Abstract
Objective
To assess whether corifollitropin-α (CFα) late-start administration (day 4) and standard administration (day 2) can obtain similar oocyte yield and live birth rate.
Study design
A randomized controlled trial.
Setting
University Hospital IVF Unit.
Patients
One hundred thirteen women undergoing IVF.
Interventions
Patients distributed in three subgroups (expected poor, normal, or high responders to FSH) were randomized into two treatment arms: (a) CFα late-start: CFα on day 4 + GnRH antagonist from day 8 + (when needed) recFSH from day 11; (b) CFα standard start: CFα on day 2 + GnRH antagonist from day 6 + (when needed) recFSH from day 9. IVF or ICSI was performed as indicated.
Results
Considering the whole study group, the late-start regimen obtained comparable oocyte yield (8.9 ± 5.6 vs. 8.8 ± 6.2; p = n.s.), cPR/started cycle (25% vs. 31.6%, p = n.s.), and cumulative live birth rate (LBR)/ovum pickup (OPU) (29.2% vs. 37.7%, p = n.s.) than the standard regimen. The outcome of the two regimens was comparable in the two subgroups of high and normal responders. Differently, in poor responders, oocyte yield was similar, but LBR/OPU was significantly lower with late-start CFα administration that caused 40% cancellation rate due to monofollicular response. ROC curves showed that the threshold AMH levels associated with cycle cancellation were 0.6 ng/ml for late-start regimen and 0.2 ng/ml for standard regimen.
Conclusion
CFα may be administered on either day 2 or day 4 to patients with expected high or normal response to FSH without compromising oocyte yield and/or live birth rate. Differently, late-start administration is not advisable for expected poor responders with AMH ≤ 0.6 ng/ml.
Trial registration
Keywords: Corifollitropin α, Controlled ovarian stimulation, Gonadotropins, In vitro fertilization, Live birth rate, Mild ovarian stimulation, Pregnancy rate
Introduction
Corifollitropin-α (CFα; Elonva®, MSD, Germany) is a synthetic recombinant glycoprotein containing a hybrid beta subunit, which provides a serum half-life of approximately 65 h during which biological activity is maintained (1). The carboxy-terminal peptide addition to the native FSH molecule maintains the binding specificity to FSH receptor and does not confer any LH activity (2), but changes its kinetics (3). As a result, a single injection of CFα can replace daily FSH injections for the first 7 days of controlled ovarian stimulation (COS) and is able to initiate and sustain multiple follicular development as required for in vitro fertilization (IVF). COS with CFα enables a simplification of IVF treatment due both to the fewer injections needed and to the reduced total FSH dose, apparently without any detrimental impact on clinical outcomes (4). Indeed, infertile patients experience a rather high level of physical and psychological distress during COS, in turn leading to a relevantly high dropout rate that significantly worsens the cumulative probability of a favourable outcome (5). The ease of using a single CFα injection instead of seven daily rFSH injections was shown to increase both patients’ satisfaction and their compliance to treatment (6).
It has been known for some time that acceptable IVF results can be obtained when COS is started in the mid-follicular phase of the menstrual cycle in the GnRH antagonist protocol, with the advantage of reducing the total number of injections as well as the risk of ovarian hyperstimulation syndrome (OHSS) (7, 8). The quick rise of circulating FSH levels after CFα administration makes it quite fit for use in late-start COS regimens, in which FSH is started on day 4 of the cycle. The idea of administering CFα on day 4 instead of day 2 as suggested by the manufacturer and applied in early studies combines the long-acting properties of the drug with the late-start stimulation philosophy, which has been addressed as “mild stimulation” in the past, and aims at performing COS with the highest tolerability.
Recent evidences in patients with normal ovarian reserve support the notion that similar clinical outcomes can be obtained when CFα is administered either on day 2 or on day 4 in the GnRH antagonist protocol (9). The aim of the present study was to test whether this approach could apply equally to all patients, independently from their ovarian reserve and from the expected response to stimulation. We designed the study in order to evaluate, in patients with expected high, normal, or poor ovarian response, whether the two ways of administering CFα would result in similar outcomes in terms of cancellation rate, total FSH consumption, oocyte yield at ovum pickup (OPU), oocyte characteristics, fertilization rate (FR), clinical pregnancy rate (cPR), and live birth rate (LBR).
Materials and methods
Patients
This prospective randomized trial (registered as NCT03816670) included 113 patients aged 18–43 years undergoing IVF to treat male or tubal-related infertility at the IVF Unit of Torino University Hospital, from April 2017 to April 2018.
Patients with the following characteristics were excluded from the study: BMI > 28, polycystic ovary syndrome; indications to IVF other than male and/or tubal factor; IVF treatment completed in the previous 2 months; history of OHSS; previous IVF cycle with more than 30 growing follicles ≥ 11 mm; presence of ovarian cyst or malignant ovarian tumour; known breast, uterus, or central nervous system cancer; systemic diseases potentially affecting ovarian response to gonadotropins.
