Abstract
Purpose
Twelve percent of women in the USA will develop invasive breast cancer in their lifetime, and that risk increases to 80% if they carry a BRCA1 or BRCA2 mutation. BRCA1/2 mutations are thought to potentially affect ovarian reserve and/or fertility.
Methods
PubMed and PubMed Central were searched for publications on ovarian reserve–related outcomes (i.e., AMH and response to controlled ovarian hyperstimulation (COH) protocols) that were reported in relation to BRCA1 and/or BRCA2 mutations from 1950 through May 2019. A meta-analysis was conducted to create forest plots and summary effect measures using Review Manager 5.3.
Results
This article reviews the 16 qualifying publications. There were several fundamental methodological differences in the study designs and outcome details reported in AMH studies. Summary statistics found no difference in AMH levels between BRCA1/2+ women as compared with controls (Z overall test effects p ≥ 0.45). Regarding responses to COH, there were overall non-significantly fewer total and mature numbers of oocytes retrieved in BRCA1/2+ cases as compared with controls (meta-analysis Z overall test effects p ≥ 0.40).
Conclusions
While the summary measures indicate no significant differences in AMH levels between BRCA1/2+ cases and controls, readers should be aware that there are significant methodological differences in the AMH reports. Additionally, the response to COH protocols does not seem to be significantly lower in BRCA1/2 mutation carriers in the existing literature. Continued research on both of these clinical parameters would be beneficial for patient counseling.
Keywords: BRCA1 mutation, BRCA2 mutation, anti mullerian hormone, ovarian reserve, in vitro fertilization, controlled ovarian hyperstimulation
Introduction
Twelve percent of women in the USA will develop invasive breast cancer in their lifetime; that risk increases to 80% in women carrying a BRCA1 or BRCA2 mutation (i.e., BRCA1/2+ status) [1]. BRCA1/2 mutations account for 5–10% of breast cancers and have a population prevalence of 1.0–2.2% in different ethnic groups [2–5]. The general population prevalence of BRCA1 mutations is estimated to be 1.2% [3] among Ashkenazi Jewish women, 0.24% [3] among Caucasian and African American women in the USA, and 0.32% [4] among Canadian women. BRCA2 mutations occur in 1% [2] of Ashkenazi Jewish women, 0.4% [5] of Caucasian (non-Ashkenazi Jewish) women, and 0.7% [4] of Canadian women.
There are four theories that biologically connect BRCA1/2 with ovarian reserve. BRCA genes, primarily BRCA1, play a role in the maintenance of double-stranded DNA breaks and telomere length [6]. BRCA1/2 mutations may thus impair ovarian reserve via (1) accumulated DNA damage secondary to inadequate double-strand DNA repair, which may cause accumulated lethal damage to oocyte DNA [7]; (2) impairment of embryogenesis [8, 9]; (3) failure to maintain telomere length [10–12]; and (4) decreased follicular pool in carriers [13–17]. It has been noted that the underlying biology of cancer and infertility is both driven by a “fundamental dysregulation of cellular renewal and differentiation” [18]. Although the quantity of oocytes is finite and declines from birth until menopause, this cell line must tightly regulate its transcription in order for embryogenesis to occur.
Markers of ovarian reserve are parameters which are aimed at estimating the number of primordial follicles remaining in a woman’s individual follicular pool, in order to predict ovarian function and/or future fertility potential [19]. Measures of ovarian reserve include day 2 or 3 antral follicle counts seen on ultrasound, day 2 or 3 circulating follicle-stimulating hormone (FSH) levels, and, more recently, circulating anti-Mullerian hormone (AMH) levels [19]. AMH is a member of the TGF-β superfamily of growth factors and is secreted by primary, secondary, and small antral follicles (< 4 mm) in the ovaries (reviewed in [20]). It is considered to be fairly constant over the course of the menstrual cycle, making it a popular marker of ovarian reserve. AMH has been successfully utilized in predicting response to controlled ovarian hyperstimulation (COH) and for dosing of gonadotropins during COH, although it is less clearly predictive of pregnancy outcomes, particularly live birth [20]. In addition to assessing hormonal markers of ovarian reserve such as AMH, ovarian function can be assessed using COH/IVF outcomes such as number of oocytes retrieved, number of blastocysts achieved, and clinical pregnancy rates. These markers can all be affected by myriad factors in addition to the patient’s ovarian reserve, and thus must be considered within the context of the patient and clinical scenario in which they are measured. Clearly, ovarian reserve is important for selecting the most appropriate clinical protocols for fertility management and for overall patient guidance/recommendations by the clinicians.
Existing research on the association between BRCA mutations and ovarian reserve and/or function is conflicting and the study designs are heterogeneous. Therefore, we performed a systematic literature review and meta-analysis as a clinical update on current knowledge about markers of ovarian reserve and/or indicators of ovarian function among women with BRCA1/2 mutations, with a particular focus on epidemiological differences in study design as a means of interpreting conflicting results. The goals were to identify any consistencies in the literature, evaluate potential explanations for any differences, and offer suggestions for future research.
Methods
Search strategy
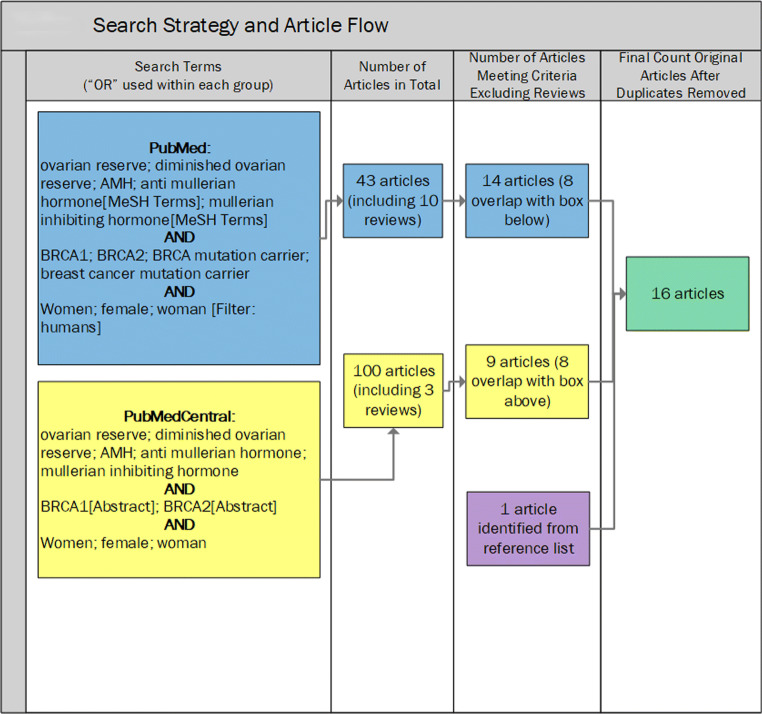
This was a systematic literature review with summary meta-analyses where possible with currently available published data. This study began with a structured approach to identify and review articles. The PubMed database was searched for publications on ovarian reserve–related outcomes (specifically, AMH and results of COH for IVF) that were reported in relation to BRCA1 and/or BRCA2 mutation carrier status. The search was run in January 2019 and subsequently updated in May 2019. The dates of available literature in the PubMed database were from 1950 through the current available at that time. The search terms used for PubMed are shown below and in Fig. 1. These search results were subsequently restricted to humans.
((ovarian reserve) OR (diminished ovarian reserve) OR AMH OR (anti Mullerian hormone[MeSH Terms]) OR (Mullerian inhibiting hormone[MeSH Terms]))
AND (BRCA1 OR BRCA2 OR (BRCA mutation carrier) OR (breast cancer mutation carrier))
AND (women OR female OR woman)
Fig. 1.
Search strategy and article flow
The terms “BRCA mutation carrier” and “breast cancer mutation carrier” did not add any additional articles and, thus, were subsequently deemed irrelevant for the search strategy. This PubMed strategy yielded 43 citations, of which 10 were classified as reviews by PubMed (Fig. 1).
In order to identify potentially relevant articles in open access journals that are not indexed in PubMed, we repeated a similar strategy in PubMed Central (PMC) in January 2019, and subsequently updated in May 2019. The search terms used for PMC were as follows:
((ovarian reserve) OR (diminished ovarian reserve) OR AMH OR (anti Mullerian hormone) OR (Mullerian inhibiting hormone))
AND (BRCA1[Abstract] OR BRCA2[Abstract])
AND (women OR female OR woman)
Readers will note differences in the two strategies. The authors discovered that they needed to limit BRCA1 and BRCA2 to the abstract, because otherwise there were 684 citations as of January 2019, most of which were ineligible due to having basic science and/or animal model study designs. With the search terms as displayed, 89 citations were identified from PMC initially (increased to 100 citations by May 2019). There were 8 overlapping relevant publications in the 2 strategies (excluding review articles) [7, 14, 15, 21–25]. The PMC search identified one additional citation included in the tables by Choi et al. [26]. One final article [27] was identified through the citations of another included article [28] (Fig. 1).
Abstracts of all identified articles were reviewed for eligibility independently by two authors (LP and BM). Eligibility required measurement and analysis of human female AMH levels and/or response to COH protocols, a case group that was restricted to women who carried the BRCA1 and/or BRCA2 mutation, and was published in English. Any discrepancies were resolved by discussion until consensus was achieved. Those publications not immediately deemed ineligible were read in their entirety for further eligibility review. Sixteen publications met our criteria [7, 14, 15, 21–33].
