Abstract
Purpose
The purpose of this study is to investigate whether progesterone (P4) levels on the day of frozen-thawed embryo transfer (FET) to a hormonally prepared endometrium correlate with pregnancy outcomes.
Methods
This is a large retrospective cohort analysis comprising of N = 2010 FETs. In these cycles, P4 levels on the day of transfer were assessed in relation to pregnancy outcomes. A threshold of 10 ng/mL was used to simulate currently accepted levels for physiological corpus luteal function. Biochemical pregnancy, clinical pregnancy, and live birth rates were compared between those with P4 levels above and below this threshold. Analyses using transfer day P4 thresholds of 5 ng/mL and 20 ng/mL were then completed to see if these could create further prognostic power.
Results
When comparing FET outcomes in relation to P4 levels < 10 ng/mL and ≥ 10 ng/mL, we observed no differences in biochemical pregnancy rates (39.53% vs. 40.98%, p = 0.52), clinical pregnancy rates (20.82 vs. 22.78, p = 0.30), and live birth rates (14.25 vs. 16.21 p = 0.23). In patients whose P4 met the threshold of 20 ng/mL, there was similarly no statistically significant improvement in pregnancy outcomes. While there was no difference for biochemical or clinical pregnancy rates, a statistically significant improvement in live birth rates was observed for those with a transfer day P4 level ≥ 5 ng/mL.
Conclusions
We demonstrated that P4 levels at or above 10 ng/mL on the day of FET do not confer a statistically significant improvement in pregnancy outcomes. P4 below 5 ng/mg was associated with lower live birth rates suggesting that there is a threshold below which it is difficult to salvage FET cycles.
Keywords: Embryo transfer, Progesterone, Assisted reproductive technology, Frozen
Introduction
Frozen-thawed embryo transfers (FETs) have been increasingly utilized in assisted reproductive technology (ART) since the implementation of more effective cryopreservation methods. As a result, endometrial preparation protocols have received greater attention as clinicians seek to optimize pregnancy outcomes. Artificial cycles (AC), involving exogenous endometrial preparation, are sometimes necessarily adopted over natural cycles for medical reasons, such as in the case of anovulation, or oocyte donation [1]. Furthermore, ACs have practical benefits over natural cycles (NC) and can be used to facilitate timely access to FETs for both clinicians and patients. In ACs, clinicians aim to reproduce the physiological hormonal milieu through sequential administration of estrogen and progesterone (P4). Hormonal replacement protocols have evolved since the initial introduction of FETs; nevertheless, clinician-dependent variations remain, with no clear consensus regarding the optimal method of artificial endometrial preparation to increase the success of FETs, or how to monitor its efficacy [2].
P4 plays a vital role in both conception and the maintenance of pregnancy; as such, it has been a central focus of investigation aimed at optimizing hormonal replacement therapy [3]. Each route of P4 administration is associated with a distinct serum and endometrial tissue response. While serum P4 levels following IM administration are greater than following PV administration, it has been demonstrated that PV administration produces greater endometrial concentrations of P4 than IM [4–6]. Ideally, P4 monitoring would be tailored to the administration method; however, given the significant practical difficulties in obtaining endometrial P4 levels, serum P4 concentrations continue to be the mainstay marker for P4 monitoring.
In accordance with physiological P4 fluctuations, the optimal serum P4 range correlated with improved pregnancy outcomes varies throughout the ART cycle stages. In the late follicular phase of ART cycles, elevated serum P4 levels have been associated with significantly poorer pregnancy rates [7–10]. However, in the mid-luteal stage, higher P4 concentrations are preferable for improved pregnancy outcomes [11–14]. It is between these two well-studied stages, the day of embryo transfer, that the literature on whether there is an optimal serum P4 range becomes more scant. While poorer pregnancy outcomes have been previously associated with lower P4 levels on the day of FET, all the studies supporting this contain small sample sizes [1, 15–17]. Furthermore, contradictory results have also been demonstrated, with P4 levels above a certain threshold on FET day being correlated with significantly lower pregnancy rates [18]. Given the heterogeneity and limited strength of the literature available in this area, it was our aim to investigate whether P4 levels on the day of embryo transfer are in fact predictive of pregnancy outcomes in artificial FET cycles. To our knowledge, this is by far the largest data set examining this topic to date.