According to the biomarkers assessed during the diagnostic workout (anti-Mullerian hormone [AMH] and antral follicle count [AFC]), enrolled patients were subgrouped as follows: (a) eHR group: expected high responders to COS (AFC > 15 and AMH > 3.5 ng/ml; n = 43); (b) eNR group: expected normal responders (AFC 7–15 and AMH 1.1–3.5 ng/ml; n = 39); (c) ePR group: expected poor responders (fulfilling at least two out of the three Bologna criteria for poor response, AMH < 1.1 ng/ml and AFC < 7; n = 31).
Approval of the study protocol was obtained by the local ethical committee, and informed consent was obtained by all recruited patients.
Controlled ovarian stimulation and oocyte collection
In the three subgroups, patients were randomized into two treatment arms using a computer-driven randomization list: in one arm, they received CFα on day 2 (standard administration recommended by the manufacturer) plus a daily subcutaneous injection of 0.25 mg GnRH antagonist (Orgalutran, MSD, Germany) from day 6, adding (when needed, according to the ovarian response) a daily supplement of rFSH (150–300 IU/day subcutaneously) from day 9; in the other arm, patients received CFα on day 4 (late-start administration) plus a daily subcutaneous injection of 0.25 mg GnRH antagonist (Orgalutran, MSD, Germany) from day 8, adding (when needed, according to the ovarian response) a daily supplement of rFSH (150–300 IU/day subcutaneously) from day 11. As a result of randomization, CFα was given on day 2 to 21 patients in the eHR group, 20 in the eNR group, and 16 in the pNR group, whereas it was administered on days 4 to 22 in women in the eHR group, 19 in the eNR group, and 15 in the ePR group. The administered dose of CFα was chosen according to the manufacturer’s recommendations: 100–150 μg for women < 36 years (100 μg when the body weight was < 70 kg, 150 μg when it was higher) and 150 μg for all women above 36 years of age.
Follicular growth was monitored by transvaginal US examination plus serial measurements of circulating estradiol (E2) concentration, which were performed every second day from day 7 of the cycle, regardless the day of CFα administration. When at least two follicles reached 18 mm in mean diameter, with appropriately corresponding E2 levels, a single subcutaneous injection of 10,000 IU hCG (Gonasi HP, IBSA, Switzerland) was administered in order to trigger ovulation. At the day of hCG administration, a venous sample was obtained in order to measure E2 and progesterone concentrations.
US-guided oocyte retrieval (OPU) was performed under local anaesthesia (paracervical block), 35–37 h after hCG injection; the aspirated follicular fluid was immediately observed under a stereomicroscope to retrieve the corresponding oocyte that was then washed in buffered medium and stored until fertilization procedure. Within 4 h of OPU, oocytes and cumulus cells were separated by gently pipetting in HEPES-buffered medium containing 80 IU/ml hyaluronidase (SynVitro Hyadase, Origio MediCult, Denmark).
Polarized light microscopy oocyte examination
Mature (metaphase II, MII) oocytes were examined using polarized light microscopy (PLM). During PLM assessment, each oocyte was placed on a glass bottom dish (Willco Wells, Amsterdam, The Netherlands) in a 10-μl drop of buffered, pre-warmed medium (Gamete, Cook, Ireland), covered with mineral oil (Culture Oil, Cook Ltd., Ireland), and kept on a 37 °C stage warmer under a microscope (CRi Oosight™, Woburn, MA, USA). PLM images of the oocytes were collected at × 400 magnification and recorded. The Oosight Meta™ software, allowing automatic zona pellucida (ZP) and meiotic spindle (MS) detection, was used to acquire and analyze data as previously described (10). The following parameters were automatically measured: average retardance, area and thickness of the inner layer of the ZP (IL-ZP), average retardance and area of the meiotic spindle (MS). In addition, the major axis of the MS was manually measured using a line scan.
Preparation of semen samples, ICSI, and fertilization assessment
Semen samples were examined to assess sperm concentration, motility, and morphology according to the World Health Organization guidelines (WHO, 2010), and were then prepared by density gradient centrifugation in order to select normally motile, morphologically normal spermatozoa. ICSI was performed on all available MII oocytes, and after 16–18 h of incubation in controlled atmosphere, normal fertilization was assessed by evaluating the presence of two pronuclei (2PN) and the extrusion of the second polar body.
In vitro embryo growth and transfer
Zygotes were placed in 4-well dishes (Thermo Scientific, Denmark) and cultured in pre-equilibrated cleavage medium (Cook Ltd., Ireland), overlain with mineral oil (Culture Oil, Cook Ltd., Ireland). Embryo morphology was evaluated using the 1–10-point scale score by Holte et al. (11), and then, it was eventually evaluated again on day 5 according to The Istanbul Consensus Workshop (12). The two best scored embryos were selected for embryo transfer (ET) on day 3. No more than two embryos were transferred in order to avoid multiple pregnancy; a single embryo was transferred on day 3 when it was the only available. If more than two good scored embryos were available on day 3, culture was prolonged and a single embryo transfer (ET) was performed on day 5, eventually vitrifying the remaining blastocysts and keeping them in liquid nitrogen until further use.
