministry of Health Malaysia guideline49.
Anthropometric measurements
Height and weight at first prenatal visit, first trimester, and second trimester were obtained from the antenatal clinic cards. Height and body weight at first prenatal visit were used to calculate early pregnancy Body Mass Index (BMI), as early pregnancy weight (kilogram) divided by the square of height (meter2), and further categorized into 4 groups: underweight (<18.5 kg/m2), normal weight (18.5–24.9 kg/m2), overweight (25.0–29.9 kg/m2) and obese (≥30.0 kg/m2)50. The GWG in the first trimester and second trimester was estimated using the difference between the first and last weight record in that trimester and then classified according to the 2009 US Institute of Medicine (IOM) guidelines, as inadequate, adequate and excessive.
Other variables
Information on demographic characteristics and obstetric history were obtained. The variables were classified as follows: age (<35, ≥35 years old), ethnicity (Malay, others), education (lower-secondary, others), occupation (housewife, working), parity (0, 1, 2, ≥3), and height (<1.56, ≥1.56 m).
Statistical analysis
All analyses were performed using SPPS version 2351. Exploratory Data Analysis (EDA) was carried out to determine the normality and homogeneity of the data. All continuous variables were normally distributed. Therefore, no transformation was performed. Basic descriptive statistics were generated such as means and standard deviations for the continuous variables, while for categorical variables, frequency, and percentage distribution.
Binary logistic regression model was used to determine the independent and combined effects of age, BMI at first prenatal visit, GWG in the first and second trimester to the risk of GDM, adjusting for covariates (ethnicity, parity gestational weeks at OGTT performed). Four factors were examined both as continuous and categorical independent variables. Categorical variables included in the analysis were age (<35, ≥35), BMI at first prenatal visit (<18.50, 18.50–24.99, ≥25.00 kg/m2), GWG at first and second trimester (inadequate, adequate and excessive), respectively. The combined effects of factors (2 risk factors, 3 risk factors, 4 risk factors) associated with the risk of GDM were further examined using binary logistic regressions. However, the study only reported the significant combined risk factors. Non GDM women served as the reference group. Adjusted odds ratios (ORs) and 95% confidence intervals (CIs) were presented. Significant level for all statistical analysis was set at p < 0.05.
Acknowledgements
The authors would like to thank all nurses and staff in MCH clinics in Seremban district, Negeri Sembilan for their support and assistance. This study was supported by a research grant from Danone Dumex (Malaysia) Shd. Bhd. Although Seremban Cohort Study (SECOST) is funded by Danone Dumex (Malaysia) Sdn. Bhd, the company does not influence the study protocol of this manuscript. As the nature of this study is more of exploratory and does not involve any testing of company product, the researchers are free to report any findings of this study.
Author contributions
Z.M.S. and H.Y.Y. conceptualized and designed the study; H.Y.Y. collected, analyzed the data and writing – original draft preparation; Z.M.S., B.N.M.Y., Z.R. contributed to the development of study protocol, read and approved the manuscript. E.V.D.B., J.B. and Y.Y.S.T. read and approved the manuscript. All authors read and approved the final version of the manuscript.
Data availability
The datasets used and analyzed during the current study are available from the corresponding author on reasonable request.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Cho, N. H. et al. IDF Diabetes Atlas: Global estimates of diabetes prevalence for 2017 and projections for 2045. Diabetes Research and Clinical Practice138, 271–281 (2018). [DOI] [PubMed]
- 2.Zhu, Y. & Zhang, C. Prevalence of Gestational Diabetes and Risk of Progression to Type 2 Diabetes: a Global Perspective. Current Diabetes Reports10.1007/s11892-015-0699-x (2016). [DOI] [PMC free article] [PubMed]
- 3.IPH. National Health and Morbidity Survey 2016 (NHMS 2016): Maternal and Child Health. (National Institutes of Health (NIH), Ministry of Health, Malaysia, 2016).
- 4.Jeganathan, R. & Karalasingam, S. D. Preliminary Report of National Obstetrics Registry, Jan 2011 – Dec 2012. (National Obstetrics Registry and the Clinical Research Centre (CRC), Ministry of Health Malaysia, 2015).
