Abstract
Purpose
Preimplantation genetic diagnosis (PGD) analysis can be challenging for couples who carry more than one genetic condition. In this study, we describe a new PGD strategy to select which embryo(s) to transfer for two clinically challenging cases. Both cases lack essential family members for linkage analysis including de novo mutation combined with reciprocal translocation.
Methods
Diverging from conventional method, we performed direct point mutation detection, quantitative analysis of gene copy number, combined with linkage analysis assisted by SNP information from single sperm (or polar bodies), thus establishing an all-in-one protocol for single embryonic cell preimplantation diagnosis for two co-existing genetic conditions (monogenic disease and chromosomal abnormality) on the NGS-based platform.
Results
Using this newly developed method, 15 embryos from two cases were screened, and two embryos were determined as free of the monogenic disease and specific chromosomal abnormalities created by the prospective father’s reciprocal translocations.
Conclusion
This novel PGD strategy could effectively select unaffected embryo(s) for couples affected with or carrying a monogenetic disease and a reciprocal chromosome translocation concurrently.
Electronic supplementary material
The online version of this article (10.1007/s10815-020-01753-2) contains supplementary material, which is available to authorized users.
Keywords: PGD, Monogenic disorders, Reciprocal translocations, NGS, Sperm haplotyping
Introduction
So far, there are 6000–8000 known monogenic diseases in the human population. Many of them are severe congenital defects, and can even lead to premature death. Furthermore, the majority of them have no effective therapeutic options [1, 2]. Reciprocal translocations arise when two non-homologous chromosomes exchange their terminal segments and are the most common form of chromosomal abnormality, occurring in virtually 1/625 to 1/500 live births [26, 32]. The vast majority of these translocation carriers are phenotypically normal, because the majority of these types of rearrangements do not result in any significant deletion or duplication of chromosomal material [26, 34]. However, high rates of formation of unbalanced gametes can cause infertility, recurrent spontaneous abortions, and may lead to affected offspring [7, 23].
For challenging cases with monogenetic diseases and reciprocal translocations running in the same family, a prenatal diagnosis with amniocytes or chorionic villi is traditionally used to directly examine the presence of monogenetic disease allele(s) with Sanger sequencing or dosage analysis by MLPA/quantitative PCR. Additionally, an examination of unbalanced translocation with cytogenetic methods like karyotyping, FISH, or CGH are conventionally recommended [11, 17]. However, due to the high risk of being pregnant with either or both genetic conditions, preimplantation genetic diagnosis (PGD) together with preimplantation genetic screening can plausibly improve pregnancy outcomes in these complicated cases. Traditional PGD detection methods include fluorescence in situ hybridization (FISH), polymerase chain reaction (PCR), multiplex quantitative PCR (qPCR), array-comparative genomic hybridization (aCGH), single nucleotide polymorphism (SNP) microarray, and karyomapping [7, 9, 14, 21, 28]. Recently, next generation screening (NGS) has emerged as a powerful tool applied in PGS or PGD [14, 17]. Each of the above methods, however, has its limitations and disadvantages on accuracy of diagnosis [7, 14, 17, 19].
Among the patients in our center, we encountered two families complicated with neurofibromatosis type II (NF2) and spinal muscular atrophy (SMA), respectively, coupled with reciprocal chromosome translocations in the prospective father. Neurofibromatosis (NF) is a relatively frequent autosomal dominant neurological disease across different ethnic groups. It includes two distinct types (Type I and Type II, respectively) [15]. NF type II (NF2) is caused by mutations of the NF2 gene located on chromosome 22q12.2 (prevalence of ~ 1/100,000) [10, 33]. Spinal muscular atrophy (SMA) is an autosomal recessive childhood onset neuromuscular disease (prevalence of ~ 1/10,000, carrier frequency of ~ 1/50) [13, 21, 22]. Two highly homologous survival motor neuronal (SMN) genes, SMN1, and SMN2, are located in the 5q13 region and only differ in three intronic and two exonic nucleotides [5, 20, 29]. Only SMN1 pathogenic variants can cause SMA [4, 22]. In 90–98% of SMA patients, homozygous deletion of exon 7 of the SMN1 gene can be detected (even affecting exon 8) [22, 24, 29].