Data collected from each article included the following: study design and analysis methods, study sample characteristics with special attention to any feature that might explain variability within the body of research on this topic, and the primary findings relevant to ovarian reserve. The literature was reviewed multiple times by the authors (LP, BM, EF, CNCM) as the tables and fields to be reported were further defined and refined. Selected article features were entered into a spreadsheet, and subsequently re-designed into its final form of tables for this manuscript. Summary observations of the findings from the body of research on these ovarian reserve outcomes were primarily made by CNCM and LP.
Forest plots and summary effect measures (Review Manager 5.3 [34]) were created for outcomes within the relevant AMH literature and separately for the COH/IVF reports, subject to limitations on the published data required for graphing purposes and summary effect size calculations. The most common data reported were means; therefore, that measurement was selected for summarization. Three articles [21, 30, 31] reported medians rather than means. Means and standard deviations (SD) were estimated for the reported median, range, and/or interquartile range values from those three articles using Wan et al. [35] (scenario 3 for the Lambertini report [31] and the Derks-Smeets report [21], scenario 1 for the van Tilborg report [30]). For the articles without data reported that could be used in the meta-analysis, the corresponding author was contacted and unpublished means/SD were obtained from two authors [14, 24]. Unit conversion from picomole per liter to nanogram per milliliter was performed as required using an online calculator for AMH values (www.unitslab.com/node/155). The AMH analyses were originally run as fixed effects models; however, the heterogeneity tests indicated that there was great variability in the findings and associated confidence intervals across publications (I2 statistics > 75%) [36]. In response, we changed to a random effects model, which subsequently used a relatively constant weight of the publications (as opposed to weighting based on sample size and variance with the fixed effects model). This was also true for the COH/IVF literature (I2 statistics > 78%).
Results
Studies of anti-Mullerian hormone levels and BRCA1/2 mutations: (1) Study designs and methodologic differences
The majority of studies assessing markers of ovarian reserve in women with BRCA1/2 mutations utilized AMH as a primary outcome variable (Table 1). Specifically, nine studies [14, 15, 22, 24, 25, 28–31] assessed AMH levels in BRCA mutation carriers in six countries between 2013 and 2019 (five studies were conducted in the USA and one each was conducted in Australia, the Netherlands, Israel, and Belgium). In total, 852 BRCA-positive cases (484 BRCA1+, 318 BRCA2+, 5 both BRCA+ and BRCA2+, and 41 unspecified) were compared with 1887 controls. The sample size within each report ranged from 19 to 319 cases and from 54 to 600 controls (Tables 2 and 3).
Table 1.
Key characteristics of the publications reporting AMH levels with BRCA1/2 mutations
| First author, year, country | Population | Did enrollment criteria allow women with a personal history of cancer | Hormonal outcome | Findings | Adjusted for | AMH sample/assay details |
|---|---|---|---|---|---|---|
| Phillips, 2016, Australia |
Age: 25–45 years Cases: 172 BRCA1+, 147 BRCA2+ (no personal history of cancer, retained both ovaries and not pregnant or breastfeeding) Controls: 216 BRCA1− from BRCA1+ families, 158 BRCA2− from BRCA2+ families (no personal history of cancer, retained both ovaries and not pregnant or breastfeeding) |
No | AMH level | BRCA1+ had, on average, 25% (95% CI 5–41%, p < 0.02) lower AMH concentrations than non-carriers and were more likely to have AMH concentrations in the lowest quartile for age (OR 1.84, 95% CI 1.11–303, p < 0.02). There was no evidence of an association between AMH concentration and BRCA2 mutation status (p < 0.94) | Age at blood draw, BMI, smoking, HC use, post-menopausal, unknown menopausal status |
Sample type: plasma, frozen Duration of freezing: mean storage 11 years without any thaws Assay: Elec system (automated) |
| Johnson, 2017, USA |
Age: 18–45 years Cases: 55 BRCA1+, 50 BRCA2+ (with a uterus and both ovaries) Controls: 26 BRCA−, 64 low-risk (with a uterus and both ovaries) |
Yes | AMH level |
BRCA2+ had AMH levels that were 33% lower than controls and an increased odds of having AMH < 1 ng/mL BRCA1+ and BRCA− women had similar AMH levels to controls When analysis was restricted to regularly menstruating women younger than 40, BRCA2+ continued to demonstrate significantly lower AMH levels and increased likelihood of low AMH. Also, in this restricted group, BRCA− women demonstrated AMH levels that were 42% lower than controls. No difference in AMH was observed among BRCA1+ |
Age, HC use, menstrual regularity, African American race |
Sample type: dried bloodspot Duration of freezing: N/A Assay: pico-AMH ELISA run by Ansh labs |
| van Tilborg, 2016, Netherlands |
Age: 18–45 years Cases: 66 BRCA1+, 57 BRCA2+, 1 BRCA1/2+ (no personal history of breast cancer or PCOS) Controls: 131 BRCA1/2− |
No | AMH level | BRCA1/2+ cases do not show a lower serum AMH level in comparison with proven non-carriers, after adjustment for potential confounders. The median AMH levels: BRCA+ 1.90 μg/L vs BRCA− 1.80 μg/L. Adjusted linear regression analysis revealed no reduction in AMH level in the carriers. Sensitivity analysis stratifying by gene suggested possible differences in BRCA1+ vs BRCA− (p = 0.08), and no difference in BRCA2+ vs BRCA− (p = 0.64) | Age, smoking, current HC use (cases were more likely to be younger, be nulliparous, and have a history of infertility) |
Sample type: frozen plasma Duration of freezing: ~ 3 years Assay: Beckman Coulter Gen II AMH ELISA kit |
| Choi, 2018, South Korea |
Age: 25–40 years Cases: 38 stage 3/4 endometriosis patients (no personal history of cancer) Controls: 31 undergoing laparoscopic surgery for benign conditions |
No | AMH level, plus BRCA1/2 mRNA expression |
Endometrial expression of BRCA1 mRNA in ovarian tissue proved significantly lower in the endometriosis group vs controls, as did ovarian expression of BRCA1 and BRCA2 mRNA Serum AMH concentration showed a significant correlation with BRCA1 mRNA expression in ovarian tissue of women with endometriosis (p = 0.03) |
No adjustment; (cases more likely to have lower BMI than controls) | Not described |
| Giordano, 2016, USA |
Age: 18–45 years Cases: 68 BRCA1+ (no personal history of cancer, ovarian surgery, or exposure to chemotherapy) Controls: 56 BRCA1− |
No | AMH level |
Women > 35 with BRCA1+ had a lower AMH, and hence ovarian reserve, than BRCA− women. BRCA1+ had a significant decline in AMH with age (p = 0.0011) BRCA1+ > 35 years had 10 times the odds of a low AMH (< 0.5 ng/mL) compared with women ≤ 35 years With adjustment for BMI, duration of BC, smoking, gravidity, parity, and age > 35, BRCA1+ was still strongly associated with a low AMH (p = 0.037) |
Age, BRCA1+, obesity, duration of HC, smoking, gravity, parity |
Sample type: frozen serum Duration of freezing: not described Assay: Beckman Coulter AMH ELISA kit |
| Michaelson-Cohen, 2014, Israel |
Age: 26–40 years Cases: 41 BRCA1/2+ (no personal history of cancer) Controls: general population normogram (n = 324) |
No | AMH level | The AMH levels of healthy BRCA1/2+ cases are similar to those of non-carrier women matched for age; therefore, their ovarian reserve is comparable. The AMH levels for most carriers were 2.71 ± 0.59 ng/m (approximately 50th percentile of normograms). These levels were similar to those in the control group, in which the AMH levels were 2.02 ± 0.12 ng/mL (p = 0.27) | Age, AMH normograms | Not described |
| Wang, 2014, USA |
Age: 18–45 years. Mean age BRCA1+ 35.5± 5.2 years, BRCA2+ 35.6 ± 6.2 years, controls 39.7 ± 3.7 years Cases: 62 BRCA1+, 27 BRCA2+ (no personal history of breast cancer) Controls: 54 women who tested negative for BRCA1/2 mutations |
No | AMH level |
BRCA1+ had a significant decrease in AMH levels compared with controls after adjusting for age and BMI (p = 0.026) BRCA1+ had a 4-fold higher odds of having AMH < 1 ng/mL compared with controls (OR = 4.22, 95% CI 1.48–12.0, p = 0.012) There was no difference in AMH levels between BRCA2+ and controls (OR = 1.38 for having AMH < 1 ng/mL, 95% CI 0.39–4.80, p = 0.499) |
Age (mean age of control group was significantly higher than case groups), BMI |