- 5.Kwapisz, J. & Bodaghi, M. Preliminary Report of National Obstetrics Registry, Jan-December 2010. (Jointly published by the National Obstetrics Registry and the Clinical Research Centre (CRC), Ministry of Health Malaysia., 2013).
- 6.Logakodie S, et al. Gestational diabetes mellitus: The prevalence, associated factors and foeto-maternal outcome of women attending antenatal care. Malaysian Fam. physician Off. J. Acad. Fam. Physicians Malaysia. 2017;12:9–17. [PMC free article] [PubMed] [Google Scholar]
- 7.DeSisto CL, Kim SY, Sharma AJ. Prevalence Estimates of Gestational Diabetes Mellitus in the United States, Pregnancy Risk Assessment Monitoring System (PRAMS), 2007–2010. Prev. Chronic Dis. 2014;11:130415. doi: 10.5888/pcd11.130415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Buckley BS, et al. Gestational diabetes mellitus in Europe: Prevalence, current screening practice and barriers to screening. A review. Diabetic Medicine. 2012;29:844–854. doi: 10.1111/j.1464-5491.2011.03541.x. [DOI] [PubMed] [Google Scholar]
- 9.Wei Y, et al. International Association of Diabetes and Pregnancy Study Group criteria is suitable for gestational diabetes mellitus diagnosis: further evidence from China. Chin. Med. J. (Engl). 2014;127:3553–3556. [PubMed] [Google Scholar]
- 10.Shimodaira M, Yamasaki T, Nakayama T. The association of maternal ABO blood group with gestational diabetes mellitus in Japanese pregnant women. Diabetes Metab. Syndr. Clin. Res. Rev. 2016;10:S102–S105. doi: 10.1016/j.dsx.2016.03.003. [DOI] [PubMed] [Google Scholar]
- 11.Chong Y-S, et al. Ethnic differences translate to inadequacy of high-risk screening for gestational diabetes mellitus in an Asian population: a cohort study. BMC Pregnancy Childbirth. 2014;14:345. doi: 10.1186/1471-2393-14-345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nguyen, C. L., Pham, N. M., Binns, C. W., Van Duong, D. & Lee, A. H. Prevalence of gestational diabetes mellitus in eastern and southeastern Asia: A systematic review and meta-analysis. Journal of Diabetes Research2018, (2018). [DOI] [PMC free article] [PubMed]
- 13.Leng, J. et al. Prevalence of gestational diabetes mellitus and its risk factors in Chinese pregnant women: A prospective population-based study in Tianjin, China. Plos One10, (2015). [DOI] [PMC free article] [PubMed]
- 14.Erem C, Kuzu UB, Deger O, Can G. Prevalence of gestational diabetes mellitus and associated risk factors in Turkish women: the Trabzon GDM Study. Arch. Med. Sci. 2015;11:724–35. doi: 10.5114/aoms.2015.53291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pirjani, R. et al. Gestational diabetes mellitus its association with obesity: a prospective cohort study. Eat. Weight Disord. - Stud. Anorexia, Bulim. Obes. 1–6 10.1007/s40519-016-0332-2 (2016). [DOI] [PubMed]
- 16.Kim, S. Y. et al. Fraction of gestational diabetes mellitus attributable to overweight and obesity by race/ethnicity, California, 2007-2009. Am. J. Public Health103, (2013). [DOI] [PMC free article] [PubMed]
- 17.Scott-Pillai, R., Spence, D., Cardwell, C., Hunter, A. & Holmes, V. The impact of body mass index on maternal and neonatal outcomes: a retrospective study in a UK obstetric population, 2004-2011. BJOG An Int. J. Obstet. Gynaecol. n/a-n/a 10.1111/1471-0528.12193 (2013). [DOI] [PubMed]
- 18.Hung T-H, Hsieh T-T. Pregestational body mass index, gestational weight gain, and risks for adverse pregnancy outcomes among Taiwanese women: A retrospective cohort study. Taiwan. J. Obstet. Gynecol. 2016;55:575–81. doi: 10.1016/j.tjog.2016.06.016. [DOI] [PubMed] [Google Scholar]
- 19.Lee, K. W. et al. Prevalence and risk factors of gestational diabetes mellitus in Asia: a systematic review and meta-analysis. BMC Pregnancy and Childbirth 18(1) (2018). [DOI] [PMC free article] [PubMed]