In this study, a novel approach was applied in two representative PGD cases. Besides monogenic diseases, both prospective fathers in these two families carry a specific reciprocal translocation. A common feature in these two cases is that the translocation found in both families started in the fathers (de novo). Therefore, haplotype information for paternal linkage analysis of the embryos cannot be derived. To solve this challenge, single sperm haplotyping is applied in this study. CNVs and SNVs information were obtained simultaneously from one embryo sample in a single sequencing run [11, 17, 31]. Combining information from single sperm haplotyping, NGS-based gene copy number quantitative analysis/pathogenic variants detection, SNP linkage analyses and chromosome aneuploidy analyses [25, 31, 32], we are able to select the appropriate embryos to transfer.
Materials and methods
Status of genetic conditions in the two study families
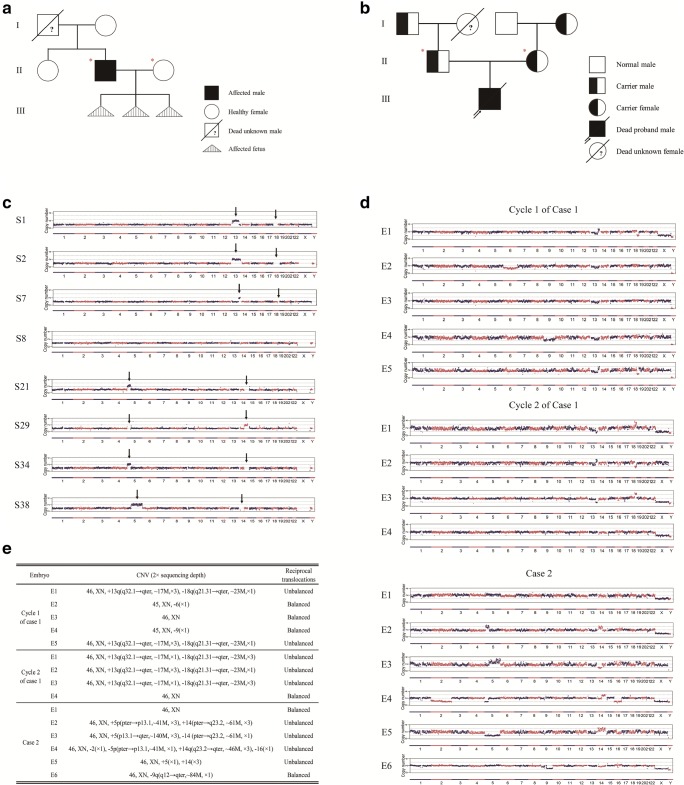
In case 1, the husband is affected with NF type II, positive for the NF2 gene mutation (NF2, c.1574 + 2 T > C). At the same time, the husband is a carrier of a reciprocal translocation between Chr13 and Chr18 [t (13; 18) (q32; q21.3)], due to which the family has had three abnormal pregnancies. Skull hypoplasia and fetal edema appeared in the fetus at 13 weeks of the first pregnancy in 2013, and 14 weeks of the second pregnancy in 2014. The latest abnormal pregnancy occurred in 2016 and resulted in embryo damage at 11 weeks. In case 2, both partners are healthy carriers of the SMN1 exons 7 and 8 deletion, and had one baby who was diagnosed with SMA and died at age 6 months. The husband has also been tested as a reciprocal translocation carrier [t (5; 14) (p15; q32)]. The pedigree charts of these two families are shown in Fig. 1a and b.
Fig. 1.