Sample type: frozen serum Duration of freezing: ~ 14 years Assay: Beckman Coulter Gen II AMH ELISA kit |
| Titus, 2013, USA |
Age: 18–42 years Cases: 15 BRCA1+, 9 BRCA2+ with breast cancer Controls: 60 BRCA− with breast cancer |
No | AMH level, plus DSB repair gene expression | DNA double-strand break (DSB) repair is a cause of aging human oocytes. DSBs accumulate in primordial follicles with age. Expression of BRCA1, but not BRCA2, declines in human oocytes. AMH was lower in BRCA+ (1.22 ± 0.92 ng/mL) compared with controls (2.23 ± 1.56 ng/mL, p = 0.0001). Stratifying by mutation, AMH was lower in BRCA1+ (1.12 ± 0.73 ng/mL, p < 0.0001) but not in BRCA2+ (1.39 ± 1.20 ng/mL, p = 0.127) compared with controls | No adjustment | Not described |
| Lambertini, Belgium, 2018 |
Age: mean age for cohort 31 years (IQR 28–33, no difference between cases and controls) Cases: 19 BRCA1+, 10 BRCA2+ with newly diagnosed early breast cancer Controls: 72 BRCA− with newly diagnosed breast cancer |
Yes | AMH level |
Lower but not significant AMH in BRCA+ (median 1.8 μg/L) vs BRCA− (median 2.6 μg/L, p = 0.109) No difference in AMH between BRCA1+ and BRCA2+ mutated patients was observed (p > 0.6) Proportion with low AMH (defined as ≤ μg/L) not different between BRCA+ (32%) and BRCA− (20%, p = 0.235) |
No adjustment; (there was no difference in age between cases and controls) | Not described |
| Gunnala (cohort 1), 2019, USA |
Age: mean at cycle start was 32.4 ± 3.6 years for cases, 35.5 ± 4.3 years in controls Cases (cohort 1): 38 BRCA1/2+ breast cancer patients prior to gonadotoxic therapy (excluded medical conditions associated with diminished ovarian reserve, or ≥ 3 months of hormone suppression, or > 40 years of age with a history of ovarian malignancy, or prior oophorectomy). Mutations in combined cohorts: 31/57 BRCA1+, 18/57 BRCA2+, 4/57 compound heterozygous BRCA1/2+, 4/57 had mutation of unknown significance Controls (cohort 1): 53 BRCA− breast cancer patients and 85 non–breast cancer patients prior to gonadotoxic therapy |
Yes | AMH level, day 3 FSH level |
Cancer cohort: no difference in AMH (adjusted p = 1.0) for BRCA+ vs non–breast cancer malignancy controls; however, FSH was borderline significantly lower in non–breast cancer malignancy controls vs BRCA+ after adjustment (p = 0.06) No difference in AMH or FSH between BRCA+ and BRCA− (p = 1.0) Non–breast cancer malignancy controls had higher E2 (p < 0.001) and shorter stimulation length (p = 0.024) than the BRCA+ cases (not controlled for in the analysis) |
Age, BMI (age significantly differed between study groups) |
Sample type: fresh serum Duration of freezing: N/A Assay: Beckman Coulter Gen II AMH ELISA kit |
| Gunnala (cohort 2), 2019, USA |
Age: mean 31.7 ± 3.1 years Cases (cohort 2): 19 BRCA1/2+ without cancer (excluded those w/ medical conditions associated with DOR, or ≥ 3 months of hormone suppression, or > 40 years of age with a history of ovarian malignancy, or prior oophorectomy). Mutations as above Controls (cohort 2): 600 women undergoing elective oocyte cryopreservation |
No | See Gunnala above |
Cancer-free cohort: neither AMH nor FSH was not statistically different between the cancer-free BRCA+ and the controls (adjusted p = 0.40 and 0.24, respectively) Controls had higher E2 (p = 0.015) and shorter stimulation length (p = 0.039) than the BRCA+ non-cancer cases (not controlled for in the analysis) |
See Gunnala above | See Gunnala above |
| Gunnala (sensitivity analysis), 2019, USA | Sensitivity analysis of n = 31 BRCA1+ vs n = 18 BRCA2+ | See Gunnala above | No difference in FSH (adjusted p = 0.6) for BRCA1+ vs BRCA2+; however, AMH was borderline significantly lower in BRCA1+ (mean 2.4 ± 1.7) vs BRCA2+ (3.6 ± 2.4) (adjusted p = 0.07) | See Gunnala above | See Gunnala above | |
Table 2.
Case and control definitions for AMH studies
| Author | Country | Year | No. of cases | No. of controls | BRCA1 only | BRCA2 only | BRCA1 and 2 | Either | % Ashkenazi Jewish for BRCA+ pts |
|---|---|---|---|---|---|---|---|---|---|
| Phillips | Australia | 2016 | 319 | 374 | 172 | 147 | Not reported | ||
| Johnson | USA | 2017 | 105 | 64 low risk + 26 BRCA- | 55 | 50 |
BRCA1/2+: 18–33% Jewish Controls: 8–12% Jewish |
||
| van Tilborg | Netherlands | 2016 | 124 | 131 | 66 | 57 | 1 | Not reported | |
| Giordano | USA | 2016 | 68 | 56 | 68 | Not reported | |||
| Michaelson-Cohen | Israel | 2014 | 41 | 324 | 41 | Not reported | |||
| Wang | USA | 2014 | 89 | 54 | 62 | 27 | BRCA1/2+ and controls combined: 70% Ashkenazi Jewish | ||
| Titus | USA | 2013 | 24 | 60 | 15 | 9 | Not reported | ||
| Lambertini | Belgium | 2018 | 25 | 60 with AMH results | 15 | 10 | Not reported | ||
| Gunnala (cohort 1) | USA | 2019 | 38 | 53 + 85 | 31 | 18 | 4 | Not reported, but author communication indicated the geographic region had “significant percentage” Jewish patients | |
| Gunnala (cohort 2) | 19 | 600 | See Gunnala above | ||||||
| Total | 852 | 1887 | 484 | 318 | 5 | 41 | |||
| MIN | 19 | 54 | 15 | 9 | 1 | 41 | |||
| MAX | 319 | 600 | 172 | 147 | 4 | 41 |
Table 3.
Case and control definitions for COH studies
| Author | Country | Year | No. of cases | No. of controls | BRCA1 only | BRCA2 only | BRCA1 and 2 | Either | % Ashkenazi Jewish for BRCA+ pts |
|---|---|---|---|---|---|---|---|---|---|
| Oktay | USA | 2010 | 12 | 68 | 12 | “A large population of people in our geographic area are of Ashkenazi Jewish origin” | |||
| Shapira | Israel | 2015 | 62 | 62 | 62 | Yes. “In the present study, a high local prevalence of BRCA1/2+ (2.5% among Ashkenazi Jews), combined with an easy access to and increased demand for IVF among carriers, allowed us to evaluate a relatively large group of both non-cancer- and cancer-affected BRCA mutation carriers, undergoing IVF treatment for PGD and fertility preservation.” | |||
| Lambertini | Belgium | 2018 | 10 | 19 | 5 | 5 | Not reported | ||
| Derks-Smeets | Netherlands, Belgium | 2017 | 43 | 175 | 20 | 23 | Not reported | ||
| Gunnala (cohort 1) | USA | 2019 | 38 | 53 + 85 | 31 | 18 | 4 | Data not reported, but author communication indicated the geographic region had “significant percentage” Jewish patients | |
| Gunnala (cohort 2) | USA | 2019 | 19 | 600 | See Gunnala above | ||||
| Turan | Turkey/USA | 2018 | 21 | 97 | 21 | Not reported | |||
| Total | 205 | 1159 | 56 | 46 | 4 | 95 | |||
| MIN | 10 | 19 | 5 | 5 | 4 | 12 | |||
| MAX | 62 | 600 | 31 | 23 | 4 | 62 |
These studies differed methodologically in several respects. Most notably, the control groups varied widely among studies (Tables 2 and 3). The definition of these comparison groups fell into four categories: (a) test-negative family members [24]; (b) test-negative breast cancer patients [25, 28, 31]; (c) test-negative women at high risk for breast cancer, but not relatives of the cases [14, 22, 28, 30]; and (d) unrelated women at low risk for breast cancer [29]. The report by Johnson et al. [15] used a control group that combined the latter two definitions. The second major methodologic difference among studies evaluated was in BRCA mutation carrier (i.e., case) eligibility criteria. Specifically, some studies included patients with a personal history of cancer [15, 28, 31], while others did not [14, 22, 24, 25, 29, 30] (Tables 2 and 3). Any cases or controls in the Johnson et al. [15] or Gunnala et al. [28] reports with a personal history of cancer were untreated at the time of their study participation. Thirdly, the populations studied have different ethnic backgrounds; for example, the proportion of study subjects with Ashkenazi or any Jewish heritage varied across the reports (e.g., 18–33% of cases and 8–12% of controls [15], 70% overall [22], and other reports noted the geographic area had a high proportion of Jewish residents [28, 29]) (Tables 2 and 3). Finally, some investigators adjusted their findings for potential confounders (varying factors considered, as noted in Table 1), while others did not report any adjustment [25, 31].
Among the nine publications on BRCA1/2 mutation carriers and AMH levels, there was some disparity in the reported data. As detailed in “Methods,” this summary analysis required conversion from medians into means for three articles, and direct contact with other study teams to obtain unpublished data. Of note, a tenth article [26] studied the correlation between serum AMH levels and the expression of BRCA1 mRNA in ovarian tissue; this study is included in Table 1, but not in any of the summary measures due to the major differences in the study designs.