ET was accomplished using the soft catheter Sydney Guardia (Cook Ltd., Ireland) after transvaginal US uterine measurement, as previously described (13). The luteal phase was supported administering 180 mg/day natural progesterone intravaginally (Crinone 8, Merck, Germany) for 15 days, starting the day after OPU. Pregnancy was assessed by serum hCG 14 days after ET and then confirmed when one or two gestational sacs were visualized by transvaginal US after two further weeks.
Outcomes and statistical analysis
Power calculation was performed according to the primary outcome measure that was the number of retrieved oocytes at OPU; in order to get 80% statistical power for an expected difference of two oocytes between treatment arms, a minimal total number of 110 IVF cycles were considered appropriate. Secondary outcomes were the following: cycle cancellation rate, total dose of exogenous FSH added to CFα, number of US plus estradiol monitoring checkpoints, E2 and progesterone concentrations at trigger, number of mature (MII) oocytes, fertilization rate, number of embryos available for ET or freezing, clinical (US-detected) pregnancy rate per started cycle (cPR/SC) and per fresh embryo transfer (cPR/ET), cumulative live birth rate per oocyte pickup (CLBR/OPU), incidence of OHSS. Adverse events, defined as any unfavourable sign, symptom, or disease that occurred during the study period, were also registered.
Statistical analysis was accomplished using the SPSS® Statistics Software. Continuous variables were reported as mean ± standard deviation (SD), whereas categorical variables as absolute or relative frequencies. At univariate analysis, unpaired t test was performed to compare cycle characteristics and clinical outcomes of the two treatment arms in the whole patients’ group and in the three subgroups separately. Significance level was set at p < 0.05.
Receiver operating characteristic (ROC) curves were generated using SAS software package (SAS Institute, Cary, NC) and were used to assess the cycle cancellation probability according to basal circulating AMH level. The area under the ROC curve (AUC) was determined to provide a numerical summary of the indicator’s performance. Cutoff points were identified for specificity and sensitivity when Youden’s index (sensitivity + specificity − 1) was maximum.
Results
The clinical characteristics of the 113 patients enrolled in the study are shown in Table 1. Considering all patients together, as well as within each subgroup, no significant difference between patients receiving either the CFα day 2 or CFα day 4 regimen was observed for age, BMI, basal endocrine and US biomarkers of ovarian reserve, smoking habit, and presence of male factor.
Table 1.
Clinical characteristics of patients subdivided in the three groups of expected poor responders (ePR), expected normal responders (eNR), and expected high responders (eHR). Considering all patients together, as well as within each subgroup, no significant difference between patients receiving either the CFα day 2 or the CFα day 4 regimen was observed for any variable
| ePR | eNR | eHR | All patients | |||||
|---|---|---|---|---|---|---|---|---|
| CFα day 2 (n = 16) | CFα day 4 (n = 15) | CFα day 2 (n = 20) | CFα day 4 (n = 19) | CFα day 2 (n = 21) | CFα day 4 (n = 22) | CFα day 2 (n = 57) | CFα day 4 (n = 56) | |
| Age (years) | 38.7 ± 3.1 | 38.7 ± 3.6 | 37.2 ± 4.3 | 37.6 ± 4.0 | 33.7 ± 4.3 | 34.7 ± 3.5 | 36.3 ± 4.5 | 36.7 ± 4.0 |
| BMI (kg/m2) | 23.1 ± 5.6 | 22.1 ± 2.3 | 22.7 ± 3.4 | 23.4 ± 3.7 | 24 ± 4.4 | 23.3 ± 3.0 | 23.3 ± 4.4 | 23.0 ± 3.1 |
| Day 3 FSH (IU/I) | 8.5 ± 3.4 | 9.5 ± 3.3 | 8.5 ± 3.0 | 7.8 ± 2.5 | 6.7 ± 1.2 | 6.5 ± 2.2 | 7.8 ± 2.7 | 7.7 ± 2.9 |
| AMH (ng/ml) | 0.7 ± 0.4 | 0.6 ± 0.3 | 2.3 ± 1.2 | 2.3 ± 0.7 | 4.7 ± 1.2 | 4.9 ± 1.4 | 2.7 ± 1.9 | 2.8 ± 2.0 |
| AFC | 6.6 ± 2.5 | 6.0 ± 2.2 | 11.0 ± 4.0 | 12.6 ± 3.2 | 19.1 ± 6.9 | 19.5 ± 5.9 | 12.7 ± 7.2 | 13.6 ± 6.9 |
| Smoking (%) | 25 (4/16) | 20 (3/15) | 25 (5/20) | 5 (1/19) | 14 (3/21) | 27 (6/22) | 21.1 (12/57) | 17.9 (10/56) |
| Male factor (%) | 44 (7/16) | 60 (9/15) | 55 (11/20) | 58 (11/19) | 52 (11/21) | 73 (16/22) | 50.9 (29/57) | 64.3 (36/56) |
Considering the whole study group, the IVF outcome (Table 2) was comparable for patients receiving CFα day 2 versus CFα day 4 regimens, both for the number of retrieved oocytes (primary outcome) and for all the secondary outcomes. In the eNR subgroup, the CFα day 4 regimen required a significantly lower number of E2 plus US checkpoints compared with the CFα day 2 regimen (2.3 ± 0.7 vs. 3 ± 0.7, respectively; p < 0.05).
Table 2.