Chromosome aneuploidy analysis of embryos. a Pedigrees of the NF type II family. Filled symbols represent affected patient of NF type II; open symbols represent wild-type individuals with respect to NF type II. Circles and squares indicate females and males, respectively. Triangles with vertical lines mean induced labor fetus with chromosome 13 and 18 abnormality. Diagonal lines represent deceased individuals and question mark means lack of related gene detection. Couple indicated by red asterisks asked for PGD treatment. b Pedigrees of this SMA combined reciprocal translocation family. Filled symbols represent affected patient of SMA; half-filled symbols represent SMA carriers; open symbols represent wild-type individuals with respect to SMA. Circles and squares indicate females and males, respectively. The arrow indicates the affected proband. Diagonal lines represent deceased individuals and question mark means lack of SMA-related gene detection. Couple indicated by red asterisks asked for PGD treatment. c CNVs of 4 randomly selected sperms each family at 2 × depth. The arrow indicates the abnormal segment of Chr 13 and Chr 18 of case 1 and Chr 5 and Chr 14 of case 2. d CNVs of the 15 embryos from the two cases at low sequencing depth (2 ×) of NGS. e CNV and reciprocal translocation summary of the 15 embryos
Blastocyst biopsy and single sperm collection
In case 1, the couple underwent two oocyte retrieval cycles. Five embryos in cycle 1 and four embryos in cycle 2 were biopsied on day 5 or 6 after fertilization at the blastocyst stage to collect trophectoderm cells for later detection. In case 2, the couple underwent one oocyte retrieval cycle. In total, six embryos at the blastocyst stage were biopsied. After washing in PBS (with 0.1% HSA), the biopsied trophectoderm (TE) cells were transferred to a 0.2-mL PCR tube filled with 5 μL lysis buffer.
Semen quality was firstly assessed by sperm concentration, motility, and morphology. Higher motile sperms (n = 10) with morphological integrity were captured and collected individually into ten separate PCR tubes for next whole genome amplification (WGA), then a portion of the WGA products was used for mutant allele analysis by PCR-Sanger sequencing. The rest of the WGA products from two sperms carrying mutant alleles and two sperm carrying wild-type alleles were selected for next NGS and haplotype analysis.
WGA, specific PCR, and NGS
We used a novel PGD strategy based on single sperm linkage analysis and MARSALA technology to inspect chromosomal abnormalities and mutation sites, and to perform linkage analysis including sperm linkage for reciprocal translocation and single gene disorders diagnosis at the same time [31]. The whole genome amplification (WGA) of lysed TE cells or sperm cells was performed using the commercial MALBAC amplification kit according to the protocols (Yikon Genomics Inc.). Then we amplified NF2 mutation loci (NF2, c.1574 + 2 T > C) in case 1, and two different sites (EX7 + 6 and IVS7 + 236) in case 2 that vary between SMN1 and SMN2, from the WGA product, using specific primers for Sanger sequencing and NGS-based quantities analysis. Specific PCR was performed at 98 °C for 30 s, 30 cycles of 15 s at 98 °C, 30 s at 58 °C, and 30 s at 72 °C, and an additional 2 min at 72 °C using tag enzyme (NEB Inc.). This PCR product was mixed with the MALBAC product (0.5–2% of MALBAC product), and the mixture was used to construct a library using the NEBNext Ultra II DNA library Prep kit (New England Biolabs, Inc.). Copy number variations (CNVs) were measured accurately at a low depth (2 ×) by using the read depth method [30, 31]. The sequencing was performed on an Illumina HiSeq X Ten platform.
Linkage analysis by MARSALA
To identify normal embryos from reciprocal translocation carrier embryos, we separated Chr13 (Chr5) and Chr18 (Chr14) haplotyping based on typical sperms’ CNVs using SNPs (Fig. 2b). For the NF type II family, we separated the NF2 wild-type allele and mutation allele based on SNPs of typical sperms S7 and S8 (Fig. 3b). Eight SNPs within 1 Mb upstream and downstream of the NF2 mutation loci were rechecked by Sanger sequencing (Fig. 3c). For the SMA family, we separated haplotypes around the SMN1 gene on Chr5 based on typical sperms and maternal family members’ SNPs information for identification of the mutant allele (Fig. 4b and c). We carefully checked SNP positions within 3 Mb upstream and downstream of the SMN1 gene, and 13 SNPs were rechecked by Sanger sequencing (Fig. 4d). For example, if the father is heterozygous C/G and the mother is homozygous C/C in one SNP marker, while the sperm inherited the mutant allele C, we can deduce that the base of C from the father is linked with the mutant allele, and the base of G is linked with the normal allele.