- 20.Morikawa M, et al. Prevalence of hyperglycemia during pregnancy according to maternal age and pre-pregnancy body mass index in Japan, 2007-2009. Int. J. Gynecol. Obstet. 2012;118:198–201. doi: 10.1016/j.ijgo.2012.04.019. [DOI] [PubMed] [Google Scholar]
- 21.Carolan M, Davey M-A, Biro MA, Kealy M. Maternal age, ethnicity and gestational diabetes mellitus. Midwifery. 2012;28:778–783. doi: 10.1016/j.midw.2011.08.014. [DOI] [PubMed] [Google Scholar]
- 22.Lawrence JM, Contreras R, Chen W, Sacks DA. Trends in the prevalence of preexisting diabetes and gestational diabetes mellitus among a racially/ethnically diverse population of pregnant women, 1999–2005. Diabetes Care. 2008;31:899–904. doi: 10.2337/dc07-2345. [DOI] [PubMed] [Google Scholar]
- 23.Khalil A, et al. Maternal age and adverse pregnancy outcome: a cohort study. Ultrasound Obs. Gynecol. 2013;42:634–643. doi: 10.1002/uog.12494. [DOI] [PubMed] [Google Scholar]
- 24.Favilli A, et al. Pregnancy outcome in women aged 40 years or more. J. Matern. Fetal. Neonatal Med. 2012;25:1260–3. doi: 10.3109/14767058.2011.643327. [DOI] [PubMed] [Google Scholar]
- 25.American Diabetes Association, A. D. et al. 2. Classification and Diagnosis of Diabetes. Diabetes Care. 2016;39(Suppl 1):S13–22. doi: 10.2337/dc16-S005. [DOI] [PubMed] [Google Scholar]
- 26.Lao TT, Ho L-F, Chan BCP, Leung W-C. Maternal Age and Prevalence of Gestational Diabetes Mellitus. Diabetes Care. 2006;29:948 LP–949. doi: 10.2337/diacare.29.04.06.dc05-2568. [DOI] [PubMed] [Google Scholar]
- 27.Chu SY, et al. Maternal obesity and risk of gestational diabetes mellitus. Diabetes Care. 2007;30:2070–2076. doi: 10.2337/dc06-2559a. [DOI] [PubMed] [Google Scholar]
- 28.Baci Y, Ustuner I, Keskin HL, Ersoy R, Avsar AF. Effect of maternal obesity and weight gain on gestational diabetes mellitus. Gynecol Endocrinol. 2013;29:133–136. doi: 10.3109/09513590.2012.730571. [DOI] [PubMed] [Google Scholar]
- 29.Dai ZY, et al. Association between gestational weight gain per trimester/total gestational weight gain and gestational diabetes mellitus. Zhonghua Liu Xing Bing Xue Za Zhi. 2016;37:1336–1340. doi: 10.3760/cma.j.issn.0254-6450.2016.10.004. [DOI] [PubMed] [Google Scholar]
- 30.Restall, A. et al. Risk factors for excessive gestational weight gain in a healthy, nulliparous cohort. J. Obes. 2014, (2014). [DOI] [PMC free article] [PubMed]
- 31.Heery, E., Kelleher, C. C., Wall, P. G. & McAuliffe, F. M. Prediction of gestational weight gain - a biopsychosocial model. Public Health Nutr. 1–11 10.1017/S1368980014001815 (2014). [DOI] [PMC free article] [PubMed]
- 32.Yong HY, Mohd Shariff Z, Koo SJ, Sa’ari NS. Pre-pregnancy body mass index, height and physical activity are associated with rate of gestational weight gain among Malaysian mothers. J. Obstet. Gynaecol. Res. 2016;42:1094–1101. doi: 10.1111/jog.13039. [DOI] [PubMed] [Google Scholar]
- 33.Cavicchia PP, et al. Proportion of gestational diabetes mellitus attributable to overweight and obesity among non-Hispanic black, non-Hispanic white, and Hispanic women in South Carolina. Matern. Child Health J. 2014;18:1919–1926. doi: 10.1007/s10995-014-1437-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hsieh, T. T. an. et al. Advanced maternal age and adverse perinatal outcomes in an Asian population. Eur. J. Obstet. Gynecol. Reprod. Biol. 10.1016/j.ejogrb.2009.08.022 (2010). [DOI] [PubMed]
- 35.Department of Statistics, M. Current Population Estimates Malaysia, 2018. Jabatan Perangkaan Malaysia (Department of Statistics, Malaysia, http://www.mida.gov.my/env3/uploads/PerformanceReport/2013/KenyataanMedia.pdf 2018).