Materials and methods
This is a large single-center retrospective cohort analysis. A standardized data set spanning 2015–2018 was analyzed. This comprised of N = 2010 frozen embryo transfers into hormonally prepared endometria. Patient characteristics, including age and BMI, were registered as routine practice in the clinic database.
The hormonal replacement protocol for artificial FETs included estradiol 2 mg BD from day 5 of the cycle. This was adjusted as required to achieve a transvaginal ultrasound measurement of endometrial thickness of ≥ 8 mm. Progesterone supplementation was then commenced PV 200 mg TID. After 5 full days of P4 replacement, embryo transfer occurred. Both E2 and P4 were continued until pregnancy testing, with beta-HCG samples taken 10 days following FET. If positive, both were continued until 12 weeks gestation; if negative or in the event of miscarriage, all medications were ceased. Serum P4 was measured on the day of FET, and if levels were less than 8 ng/mL, then P4 replacement was increased at the discretion of the treating clinician.
Embryos were derived from IVF or intracytoplasmic sperm injection (ICSI) cycles. The vitrification and warming protocols on either D5 or D6 were performed as per the now-standard method first described by Sifer et al. [19]. The quality of each blastocyst was recorded on day of freeze as per the Gardner classification [20]. A high quality (Q+) transfer was considered to occur where a blastocyst had an expansion grade of 4 or higher (expanded blastocyst to hatched blastocyst), an inner cell mass grade of A or B, and a trophectoderm grade of A or B, i.e., 4BB or superior.
Similar to significant previous studies in the area, a day of transfer P4 threshold of 10 ng/mL (31.8 nmol/L) was used to simulate the currently accepted level for physiological corpus luteal function, with FET outcomes compared between patients measuring above and below this threshold [1, 21, 22]. A total of 807 FETs were identified in patients with a P4 level below 10 ng/mL and 1203 FETs in patients with P4 levels at or above 10 ng/mL. Additionally, analysis was further completed looking at day of transfer P4 thresholds of 5 ng/mL and 20 ng/mL to see if these could create further prognostic power for clinicians attempting FET.
In these artificial cycles, serum P4 levels on the day of transfer were assessed in relation to pregnancy outcomes. The main outcomes measured were biochemical pregnancy (beta human chorionic gonadotropin ≥ 5), ultrasound diagnosed clinical pregnancy (here defined as the presence of a fetal heart beat), and live birth rates.
Univariate analyses of patient characteristics and pregnancy outcomes were compared between these cohorts using the Chi-squared test, or the student’s t test for continuous variables. A multivariate logistic regression, controlling for factors including female age at the time of egg collection, day 5 or day 6 blastocyst, and blastocyst quality at freeze, further evaluated the relationship between P4 levels and outcomes.
This study had local human research and ethics committee approval, HREC ID 71/19-MIVF.
Results
A total of 2010 FETs were included in the available data set. The characteristics of patients were compared between the two cohorts of those that recorded P4 levels ≥ 10 on day of embryo transfer, versus those that did not (Table 1). The baseline characteristics of the two cohorts were not significantly different with the single exception of previous clinical pregnancy, i.e., whether a patient previously conceived an ultrasound diagnosed pregnancy (p = 0.035). Cycles using donor oocytes were excluded from this cohort; thus, all transfers were homologous. Furthermore, endometrial thickness was similar in both groups, and all FETs occurred into normal endometrial cavities with no polyps of submucosal fibroids present.
Table 1.