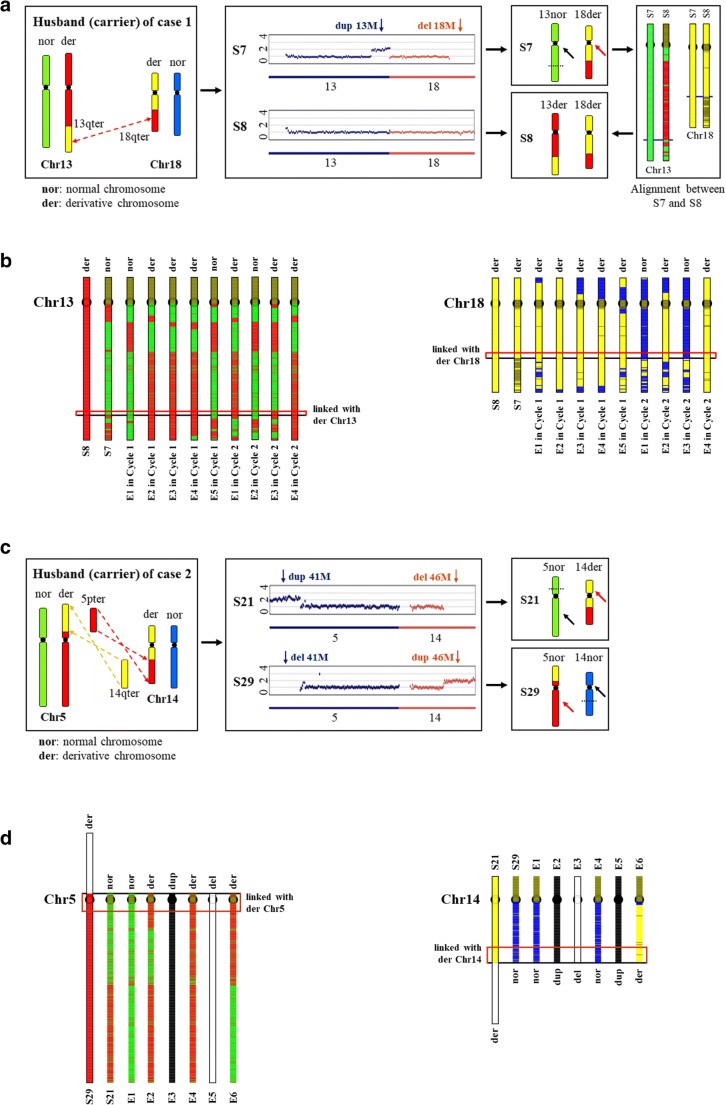
Fig. 2.
Identification of normal embryos from reciprocal translocation carrier embryos. a The reciprocal translocation schematic illustration of the husband between Chr 13 and Chr 18 and haplotype of two typical sperms deduced from their CNVs of case 1. S7, which inherited derivative Chr18, is regarded as a proband for analysis of Chr18. S8, which inherited derivative Chr13 and Chr18 deducing from its CNV and comparison with S7, is regarded as a proband for analysis of Chr13 and Chr18. nor, normal chromosome around breakpoint; der, derivative chromosome around breakpoint; pter, chromosome p arm terminal; qter, chromosome q arm terminal. Black arrows indicate normal chromosomes. Red arrows indicate derivative chromosomes. Dotted lines represent breakpoint. b Haplotype of paternal inherited chromosomes for each embryo of case 1. Color differences (red vs. green, yellow vs. blue) of the region around breakpoint positions (red boxes indicated) were used to determine the inherited chromosomes. Red and green represent derivative Chr13 and normal Chr13, respectively. Yellow and blue represent derivative Chr18 and