IVF outcome of patients subdivided in the three groups of expected poor responders (ePR), expected normal responders (eNR), and expected high responders (eHR). Monitoring checkpoints (E2 + US) were significantly fewer in the eNR group with day 4 regimen (*p < 0.05). The “freeze-all” strategy, applied to lower the OHSS risk in the eHR group, was significantly more frequent with day 2 regimen (*p < 0.05). No significant differences between patients receiving CFα day 2 or CFα day 4 regimen were observed for all other variables considering all patients together as well as those in subgroups eHR and eNR. In the ePR subgroup, the clinical pregnancy rate per started cycle and per ET, as well as the cumulative live birth rate per OPU, were significantly higher for CFα day 2 regimen (**p < 0.04)
| ePR | eNR | eHR | All patients | |||||
|---|---|---|---|---|---|---|---|---|
| CFα day 2 (n = 16) | CFα day 4 (n = 15) | CFα day 2 (n = 20) | CFα day 4 (n = 19) | CFα day 2 (n = 21) | CFα day 4 (n = 22) | CFα day 2 (n = 57) | CFα day 4 (n = 56) | |
| Total rFSH added (IU) | 840 ± 546 | 600 ± 543 | 660 ± 361 | 473 ± 334 | 314 ± 205 | 293 ± 206 | 1871 ± 487 | 1736 ± 462 |
| Days of GnRH antag | 4.7 ± 2.2 | 4.5 ± 2.1 | 5.9 ± 1.9 | 4.7 ± 1.9 | 5.7 ± 2.0* | 5.5 ± 1.8 | 5.5 ± 2.0 | 4.8 ± 1.9 |
| Monitoring checkpoints | 2.6 ± 0.97 | 2.8 ± 0.8 | 3 ± 0.7 | 2.3 ± 0.7* | 2.8 ± 0.8 | 2.8 ± 0.7 | 2.8 ± 0.8 | 2.7 ± 0.7 |
| E2 at trigger (pg/ml) | 515 ± 294 | 858 ± 820 | 1493 ± 1107 | 1197 ± 568 | 1564 ± 881 | 1782 ± 999 | 1244 ± 963 | 1336 ± 898 |
| Pg at trigger (ng/ml) | 0.5 ± 0.5 | 0.5 ± 0.2 | 0.5 ± 0.3 | 0.6 ± 0.7 | 0.9 ± 0.3 | 0.8 ± 0.3 | 0.5 ± 0.4 | 0.6 ± 0.4 |
| Cancellation rate (%) | 18 (3/16) | 40 (6/15) | 5 (1/20) | 5 (1/19) | 0 (0/21) | 4 (1/22) | 7.0 (4/57) | 14.3 (8/56) |
| Freeze all (high OHSS risk) (%) | 0/16 | 0/15 | 0/20 | 0/19 | 38 (8/21)* | 9 (2/22) | 14 (8/57) | 3.6 (2/56) |
| Retrieved oocytes/OPU | 4.3 ± 3.3 | 4.2 ± 2.9 | 7.3 ± 5.2 | 6.7 ± 4.0 | 12.8 ± 6.1 | 12.8 ± 5.1 | 8.8 ± 6.2 | 8.9 ± 5.6 |
| MII oocytes/OPU | 3.6 ± 3.0 | 3.1 ± 2.6 | 6.0 ± 3.7 | 5.1 ± 3.3 | 10.1 ± 4.7 | 10.3 ± 4.4 | 7.1 ± 4.8 | 7.0 ± 4.7 |
| Fertilization Rate (%) | 59.7 ± 29.1 | 65.1 ± 32.9 | 72.9 ± 28.5 | 65.4 ± 31.3 | 71.1 ± 28.1 | 70.1 ± 26.4 | 69.4 ± 28.3 | 67.5 ± 28.9 |
| Transferred embryos | 1.2 ± 0.8 | 1 ± 0.6 | 1.5 ± 0.8 | 1.4 ± 0.8 | 1.6 ± 1 | 1.6 ± 0.9 | 1.5 ± 1 | 1.5 ± 0.9 |
| Mean embryo score | 6.2 ± 2.9 | 5.3 ± 3.1 | 7.2 ± 2.5 | 7 ± 2.8 | 8.8 ± 3.1 | 8.4 ± 3.7 | 7.9 ± 3.5 | 7.5 ± 3.9 |
| Clinical PR/SC (%) | 18.7 (3/16)** | 0 (0/15) | 25 (5/20) | 15.8 (3/19) | 47.6 (10/21) | 50.0 (11/22) | 31.6 (18/57) | 25 (14/56) |
| Clinical PR/fresh ET (%) | 27.2 (3/11)** | 0 (0/6) | 26.3 (5/19) | 16.7 (3/18) | 69.2 (9/13) | 57.9 (11/19) | 39.5 (17/43) | 31.1 (14/45) |
| Cumulative LBR/OPU (%) | 23.1 (3/13)** | 0 (0/9) | 26.3 (5/19) | 16.7 (3/18) | 57.1 (12/21) | 52.4 (11/21) | 37.7 (20/53) | 29.2 (14/48) |
The clinical PR per started cycle and per ET, as well as the cumulative LBR/OPU (fresh plus frozen/thawed embryo transfers), were comparable for patients allocated to either regimen (Table 2). However, when the ePR subgroup was considered separately, both the cPR/SC, the cPR/ET, and the cumulative LBR/OPU were significantly higher for patients receiving CFα day 2 regimen with respect to those receiving CFα day 4 regimen (p = 0.04) (Table 2). Of note, the cancellation rate due to monofollicular response was more than doubled in patients of the ePR group receiving CFα day 4 regimen than in their counterparts (40% vs. 18%, Table 2).