Characteristics of cycles according to the serum P4 threshold of 10 ng/mL on day of FET
| P4 < 10, N = 807 cycles | P4 ≥ 10, N = 1203 cycles | p value | |
|---|---|---|---|
| Age (years – mean – via t test) | 33.73 | 34.04 | 0.112 |
| < 30 | 27.94 | 27.84 | 0.6094 |
| 30 < 35 | 32.51 | 32.67 | 0.0919 |
| 35 < 40 | 37.11 | 37.25 | 0.2442 |
| ≥ 40 | 42.17 | 41.93 | 0.2942 |
| BMI (mean) | 26.09 | 25.76 | 0.2218 |
| Previous clinical pregnancy | 227 (29.13%) | 288 (23.94%) | 0.035 |
| IVF vs. ICSI | 0.497 | ||
| IVF | 32.03% | 33.47% | |
| ICSI | 67.97% | 66.53% | |
|
Q*/4BB or superior Endometrial thickness (mean in mm) |
57.46% 8.97 |
55.95%% 8.90 |
0.500 0.196 |
| Blastocyst expansion grade at freeze^ | 0.177 | ||
| 5 and 6, or hatching blastocyst and hatched blastocyst | 79 (9.79%) | 99 (8.23%) | |
| 4, or expanded blastocyst | 421 (52.17%) | 614 (51.04%) | |
| 3, or full blastocyst | 200 (24.78%) | 291 (24.19%) | |
| 2, blastocyst or lower | 107 (13.26%) | 199 (16.54%) | |
| Trophectoderm grade at freeze^ | 0.22 | ||
| A | 354 (47.52%) | 495 (44.92%) | |
| B | 336 (45.10%) | 539 (48.91%) | |
| C | 55 (7.38%) | 68 (6.17%) | |
| Inner cell mass grade at freeze^ | 0.11 | ||
| A and B | 15 (2.04%) | 12 (1.10%) | |
| C | 722 (97.96%) | 1077 (98.90%) | |
| Embryo stage at freeze | 0.73 | ||
| Day 5 | 509 (63.07%) | 768 (63.84%) | |
| Day 6 | 298 (36.93%) | 435 (36.16%) |
^Gardner blastocyst grading system [20]
There was a wide range of observed P4 levels on the day of FET (0.5–73.7 ng/mL), with a mean value of 12.33 ± 6.70 ng/mL (Figure 1).
Fig. 1.
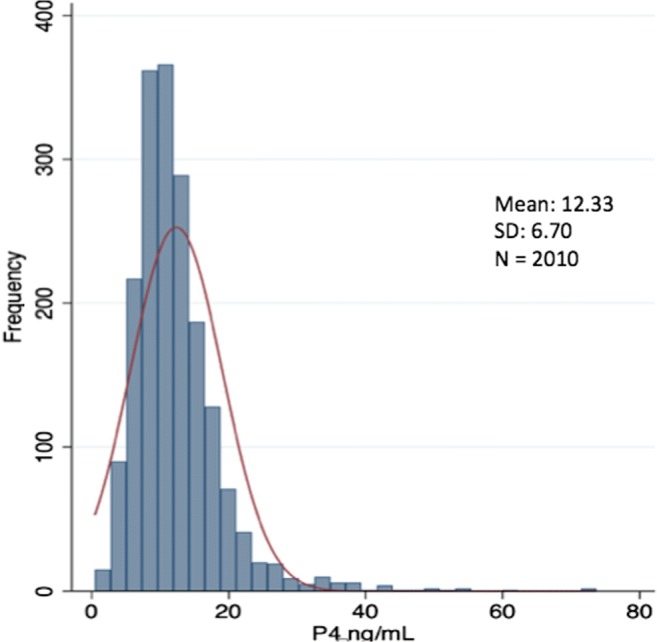
Frequency distribution of P4 levels on day of FET
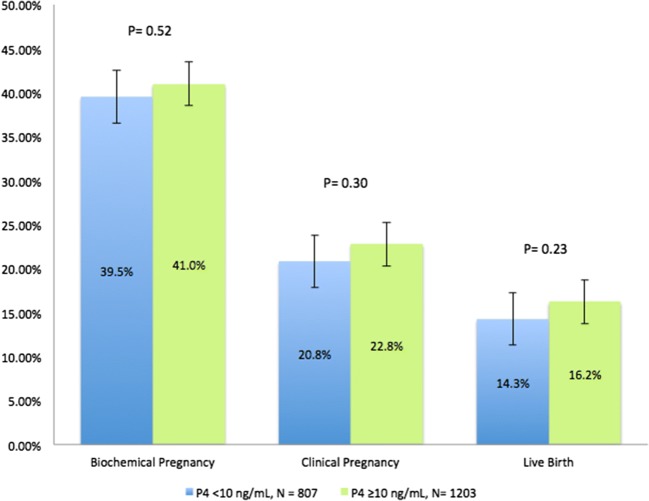
We observed no statistically significant difference in pregnancy outcomes between these two cohorts (P4 on day of embryo transfer < 10 ng/mL versus ≥ 10 ng/mL). This was uniformly demonstrated across biochemical pregnancy rates (39.53% vs. 40.98%, p = 0.516), clinical pregnancy rates (20.82 vs. 22.78, p = 0.299), and live birth rates (14.25 vs. 16.21 p = 0.233) (Figure 2).