normal Chr18, respectively. c The reciprocal translocation schematic illustration of the husband between Chr 5 and Chr 14 and haplotype of two typical sperms deduced from their CNVs of case 2. S29, which inherited derivative Chr5, is regarded as a proband for analysis of Chr5. S21, which inherited derivative Chr14, is regarded as a proband for analysis of Chr14. nor, normal chromosome around breakpoint; der, derivative chromosome around breakpoint; pter, chromosome p arm terminal; qter, chromosome q arm terminal. Black arrows indicate normal chromosomes. Red arrows indicate derivative chromosomes. Dotted lines represent breakpoint. d Haplotype of paternal inherited chromosomes for each embryo of case 2. Color differences (red vs. green, yellow vs. blue) of the region around breakpoint positions (red boxes indicated) were used to determine the inherited chromosomes. Red and green represent derivative Chr5 and normal Chr5, respectively. Yellow and blue represent derivative Chr14 and normal Chr14, respectively. White regions indicate deletion of this part of chromosome. Dup, duplication; del, deletion
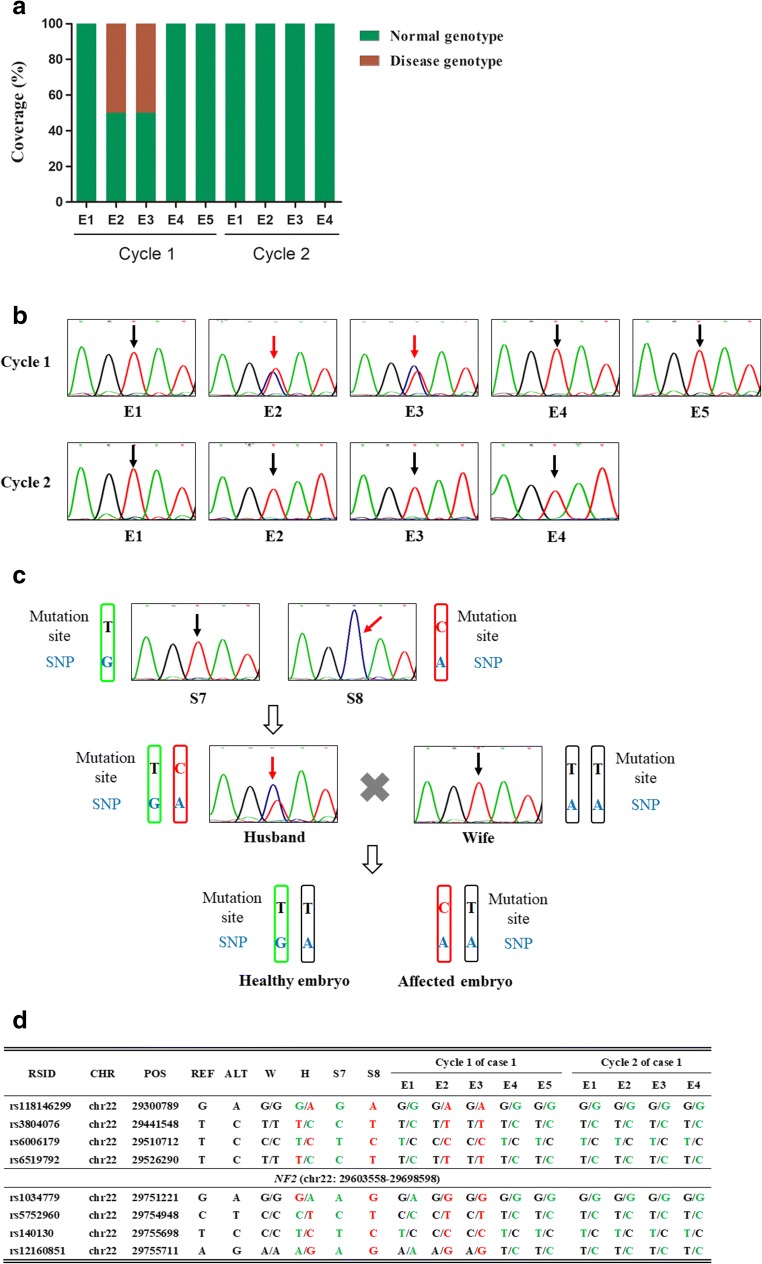
Fig. 3.
