Abstract
Fish are parasitized by several species of crustaceans, including Cymothoidae and Corallanidae. The aim of this study was to investigate the crustacean parasite fauna in Anableps anableps, Amphiarius rugispinis, Bagre bagre, Cathorops spixii, Cynoscion acoupa, Centropomus undecimalis, Macrodon ancylodon, Mugil curema, Megalops atlanticus, Pseudachenipterus nodosus, Plagioscion squamosissimus, Platystacus cotylephorus, Sciades passany, Sciades herzbergii, and Hypostomus ventrimaculata from the coast of the State of Amapá, eastern Amazon. In addition, an update on the geographic distribution of Nerocila acuminata in Brazilian Amazon is present. A total of 204 fish were examined and prevalence was 16.17%. A total of 185 Excorallana longicornis and Nerocila acuminata were collected and E. longicornis was the most frequent parasite species. The community of parasitic crustaceans in fish species from the coast of the State of Amapá consisted of two species of isopods, E. longicornis and N. acuminata, which are new records for nine host species here studied. Lastly, this is the first record of Nerocila acuminata for Brazil, besides the first report of E. longicornis for M. curema, C. acoupa, H. vetrimaculata, A. anableps, A. rugispinis, C. spixii and S. herzbergii; as well as N. acuminata for A. anableps, P. nodosus, A. rugispinis, C. spixii and M. atlanticus.
Keywords: Brackish fish, Crustaceans, Ectoparasites, Infestation
Introduction
The Brazilian coast is approximately 8500 km in length with specific geological, sedimentological, hydrographic and climatic features, and is divided in five coastal regions. Among them, the northern coast of Brazil covers 2500 km between the mouths of Oiapoque and Parnaíba rivers, in the states of Amapá and Maranhão, respectively (Ekau and Knoppers 1999; Brasil 2007). This region comprises a great estuarine and mangrove complex that provides food biomass production and habitat for colonization by various fish species. Currently, 925 fish species are known for the northern coast of Brazil and 73% Brazilian coastal fish may be found in this region (Menezes et al. 2003; Marceniuk et al. 2017). Thus, fish diversity in the northern coast is high and many species are largely explored by riverine communities for feeding and by fishing industry. However, the parasitic fauna of Brazilian coastal fish from the northern region is poorly understood and several species of parasites have not been studied or have been little addressed.
In general, fish are parasitized by several species of ectoparasites, which include the crustaceans (Thatcher 2006). In fish, here are 5400 species of parasitic crustaceans distributed in three taxa: Isopoda Latreille, 1871, Branchiura Thorell, 1818 and Copepoda Milne Edwards, 1940 (Frye 1968; Poly 2008; Tavares-Dias et al. 2015; Misganaw and Getu 2016). Isopods are ectoparasites that have been recorded in freshwater, brackish and marine fish, infesting mainly the oral cavity, tegument and gills of hosts (Frye 1968; Thatcher 1993; Smit et al. 2014; Gentil-Vasconcelos and Tavares-Dias 2015; Junoy 2016). They are blood-feeding and vectors of haemogregarines: blood-borne parasites (Davies and Smit 2001; Davies et al. 2004; Esteves-Silva et al. 2019). Isopods can cause tissue, osmoregulatory and respiratory damages, histopathological alterations and secondary infections caused by bacteria and fungi, besides a reduction in growth and reproduction leading to mortality of farmed and wild fish populations resulting in economic losses in aquaculture and fishing (Azevedo et al. 2006; Ravichandran et al. 2010; Rameshkumar and Ravichandran 2014; Tavares-Dias et al. 2014).
Parasitic isopods may establish complex associations with fish and present a wide geographical distribution (Tavares-Dias et al. 2015). Although fish can be parasitized by five families of isopods, only Cymothoidae, Gnathiidae and Corallanidae species have been reported infesting fish from the Brazilian Amazon. This region is a hotspot of biodiversity for parasitic crustaceans with 13 species recorded. Cymothoidae, the most species-rich family of parasitic isopods have preference for infection sites and specificity of hosts. The genera Riggia, Artystone, Braga, Vanamea, Anphira and Nerocila are the most common Cymothoidae found in fish from the Amazon region (Luque et al. 2013; Tavares-Dias et al. 2015). Among them, Nerocila is the only genus that harbors species occurring in brackish environments, while other genera have been found exclusively in freshwater fish species (Thatcher 2006; Tavares-Dias et al. 2015; Gueretz et al. 2018). On the other hand, Corallanidae species comprises six genera and 67 species known, but only the genera Lanocira, Alcirona and Excorallana occur in the tropical region. Excorallana is the largest genus of the family Corallanidae composed of 27 freshwater and marine species in tropical and temperate regions from the New World (Silva and Souza-Filho 2017) with exception of E. oculata that is restricted to eastern and western Atlantic coast (Delaney 1989). Species of the genera Lanocira, Alcirona and Excorallana may be opportunistic ectoparasites and only Excorallana species have been recorded in fish species from the Amazon (Delaney 1989; Luque et al. 2013), demonstrating that more parasitological surveys should be performed in this region.
Despite the ecological and economical importance of parasites in wild fish populations, the parasitic crustacean fauna has been neglected and underestimated in Brazil. Only 279 fish species are known to be infected with parasitic crustaceans and few records have been carried out in Brazilian fish (Luque et al. 2013), and only few species of these parasites have been recorded in fish species from the Amazon basin system (Table 1). Thus, the aim of this study was to investigate the parasitic crustacean fauna in 15 fish species at the Maracá-Jipioca Ecological Station, in the coastal region of the State of Amapá (Brazil), and in addition, to present a checklist of the parasitic Isopod species in fish from the Brazilian Amazon, as well as an update on the geographic distribution of Nerocila acuminata.