Fig. 2.
Pregnancy outcomes by P4 threshold of 10 ng/mL
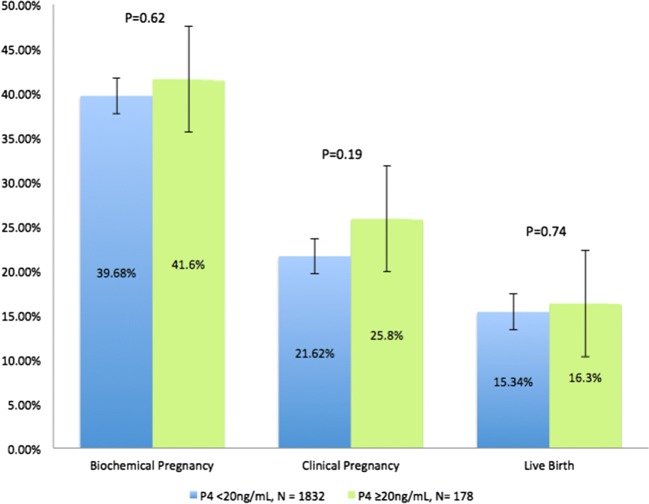
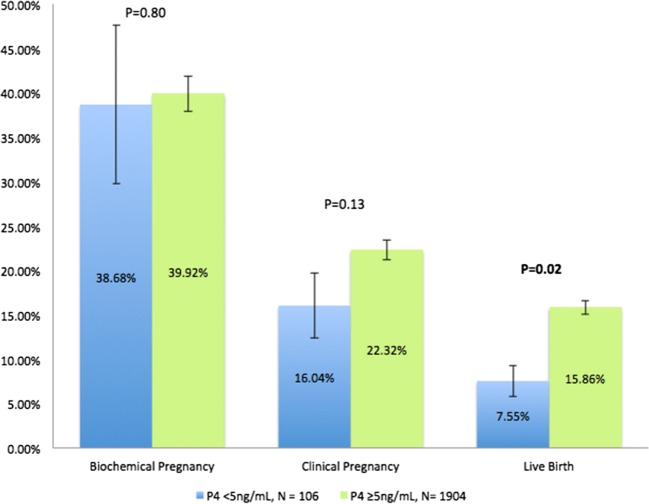
Further analysis was subsequently completed, assessing pregnancy outcomes following P4 thresholds on day of FET of 20 ng/mL and 5 ng/mL (Figs. 3 and 4, respectively). No statistically significant differences in pregnancy outcomes (in either direction) were observed in those patients whose day of transfer P4 met the threshold of 20 ng/mL. However, while there was no difference for biochemical pregnancy or clinical pregnancy rates, a statistically significant improvement in live birth rates was observed for those with a day of transfer P4 level ≥ 5 ng/mL compared to those with a level < 5 ng/mL (15.86% vs. 7.55% respectively, p = 0.02).
Fig. 3.
Pregnancy outcomes by P4 threshold of 20 ng/mL
Fig. 4.
Pregnancy outcomes by P4 threshold of 5 ng/mL
Additionally, a multivariate logistic regression was performed, controlling for variables including female age, D5 vs. D6 blastocyst, the blastocyst expansion grade (as a marker for the full Gardner classification of embryo quality), and previous clinical pregnancy. Age, D5 vs. D6 blastocyst and embryo quality were significantly associated with pregnancy outcomes. However, multivariate analysis failed to demonstrate a significant result for P4 levels above 10 ng/mL on day of FET as an independent prognostic factor for pregnancy outcomes (Table 2). A second multivariate analysis using the P4 threshold of 5 ng/mL demonstrated statistically significant improvements in live birth rates with P4 above this level, but like the primary analysis using this P4 value, no differences were noted in biochemical or clinical pregnancy rates (Table 3).