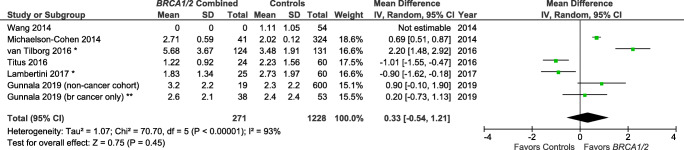
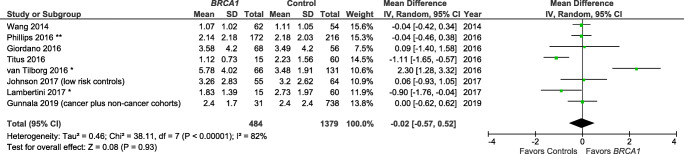
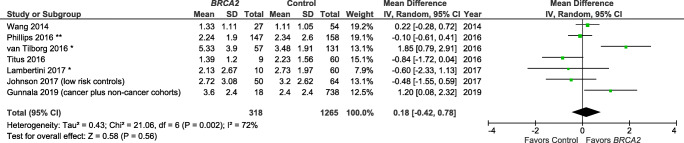
Studies also varied in the reporting of outcome variables by BRCA mutation type (Table 1). Specifically, several publications [15, 22, 24] reported results for BRCA1 and BRCA2 individually, but not for BRCA1/2 as a combined group. There are, therefore, only 5 studies included in the summary measure for BRCA1/2 and AMH (Fig. 2) [25, 28–31]. There are a total of eight and seven studies included in the summary measures for the individual BRCA1 mutation (Fig. 3) and BRCA2 mutation (Fig. 4), respectively.
Fig. 2.
Studies of AMH (ng/mL) for BRCA1 and/or BRCA2 mutation carriers vs controls. *Mean/SD calculated based upon reported median/interquartile range. **Control population with breast cancer history used for this meta-analysis. Contributing studies are ordered by ascending year of publication and relative weight (%) of study. By individual study, the mean difference in AMH levels between cases and controls is illustrated by a small green square and horizontal line (difference and 95% confidence interval (CI) estimate, respectively) using an inverse variance (IV) statistical method; relative weights of individual studies are illustrated by the area of the green squares
Fig. 3.
Meta-analysis of AMH (ng/mL) for BRCA1 mutation carriers vs controls. *Mean/SD calculated based upon reported median/interquartile range. **Converted from pmol/L. Contributing studies are ordered by ascending year of publication and relative weight (%) of study. By individual study, the mean difference in AMH levels between cases and controls is illustrated by a small green square and horizontal line (difference and 95% confidence interval (CI) estimate, respectively) using an inverse variance (IV) statistical method; relative weights of individual studies are illustrated by the area of the green squares
Fig. 4.
Meta-analysis of AMH (ng/mL) for BRCA2 mutation carriers vs controls. *Mean/SD calculated based upon reported median/interquartile range. **Converted from pmol/L. Contributing studies are ordered by ascending year of publication and relative weight (%) of study. By individual study, the mean difference in AMH levels between cases and controls is illustrated by a small green square and horizontal line (difference and 95% confidence interval (CI) estimate, respectively) using an inverse variance (IV) statistical method; relative weights of individual studies are illustrated by the area of the green squares
Finally, the AMH assays utilized varied among studies (Table 1). Three utilized the Beckman Coulter AMH Elisa kit using frozen serum samples [14, 22, 30], one used a picoAMH ELISA using a dried bloodspot [15], and one used the Elec automated system using frozen samples [24]. One report used AMH levels reported through usual clinical care lab orders [28], as communicated directly from the author (21 May 2019). Three studies did not specify which assay was utilized [25, 29, 31].
Studies of anti-Mullerian hormone levels and BRCA1/2 mutations: (2) Comparison of the reported findings
The meta-analysis indicated that BRCA1/2+ was not associated with a lower AMH level as compared with controls (Z overall test effect non-significant, p = 0.45; Fig. 2), based on five reports [25, 28–31]. Similarly, BRCA1+ was not associated with a lower AMH (Z overall test effect non-significant, p = 0.93; Fig. 3), based on eight reports [14, 15, 22, 24, 25, 28, 30, 31]. Finally, for BRCA2 cases alone, the summary measures indicate that BRCA2+ is also not associated with a lower AMH (Z overall test effect, p = 0.56; Fig. 4), based on seven reports [15, 22, 24, 25, 28, 30, 31]. For all three of these analyses, there was a high degree of variability across the publications (heterogeneity measure I2 ranged from 72 to 93%).
Studies of controlled ovarian hyperstimulation response and BRCA1/2 mutations: (1) Study designs and methodologic differences
There were six studies assessing response to COH (in preparation for IVF) as a marker of ovarian reserve in women with BRCA1/2 mutations (Table 4) [7, 21, 27, 28, 31, 32]. These reports spanned 2010–2019 in five countries (USA, Netherlands, Israel, Belgium, and Turkey). In total, 205 BRCA-positive women (reported separately by BRCA1 and BRCA2 mutations in half of the reports) were compared with 1159 control women. The sample size ranged from 10 to 62 cases and from 19 to 600 controls (Tables 2, 3 and 5).
Table 4.
Key characteristics of the publications reporting response to controlled ovarian hyperstimulation to IVF in women with BRCA1/2 mutations
| First author, year, country | Population | Did enrollment criteria allow women with a personal history of cancer | Outcome | Findings | Adjusted for |
|---|---|---|---|---|---|
| Oktay, 2010, USA |
Age: < 38 years Cases: 12 BRCA1/2+ breast cancer patients undergoing oocyte/embryo cryopreservation (no prior ovarian surgery) Controls: 33 BRCA1/2− and 35 BRCA unknown breast cancer patients undergoing oocyte/embryo cryopreservation (no prior ovarian surgery) |
Yes | Oocyte yield, incidence of low response to ovarian stimulation (retrieval of four or fewer oocytes) |
In BRCA+ patients, low ovarian response rate was significantly higher compared with BRCA− patients (33.3 vs 3.3%; p = .014) and with BRCA-untested women (2.9%; p = .012) All BRCA+ low responders had BRCA1 mutations, but low response was not encountered in women who were only BRCA2+ Compared with controls, BRCA1+ women (but not BRCA2+ women) produced lower numbers of eggs (7.4 [95% CI, 3.1 to 17.7] vs 12.4 [95% CI, 10.8 to 14.2]; p = .025) and had as many as 38.3 times the odds ratio of low response (95% CI, 4.1 to 353.4; p = .001) |
Age, total FSH dose, BMI |
| Shapira, 2015, Israel |
Age: average 32 ± 3.58 years Cases: 62 BRCA1/2+ breast cancer patients prior to chemotherapy Controls: 62 matched BRCA1/2− breast cancer patients prior to chemotherapy |
Yes | Oocyte yield, poor response rate, number of zygotes, pregnancy rates |
Number of stimulation days, total stimulation dose, number of zygotes, fertilization rates, and conception rates were comparable between carriers and non-carriers Their cycles resulted in comparable oocyte yield (13.75 vs 14.75; p = 0.49) and low response rates (8.06% vs 6.45%; p = 0.73) Both healthy and cancer-affected BRCA+ carriers demonstrated normal ovarian response in IVF cycles |
BRCA+ cases were matched with BRCA− controls by age, IVF protocol, IVF unit, and cancer disease status. Possible confounders (e.g., number of stimulation days and stimulation dosage) were adjusted in the multivariate models |
| Lambertini, 2018, Belgium |
Age: mean age for cohort 31 years (IQR 28–33, no difference between cases and controls) Cases: 19 BRCA1+, 10 BRCA2+ newly diagnosed early breast cancer patients Controls: 72 BRCA1/2− newly diagnosed early breast cancer patients |
Yes |
Oocyte cryopreservation outcomes (ex. no. of oocytes) Ovarian tissue cryopreservation outcomes (ex. no. of oocytes per mm2) |
Reduced reproductive potential and performance of cryopreservation strategies was observed in BRCA+ breast cancer patients BRCA+ pts retrieved (6.5 vs 9; p = .145) and preserved (3.5 vs 6; p = 0.12) fewer oocytes, had higher poor response rates (4 vs 2; p = 0.15), and had fewer oocytes with ovarian tissue cryopreservation (9 vs 28; p = 0.15) No difference between BRCA1+ and BRCA2+ cases was observed |
No adjustment |
| Derks-Smeets, 2017, Netherlands & Belgium |
Age: < 43 years Cases: 20 BRCA1+, 23 BRCA2+ (invasive (breast) cancer up to 2 years prior to IVF/PGD treatment, ovarian surgery, chemotherapy, pelvic radiation, PCOS, and known reduced ovarian reserve) Controls: 174 couples who underwent PGT-M for an autosomal dominant or recessive disorder not known to be associated with decreased ovarian reserve |
No | No. of mature oocytes |
Response to stimulation (adjusted median no. of mature oocytes) was reduced in BRCA1+ (vs controls, p = 0.02) but not in BRCA2+ (vs controls, p = 0.50); the combined case group had reduced response to stimulation vs controls (p = 0.04) Although oocyte yield was in correspondence to a normal response in all sub-groups, this finding points to a possible negative influence of the BRCA1 gene on ovarian reserve |
Age, BMI, treatment center, type of gonadotropin administered (FSH or hMG), total dose of gonadotropin administered |
| Gunnala (cohort 1), 2019, USA |