Table 1.
Parasitic isopods of freshwater teleost fish in the Brazilian Amazon
| Host species | Parasite species | SI | Localities | References |
|---|---|---|---|---|
| Pseudoplatystoma punctifer | Cymothoidae gen. sp. | Gills | Negro and Solimões rivers (AM) | Lopes et al. (2009) |
| Acestrorhynchus microlepis | Braga amapaensis | Mouth | Araguari River (AP) | Thatcher (1996) |
| Pygocentrus nattereri | Anphira branchialis | Gills | Maracá Island (RR) and Manaus River (AM) | Thatcher (1993) |
| Serrasalmus sp. | Anphira branchialis | Gills | Maracá Island (RR) and Manaus River (AM) | Thatcher (1993) |
| Serrasalmus spilopleura | Anphira branchialis | Gills | Maracá Island (RR) and Manaus River (AM) | Thatcher (1993) |
| Triportheus flavus | Anphira junki | Gills and tegument | Manaus (AM) | Araujo and Thatcher (2003) |
| Triportheus albus | Anphira junki | Gills and tegument | Manaus (AM) | Araujo and Thatcher (2003) |
| Pygocentrus nattereri | Anphira branchialis | Gills | Piranha Lake (AM) | Vital et al. (2011) |
| Cichla temensis | Braga cichlae | Mouth | Negro River (AM) | Araujo et al. (2009) |
| Ageneiosus uyacalensis | Excorallana sp. | Tegument | Amazon River (AM and PA) | Thatcher (2006) |
| Nannostomus beckfordi | Artystone minima | Body cavity | Negro River | Thatcher and Carvalho (1988) |
| Serrasalmus spilopleura | Vanamea symmetrica | Mouth | Tocantins and Araguaia rivers (PA) | Thatcher (1993) |
| Serrasalmus elongatus | Vanamea symmetrica | Mouth | Tocantins and Araguaia rivers (PA) | Thatcher (1993) |
| Ossubtus xinguense | Anphira xinguensis | Gills | Xingu River (PA) | Thatcher (1995) |
| Hoplias malabaricus | Braga patagonica | Gills | Igarapé Fortaleza River (AP) | Alcântara and Tavares-Dias (2015) |
| Plasgioscion squamosissimus | Braga patagonica | Mouth and gills | Negro and Solimões rivers (AM) | Tavares-Dias et al. (2014) |
| Pygocentrus nattereri | Braga patagonica | Mouth and gills | Solimões River (AM) | Tavares-Dias et al. (2014) |
| Colossoma macropomum | Braga patagonica | Mouth and gills | Solimões River (AM) | Tavares-Dias et al. (2014) |
| Serrasalmus sp. | Braga patagonica | Mouth and gills | Solimões River (AM) | Tavares-Dias et al. (2014) |
| Serrasalmus rhombeus | Braga patagonica | Mouth and gills | Solimões River (AM) | Tavares-Dias et al. (2014) |
| Mylossoma duriventre | Braga patagonica | Mouth and gills | Solimões River (AM) | Tavares-Dias et al. (2014) |
| Brycon amazonicus | Braga patagonica | Mouth and gills | Solimões River (AM) | Tavares-Dias et al. (2014) |
| C Chaetobranchopsis orbicularis | Braga patagonica | Mouth and gills | Solimões River (AM) | Tavares-Dias et al. (2014) |
| Hydrolycus scombreoides | Braga patagonica | Mouth and gills | Solimões River (AM) | Tavares-Dias et al. (2014) |
| Colossoma macropomum | Braga patagonica | Tegument | Macapá city (AP) | Dias et al. (2015) |
| Chaetobranchus flavescens | Braga patagonica | Gills | Igarapé Fortaleza River (AP) | Tavares-Dias et al. (2018) |
| Acestrorhynchus falcatus | Braga patagonica | Gills | Igarapé Fortaleza River (AP) | Hoshino et al. (2016) |
| Colossoma macropomum | Braga patagonica | − | Macapá city (AP) | Dias and Tavares-Dias (2015) |
| Serrasalmus altispinis | Anphira branchialis | Gills | Solimões River (AM) | Murrieta-Morey et al. (2016) |
| Serrasalmus altispinis | Anphira branchialis | Gills | Solimões River (AM) | Murrieta-Morey et al. (2016) |
| Curimata incompta | Braga patagonica | Gills and fins | Igarapé Fortaleza River (AP) | Neves et al. (2015) |
| Chaetobranchus flavescens | Braga patagonica | − | Igarapé Fortaleza River (AP) | Bittencourt et al. (2014) |
| Colossoma macropomum | Braga patagonica | Fins | Jari River (PA) | Gonçalves et al. (2018) |
| Acestrorhynchus falcirostris | Excorallana berbicensis | Mouth, gills and tegument | Araguari River (AP) | Gentil-Vasconcelos and Tavares-Dias (2015) |
| Ageneiosus uyacalensis | Excorallana berbicensis | Mouth, gills and tegument | Araguari River (AP) | Gentil-Vasconcelos and Tavares-Dias (2015) |
| Geophagus proximus | Excorallana berbicensis | Mouth, gills and tegument | Araguari River (AP) | Gentil-Vasconcelos and Tavares-Dias (2015) |
| Hemiodus unimaculatus | Excorallana berbicensis | Mouth, gills and tegument | Araguari River (AP) | Gentil-Vasconcelos and Tavares-Dias (2015) |
| Serrasalmus gibbus | Excorallana berbicensis | Tegument | Araguari River (AP) | Gentil-Vasconcelos and Tavares-Dias (2015) |