Table 2.
Multivariate logistic regression analysis of factors related to pregnancy outcomes, P4 threshold 10 ng/mL
| Adjusted OR (95% CI) | p value | |
|---|---|---|
| Biochemical Pregnancy | ||
| P4 ≥ 10 ng/mL | 1.14 (0.94; 1.37) | 0.19 |
| Age (years) | 0.94 (0.92; 0.96) | 0.00 |
| D5 blastocyst | 2.23 (1.81; 2.74) | 0.00 |
| Blastocyst expansion grade^ | 0.67 (0.60; 0.74) | 0.00 |
| Previous clinical pregnancy | 1.34 (1.08; 1.66) | 0.007 |
| Clinical pregnancy | ||
| P4 ≥ 10 ng/mL | 1.20 (0.96; 1.50) | 0.11 |
| Age (years) | 0.94 (0.91; 0.96) | 0.00 |
| D5 blastocyst | 2.11 (1.64; 2.70) | 0.00 |
| Blastocyst expansion grade^ | 0.65 (0.57; 0.74) | 0.00 |
| Previous clinical pregnancy | 1.20 (0.94; 1.54) | 0.152 |
| Live birth | ||
| P4 ≥ 10 ng/mL | 1.22 (0.95; 1.58) | 0.12 |
| Age (years) | 0. 92 (0.89; 0.95) | 0.00 |
| D5 blastocyst | 1.77 (1.33, 2.35) | 0.00 |
| Blastocyst expansion grade^ | 0.75 (0.65; 0.87) | 0.00 |
| Previous clinical pregnancy | 0.96 (0.72; 1.29) | 0.805 |
^ as surrogate marker of embryo quality
Table 3.
Multivariate logistic regression analysis of factors related to pregnancy outcomes, P4 threshold 5 ng/mL
| Adjusted OR (95% CI) | p value | |
|---|---|---|
| Biochemical pregnancy | ||
| P4 ≥ 5 ng/mL | 1.18 (0.77; 1.78) | 0.45 |
| Age (years) | 0.94 (0.92; 0.96) | 0.00 |
| D5 blastocyst | 2.23 (1.81; 2.74) | 0.00 |
| Blastocyst expansion grade^ | 0.67 (0.60; 0.74) | 0.00 |
| Previous clinical pregnancy | 1.34 (1.08; 1.66) | 0.007 |
| Clinical pregnancy | ||
| P4 ≥ 5 ng/mL | 1.65 (0.96; 2.83) | 0.071 |
| Age (years) | 0.94 (0.91; 0.96) | 0.00 |
| D5 Blastocyst | 2.11 (1.65; 2.71) | 0.00 |
| Blastocyst expansion grade^ | 0.65 (0.57; 0.74) | 0.00 |
| Previous clinical pregnancy | 1.19 (0.93; 1.53) | 0.167 |
| Live birth | ||
| P4 ≥ 5 ng/mL | 2.48 (1.19; 5.20) | 0.016 |
| Age (years) | 0. 92 (0.89; 0.95) | 0.00 |
| D5 blastocyst | 1.79 (1.35, 2.37) | 0.00 |
| Blastocyst expansion grade^ | 0.75 (0.65; 0.87) | 0.00 |
| Previous clinical pregnancy | 0.96 (0.72; 1.29) | 0.787 |
^as surrogate marker of embryo quality.
Discussion
This study shows that P4 levels above 10 ng/mL on day of FET are not a significant factor in predicting pregnancy outcomes. A P4 threshold of 10 ng/mL was identified based on previous studies in this area that determined such a P4 level to be an indicator of adequate corpus luteal function during the luteal phase [21, 22]. Univariate analyses comparing FETs with P4 levels that reached this threshold with those that did not demonstrated no significant differences in biochemical pregnancy, clinical pregnancy, and live birth rates between the two cohorts. Subsequent multivariate analyses, controlling for potential confounders such as female age and embryo quality, further supported this negative finding. This result contradicts what little literature is currently available.