Age: mean at cycle start was 32.4 ± 3.6 years for cases, 35.5 ± 4.3 years in controls Cases (cohort 1): 38 BRCA1/2+ breast cancer patients prior to gonadotoxic therapy (excluded medical conditions associated with diminished ovarian reserve, or ≥ 3 months of hormone suppression, or > 40 years of age with a history of ovarian malignancy, or prior oophorectomy). Mutations in combined cohorts: 31/57 BRCA1+, 18/57 BRCA2+, 4/57 compound heterozygous BRCA1/2+, 4/57 had mutation of unknown significance Controls (cohort 1): 53 BRCA− breast cancer patients and 85 non–breast cancer patients prior to gonadotoxic therapy |
Yes | AFC, no. of mature oocytes, no. of total harvested oocytes. Secondary endpoints: starting gonadotropin dose, peak E2 levels during stimulation, total gonadotropins use and days of stimulation |
Cancer cohort: no difference in AFC, total no. of harvested oocytes and no. of mature cryo oocytes between BRCA+ and either control group (p = 1.0) Non–breast cancer malignancy controls had higher E2 (95% CI, − 1364.1 to − 728.6; p < 0.001) and shorter stimulation length (95% CI, 0.3 to 2.1; p = 0.024) than the BRCA+ cases (not controlled for in the analysis) |
Age, BMI; (age significantly different: The mean age at the start of the cycle was 31.7 non-cancer BRCA+ vs 36.6 elective oocyte freezing (p < 0.001)) |
| Gunnala (cohort 2), 2019, USA |
Age: mean 31.7 ± 3.1 years Cases (cohort 2): 19 BRCA1/2+ without cancer (excluded those w/ medical conditions associated with DOR, or ≥ 3 months of hormone suppression, or > 40 years of age with a history of ovarian malignancy, or prior oophorectomy). Mutations as above Controls (cohort 2): 600 women undergoing elective oocyte cryopreservation |
No | See Gunnala above |
Cancer-free cohort: cancer-free BRCA+ had a higher AFC and higher no. of mature cryo oocytes than the controls (adjusted p = 0.025 and 0.49, respectively), while there was no difference in total no. of harvested oocytes between cancer-free BRCA+ and controls (95% CI, − 1.3 to 5.9; p = 0.20) Controls had higher E2 (95% CI, 104.2 to 968.6; p = 0.015) and shorter stimulation length (95% CI, 0.05 to 1.8; p = 0.039) than the BRCA+ non-cancer cases (not controlled for in the analysis) |
See Gunnala above |
| Gunnala (sensitivity analysis), 2019, USA | Sensitivity analysis of n = 31 BRCA1+ vs n = 18 BRCA2+ | Yes | See Gunnala above | No difference in AFC (adjusted p = 0.66), total no. of harvested oocytes (adjusted p = 0.57) and no. mature cryo oocytes (adjusted p = 0.84) between BRCA1+ vs BRCA2+ | See Gunnala above |
| Turan, 2018, Turkey and USA |
Age: < 40 years Cases: 21 BRCA1/2+ breast cancer patients Controls: 97 BRCA− or BRCA unknown breast cancer patients |
Yes | No. of oocytes, no. of mature oocytes, fertilization rate, no. of embryos frozen | In women with breast cancer undergoing fertility preservation, the presence of BRCA+ is associated with lower oocyte yield (11 vs 16.4; p = 0.002 [95% CI, − 10.6 to − 2.5]), embryo yield (5.1 vs 8.2; p = .003 [95% CI, − 7.1 to − 1.5]) and fertilization rate (74 vs 79.3; p = .053 [95% CI, − 20.1 to 0.2]) | Age, BMI |
Table 5.
Studies assessing alternative measures of ovarian reserve
| First author, year, country | Population | Did enrollment criteria allow women with a personal history of cancer | Outcome | Findings | Adjusted for |
|---|---|---|---|---|---|
| Lin, USA, 2017 |
Age: 24–40 years Cases: 13 BRCA1+, 5 BRCA2+ women undergoing oophorectomy (no personal history of breast cancer) Controls: 12 organ donor cadaver ovaries |
No | Primordial follicle density, and percentage of DNA double-strand break (DSB)–positive primordial follicle oocytes |
Women with BRCA mutations have diminished ovarian reserve as well as accelerated primordial follicle loss and oocyte DNA damage BRCA1 and BRCA2 mutations were associated with lower primordial follicle density compared with controls BRCA1 mutations were associated with higher DNA DSBs than controls The rates of follicle decline and DNA DSB accumulation appeared to be accelerated, particularly in primordial follicle oocytes of BRCA mutation carriers over age 30 years |
Age |
| Pavone, USA, 2014 |
Age: 18–51 Cases: 35 BRCA1/2+ or with a strong family history of breast and/or ovarian cancer undergoing oophorectomy Controls: 99 = (20 with ovarian cancer) + (69 with benign conditions) + (10 pregnant or immediately postpartum) |
Yes | Follicle count |
Patients undergoing risk-reducing surgery had significantly decreased follicle count compared with physiologic control Patients with cancer had significantly decreased counts compared with all other groups There were no differences within the benign cohort |
Age |
The studies in this section of the analysis were, overall, methodologically similar. In contrast to the AMH studies, most of the COH/IVF studies (five of the six publications) enrolled mutation carriers (cases) with a personal history of cancer. Exceptions included one of the cohorts in the Gunnala et al. report [28], and Derks-Smeets [21]. Also, in contrast to the AMH articles, all except one of the COH/IVF reports [31] did adjust their findings for potential confounders (varying factors considered, as noted in Table 4; age and BMI were frequently controlled for).
Studies of controlled ovarian hyperstimulation response and BRCA1/2 mutations: (2) Comparison of the reported findings and potential confounding by the medication protocol
Among studies investigating women with BRCA1/2 mutations and their response to COH—either for fertility preservation or for IVF with preimplantation genetic testing for monogenic disease (PGT-M)—four studies were supportive of an association between BRCA1/2 mutations and a reduced response to COH/IVF [7, 21, 27, 31], while two did not definitively confirm this association [28, 32]. Supportive study no. 1 reported fewer median mature oocytes retrieved in BRCA1/2 mutation patients overall (adjusted p = 0.04, n = 174 controls), with a subgroup analysis demonstrating that this difference was specific to BRCA1 mutation carriers (adjusted p = 0.02, n = 20), but not to BRCA2 mutation carriers (adjusted p = 0.50, n = 23; Netherlands and Belgium) [21]. Supportive study no. 2 reported a similar association in a combined BRCA1/2 case group (33% low ovarian response in n = 12 BRCA+ vs 3% in n = 33 non-carriers; USA) [7]. Supportive study no. 3 reported a non-significant reduced response to COH for oocyte cryopreservation, and a greater likelihood to have fewer than four oocytes retrieved among BRCA1/2 mutation carriers (n = 10) versus non-carriers (n = 19) in Belgium (p < 0.15) [31]. Supportive study no. 4 reported fewer total and mature oocytes retrieved in BRCA1/2 mutation carriers with breast cancer as compared with test-negative controls with breast cancer (adjusted p values < 0.01, n = 21 cases; USA and Turkey) [27]. Non-supportive study no. 1 (Israel) [32] found no difference in response to COH for IVF between BRCA1/2 mutation carriers and non-carriers (n = 62/62). Finally, ambiguous results were reported by Gunnala et al. [28]; there was a significant difference noted in the number of mature oocytes retrieved (adjusted p = 0.01), but not in the total number of oocytes retrieved (adjusted p = 0.12) in BRCA1/2 mutation carriers as compared with non-carriers in both a cancer patient cohort and a non-cancer patient cohort (57 total carriers and 738 total controls; USA).
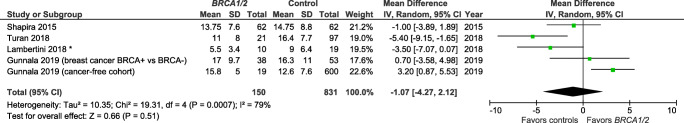
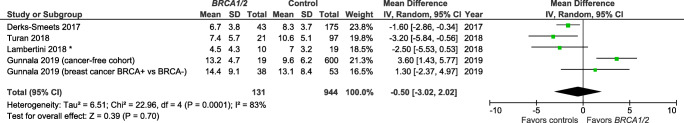
The meta-analysis could only include four of the six studies with comparable outcomes for analysis (means/SD or medians). For both outcomes analyzed (i.e., total number of retrieved oocytes, Fig. 5; and number of mature oocytes retrieved, Fig. 6), the summary measures indicate non-significantly fewer total oocytes and fewer mature oocytes retrieved among BRCA1 and/or BRCA2 mutation carriers, as compared with controls, with overall test effects of Z = 0.85 (p = 0.40) and Z = 059 (p = 0.56), respectively. For both COH/IVF meta-analyses, there was a high degree of variability across the publications (heterogeneity measure I2 ranged from 79 to 83%).
Fig. 5.
Meta-analysis of BRCA1/2 cases vs controls for number of total retrieved oocytes after controlled ovarian hyperstimulation. *Mean/SD calculated based upon reported median/interquartile range. Contributing studies are ordered by ascending year of publication and relative weight (%) of study. By individual study, the mean difference in oocyte count between cases and controls is illustrated by a small green square and horizontal line (difference and 95% confidence interval (CI) estimate, respectively) using an inverse variance (IV) statistical method; relative weights of individual studies are illustrated by the area of the green squares
Fig. 6.