| Psectrogaster falcata | Excorallana berbicensis | Mouth, gills and tegument | Araguari River (AP) | Gentil-Vasconcelos and Tavares-Dias (2015) |
| Arapaima gigas | Braga nasuta | Body surface | Parauapebas (PA) | Jesus et al. (2017) |
| Serrasalmus altispinis | Braga patagonica | Mouth | Amazon River (AP) | Oliveira et al. (2017) |
| Leporinus friderici | Braga fluviatilis | Mouth | Amazon River (AP) | Oliveira et al. (2017) |
| Peprilus paru | Braga patagonica | Mouth | São João de Pirabas (PA) | Chagas et al. (2015) |
| Anableps anableps | Gnathiidae gen. sp. | Gills | Atlantic Ocean (PA) | Diniz et al. (2008) |
| Mugil gaimardianus | Gnathiidae gen. sp. | Gills | Atlantic Ocean (PA) | Diniz et al. (2008) |
| Conodon nobilis | Gnathiidae gen. sp. | Gills | Atlantic Ocean (PA) | Diniz et al. (2008) |
| Cetengraulis edentulus | Gnathiidae gen. sp. | Gills | Atlantic Ocean (PA) | Diniz et al. (2008) |
| Arius phrygiatus | Gnathiidae gen. sp. | Gills | Atlantic Ocean (PA) | Diniz et al. (2008) |
| Hydrolycus sp. | Excorallana sp. | Body surface | Xingu River (PA) | Magalhães et al. (2018) |
| Metynnis lippincottianus | Anphira xinguensis | Gills | Xingu River (PA) | Magalhães et al. (2018) |
| Metynnis hypsauchen | Anphira xinguensis | Gills | Xingu River (PA) | Magalhães et al. (2018) |
| Metynnis altidorsalis | Anphira xinguensis | Gills | Xingu River (PA) | Magalhães et al. (2018) |
| Ancistrus sp. | Riggia puyensis | Abdomen | Xingu River (PA) | Magalhães et al. (2018) |
SI site of infection, AP state of Amapá, AM state of Amazonas, PA state of Pará, RR State of Roraima
Material and methods
Fish and locality of collection
From March 2017 to July 2018, 15 fish species (Table 2) were collected at the Maracá-Jipioca Ecological Station, State of Amapá, northern coast of Brazil (1°57′5″ N, 50°30′51″ W), using gill nets of different mesh sizes. The Maracá-Jipioca Ecological Station has an area of 75,000 km2, located on the Atlantic coast, separated from the State of Amapá by the Caraporis channel and 310 km far from the city of Macapá. It is a full protection conservation unit that comprises three islands: Maracá-Norte and Maracá-Sul insulated by Stream Canal do Inferno and Jipioca Island (Xavier and Boss 2011).
Table 2.
Length (cm) and Weight (g) of fish from the Maracá-Jipioca Ecological Station, coastal region from the State of Amapá (Brazil)
| Family/host species | N | Length (cm) | Weight (g) |
|---|---|---|---|
| Anablepidae | |||
| Anableps anableps | 25 | 34.1 ± 7.7 | 804.0 ± 401.2 |
| Auchenipteridae | |||
| Pseudauchenipterus nodosus | 2 | 32.5 ± 1.4 | 299.0 ± 57.9 |
| Aspredinidae | |||
| Platystacus cotylephorus | 2 | 38.7 ± 7.2 | 151.0 ± 655.6 |
| Ariidae | |||
| Amphiarius rugispinis | 6 | 39.8 ± 3.9 | 827.9 ± 329.7 |
| Bagre bagre | 4 | 37.3 ± 2.4 | 318.5 ± 52.5 |
| Cathorops spixii | 5 | 28.0 ± 2.1 | 229.2 ± 37.51 |
| Sciades herzbergii | 49 | 41.9 ± 8.4 | 1052.0 ± 720.6 |
| Sciades passany | 3 | 37.3 ± 7.9 | 1538.0 ± 743.9 |
| Centropomidae | |||
| Centropomus undecimalis | 4 | 38.5 ± 2.5 | 469.7 ± 78.1 |
| Loricariidae | |||
| Hypostomus vetromaculata | 8 | 52.3 ± 9.8 | 880.7 ± 755.3 |
| Megalopidae | |||
| Megalopus atlanticus | 5 | 49.8 ± 8.7 | 2154.0 ± 802.3 |
| Mugilidae | |||
| Mugil curema | 66 | 47.6 ± 4.7 | 1116.0 ± 464.4 |
| Sciaenidae | |||
| Cynoscion acoupa | 15 | 42.8 ± 8.2 | 1370.0 ± 2104.0 |
| Macrodon ancylodon | 3 | 33.6 ± 0.1 | 311.7 ± 24.1 |
| Plagioscion squamosissimus | 7 | 39.8 ± 7.3 | 877.4 ± 625.9 |
Parasite sampling procedures
After collection, all fishes were weighed (g) and measured for total length (cm). Mouth, nostrils, opercula, gills, abdominal cavity and tegument of each fish was analyzed for the presence of parasitic crustaceans using a stereomicroscope. The crustaceans found were fixed in alcohol (70%), and then preserved in 70% ethyl with 10% glycerin (Eiras et al. 2006). The ecological descriptors used followed the recommendations of Bush et al. (1997) and Eiras et al. (2006). Parasites were identified in accordance with Castro (1960), Thatcher (2006) and Silva and Souza-Filho (2017).
A review on Isopoda in fish species from the Brazilian Amazon was performed by searching databases (SciELO, ISI, Scopus, Science Direct, Zoological Records, CAB Abstracts databases and Google Scholar), and available data regarding these parasites were added to Table 1.