Diagnosis and confirmation of NF type II of nine embryos from two cycles. a Result of NGS sequencing the targeted mutation site in the NF2 gene. E2, and E3 of cycle 1 are affected embryos. b Sanger sequencing of NF2 mutation site of nine embryos. Black arrows indicate wild-type embryos. Red arrows indicate affected embryos. c Paternal SNP analysis schematic depending on normal and mutant sperms. The black “T” means S7 carrying wild-type allele and the blue “G” indicates the base associated with wild-type allele of NF2 gene. The red “C” means S8 carrying mutation allele and the blue “A” indicates the base linked with mutation allele of NF2 gene. Embryo carrying blue “G” means it inherited wild-type allele while embryo carrying blue “A” means it inherited mutation allele. d Results of linkage analyses of the nine embryos. Four upstream and four downstream SNP markers were selected to identify the disease-carrying allele in each embryo. RSID, reference SNP cluster ID; CHR, chromosome number; POS, genomic position; REF, reference allele of the SNPs; ALT, alternative allele of the SNPs; W, wife; H, husband
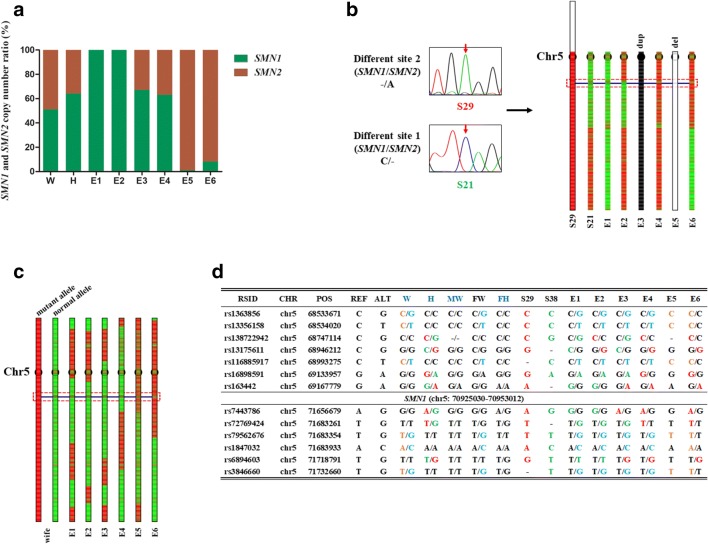
Fig. 4.
Diagnosis and confirmation of SMA of six embryos. a NGS results of copy number ratio of SMN1 and SMN2 by sequencing different site 1. E5 and E6 are affected embryos while the other embryos have at least one copy of SMN1. W, wife; H, husband. b Haplotype of six embryos for paternal linkage analysis depending on S29 (disease-carrying allele) and S21 (normal allele). E1 and E2 inherited the normal allele, while E4 and E6 inherited the disease-carrying allele. E5 lacked the paternal chromosome 5 q arm. E3 inherited both the normal allele and disease-carrying allele from the father. c Haplotype of six embryos for maternal linkage analysis depending on mother’s parents. The mother’s mutant allele was inherited from her mother and normal allele from her father. E1, E2, E3, and E4 inherited the normal allele from the mother, while E5 and E6 inherited the disease-carrying allele. d Results of linkage analyses of the six embryos based on SNP. Seven upstream and six downstream SNP markers were selected to identify the disease-carrying allele in each embryo. RSID, reference SNP cluster ID; CHR, chromosome number; POS, genomic position; REF, reference allele of the SNPs; ALT, alternative allele of the SNPs; W, wife; H, husband; MW, mother of the wife; FW, father of the wife; FH, father of the wife. Adult carriers labeled in dark blue. - represents the alleles that are not covered by single-cell low-depth sequencing and Sanger sequencing
NGS-based quantitative analysis
This analysis was designed for the SMA family to verify the copy number of SMN1. We incubated equally 8 ng MALBAC product of each biopsied sample for specific PCR, and the PCR product was mixed with the MALBAC product (0.5–2% of MALBAC product) in a specific quantity. We stringently controlled quantities of each sample in every step to ensure that sequencing quantities of each sample were equal, and that the amplification efficiency of SMN1 and SMN2 are approximately identical. NGS sequencing can distinguish and count SMN1 fragments and SMN2 fragments. From the above, we can directly obtain copy numbers of SMN1 and SMN2 through normalizing and comparing (Fig. 4a).