A further analysis looking at P4 threshold of 5 ng/mL on day of FET did show that while cycles below this threshold produced equivalent biochemical and clinical pregnancy rates, there was a higher live birth rate observed in cycles starting out with serum P4 levels > 5 ng/mL.
Prior evidence in this area has demonstrated a positive correlation between P4 levels on the day of embryo transfer and pregnancy outcomes; however, in reviewing these findings, some inherent limitations demonstrated the need for further investigation. Similar to our study design, the majority of reports have been retrospective in nature; however, unlike the current study, prior literature has been additionally limited by small sample sizing [1, 15–18]. In line with the value chosen here, a number of papers used a P4 threshold of 10 ng/mL [1, 17] with one prospective study using a similar threshold of 9.2 ng/mL [15]. These studies illustrated a beneficial effect for FET outcomes where P4 reaches these thresholds. There are two papers that utilized 20 ng/mL as a threshold for analysis; while one found that results commensurate with the abovementioned studies [16], the other demonstrated reduced ongoing pregnancy and live birth rates associated with P4 levels above this threshold [18]. Another limitation of available studies in this area is the lack of uniformity in P4 replacement protocols as well as a limited understanding of the extent of correlation between serum and endometrial P4 concentrations.
In artificial cycles, P4 replacement is needed to simulate physiological hormonal function to augment endometrial receptivity; however, there is still no consensus on the optimal route of P4 administration and whether serum P4 is a reliable marker [2]. P4 improves endometrial receptivity through the regulation of cytokines, resulting in both improved trophoblast function at the site of implantation and the suppression of a maternal immunologic response against the trophoblast cells [23]. Furthermore, under the influence of P4, stromal cells produce several growth factors that help maintain the pregnancy until the placenta takes over P4 production at around 10 weeks gestation [24]. Serum and endometrial P4 concentrations correlate well in the setting of endogenous P4 or exogenous IM administration [25, 26]. However, IM injection of P4 results in significantly higher mean serum P4 levels than PV but possibly lower endometrial concentrations [4–6]. This is postulated to be the result of the uterine first-pass effect [27]. Additionally, peak serum P4 levels following PV administration demonstrated high inter-individual variability likely secondary to variations in vaginal absorption, vagina mucosal surface area, and differences in the vaginal microbiome [1, 28, 29]. Thus it becomes difficult to equate endometrial P4 action with serum P4 levels, particularly where PV luteal phase support is used. This is one potential explanation for the current study’s findings.
Another possible theory to explain these findings could be that our cohort received adequate P4 replacement therapy to mitigate the negative effect of lower P4 levels during the luteal phase. Similar to other studies, it was standard practice in our center to increase P4 replacement if day of transfer levels were deemed poor (< 8 ng/mL). As such, even though P4 may have been inadequate on day of transfer, this was potentially remedied by additional P4 administration, reinforcing that intervention might still be possible beyond the day of transfer. With respect to the positive association between live birth rates and P4 levels ≥ 5 ng/mL, it may be that additional P4 administration cannot mitigate the effects of an initially very low circulating level of P4 that falls below this threshold. An admitted limitation of this study is that additional replacement was not standardized and the efficacy of this was not monitored with further serum P4 levels. While a single P4 measure on day of transfer in isolation may not be helpful for predicting pregnancy outcomes, is it possible that serial measurements of P4 may be useful? This will be an important avenue for future investigation.