Results
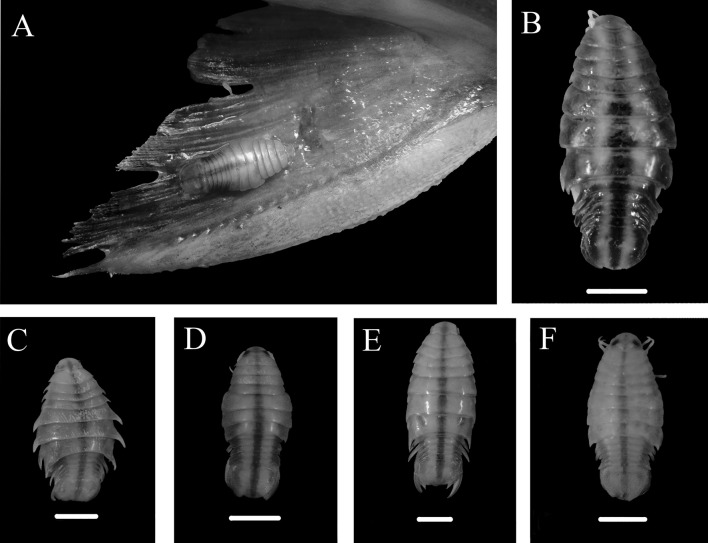
The Table 1 show the isopod species of different families, infection site and locality reported for freshwater fish species from the Amazon river system. Of 15 fish species, a total of 204 specimens were examined, samples number, weight and length are reported (Table 2). The parasitic prevalence was 16.17% and a total of 185 Excorallana longicornis and Nerocila acuminata (Fig. 1) were collected. Excorallana longicornis was the most frequent parasite. In nine fish species, the infestation by these isopods varied from 4.1 to 50.0% (Table 3).
Fig. 1.
Specimens of Nerocila acuminata in fish from the Maracá-Jipioca Ecological Station, coastal region from the State of Amapá, northern Brazil. Male specimen attached to the pectoral fin of Amphiarius rugispinis (A), ‘acuminata form’, ovigerous female found in Anablepsanableps (B), ‘aster form’, non-ovigerous female found in Cathorops spixii (C), male specimen found in A. rugispinis (D), ‘acuminata-aster intermediate form’, ovigerous female found in Megalops atlanticus (E) and male found in Pseudauchenipterus nodosus (F)
Table 3.
Infection by isopod species in fish from the Maracá-Jipioca Ecological Station, coastal region from the State of Amapá (Brazil)
| Host species | Parasite species | Site of infection | EF/PF | P (%) | MI | MA | TNP |
|---|---|---|---|---|---|---|---|
| Mugil curema | Excorallana longicornis | Tegument, mouth and fins | 66/11 | 16.7 | 5.6 | 0.94 | 62 |
| Cynoscion acoupa | Excorallana longicornis | Tegument and fins | 15/6 | 40.0 | 16.3 | 6.53 | 98 |
| Hypostomus vetrimaculata | Excorallana longicornis | Tegument | 8/2 | 25.0 | 1 | 0.25 | 2 |
| Anableps anableps | Excorallana longicornis | Tegument | 25/5 | 20.0 | 2.8 | 0.56 | 14 |
| Nerocila acuminata | Pectoral fin | 25/1 | 4.0 | 1 | 0.04 | 1 | |
| PSses Pseudauchenipterus nodosus | Nerocila acuminata | Caudal fin | 2/1 | 50.0 | 1 | 0.50 | 1 |
| Amphiarius rugispinis | Excorallana longicornis | Tegument | 8/1 | 16.7 | 1 | 16.67 | 1 |
| Nerocila acuminata | Caudal fin | 8/1 | 16.7 | 1 | 16.67 | 1 | |
| Cathorops spixii | Excorallana longicornis | Caudal fin | 5/1 | 20.0 | 1 | 0.20 | 1 |
| Nerocila acuminata | Tegument | 5/1 | 20.0 | 1 | 0.20 | 1 | |
| Sciades herzbergii | Excorallana longicornis | Tegument | 49/2 | 4.1 | 1 | 0.04 | 2 |
| Megalops atlanticus | Nerocila acuminata | Tegument | 5/1 | 20.0 | 1 | 0.20 | 1 |
EF examined fish, PF parasitized fish, P prevalence, MI mean intensity, MA mean abundance, TNP total number of parasites
Discussion
Excorallana longicornis and N. acuminata were found in nine host species of the present study, but E. longicornis was the most frequent isopod. However, both isopods have not been reported in freshwater fish species from the Amazon River system, which were infected by species of four species of Braga, three Anphira, one Excorallana, one Asotana, one Artystone, one Vanamea and one Riggia (Table 1). Therefore, this is the first record of E. longicornis for Amphiarius rugispinis, Mugil curema, Cynoscion acoupa, Hypostomus vetrimaculata, Anableps anableps and Sciades herzbergii, as well as N. acuminata for A. anableps, A. rugispinis, Pseudauchenipterus nodosus, Cathorops spixii and Megalops atlanticus. For Acestrorhynchus falcirostris, Ageneiosus ucayalensis, Geophagus proximus, Hemiodus unimaculatus, Psectrogaster falcata and Serrassalmus gibbus from the Araguari River basin, in the State of Amapá, it has been reported Excorallana berbicensis (Gentil-Vasconcelos and Tavares-Dias 2015). An unidentified species of Excorallana was reported for Ageneiosus inermis from the States of Amazonas and Pará (Thatcher 2006). In general, Excorallana spp. occurs from the intertidal zone to great depths in different environments, mainly E. longicornis that was collected in mangroves. Excorallana spp. may emerge from cryptic habitats as demersal plankton communities and eventually prey on microcrustaceans or temporarily parasitize fish (Delaney 1989). We believe that E. longicornis may parasitize a broad range of fish when perhaps migrates vertically in the water column.