No cases of OHSS were observed. However, 10 patients in the eHR group (8 cases for the CFα day 2 regimen vs. 2 cases for the CFα day 4 regimen, p < 0.05) had E2 peak concentrations above 3000 pg/ml and/or more than 15 follicles > 11 mm (mean diameter) at OPU. In order to minimize the risk of OHSS, a “freeze-all embryos” strategy was adopted in these cases, postponing ET to a subsequent spontaneous cycle.
Polarized light microscopy (PLM) was used to analyze the birefringent structures (zona pellucida and meiotic spindle) of 653 mature oocytes (79 in the ePR group, 181 in the eNR group, 393 in the eHR group): no significant differences between oocytes obtained with either CFα regimen were observed, in any of the three subgroups of patients (Table 3).
Table 3.
Morphological features measured by polarized light microscopy (PLM) on the oocytes of the three groups of expected poor responders (ePR), expected normal responders (eNR), and expected high responders (eHR). In all groups, n refers to the number of analyzed oocytes; there was no significant difference between patients receiving CFα day 2 or CFα day 4 regimen for all measured variables
| ePR | eNR | eHR | ||||
|---|---|---|---|---|---|---|
| CFα day 2 (n = 50) | CFα day 4 (n = 29) | CFα day 2 (n = 86) | CFα day 4 (n = 95) | CFα day 2 (n = 197) | CFα day 4 (n = 196) | |
| IL-retardance (nm) | 1.9 ± 0.7 | 2.0 ± 0.5 | 2.0 ± 0.6 | 2.1 ± 0.6 | 2 ± 0.6 | 2 ± 0.6 |
| IL-area (μm2) | 2978 ± 655 | 3148 ± 486 | 3090 ± 532 | 3225 ± 562 | 3017 ± 644 | 2887 ± 569 |
| IL-thickness (μm) | 5.5 ± 1.6 | 5.7 ± 1.3 | 5.8 ± 1.3 | 5.9 ± 1.3 | 5.6 ± 1.5 | 5.3 ± 1.4 |
| MS-retardance (nm) | 1.6 ± 0.3 | 1.4 ± 0.4 | 1.7 ± 0.5 | 1.6 ± 0.4 | 1.7 ± 0.4 | 1.5 ± 0.3 |
| MS-area (μm2) | 86 ± 17.5 | 79.3 ± 14.2 | 86.1 ± 22.2 | 87 ± 19.9 | 85.5 ± 16.9 | 79.3 ± 16.1 |
| MS-axis length (μm) | 11.5 ± 1.9 | 11.7 ± 1.7 | 11.9 ± 2.1 | 12 ± 2.0 | 11.8 ± 1.7 | 11.2 ± 1.8 |
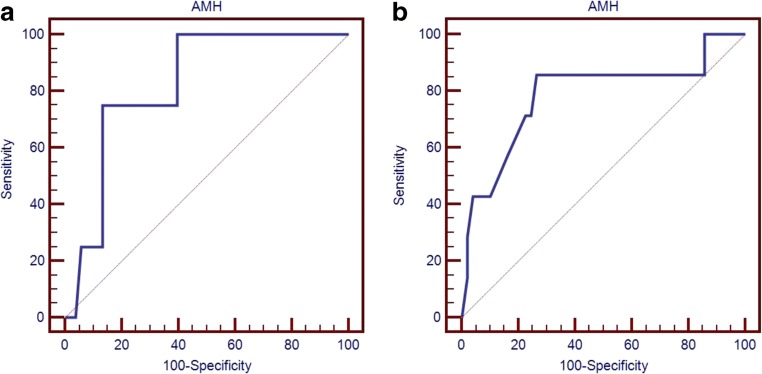
Receiver operating characteristic (ROC) curves were applied to assess the probability of cycle cancellation according to basal circulating AMH level. The threshold AMH level below which a high probability of cycle cancellation was observed, according to maximization of the Youden index, was 0.2 ng/ml for the CFα day 2 regimen (specificity 94%; AUC = 0.82; 95% CI 0.69–0.91; p = 0.0002) and 0.6 ng/ml for the CFα day 4 regimen (specificity 90%; AUC = 0.79; 95% CI 0.66–0.88; p = 0.014) (Fig. 1a, b).
Fig. 1.