In conclusion, we demonstrated that serum P4 levels at or above 10 ng/mL on the day of FET are not associated with statistically significant improvements in biochemical pregnancy, clinical pregnancy, or live birth rates. It could be that increasing P4 replacement following low P4 levels on transfer day mitigated the negative effect of low initial P4 concentrations. However, P4 below 5 ng/mg was associated with worse live birth rates, suggesting that there is a threshold below which increasing P4 replacement after embryo transfer cannot be expected to prevent pregnancy loss. Additional research is clearly needed in this area to further characterize ideal P4 replacement as well as the role of P4 monitoring in artificial cycles.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Cedrin-Durnerin I, et al. Serum progesterone concentration and live birth rate in frozen-thawed embryo transfers with hormonally prepared endometrium. Reprod BioMed Online. 2019;38(3):472–480. doi: 10.1016/j.rbmo.2018.11.026. [DOI] [PubMed] [Google Scholar]
- 2.van der Linden M, et al. Luteal phase support for assisted reproduction cycles. Cochrane Database Syst Rev. 2015;7. [DOI] [PMC free article] [PubMed]
- 3.Taraborrelli S. Physiology, production and action of progesterone. Acta Obstet Gynecol Scand. 2015;94:8–16. doi: 10.1111/aogs.12771. [DOI] [PubMed] [Google Scholar]
- 4.Miles RA, Paulson RJ, Lobo RA, Press MF, Dahmoush L, Sauer MV. Pharmacokinetics and endometrial tissue levels of progesterone after administration by intramuscular and vaginal routes: a comparative study. Fertil Steril. 1994;62(3):485–490. doi: 10.1016/S0015-0282(16)56935-0. [DOI] [PubMed] [Google Scholar]
- 5.Paulson RJ, Collins MG, Yankov VI. Progesterone pharmacokinetics and pharmacodynamics with 3 dosages and 2 regimens of an effervescent micronized progesterone vaginal insert. J Clin Endocrinol Metab. 2014;99(11):4241–4249. doi: 10.1210/jc.2013-3937. [DOI] [PubMed] [Google Scholar]
- 6.De Ziegler D, et al. The first uterine pass effect. Ann N Y Acad Sci. 1997;828:291–299. doi: 10.1111/j.1749-6632.1997.tb48550.x. [DOI] [PubMed] [Google Scholar]
- 7.Racca A, Santos-Ribeiro S, de Munck N, Mackens S, Drakopoulos P, Camus M, Verheyen G, Tournaye H, Blockeel C. Impact of late-follicular phase elevated serum progesterone on cumulative live birth rates: is there a deleterious effect on embryo quality? Hum Reprod. 2018;33(5):860–868. doi: 10.1093/humrep/dey031. [DOI] [PubMed] [Google Scholar]
- 8.Esteves SC, et al. Association between progesterone elevation on the day of human chronic gonadotropin trigger and pregnancy outcomes after fresh embryo transfer in in vitro fertilization/intracytoplasmic sperm injection cycles. Front Endocrinol (Lausanne) 2018;9:201. doi: 10.3389/fendo.2018.00201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ibrahim Y, et al. Elevated progesterone and its impact on birth weight after fresh embryo transfers. J Assist Reprod Genet. 2017;34(6):759–764. doi: 10.1007/s10815-017-0920-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lawrenz B, Fatemi HM. Effect of progesterone elevation in follicular phase of IVF-cycles on the endometrial receptivity. Reprod BioMed Online. 2017;34(4):422–428. doi: 10.1016/j.rbmo.2017.01.011. [DOI] [PubMed] [Google Scholar]
- 11.Yovich JL, Conceicao JL, Stanger JD, Hinchliffe PM, Keane KN. Mid-luteal serum progesterone concentrations govern implantation rates for cryopreserved embryo transfers conducted under hormone replacement. Reprod BioMed Online. 2015;31(2):180–191. doi: 10.1016/j.rbmo.2015.05.005. [DOI] [PubMed] [Google Scholar]
- 12.Solomon HF, Sneeringer RM, Penzias AS. Prediction of pregnancy outcome by serum progesterone level after assisted reproductive technology. Fertil and Steril. 2008;90:Supp 231. doi: 10.1016/j.fertnstert.2008.07.561. [DOI] [Google Scholar]
- 13.Arce JC, Balen A, Platteau P, Pettersson G, Andersen AN. Mid-luteal progesterone concentrations are associated with live birth rates during ovulation induction. Reprod BioMed Online. 2011;22(5):449–456. doi: 10.1016/j.rbmo.2011.01.006. [DOI] [PubMed] [Google Scholar]