Infestation levels by E. longicornis in fish of this study were low and similar to that found by Gentil-Vasconcelos and Tavares-Dias (2015) in freshwater fish from the Araguari River, in the State of Amapá. In addition, infestation levels by N. acuminata were also low, and similar to that described by Er and Kayiş (2015) for fish from the eastern Black Sea infested by Nerocila spp. In Brazil, Nerocila Leach, 1818 was reported on Mugil liza from the State of Santa Catarina (Gueretz et al. 2018).
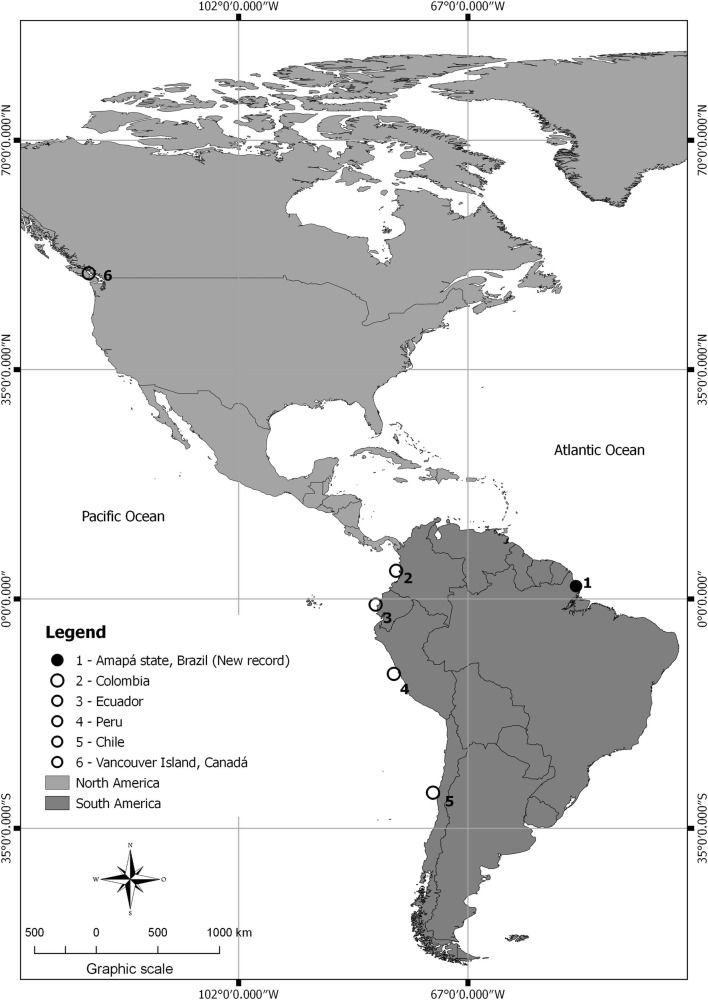
Nerocila acuminata, a Cymothoidae with a wide geographic distribution (Yamauchi and Nagasawa 2012), is known to infest mainly species of Engraulidae, Atherinidae, Serranidae, Mugilidae and Embiotocidae (Brusca 1981) from Chile, Colombia and Ecuador (Brusca 1981; Luque et al. 2013). Further, recently, N. acuminata has been reported on speckled Pseudobatos glaucostigma in the Gulf of California, Mexico (Carrillo-colín et al. 2016) and on Stellifer erycimba in Honduras (Salgado et al. 2015; Carrillo-colín et al. 2016).
Cymothoidae originated in the ocean and afterwards expanded to freshwater and brackish habitats at least twice in the evolutionary history by host shifting. Hence, these isopods present various types of attachment in hosts that evolved first from an ancestor with opercular cavity-dwelling habit. These isopods have a broad range of hosts and site specificity. Many species of flesh-burrowing parasites are more host-specific than those infesting the body surface, which parasitize several host fish species of different families (Hata et al. 2017). Nerocila acuminata and E. longicornis were found attached to the external surfaces of some fish in the present study, because they have a low host-specificity. This is the first report of N. acuminata for Brazilian fish (Fig. 2), and that this is a ubiquitous and cosmopolitan isopod that has been not studied in fish from Brazil.
Fig. 2.
Updated distribution map of occurrence for Nerocila acuminata
The zoogeographical distribution pattern of parasitic crustaceans depends on several factors including host-parasite interactions. In addition, biology and ecology of both host and parasites are also involved in the geographical distribution of parasites, such as host specificity and latitudinal diversity gradient (Tavares-Dias et al. 2015).
Conclusions
The community of parasitic crustaceans in fish species from the coastal region of the State of Amapá consisted of two species of isopods, E. longicornis and N. acuminata. These parasitic isopods may establish complex associations with hosts, and N. acuminata has a wide geographical distribution.
Acknowledgements
The authors thank the National Council for Scientific and Technological Development (Conselho Nacional de Desenvolvimento Científico e Tecnológico, CNPq), Brazil, for the research productivity grant to PhD. M. Tavares-Dias (# 303013/2015-0) and to ESEC Maracá-Jipióca chief Iranildo Coutinho and fieldwork team for the help during surveys.
Compliance with ethical standards
Conflict of interest
Authors declare that there is no conflict of interest regarding the publication of this paper.
Ethical approval
All procedures involving animals were authorized by the ICMBio (# 59031-1) and this study was approved by the Ethics Committee on the Use of Animals of the Embrapa Amapá (# 014/2018).