Meta-analysis of BRCA1/2 cases vs controls for number of total number of mature oocytes retrieved after controlled ovarian hyperstimulation. *Mean/SD calculated based upon reported median/interquartile range. Contributing studies are ordered by ascending year of publication and relative weight (%) of study. By individual study, the mean difference in oocyte count between cases and controls is illustrated by a small green square and horizontal line (difference and 95% confidence interval (CI) estimate, respectively) using an inverse variance (IV) statistical method; relative weights of individual studies are illustrated by the area of the green squares
Of note, the stimulation protocol used for COH in the studies assessed here did vary in two respects. First, in terms of the IVF stimulation protocols, two studies utilized a GnRH agonist protocol [7, 21], two used a GnRH antagonist protocol [27, 31], and two used a mix of the two [28, 32]. Additionally, cancer patients in all studies assessed were treated with medication during their stimulation to lower overall estradiol levels. Of these, 2/2 studies using a GnRH agonist protocol reported that BRCA mutations are associated with decreased oocyte yield [7, 21], while 2/2 studies using a GnRH antagonist protocol reached the opposite conclusion [27, 31]. The two studies using mixed protocols yielded no association between BRCA mutations and decreased IVF yield [28, 32].
As discussed by Gunnala et al. [28], the use of estradiol-lowering agents in cancer patients could also confound results when comparing with non-cancer patients. Of the five studies that involved patients with cancer, four treated all cancer patients with an estradiol-lowering agent. Three studies employed letrozole [7, 28, 31], and one used tamoxifen [32]. Half of these studies reported an association between BRCA mutations and decreased oocyte yield [7, 31] and half did not [28, 32]. One study assessed the use of letrozole versus not in patients with BRCA-associated breast cancers, as well as other cancers [27]. In contradiction to all of the other studies assessed here, this group reported a higher oocyte yield in the letrozole group.
Other studies of ovarian reserve and BRCA1/2 mutations
Two additional studies utilized alternative methods of assessing ovarian reserve not accessible in clinical practice: assessment of pathological specimens after oophorectomy. These two studies both reported reduced follicle counts in women with BRCA1/2 mutations. Follicle counts were significantly lower among both women undergoing oophorectomy for ovarian cancer (n = 20) and women undergoing risk-reducing oophorectomy for a genetic disposition to cancer (n = 35, of whom n = 15 had a BRCA1 mutation), as compared with those having this surgery for benign conditions (n = 35) (USA) [33]. Ovaries from BRCA1 and BRCA2 mutation carriers (n = 18) had lower primordial follicle densities than those from controls (n = 12, 11 vs 44 follicles/mm3; p = 0.0002; USA) [23]. Subgroup analyses upheld this finding for BRCA1 and BRCA2 individually.
Discussion
Here, we present a systematic review of the existing literature exploring indicators of ovarian reserve among women with detrimental BRCA1 and/or BRCA2 mutations. Most studies on this topic compared AMH levels and/or response to COH for IVF. Based on the existing publications, there does not appear to be a significant association between BRCA1/2 mutation status and AMH levels, although readers should be aware of the multiple types of methodological differences in the AMH articles and future studies may yield different conclusions. Overall, the existing literature suggests that oocyte yield is not significantly lower in BRCA1/2 mutation carriers. We propose that some of the variability in study findings can be attributed to epidemiological differences in study design and populations studied. We will also suggest that only certain mutations in the BRCA1 or BRCA2 alleles may be associated with decreased ovarian reserve and/or function, and that some of those may be held by individuals of Ashkenazi Jewish ancestry, specifically.
Summary comments regarding BRCA1/2 mutations and AMH levels
We propose that some of the differences in study findings with respect to AMH levels can be attributed to differences in the populations studied. As mentioned earlier, among the nine studies assessing AMH levels in BRCA1/2+ patients, we identified four different types of control groups across studies.
Only one group assessed AMH levels among women with detrimental BRCA mutations as compared with their test-negative blood relatives [24]. This would control for family effects. In that study, the authors reported reduced AMH levels among BRCA1+ family members as compared with their BRCA1 mutation–negative relatives (by 25% (95% CI 5–41%, p < 0.02)). A similar finding was observed for BRCA2.
Four studies included cancer patients in their case definition. Lambertini et al. [31] compared baseline AMH levels between unrelated BRCA-positive and BRCA-negative women with newly diagnosed breast cancer. In this study, the BRCA+ group included both BRCA1/2 together. No difference was found between BRCA mutation carrier and non-carrier cancer patients. Similarly, Gunnala et al. [28] compared AMH levels between breast cancer patients with and without BRCA1/2 mutations and found no differences between unrelated BRCA-positive and BRCA-negative cancer patients. This group also compared AMH levels between these two groups and BRCA+ women without cancer, finding again a lack of association between lower AMH levels in women with BRCA1/2 mutations without cancer and lower AMH levels as compared with BRCA-negative cancer patients. Of note, Johnson et al. [15] also included patients with cancer (without prior chemotherapy or radiation) in their case definition, although only nine subjects (4.6%) had a cancer diagnosis.
In contrast to Lambertini et al. [31] and Gunnala et al. [28], Titus et al. [25] compared AMH levels between BRCA-positive and BRCA-negative patients with breast cancer, and did report a significant difference in AMH levels between combined BRCA1/2-positive versus BRCA1/2-negative women. However, when subdivided by mutation, a significantly lower AMH level was seen only in BRCA1+ women as compared with BRCA1− women.
A third group of studies compared BRCA1/2-positive women with unrelated BRCA-negative women at high risk of breast cancer (i.e., based on a family history of BRCA mutations or family cancer history). Similar to previous studies, two of three of these studies reported that BRCA1 mutations were associated with a lower AMH levels as compared with high-risk BRCA-negative controls [14, 22], and two of two studies assessing BRCA2 mutations showed no difference in AMH levels with controls [22, 30]. Finally, similar to Lambertini et al. and Gunnala et al. [28, 31], two of two studies comparing BRCA1/2 mutation carriers as a whole to non-carrier high-risk individuals showed no difference in AMH levels [28, 30].
Finally, two studies compared BRCA+ women with low-risk, unrelated control groups. Michaelson-Cohen et al. [29] found no difference in AMH levels between healthy BRCA1/2 carriers as a whole and general population normograms. Johnson et al. [15] compared BRCA1 and BRCA2 carriers separately with low-risk controls (in addition to BRCA-negative women, as described above). In contrast to previous studies, Johnson et al. [15] noted lower AMH levels in BRCA2 carriers, but not BRCA1 carriers, as compared with controls.
Thus, we observed both inconsistent findings between different types of control group, as well as discordant findings among studies using similar control groups, indicating that the comparison group (i.e., related, unrelated, cancer/no, high/low risk of cancer) cannot explain all the variability in study findings. In this review, we attempted to reconcile these differences using a random effects model. Overall, our summary measures did not indicate that BRCA1 or BRCA2 mutations are associated with lower AMH levels.
Summary comments regarding BRCA1/2 and controlled ovarian hyperstimulation for IVF
Five studies assessed the response to controlled ovarian hyperstimulation during in vitro fertilization, often for fertility preservation [21, 25, 31, 32, 37]. It is important to note that, in these studies, COH and oocyte retrieval were performed either for fertility preservation for a cancer diagnosis or for a known BRCA1/2 mutation in a healthy woman. In the latter group, one would presume that the subjects in question do not have any known infertility, and thus, outcomes should be comparable with fertile women, or with other healthy women who are at risk of passing a genetic disease to their offspring—and thus pursuing in vitro fertilization for purposes of performing preconception genetic diagnosis (PGT-M) with selective transfer of unaffected embryos.
In contrast to the studies assessing AMH levels, the publications assessing the response to COH more consistently suggest that BRCA mutation carriers have significantly fewer oocytes retrieved during IVF cycles, although this was not found to be significant on meta-analysis summary statistics. Among women undergoing fertility preservation for newly diagnosed cancer, Lambertini et al. [31] observed fewer oocytes cryopreserved, a higher poor response rate, and fewer oocytes in ovarian tissue harvested for cryopreservation, among BRCA1- and BRCA2-positive women (n = 19 BRCA1, n = 10 BRCA2) as compared with BRCA1/2− controls. This was also reported among BRCA1/2 mutation carriers without a cancer diagnosis. For example, the number of mature oocytes retrieved was lower in those undergoing IVF with PGT-M for BRCA1 mutations, as compared with patients undergoing IVF with PGT-M for other diseases [21]. Other studies report conflicting data. One study of BRCA-positive and BRCA-negative women, most without, but some with, breast cancer, demonstrated no differences in IVF outcomes between carriers and healthy controls [32].
Despite the relative consistency in the literature regarding response to COH in women with BRCA1/2 mutations, it is prudent to consider potential differences within and between studies on the specific COH protocol used. In the reports included here, the type of protocol may have confounded differing conclusions. Specifically, studies employing solely GnRH agonist protocols noted a decreased oocyte yield among BRCA mutation carriers in IVF. In contrast, those employing exclusively GnRH antagonist protocols reported no difference in oocyte yield. Additionally, inconsistent findings on this topic were reported by studies that used an estradiol-lowering agent. Thus, it is possible that differences in study findings are partially due to differing COH protocols.