ROC curves showing the probability of cycle cancellation according to basal circulating AMH for CFα day 2 (a) or day 4 regimens (b). The ROC curve for the CFα day 2 regimen (a) showed an AUC of 0.82 with specificity 94% for a threshold AMH level of 0.2 ng/ml, whereas the ROC curve for the CFα day 4 regimen (b) showed an AUC of 0.79 with specificity 90% for a threshold AMH level of 0.6 ng/ml. ROC, receiver operating characteristic; AUC, area under the curve
Discussion
The administration of a single dose of CFα represents an effective alternative to daily injections of rFSH in COS. Comparable results in terms of live birth rate, ongoing pregnancy rate, miscarriage rate, and multiple pregnancy rate were reported with the use of CFα versus daily rFSH, at least in patients with normal ovarian response (14). An increased risk of OHSS in patients defined as high responders was also reported (15), but on the other hand, CFα was suggested to offer a potential advantage in patients whose ovarian reserve is limited (16). Overall, a flexible use of CFα during the follicular phase of the ovarian cycle could potentially widen the spectrum of patients using the medication and limit undesirable effects while maintaining the advantages associated with a CFα peculiar pharmacokinetic.
The current study represents the first RCT in which ovarian stimulation with CFα was initiated on different days of the menstrual cycle (day 2 vs. day 4), and enrolled patients covered all the range of ovarian reserve (expected poor responders according to the Bologna criteria, normal and high responders). When all patients were considered together, the main finding of the present study was that administering CFα according to either standard or late-start protocol did not result in different oocyte yields or morphological oocyte quality, as appearing from conventional plus PLM evaluation. Also, the clinical outcomes of IVF cycle, including the cumulative LBR/OPU, were similar when the late-start administration regimen was compared with the regimen recommended by the manufacturer. Similar findings were previously reported in a small group of patients with normal ovarian reserve and expected normal ovarian response to stimulation (9), but the present study confirms those observations also in a larger group of patients, including women with expected poor and high ovarian responses.
However, we observed significant differences in clinical outcomes when the analysis was performed within each subgroup of patients. Indeed in patients with expected poor response, although the oocyte yield was similar independently from the day of CFα administration, the clinical PR per started cycle and per ET, as well as the cumulative LBR, were disappointing for day 4 CFα regimen and significantly lower than those observed with day 2 regimen. In expected poor responders, the cancellation rate due to monofollicular response was significantly higher in the late-start arm, at variance with the other two types of patient, in which comparable cancellation rates with day 4 or day 2 regimens were registered. Moreover, the proportion of expected poor responders who reached OPU and ET in the day 4 arm never obtained a clinical pregnancy. This negative result does not seem to be linked to the number of retrieved oocytes, the proportion of MII oocytes, or their morphological appearance; given the limited possibility of correctly assessing oocyte competence using morphological techniques (including PLM), it cannot be excluded that late-start regimen could have negatively affected oocyte development in poor responders. CFα day 2 regimen obtained fairly good results in our poor responder group, confirming what observed in another recent study, that reported the equivalence between CFα day 2 standard administration regimen and daily recombinant FSH in poor responders, at least when the antral follicular count is above 5 (17).
Also, ROC curves showed that the threshold AMH level implying a high probability of cycle cancellation (90% specificity) was different for the two administration regimens: 0.6 ng/ml for the late-start regimen versus 0.2 ng/ml for the standard regimen. The most likely explanation for such a finding is that in patients with poor ovarian reserve, follicle recruitment occurs very early in the follicular phase, sometimes even during the late luteal phase of the preceding menstrual cycle (18). In these cases, the potential advantages of an early strong rise in circulating FSH associated with the use of CFα would be nullified, since the selection of a single dominant follicle would already be completed on day 4 of the menstrual cycle. Whatever the reason behind such disappointing outcome, the results of the present study suggest that the use of CFα in patients with a severely compromised ovarian reserve (AMH ≤ 0.6) should be limited to the early follicular phase (day 2).
As far as high responders are concerned, benefits and possible disadvantages of day 4 CFα administration should be carefully balanced, in particular as regards the risk of developing OHSS. A previous pilot study comparing the administration of CFα on day 2 versus day 4 in young expected normal responders showed that the proportion of patients with more than 20 follicles > 11 mm in diameter the day of ovulation trigger was higher in the day 4 group, raising the concern that late-start regimen could be associated with a higher risk of OHSS (9). The results obtained in our larger series do not confirm that finding: neither the number of follicles above 11 mm diameter nor peak E2 levels were significantly higher in the day 4 CFα group than in the day 2 CFα group. Of note, 11 patients in our eHR group were managed with a “freeze-all” policy in order to reduce the OHSS risk, but the presence of a OHSS risk was more than doubled for patients receiving day 2 CFα (8 vs. 3, p < 0.05). Although the sample size is too small to drive conclusive indications, the day 4 administration of CFα in high responders seems a safer alternative to the traditional protocol and could be recommendable.