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Alcântara NM, Tavares-Dias M. Structure of the parasites communities in two Erythrinidae fish from Amazon river system (Brazil) Rev Bras Parasitol Veterinária. 2015;24:183–190. doi: 10.1590/s1984-29612015039. [DOI] [PubMed] [Google Scholar]
- Araujo CS, Thatcher VE. Anphira n. sp. (Isopoda, Cymothoidae) a gill chamber parasite of Triportheus albus and T. flavus (Pisces) in the Brazilian Amazon. Amazoniana. 2003;17:283–290. [Google Scholar]
- Araujo CSO, Barros MC, da Gomes ALS, et al. Parasitas de populações naturais e artifciais de tucunaré (Cichla spp.) Rev Bras Parasitol Vet. 2009;18:34–38. doi: 10.4322/rbpv.01801006. [DOI] [PubMed] [Google Scholar]
- Azevedo JDS, Da Silva LG, Bizerril CRSF, Dansa-Petretski MA, Lima NRW. Infestation pattern and parasitic castration of the crustacean Riggia paranensis (Crustacea: Cymothoidea) on the fresh water fish Cyphocharax gilbert (Teleostei: Curimatidae) Neotrop Ichthyol. 2006;4:363–369. doi: 10.1590/S1679-62252006000300008. [DOI] [Google Scholar]
- Bittencourt LS, Pinheiro DA, Cárdenas MQ, Fernandes BM, Tavares-Dias M. Parasites of native Cichlidae populations and invasive Oreochromis niloticus (Linnaeus, 1758) in tributary of Amazonas River (Brazil) Rev Bras Parasitol Veterinária. 2014;23:44–54. doi: 10.1590/s1984-29612014006. [DOI] [PubMed] [Google Scholar]
- BRASIL. Ministério do Meio Ambiente—MMA (2007) Áreas Prioritárias para a Conservação, Uso Sustentável e Repartição de Benefícios da Biodiversidade Brasileira: Atualização—Portaria MMA n. 09, de 23 de janeiro de 2007. Série Biodiversidade 31, pp 1–300
- Brusca RC. A monograph on the Isopoda Cymothoidae (Crustacea) of the eastern Pacific. Zool J Linn Soc. 1981;73:117–199. doi: 10.1111/j.1096-3642.1981.tb01592.x. [DOI] [Google Scholar]
- Bush AO, Lafferty KD, Lotz JM, Shostak AW, et al. Parasitology meets ecology on its own terms: Margolis et al. revisited. J Parasitol. 1997;83:575–583. doi: 10.2307/3284227. [DOI] [PubMed] [Google Scholar]
- Carrillo-colín LD, Lara-Mendoza RE, Márquez-Farías JF. Nerocila acuminata (Crustacea : Isopoda : Cymothoidae), ectoparasite of speckled guitarfish Pseudobatos glaucostigma ( Elasmobranchii ) from southeastern Gulf of California, Mexico. Cienc Pesq. 2016;24:139–143. [Google Scholar]
- Castro AL. Quatro espécies novas, brasileiras, Excorallana Stebbing, 1904 (Isopoda, Excorallanidae) Arq do Mus Nac. 1960;50:61–77. [Google Scholar]
- Chagas RA, Barros MRF, Salimos RKC, Santos WCR, Herrmann M. Ocorrência de Braga patagonica (Isopoda, Cymothoidae) parasitando Peprilus paru (Osteichthyes: Stromateidae) em águas costeiras tropicais de São João de Pirabas, Pará, norte do Brasil. Acta Fish Aquat Res. 2015;3:1–9. [Google Scholar]
- Davies AJ, Smit NJ. The life cycle of Haemogregarina bigemina (Adeleina: Haemogregarinidae) in South African hosts. Folia Parasitol. 2001;48:169–177. doi: 10.14411/fp.2001.029. [DOI] [PubMed] [Google Scholar]
- Davies AJ, Smit NJ, Hayes PM, Seddon AM, Wertheim D. Haemogregarina bigemina (Protozoa: Apicomplexa: Adeleorina)—past, present and future. Folia Parasitol. 2004;51:99–108. doi: 10.14411/fp.2004.015. [DOI] [PubMed] [Google Scholar]
- Delaney PM. Phylogeny and biogeography of the marine isopod family Corallanidae (Crustacea, Isopoda, Flabellifera) Nat Hist Mus. 1989;409:1–75. [Google Scholar]
- Dias MKR, Tavares-Dias M. Seasonality affects the parasitism levels in two fish species in the eastern Amazon region. J Appl Ichthyol. 2015;31:1049–1055. doi: 10.1111/jai.12865. [DOI] [Google Scholar]
- Dias MKR, Neves LR, Marinho RGB, Tavares-Dias M. Parasitic infections in tambaqui from eight fish farms in Northern Brazil. Arq Bras Med Vet e Zootec. 2015;67:1070–7076. doi: 10.1590/1678-4162-7592. [DOI] [Google Scholar]
- Diniz DG, Varella JEA, Guimarães MDF, et al. A note on the occurrence of praniza larvae of Gnathiidae (Crustacea, Isopoda) on fishes from Northeast of Pará, Brazil. An Acad Bras Cienc. 2008;80:657–664. doi: 10.1590/S0001-37652008000400007. [DOI] [PubMed] [Google Scholar]
- Eiras JDC, Takemoto RM, Pavanelli GC. Métodos de estudo e técnicas laboratoriais em parasitologia de peixes. Maringá: Eduem; 2006. [Google Scholar]
- Ekau W, Knoppers B. An introduction to the pelagic system of the Northeast and East Brazilian shelf. Arch Fish Mar Res. 1999;47:5–24. [Google Scholar]
- Er A, Kayiş Ş. Intensity and prevalence of some crustacean fish parasites in Turkey and their molecular identification. Turk J Zool. 2015;39:1142–1150. doi: 10.3906/zoo-1409-35. [DOI] [Google Scholar]