Supportive findings with related outcomes
Although BRCA1/2 mutations are not consistently associated either with lower AMH or with less optimal COH outcomes across all the existing literature in women, there are some related clinical data of interest. First, two studies have assessed ovarian reserve on pathologic specimens of BRCA1/2+ patients (Table Misc Rpts). Lin et al. [23] noted lower primordial follicle density in BRCA1/2+ ovarian tissue specimens, as well as higher double-stranded breaks in DNA, as compared with controls. Similarly, Pavone et al. [33] found that individuals with BRCA1/2+ mutations undergoing risk-reducing oophorectomies had lower follicle counts in ovary specimens as compared with controls undergoing oophorectomy for non-risk-reducing/oncologic reasons. Second, on a different note, age at menopause, which can be considered a surrogate marker for ovarian reserve, may differ in women with BRCA1/2 mutations. Specifically, five studies report an earlier age of natural menopause in BRCA1/2 mutation carriers [13–17], with sample sizes ranging from 81 [17] to 908 [13] carriers.
Potential influence of genetic variants
The qualifying studies in this review as a whole do not account for the diversity of BRCA1/2 mutations and the importance of considering the ethnic background of individuals studied, in order to more clearly interpret results of these types of investigations. To date, over 20,000 variants of the BRCA genes have been identified [38], and there are multiple mutations in the BRCA1 and BRCA2 genes which are common among individuals of different ethnic groups (e.g., Ashkenazi Jew, North African, Balkan, Russian) [39]; thus, studies in different countries or of different groups may have variable results because they are inherently comparing individuals with different variants in the BRCA1 and BRCA2 alleles.
Notably, in studies in which the authors specifically note that the population studied was predominately Ashkenazi Jewish, the results do tend to indicate a difference in ovarian reserve as measured by AMH among BRCA1/2 mutation carriers—a finding not seen in the aforementioned studies of more ethnically diverse populations of unrelated women. For example, Wang et al. [22] reported that, in a population of roughly 70% Ashkenazi Jewish women, BRCA1 mutations were associated with AMH levels which were 0.52 ng/mL lower than non-family BRCA-negative women, with a 4-fold higher odds of having an AMH < 1 ng/mL in BRCA1 carriers (OR 4.22, CI 1.48–12.0, p = 0.012). Similarly, a study of cancer patients undergoing fertility preservation, among whom “a large proportion” were Ashkenazi Jews, reported a significantly higher low ovarian response rate and fewer oocytes produced among BRCA1-positive patients [7]. In contrast, in a study involving a less significant proportion of Ashkenazi Jewish women (32.7% of BRCA1 carriers and 18% of BRCA2 carriers), BRCA2 mutations were associated with a lower AMH levels as compared with low-risk control women [15]. This latter study noted that the proportion of BRCA2 carriers was higher than that in many previous studies, increasing power to detect a difference, and that patients’ conditions such as PCOS which may impact AMH levels were excluded.
Strengths, limitations, future directions, and summary observations
Our study has several strengths. First, it provides a more recent update to the Oktay et al. [37] review on the topic with a new focus on epidemiological differences among studies, as well as a clinical focus on the utility of AMH as a marker for ovarian reserve in BRCA-positive patients. Our review highlights several new epidemiological explanations for the differences in findings within the literature regarding ovarian reserve markers and ovarian response in women with BRCA mutations.
This systematic review and meta-analysis is limited primarily by the methodological differences among studies evaluated.
Future studies will be needed to follow individuals prospectively to assess intraindividual AMH values over time, to correlate AMH values with ovarian response, and to perhaps assess alternative predictive factors for IVF response and/or fecundability in this population of women. Clearly, the use of specific COH protocols may influence the varying reports of any differences in the response to ovarian stimulation regimens by BRCA1/2 mutation. Future research on potential impact by specific BRCA1/2 variant may also shed light on patient response to COH and/or their likely AMH levels.
Conclusions
In conclusion, our review indicates that the response to COH protocols is not significantly lower in BRCA1/2 mutation carriers in the existing literature, and there does not appear to be a significant association between BRCA1/2 mutation status and AMH levels (albeit there were important methodological differences in the AMH articles). Continued research on both of these clinical parameters would be greatly beneficial for counseling of these patients, given the limitations of the current sample sizes and the wide variation in the AMH study designs. BRCA1, and less so BRCA2, mutation carriers, and particularly those with a cancer diagnosis or Ashkenazi Jewish heritage, may have impaired reproduction function. Furthermore, it serves as a caution for providers in counseling patients that AMH levels may not be predictive of ovarian function or COH/IVF outcomes in all carriers of BRCA mutations, as AMH levels appear to be only significantly different among certain ethnic or genetically related subgroups.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Breastcancer.org. U.S. Breast Cancer Statistics Ardmore, PA2018 [Available from: http://www.breastcancer.org/symptoms/understand_bc/statistics.
- 2.Oddoux C, Struewing JP, Clayton CM, Neuhausen S, Brody LC, Kaback M, Haas B, Norton L, Borgen P, Jhanwar S, Goldgar D, Ostrer H, Offit K. The carrier frequency of the BRCA2 6174delT mutation among Ashkenazi Jewish individuals is approximately 1% Nat Genet. 1996;14(2):188–190. doi: 10.1038/ng1096-188. [DOI] [PubMed] [Google Scholar]
- 3.Whittemore AS, Gong G, John EM, McGuire V, Li FP, Ostrow KL, et al. Prevalence of BRCA1 mutation carriers among U.S. non-Hispanic Whites. Cancer Epidemiol Biomark Prev. 2004;13(12):2078–2083. [PubMed] [Google Scholar]
- 4.Risch HA, McLaughlin JR, Cole DE, Rosen B, Bradley L, Fan I, Tang J, Li S, Zhang S, Shaw PA, Narod SA. Population BRCA1 and BRCA2 mutation frequencies and cancer penetrances: a kin-cohort study in Ontario, Canada. J Natl Cancer Inst. 2006;98(23):1694–1706. doi: 10.1093/jnci/djj465. [DOI] [PubMed] [Google Scholar]
- 5.Malone KE, Daling JR, Doody DR, Hsu L, Bernstein L, Coates RJ, Marchbanks PA, Simon MS, McDonald J, Norman SA, Strom BL, Burkman RT, Ursin G, Deapen D, Weiss LK, Folger S, Madeoy JJ, Friedrichsen DM, Suter NM, Humphrey MC, Spirtas R, Ostrander EA. Prevalence and predictors of BRCA1 and BRCA2 mutations in a population-based study of breast cancer in White and Black American women ages 35 to 64 years. Cancer Res. 2006;66:8297–8308. doi: 10.1158/0008-5472.CAN-06-0503. [DOI] [PubMed] [Google Scholar]
- 6.de la Noval BD. Potential implications on female fertility and reproductive lifespan in BRCA germline mutation women. Arch Gynecol Obstet. 2016;294(5):1099–1103. doi: 10.1007/s00404-016-4187-6. [DOI] [PubMed] [Google Scholar]
- 7.Oktay K, Kim JY, Barad D, Babayev SN. Association of BRCA1 mutations with occult primary ovarian insufficiency: a possible explanation for the link between infertility and breast/ovarian cancer risks. J Clin Oncol. 2010;28(2):240–244. doi: 10.1200/JCO.2009.24.2057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.de la Hoya M, Fernandez JM, Tosar A, Godino J, Sanchez de Abajo A, Vidart JA, et al. Association between BRCA1 mutations and ratio of female to male births in offspring of families with breast cancer, ovarian cancer, or both. Jama. 2003;290(7):929–931. doi: 10.1001/jama.290.7.929. [DOI] [PubMed] [Google Scholar]
- 9.Scully R, Livingston DM. In search of the tumour-suppressor functions of BRCA1 and BRCA2. Nature. 2000;408(6811):429–432. doi: 10.1038/35044000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ballal RD, Saha T, Fan S, Haddad BR, Rosen EM. BRCA1 localization to the telomere and its loss from the telomere in response to DNA damage. J Biol Chem. 2009;284(52):36083–36098. doi: 10.1074/jbc.M109.025825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.French JD, Dunn J, Smart CE, Manning N, Brown MA. Disruption of BRCA1 function results in telomere lengthening and increased anaphase bridge formation in immortalized cell lines. Genes Chromosom Cancer. 2006;45(3):277–289. doi: 10.1002/gcc.20290. [DOI] [PubMed] [Google Scholar]