Another issue is that the administration of CFα later in the follicular phase could raise some concerns about the possibility to increase progesterone (P4) levels at ovulation triggering, especially in high responders, with detrimental effects on endometrial receptivity (19, 20). In our trial, similar serum P4 levels were observed the day of trigger with either regimen, irrespective of the expected ovarian responsiveness. CFα administration implies a gradual decrease of FSH activity after injection (21); granulosa cells respond with apoptosis or atresia to this condition, resulting in lower P4 production and serum concentrations at hCG trigger. In fact, it was previously reported in high responders with AMH > 3.3 ng/ml that serum P4 level the day of hCG injection was significantly lower after CFα stimulation than after daily rFSH administration (22). A further point to be considered is the reduction of FSH consumption with the use of late-start CFα regimen; indeed, a significant reduction of rFSH added at the end of the follicular phase was previously reported after CFα administration on day 4 (9). Even in the present study, the total amount of rFSH added after CFα was higher in the day 2 regimen. Furthermore, the number of days in which a GnRH antagonist was added was lower in the day 4 group, reaching statistical significance in the expected normal responders. The late administration of CFα, in fact, allows to add GnRH antagonist from day 8 of the cycle instead than from day 5, as suggested when CFα is given with the standard regimen (23, 24), reducing the overall number of injections needed to complete COS.
Lastly, the day 4 regimen allowed also a significant reduction in the number of checkpoints during COS (E2 measurement plus transvaginal US monitoring), positively affecting the indirect costs of the cycle and the daily impact on the patients’ life organization.
In conclusion, this randomized controlled trial shows that CFα has a remarkable flexibility of use in COS, as it may be administered either on day 2 or on day 4 to patients with expected high or normal response without compromising oocyte yield and/or live birth rate, and possibly limiting the risk of OHSS and the impact on the daily patient’s life. Conversely, late-start administration should be avoided in expected poor responders with AMH ≤ 0.6 ng/ml, due to the high risk of cycle cancellation and the significantly poorer outcome compared with standard stimulation.
Compliance with ethical standards
Approval of the study protocol was obtained by the local ethical committee, and informed consent was obtained by all recruited patients.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Change history
5/22/2020
The original article unfortunately has a missing acknowledgement.
References
- 1.Fares FA, Suganuma N, Nishimori K, LaPolt PS, Hsueh AJ, Boime I. Design of a long-acting follitropin agonist by fusing the C-terminal sequence of the chorionic gonadotropin beta subunit to the follitropin beta subunit. Proc Natl Acad Sci U S A. 1992;89(10):4304–4308. doi: 10.1073/pnas.89.10.4304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.LaPolt PS, Nishimori K, Fares FA, Perlas E, Boime I, Hsueh AJ. Enhanced stimulation of follicle maturation and ovulatory potential by long acting follicle-stimulating hormone agonists with extended carboxyl-terminal peptides. Endocrinology. 1992;131(6):2514–2520. doi: 10.1210/endo.131.6.1446593. [DOI] [PubMed] [Google Scholar]
- 3.Fauser BCJM, Mannaerts BMJL, Devroey P, Leader A, Boime I, Baird DT. Advances in recombinant DNA technology: corifollitropin alfa, a hybrid molecule with sustained follicle-stimulating activity and reduced injection frequency. Hum Reprod Update. 2009;15(3):309–321. doi: 10.1093/humupd/dmn065. [DOI] [PubMed] [Google Scholar]
- 4.Revelli A, Pittatore G, Casano S, Canosa S, Evangelista F, Benedetto C. Efficacy and safety of late-start Corifollitropin-alfa administration for controlled ovarian hyperstimulation in IVF: a cohort, case-control study. J Assist Reprod Genet. 2015;32(3):429–434. doi: 10.1007/s10815-014-0426-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brandes M, van der Steen JOM, Bokdam SB, Hamilton CJCM, de Bruin JP, Nelen WLDM, Kremer JA. When and why do subfertile couples discontinue their fertility care? A longitudinal cohort study in a secondary care subfertility population. Hum Reprod. 2009;24(12):3127–3135. doi: 10.1093/humrep/dep340. [DOI] [PubMed] [Google Scholar]
- 6.Nargund G, Frydman R. Towards a more physiological approach to IVF. Reprod BioMed Online. 2007;14(5):550–552. doi: 10.1016/S1472-6483(10)61043-7. [DOI] [PubMed] [Google Scholar]
- 7.Verberg MFG, Macklon NS, Nargund G, Frydman R, Devroey P, Broekmans FJ, Fauser BC. Mild ovarian stimulation for IVF. Hum Reprod Update. 2009;15(1):13–29. doi: 10.1093/humupd/dmn056. [DOI] [PubMed] [Google Scholar]
- 8.Heijnen EM, Eijkemans MJ, De Klerk C, Polinder S, Beckers NG, Klinkert ER, et al. A mild treatment strategy for in-vitro fertilisation: a randomised non-inferiority trial. Lancet. 2007;369(9563):743–749. doi: 10.1016/S0140-6736(07)60360-2. [DOI] [PubMed] [Google Scholar]