- Esteves-Silva PH, da Silva MRL, O’Dwyer LH, Tavares-Dias M, Viana LA. Haemogregarina daviesensis sp. nov. (Apicomplexa: Haemogregarinidae) from South American lungfish Lepidosiren paradoxa (Sarcopterygii: Lepidosirenidae) in the eastern Amazon region. Parasitol Res. 2019;10:2773–2779. doi: 10.1007/s00436-019-06430-7. [DOI] [PubMed] [Google Scholar]
- Frye G. The parasitic Crustacea of African freshwater fishes; their biology and distribution. J Zool. 1968;156:45–95. doi: 10.1111/j.1469-7998.1968.tb08578.x. [DOI] [Google Scholar]
- Gentil-Vasconcelos HC, Tavares-Dias M. First study on infestation of Excorallana berbicensis (Isopoda: Corallanidae) on six fishes in a reservoir in Brazilian Amazon during dry and rainy seasons. Lat Am J Aquat Res. 2015;43:936–943. doi: 10.3856/vol43-issue5-fulltext-13. [DOI] [Google Scholar]
- Gonçalves BB, Oliveira MSB, Borges WF, Santos GG, Tavares-Dias M. Diversity of metazoan parasites in Colossoma macropomum (Serrasalmidae) from the lower Jari River, a tributary of the Amazonas River in Brazil. Acta Amaz. 2018;48:211–216. doi: 10.1590/1809-4392201704371. [DOI] [Google Scholar]
- Gueretz JS, Cardoso L, Martins ML, Souza AP. Nerocila sp. (Isopoda: Cymothoidae) parasitizing Mugil liza (Teleostei: Mugilidae) in São Francisco do Sul, Santa Catarina, Brazil. Biotemas. 2018;31:41–44. doi: 10.5007/2175-7925.2018v31n1p41. [DOI] [Google Scholar]
- Hata H, Sogabe A, Tada S, Nishimoto R. Molecular phylogeny of obligate fish parasites of the family Cymothoidae (Isopoda, Crustacea): evolution of the attachment mode to host fish and the habitat shift from saline water to freshwater. Mar Biol. 2017;164:1–15. doi: 10.1007/s00227-017-3138-5. [DOI] [Google Scholar]
- Hoshino MDFG, Neves LR, Tavares-Dias M. Parasite communities of the predatory fish, Acestrorhynchus falcatus and Acestrorhynchus falcirostris, living in sympatry in Brazilian Amazon. Rev Bras Parasitol Veterinária. 2016;25:207–216. doi: 10.1590/s1984-29612016038. [DOI] [PubMed] [Google Scholar]
- Jesus EC, Cardoso L, Ferreira TH, Martins ML, Rodrigues MDN. Braga nasuta (Cymothoidae): an ectoparasite of the Giant Amazonian fish Arapaima gigas (Osteoglossidae) fingerlings cultured in the Amazon region in Northern Brazil. Acta Sci Biol Sci. 2017;39:507–511. doi: 10.4025/actascibiolsci.v39i4.35080. [DOI] [Google Scholar]
- Junoy J. Parasitism of the isopod Artystone trysibia in the fish Chaetostoma dermorhynchum from the Tena River (Amazonian region, Ecuador) Acta Trop. 2016;153:36–45. doi: 10.1016/j.actatropica.2015.10.006. [DOI] [PubMed] [Google Scholar]
- Lopes L, Varella A, Malta J. Metazoan parasites of Pseudoplatystoma punctifer (Linnaeus, 1766) and Pseudoplatystoma tigrinum (Spix & Agassiz, 1829) (Siluriformes: Pimelodidae) of the central Amazon basin, Brazil. Biol Geral e Exp. 2009;9:3–15. [Google Scholar]
- Luque JL, Vieira FM, Takemoto RM, Pavanelli GC, Eiras JC. Checklist of Crustacea parasitizing fishes from Brazil. Check List. 2013;9:1449–1470. doi: 10.15560/9.6.1449. [DOI] [Google Scholar]
- Magalhães C, Robles R, Souza-Carvalho EA, Malta JCO, Mantellato FL. Annotated checklist of parasitic and decapod crustaceans from the middle and lower Xingu (Amazon Basin) above and below the Belo Monte dam complex, Pará State, Brazil. Proc Acad Nat Sci Philadelphia. 2018;166:1–34. doi: 10.1635/053.166.0110. [DOI] [Google Scholar]
- Marceniuk AP, Caires RA, Rotundo MM, et al. The icthyofauna (Teleostei) of the Rio Caeté estuary, northeast Pará, Brazil, with a species identification key from northern Brazilian coast. Panam J Aquat Sci. 2017;12:31–79. [Google Scholar]
- Menezes NA, Buckup PA, Figueiredo JL, Moura RL. Catálogo das Espécies de Peixes Marinhos do Brasil. São Paulo: Museu de Zoologia da Universidade de São Paulo; 2003. [Google Scholar]
- Misganaw K, Getu A. Review on Major Parasitic Crustacean in Fish. Fish Aquac J. 2016;7:1–6. [Google Scholar]
- Murrieta-Morey GA, Sanatana HP, Malta JCO. As espécies de Isopoda (Crustacea: Cymothoidea) parasitas de Serrasalmus altispinis Merckx, Jégu & Santos, 2000 (Characiformes: Serrasalmidae) coletadas em lagos de várzea da Amazônia, Brasil. Folia Amaz. 2016;25:145–152. doi: 10.24841/fa.v25i2.398. [DOI] [Google Scholar]