- 14.Blakemore JK, Kofinas JD, McCulloh D, Grifo J. Serum progesterone trend after day of transfer predicts live birth in fresh IVF cycles. J Assist Reprod Genet. 2017;34(3):339–343. doi: 10.1007/s10815-016-0859-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Labarta E, et al. Low serum progesterone on the day of embryo transfer is associated with a diminished ongoing pregnancy rate in oocyte donation cycles after artificial endometrial preparation: a prospective study. Hum Reprod. 2017;32(12):2437–2442. doi: 10.1093/humrep/dex316. [DOI] [PubMed] [Google Scholar]
- 16.Brady PC, Kaser DJ, Ginsburg ES, Ashby RK, Missmer SA, Correia KF, Racowsky C. Serum progesterone concentration on day of embryo transfer in donor oocyte cycles. J Assist Reprod Genet. 2014;31(5):569–575. doi: 10.1007/s10815-014-0199-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tihomirova T, et al. Measurement of serum progesterone levels on the day of embryo transfer is a useful tool in prediction of successful pregnancy. Annuaire de l'Afrique du Nord. 2018;103:53–59. [Google Scholar]
- 18.Kofinas JD, Blakemore J, McCulloh D, Grifo J. Serum progesterone levels greater than 20 ng/dl on day of embryo transfer are associated with lower live birth and higher pregnancy loss rates. J Assist Reprod Genet. 2015;32(9):1395–9. doi: 10.1007/s10815-015-0546-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sifer C, et al. Outcome of embryo vitrification compared to slow freezing process at early cleavage stages. Report of the first French birth. Gynecol Obstet Fertil. 2012;40(3):158–161. doi: 10.1016/j.gyobfe.2011.10.004. [DOI] [PubMed] [Google Scholar]
- 20.Gardner, D. and W. Schoolcraft, In vitro culture of human blastocyst, in Towards Reproductive Certainty: Fertility and Genetics Beyond 1999, J. R and M. D, Editors., Parthenon Publishing Carnforth: UK. 1999 p. 378–388.
- 21.Hull MG, Savage PE, Bromham DR, Ismail AA, Morris AF. The value of a single serum progesterone measurement in the midluteal phase as a criterion of a potentially fertile cycle ("ovulation") derived form treated and untreated conception cycles. Fertil Steril. 1982;37(3):355–360. doi: 10.1016/S0015-0282(16)46095-4. [DOI] [PubMed] [Google Scholar]
- 22.Jordan J, et al. Luteal phase defect: the sensitivity and specificity of diagnostic methods in common clinical use. Fertil Steril. 1994;62(1):54–62. doi: 10.1016/S0015-0282(16)56815-0. [DOI] [PubMed] [Google Scholar]
- 23.Kumar P, Magon N. Hormones in pregnancy. Niger Med J. 2012;53(4):179–183. doi: 10.4103/0300-1652.107549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Spencer TE, Bazer FW. Biology of progesterone action during pregnancy recognition and maintenance of pregnancy. Front Biosci. 2002;7:d1879–d1898. doi: 10.2741/spencer. [DOI] [PubMed] [Google Scholar]
- 25.Bayard F, Louvet JP, Monrozies M, Boulard A, Pontonnier G. Endometrial progesterone concentrations during the menstrual cycle. J Clin Endocrinol Metab. 1975;41(2):412–414. doi: 10.1210/jcem-41-2-412. [DOI] [PubMed] [Google Scholar]
- 26.Carson RL, Ganjam VK, Hudson RS, McLeod C, Kwapien R. Plasma and endometrial progesterone content following exogenous progesterone administration in mares. Theriogenology. 1983;19(2):235–241. doi: 10.1016/0093-691X(83)90009-2. [DOI] [PubMed] [Google Scholar]
- 27.Cicinelli E, et al. Direct transport of progesterone from vagina to uterus. Obstet Gynecol. 2000;95(3):403–406. doi: 10.1016/s0029-7844(99)00542-6. [DOI] [PubMed] [Google Scholar]
- 28.Nahoul K, Dehennin L, Jondet M, Roger M. Profiles of plasma estrogens, progesterone and their metabolites after oral or vaginal administration of estradiol or progesterone. Maturitas. 1993;16(3):185–202. doi: 10.1016/0378-5122(93)90064-O. [DOI] [PubMed] [Google Scholar]
- 29.Archer DF, et al. Initial and steady-state pharmacokinetics of a vaginally administered formulation of progesterone. Am J Obstet Gynecol. 1995;173(2):471–7 discussion 477–8. [DOI] [PubMed]