- 12.McPherson JP, Hande MP, Poonepalli A, Lemmers B, Zablocki E, Migon E, Shehabeldin A, Porras A, Karaskova J, Vukovic B, Squire J, Hakem R. A role for Brca1 in chromosome end maintenance. Hum Mol Genet. 2006;15(6):831–838. doi: 10.1093/hmg/ddl002. [DOI] [PubMed] [Google Scholar]
- 13.Finch A, Valentini A, Greenblatt E, Lynch HT, Ghadirian P, Armel S, Neuhausen SL, Kim-Sing C, Tung N, Karlan B, Foulkes WD, Sun P, Narod S, Hereditary Breast Cancer Study Group Frequency of premature menopause in women who carry a BRCA1 or BRCA2 mutation. Fertil Steril. 2013;99(6):1724–1728. doi: 10.1016/j.fertnstert.2013.01.109. [DOI] [PubMed] [Google Scholar]
- 14.Giordano S, Garrett-Mayer E, Mittal N, Smith K, Shulman L, Passaglia C, Gradishar W, Pavone ME. Association of BRCA1 mutations with impaired ovarian reserve: connection between infertility and breast/ovarian cancer risk. J Adolesc Young Adult Oncol. 2016;5(4):337–343. doi: 10.1089/jayao.2016.0009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Johnson L, Sammel MD, Domchek S, Schanne A, Prewitt M, Gracia C. Antimullerian hormone levels are lower in BRCA2 mutation carriers. Fertil Steril. 2017;107(5):1256–65.e6. doi: 10.1016/j.fertnstert.2017.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lin WT, Beattie M, Chen LM, Oktay K, Crawford SL, Gold EB, Cedars M, Rosen M. Comparison of age at natural menopause in BRCA1/2 mutation carriers with a non-clinic-based sample of women in northern California. Cancer. 2013;119(9):1652–1659. doi: 10.1002/cncr.27952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rzepka-Gorska I, Tarnowski B, Chudecka-Glaz A, Gorski B, Zielinska D, Toloczko-Grabarek A. Premature menopause in patients with BRCA1 gene mutation. Breast Cancer Res Treat. 2006;100(1):59–63. doi: 10.1007/s10549-006-9220-1. [DOI] [PubMed] [Google Scholar]
- 18.Hotaling JM, Laufer N, Rosenwaks Z. Introduction: cancer biomarkers and fertility. Fertil Steril. 2018;109(1):4–5. doi: 10.1016/j.fertnstert.2017.12.009. [DOI] [PubMed] [Google Scholar]
- 19.Podfigurna A, Lukaszuk K, Czyzyk A, Kunicki M, Maciejewska-Jeske M, Jakiel G, Meczekalski B. Testing ovarian reserve in pre-menopausal women: why, whom and how? Maturitas. 2018;109:112–117. doi: 10.1016/j.maturitas.2017.11.014. [DOI] [PubMed] [Google Scholar]
- 20.Victoria M, Labrosse J, Krief F, Cedrin-Durnerin I, Comtet M, Grynberg M. Anti Mullerian hormone: more than a biomarker of female reproductive function. J Gynecol Obstet Hum Reprod. 2019;48(1):19–24. doi: 10.1016/j.jogoh.2018.10.015. [DOI] [PubMed] [Google Scholar]
- 21.Derks-Smeets IAP, van Tilborg TC, van Montfoort A, Smits L, Torrance HL, Meijer-Hoogeveen M, et al. BRCA1 mutation carriers have a lower number of mature oocytes after ovarian stimulation for IVF/PGD. J Assist Reprod Genet. 2017. [DOI] [PMC free article] [PubMed]
- 22.Wang ET, Pisarska MD, Bresee C, Chen YD, Lester J, Afshar Y, Alexander C, Karlan BY. BRCA1 germline mutations may be associated with reduced ovarian reserve. Fertil Steril. 2014;102(6):1723–1728. doi: 10.1016/j.fertnstert.2014.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lin W, Titus S, Moy F, Ginsburg ES, Oktay K. Ovarian aging in women with BRCA germline mutations. J Clin Endocrinol Metab. 2017;102(10):3839–3847. doi: 10.1210/jc.2017-00765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Phillips K-A, Collins IM, Milne RL, McLachlan SA, Friedlander M, Hickey M, Stern C, Hopper JL, Fisher R, Kannemeyer G, Picken S, Smith CD, Kelsey TW, Anderson RA, Kathleen Cuningham Consortium for Research into Familial Breast Cancer (kConFab) Anti-Müllerian hormone serum concentrations of women with germline BRCA1 or BRCA2 mutations. Hum Reprod. 2016;31(5):1126–1132. doi: 10.1093/humrep/dew044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Titus S, Li F, Stobezki R, Akula K, Unsal E, Jeong K, et al. Impairment of BRCA1-related DNA double-strand break repair leads to ovarian aging in mice and humans. Sci Transl Med. 2013;5(172):172ra21. doi: 10.1126/scitranslmed.3004925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Choi YS, Park JH, Lee JH, Yoon JK, Yun BH, Park JH, et al. Association between impairment of DNA double strand break repair and decreased ovarian reserve in patients with endometriosis. Front Endocrinol. 2018;9:772. doi: 10.3389/fendo.2018.00772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Turan V, Bedoschi G, Emirdar V, Moy F, Oktay K. Ovarian stimulation in patients with cancer: impact of letrozole and BRCA mutations on fertility preservation cycle outcomes. Reprod Sci. 2018;25(1):26–32. doi: 10.1177/1933719117728800. [DOI] [PubMed] [Google Scholar]
- 28.Gunnala V, Fields J, Irani M, D’Angelo D, Xu K, Schattman G, Rosenwaks Z. BRCA carriers have similar reproductive potential at baseline to noncarriers: comparisons in cancer and cancer-free cohorts undergoing fertility preservation. Fertil Steril. 2019;111(2):363–371. doi: 10.1016/j.fertnstert.2018.10.014. [DOI] [PubMed] [Google Scholar]
- 29.Michaelson-Cohen R, Mor P, Srebnik N, Beller U, Levy-Lahad E, Eldar-Geva T. BRCA mutation carriers do not have compromised ovarian reserve. Int J Gynecol Cancer. 2014;24(2):233–237. doi: 10.1097/IGC.0000000000000058. [DOI] [PubMed] [Google Scholar]
- 30.van Tilborg TC, Derks-Smeets IA, Bos AM, Oosterwijk JC, van Golde RJ, de Die-Smulders CE, van der Kolk L, van Zelst-Stams W, Velthuizen ME, Hoek A, Eijkemans MJ, Laven JS, Ausems MG, Broekmans FJ. Serum AMH levels in healthy women from BRCA1/2 mutated families: are they reduced? Hum Reprod. 2016;31(11):2651–2659. doi: 10.1093/humrep/dew242. [DOI] [PubMed] [Google Scholar]
- 31.Lambertini M, Goldrat O, Ferreira AR, Dechene J, Azim HA, Jr, Desir J, et al. Reproductive potential and performance of fertility preservation strategies in BRCA-mutated breast cancer patients. Ann Oncol. 2018;29(1):237–243. doi: 10.1093/annonc/mdx639. [DOI] [PubMed] [Google Scholar]
- 32.Shapira M, Raanani H, Feldman B, Srebnik N, Dereck-Haim S, Manela D, Brenghausen M, Geva-Lerner L, Friedman E, Levi-Lahad E, Goldberg D, Perri T, Eldar-Geva T, Meirow D. BRCA mutation carriers show normal ovarian response in in vitro fertilization cycles. Fertil Steril. 2015;104(5):1162–1167. doi: 10.1016/j.fertnstert.2015.07.1162. [DOI] [PubMed] [Google Scholar]
- 33.Pavone ME, Hirshfeld-Cytron J, Tingen C, Thomas C, Thomas J, Lowe MP, et al. Human ovarian tissue cortex surrounding benign and malignant lesions. Reprod Sci. 2014;21(5):582–589. doi: 10.1177/1933719113506498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.The_Nordic_Cochrane_Centre. Review Manager (RevMan). Version 5.3 ed. Copenhagen: The Cochrane Collaboration; 2014.
- 35.Wan X, Wang W, Liu J, Tong T. Estimating the sample mean and standard deviation from the sample size, median, range and/or interquartile range. BMC Med Res Methodol. 2014;14:135. doi: 10.1186/1471-2288-14-135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. Bmj. 2003;327(7414):557–560. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Oktay K, Turan V, Titus S, Stobezki R, Liu L. BRCA mutations, DNA repair deficiency, and ovarian aging. Biol Reprod. 2015;93(3):67. doi: 10.1095/biolreprod.115.132290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cline MS, Liao RG, Parsons MT, Paten B, Alquaddoomi F, Antoniou A, Baxter S, Brody L, Cook-Deegan R, Coffin A, Couch FJ, Craft B, Currie R, Dlott CC, Dolman L, den Dunnen J, Dyke SOM, Domchek SM, Easton D, Fischmann Z, Foulkes WD, Garber J, Goldgar D, Goldman MJ, Goodhand P, Harrison S, Haussler D, Kato K, Knoppers B, Markello C, Nussbaum R, Offit K, Plon SE, Rashbass J, Rehm HL, Robson M, Rubinstein WS, Stoppa-Lyonnet D, Tavtigian S, Thorogood A, Zhang C, Zimmermann M, BRCA Challenge Authors. Burn J, Chanock S, Rätsch G, Spurdle AB. BRCA challenge: BRCA exchange as a global resource for variants in BRCA1 and BRCA2. PLoS Genet. 2018;14(12):e1007752. doi: 10.1371/journal.pgen.1007752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Barnes-Kedar I, Bernstein-Molho R, Ginzach N, Hartmajer S, Shapira T, Magal N, Kalis ML, Peretz T, Shohat M, Basel-Salmon L, Friedman E, Bazak L, Goldberg Y. The yield of full BRCA1/2 genotyping in Israeli high-risk breast/ovarian cancer patients who do not carry the predominant mutations. Breast Cancer Res Treat. 2018;172(1):151–157. doi: 10.1007/s10549-018-4887-7. [DOI] [PubMed] [Google Scholar]