- Neves LR, Braga ECR, Tavares-Dias M. Diversity of parasites in Curimata incompta (Curimatidae), a host from Amazon river system in Brazil. J Parasit Dis. 2015;40:1296–1300. doi: 10.1007/s12639-015-0674-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliveira MSB, Corrêa LL, Ferreira DO, Neves LR, Tavares-Dias M. Records of new localities and hosts for crustacean parasites in fish from the eastern Amazon in northern Brazil. J Parasit Dis. 2017;41:565–570. doi: 10.1007/s12639-016-0852-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poly WJ. Global diversity of fishlice (Crustacea: Branchiura: Argulidae) in freshwater. Hydrobiologia. 2008;595:209–212. doi: 10.1007/s10750-007-9015-3. [DOI] [Google Scholar]
- Rameshkumar G, Ravichandran S. Problems caused by isopod parasites in commercial fishes. J Parasit Dis. 2014;38:138–141. doi: 10.1007/s12639-012-0210-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ravichandran S, Rameshkumar G, Balasubramanian T. Infestation of isopod parasites in commercial marine fishes. J Parasit Dis. 2010;34:97–98. doi: 10.1007/s12639-010-0014-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salgado AI, Merida JE, Cruz GA. Los isópodos Cymothoa exigua y Nerocila acuminata (Isopoda: Cymothoidae), ectoparásitos de Parapsettus panamensis (Ephippidae), Chloroscombrus orqueta (Carangidae) y Stellifer ericymba (Sciaenidae) del Pacífico de Honduras. Cuad Investig. 2015;7:301–304. [Google Scholar]
- Silva ES, Souza-Filho JF. Species of Excorallana (Isopoda, Corallanidae) from northern and northeastern Brazil, with description of a new species, Excorallana lemoscastroi sp. nov. Nauplius. 2017;25:e2017026. doi: 10.1590/2358-2936e2017026. [DOI] [Google Scholar]
- Smit NJ, Bruce NL, Hadfield KA. Global diversity of fish parasitic isopod crustaceans of the family Cymothoidae. Int J Parasitol Parasites Wildl. 2014;3:188–197. doi: 10.1016/j.ijppaw.2014.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tavares-Dias M, Araújo CSO, Barros MS, Viana GM. New Hosts and distribution records of Braga patagonica, a parasite Cymothoidae of fishes from the Amazon. Braz J Aquat Sci Technol. 2014;18:91–97. doi: 10.14210/bjast.v18n1.p91-97. [DOI] [Google Scholar]
- Tavares-Dias M, Dias-júnior MBF, Florentino AC, Silva LMA, Cunha AC. Distribution pattern of crustacean ectoparasites of freshwater fish from Brazil. Braz J Vet Parasitol. 2015;24:136–147. doi: 10.1590/S1984-29612015036. [DOI] [PubMed] [Google Scholar]
- Tavares-Dias M, Gonçalves RA, Oliveira MSB, Neves LR. Parasites community in Chaetobranchus flavescens heckel, 1840, (Cichliformes: Cichlidae) from the eastern Amazon, Brazil. Bol do Inst Pesca. 2018;44:10–16. doi: 10.20950/1678-2305.2018.262. [DOI] [Google Scholar]
- Thatcher VE. Anphira branchialis gen. et sp. nov. (Crustacea, Isopoda, Cymothoidae) a gill cavity parasite of piranhas (Serrasalmus spp.) in the brazilian Amazon. Acta Amaz. 1993;23:297–307. doi: 10.1590/1809-43921993233307. [DOI] [Google Scholar]
- Thatcher VE. Anphira xinguensis sp. nov. (Isopoda, Cymothoidae) a gill chamber parasite of an Amazonian serrasalmid fish, Ossubtus xinguense Jégu, 1992. Amazoniana. 1995;13:293–303. [Google Scholar]
- Thatcher VE. Braga amapaensis n. sp. (Isopoda: Cymothoidae) a mouth cavity parasite of the Amazonian fish, Acesteorhynchus guyanensis Menezes, with a redefinition of the genus Braga. Amazoniana. 1996;14:121–129. [Google Scholar]
- Thatcher VE. Amazon Fish Parasites. 2a. Sofia, Moscow: Pensoft Publishers; 2006. [Google Scholar]
- Thatcher VE, Carvalho ML. Artystone minima n. sp. (Isopoda, Cymothoidae) a body cavity parasite of the pencil fish (Nannostomus beckfordi Guenther) from the Brazilian Amazon. Amazoniana. 1988;10:255–265. [Google Scholar]
- Vital JF, Varella AMB, Porto DB, de Malta JCO. Sazonalidade da fauna de metazoários de Pygocentrus nattereri (Kner, 1858) no lago Piranha (Amazonas, Brasil) e a avaliação de seu potencial como indicadora da saúde do ambiente. Biota Neotrop. 2011;11:199–204. doi: 10.1590/s1676-06032011000100021. [DOI] [Google Scholar]
- Xavier BF, Boss RL. Região Norte: Estação Ecológica Maracá-Jipioca. In: Valente RM, Silva JMC, Straube CF, Nascimento JLX, editors. Conservação de Aves Migratórias Neárticas no Brasil. Belém, Pará: Conservação Internacional; 2011. pp. 28–32. [Google Scholar]
- Yamauchi T, Nagasawa K. Redescription of the fish parasite Nerocila japonica Schioedte & Meinert, 1881 (Crustacea: Isopoda: Cymothoidae), with comments on previous records of N. acuminata in Japanese waters. Syst Parasitol. 2012;81:147–157. doi: 10.1007/s11230-011-9336-5. [DOI] [PubMed] [Google Scholar]