- 36.Institute for Public Health (IPH). National Health and Morbidity Survey 2014: Malaysian Adult Nutrition Survey (MANS) Vol. II. (National Institutes of Health, Ministry of Health Malaysia., 2014).
- 37.Gunderson EP. Childbearing and Obesity in Women: Weight Before, During, and After Pregnancy. Obstetrics and Gynecology Clinics of North America. 2009;36:317–332. doi: 10.1016/j.ogc.2009.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Li N, et al. Maternal prepregnancy body mass index and gestational weight gain on pregnancy outcomes. Plos One. 2013;8:e82310. doi: 10.1371/journal.pone.0082310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Dong B, et al. The effect of pre-pregnancy body mass index and excessive gestational weight gain on the risk of gestational diabetes in advanced maternal age. Oncotarget. 2017;8:58364–58371. doi: 10.18632/oncotarget.17651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mathews, T. J. & Hamilton, B. E. Mean age of mother, 1970-2000. Natl. Vital Stat. Rep. 10.1210/en.2002-220993 (2002). [PubMed]
- 41.Vincenzo, D. T. Age-related impairment of pancreatic beta-cell function: Pathophysiological and cellular mechanisms. Frontiers in Endocrinology5 (2014). [DOI] [PMC free article] [PubMed]
- 42.Stock EO, Redberg R. Cardiovascular Disease in Women. Curr. Probl. Cardiol. 2012;37:450–526. doi: 10.1016/j.cpcardiol.2012.07.001. [DOI] [PubMed] [Google Scholar]
- 43.Lin P-C, et al. The risk factors for gestational diabetes mellitus: A retrospective study. Midwifery. 2016;42:16–20. doi: 10.1016/j.midw.2016.09.008. [DOI] [PubMed] [Google Scholar]
- 44.Papachatzi E, Dimitriou G, Dimitropoulos K, Vantarakis A. Pre-pregnancy obesity: Maternal, neonatal and childhood outcomes. Journal of Neonatal-Perinatal Medicine. 2013;6:203–216. doi: 10.3233/NPM-1370313. [DOI] [PubMed] [Google Scholar]
- 45.Sivan E, Chen X, Homko CJ, Reece EA, Boden G. Longitudinal study of carbohydrate metabolism in healthy obese pregnant women. Diabetes Care. 1997;20:1470–1475. doi: 10.2337/diacare.20.9.1470. [DOI] [PubMed] [Google Scholar]
- 46.Torloni MR, et al. Prepregnancy BMI and the risk of gestational diabetes: a systematic review of the literature with meta-analysis. Obes. Rev. 2009;10:194–203. doi: 10.1111/j.1467-789X.2008.00541.x. [DOI] [PubMed] [Google Scholar]
- 47.Bastard JP, et al. Elevated levels of interleukin 6 are reduced in serum and subcutaneous adipose tissue of obese women after weight loss. J. Clin. Endocrinol. Metab. 2000;85:3338–42. doi: 10.1210/jcem.85.9.6839. [DOI] [PubMed] [Google Scholar]
- 48.Voerman E, et al. Association of Gestational Weight Gain With Adverse Maternal and Infant Outcomes. Jama. 2019;321:1702. doi: 10.1001/jama.2019.3820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ministry of Health Malaysia. Perinatal Care Manual 3rd Edition. (Division of Family Health Development, MOH, 2013).
- 50.WHO. Physical status: The Use and Interpretation of Anthropometry. Report of a WHO Expert Committee. (WHO Technical Report Series No. 854., 1995). [PubMed]
- 51.IBM Corp. Released. IBM SPSS Statistics for Windows, Version 23.0. 2015 (2013).
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and analyzed during the current study are available from the corresponding author on reasonable request.