- 9.Blockeel C, Polyzos NP, Derksen L, De Brucker M, Vloeberghs V, van de Vijver A, et al. Administration of corifollitropin alfa on day 2 versus day 4 of the cycle in a GnRH antagonist protocol: a randomized controlled pilot study. Hum Reprod. 2014;29(7):1500–1507. doi: 10.1093/humrep/deu105. [DOI] [PubMed] [Google Scholar]
- 10.Molinari E, Evangelista F, Racca C, Cagnazzo C, Revelli A. Polarized light microscopy-detectable structures of human oocytes and embryos are related to the likelihood of conception in IVF. J Assist Reprod Genet. 2012;29(10):1117–1122. doi: 10.1007/s10815-012-9840-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Holte J, Berglund L, Milton K, Garello C, Gennarelli G, Revelli A, Bergh T. Construction of an evidence-based integrated morphology cleavage embryo score for implantation potential of embryos scored and transferred on day 2 after oocyte retrieval. Hum Reprod. 2007;22(2):548–557. doi: 10.1093/humrep/del403. [DOI] [PubMed] [Google Scholar]
- 12.Alpha Scientists in Reproductive Medicine and ESHRE Special Interest Group of Embryology The Istanbul consensus workshop on embryo assessment: proceedings of an expert meeting. Hum Reprod. 2011;26(6):1270–1283. doi: 10.1093/humrep/der037. [DOI] [PubMed] [Google Scholar]
- 13.Revelli A, Rovei V, Dalmasso P, Gennarelli G, Racca C, Evangelista F, Benedetto C. Large randomized trial comparing transabdominal ultrasound-guided embryo transfer with a technique based on uterine length measurement before embryo transfer. Ultrasound Obstet Gynecol. 2016;48(3):289–295. doi: 10.1002/uog.15899. [DOI] [PubMed] [Google Scholar]
- 14.Cozzolino M, Vitagliano A, Cecchino GN, Ambrosini G, Garcia-Velasco JA. Corifollitropin alfa for ovarian stimulation in in vitro fertilization: a systematic review and meta-analysis of randomized controlled trials. Fertil Steril. 2019;111(4):722–733. doi: 10.1016/j.fertnstert.2018.11.047. [DOI] [PubMed] [Google Scholar]
- 15.Fensore S, Di Marzio M, Tiboni GM. Corifollitropin alfa compared to daily FSH in controlled ovarian stimulation for in vitro fertilization: a meta-analysis. J Ovarian Res. 2015;8:33. doi: 10.1186/s13048-015-0160-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Polyzos NP, De Vos M, Corona R, Vloeberghs V, Ortega-Hrepich C, Stoop D, et al. Addition of highly purified HMG after corifollitropin alfa in antagonist-treated poor ovarian responders: a pilot study. Hum Reprod. 2013;28(5):1254–1260. doi: 10.1093/humrep/det045. [DOI] [PubMed] [Google Scholar]
- 17.Andrisani A, Marin L, Ragazzi E, Donà G, Bordin L, Dessole F, et al. Is corifollitropin alfa effective in controlled ovarian stimulation among all poor ovarian responders? A retrospective comparative study. Gynecol Endocrinol. 2019:1–5. [DOI] [PubMed]
- 18.Boots CE, Meister M, Cooper AR, Hardi A, Jungheim ES. Ovarian stimulation in the luteal phase: systematic review and meta-analysis. J Assist Reprod Genet. 2016;33(8):971–980. doi: 10.1007/s10815-016-0721-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kolibianakis EM, Bourgain C, Papanikolaou EG, Camus M, Tournaye H, Van Steirteghem AC, et al. Prolongation of follicular phase by delaying hCG administration results in a higher incidence of endometrial advancement on the day of oocyte retrieval in GnRH antagonist cycles. Hum Reprod. 2005;20(9):2453–2456. doi: 10.1093/humrep/dei069. [DOI] [PubMed] [Google Scholar]
- 20.Bosch E, Labarta E, Crespo J, Simón C, Remohí J, Jenkins J, Pellicer A. Circulating progesterone levels and ongoing pregnancy rates in controlled ovarian stimulation cycles for in vitro fertilization: analysis of over 4000 cycles. Hum Reprod. 2010;25(8):2092–2100. doi: 10.1093/humrep/deq125. [DOI] [PubMed] [Google Scholar]
- 21.Fauser BCJM, Alper MM, Ledger W, Schoolcraft WB, Zandvliet A, Mannaerts BMJL, Engage Investigators Pharmacokinetics and follicular dynamics of corifollitropin alfa versus recombinant FSH during ovarian stimulation for IVF. Reprod BioMed Online. 2010;21(5):593–601. doi: 10.1016/j.rbmo.2010.06.032. [DOI] [PubMed] [Google Scholar]
- 22.Lee T-H, Tzeng S-L, Lee C-I, Chen H-H, Huang C-C, Chen S-U, Lee MS. Association of progesterone production with serum anti-Müllerian hormone levels in assisted reproductive technology cycles with corifollitropin alfa. PLoS One. 2018;13(11):e0206111. doi: 10.1371/journal.pone.0206111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Devroey P, Boostanfar R, Koper NP, Mannaerts BMJL, Ijzerman-Boon PC, Fauser BCJM, ENGAGE Investigators A double-blind, non-inferiority RCT comparing corifollitropin alfa and recombinant FSH during the first seven days of ovarian stimulation using a GnRH antagonist protocol. Hum Reprod. 2009;24(12):3063–3072. doi: 10.1093/humrep/dep291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Corifollitropin alfa Ensure Study Group Corifollitropin alfa for ovarian stimulation in IVF: a randomized trial in lower-body-weight women. Reprod BioMed Online. 2010;21(1):66–76. doi: 10.1016/j.rbmo.2010.03.019. [DOI] [PubMed] [Google Scholar]