Results
Sperm detection and chromosome aneuploidy analysis
In case 1, after the NF type II family had three abnormal pregnancies, we tested the couple for chromosomal abnormalities. It was confirmed that the husband was a carrier of a reciprocal translocation between Chr13 and Chr18 [t (13; 18) (q32; q21.3)], as well as the NF2 gene pathogenic variant (NF2, c.1574 + 2 T > C) (Fig. 1a). In case 2, both husband and wife carried pathogenic variants (SMN1, exons 7 and 8 deletion) and their child was fatally affected with SMA (Fig. 1b). Lacking the genetic information of an essential family member for linkage analysis (Fig. 1a, b), single sperm was used for paternal linkage analysis and to assist in identification of pathogenic variant sites. Before undergoing the PGD cycle, four sperms from each case (S1, S2, S7, and S8 in case 1; and S21, S29, S34, and S38 in case 2) were selected and amplified separately, followed by pathogenic variant detection and NGS to distinguish mutant alleles from wild-type alleles in each family. S1 and S7 each carry the wild-type allele by amplification of NF2 mutation site, whereas S2 and S8 each carry the pathogenic variant allele (Fig. 1c). S29 carries the disease-causing allele by amplification of the SMN1 and SMN2 different site 2, while S21 carries the wild-type allele by amplification of the SMN1 and SMN2 different site 1. Unexpectedly, we observed chromosome aneuploidy with Chr5 and Chr14 in each sperm from case 2 (Fig. 1c). Based on this observation, we speculated that the husband could be a reciprocal translocation carrier. To confirm this speculation, we performed FISH (fluorescence in situ hybridization), and the father was diagnosed as a carrier of a reciprocal translocation between Chr5 and Chr14 [t (5; 14) (p15; q32)]. Both of these exceptional cases involved a single gene disorder and reciprocal translocation, meanwhile both cases lack essential family members required for conventional paternal linkage analysis. To perform PGD for these complicated cases, we employed the new testing strategy mentioned above.
Chromosome CNV analysis of fifteen embryos from 3 cycles showed that E3 in cycle 1 of case 1, E4 in cycle 2 of case 1, and E1 in case 2 were balanced, and no other chromosomal abnormalities were observed, whereas chromosomal aneuploidies were observed in other embryos. The majority of the abnormalities occurred in translocation chromosomes, including E1 (unbalanced) and E5 (unbalanced) in cycle 1 of case 1; E1 (unbalanced), E2 (unbalanced), and E3 (unbalanced) in cycle 2 of case 1; and E2 (unbalanced), E3 (unbalanced), E4 (unbalanced and monosomy 2, 16), and E5 (unbalanced) in case 2. Meanwhile, other chromosomal abnormalities occurred, including E2 (monosomy 6) and E4 (monosomy 9) in cycle 1 of case 1 and E6 (deletion in part of chromosome 9) in case 2 (Fig. 1d, e).
Distinguishing wild-type embryos from reciprocal translocation carrier embryos
Through CNV analysis, embryos with abnormal chromosome copy numbers were identified, but it was difficult to distinguish between wild-type and translocation carrier embryos. To overcome this challenge, we chose an additional chromosome haplotyping method based on single sperm CNV analysis [32]. Two sperms were selected for haplotyping analyses that inherit different types of translocation chromosomes caused by reciprocal translocation in each family. In case 1, Fig. 2a illustrates the translocation events between the Chr13 q arm and Chr18 q arm of the male carrier. According to the CNV result of S7, we can deduce that S7 inherited normal Chr13 and derivative Chr18. Other than S7, S8 had no obvious copy number abnormality, so we sought to deduce whether S8 carried normal Chr13 and Chr18 or derivative Chr13 and Chr18. Compared with S7, we inferred that S8 carried derivative Chr13 and Chr18 (Fig. 2a). From CNV analysis, we know that Chr13 and Chr18 of E2, E3, and E4 of cycle 1, and E4 of cycle 2 were amphidiploid (Fig. 1e). Haplotypes of embryos and alignment between embryos and S8 told us that all four embryos were reciprocal translocation carriers (Fig. 2b). Using E4 of cycle 2 as an example, which is consistent with S8 in the red region linked with derivative Chr13 and Chr18, we deduced that S8 carried derivative Chr13 and Chr18. Figure 2c illustrates the translocation events between the Chr5 p arm and Chr14 q arm of the male carrier of case 2. Through sperm CNV and translocation patterns, we can deduce which chromosomes were present in the sperm. S21 inherited normal Chr5 and derivative Chr14, and can provide partial (yellow region indicated by red arrow) haplotype linkage information of derivative Chr14. Likewise, S29 inherited derivative Chr5 and normal Chr14, and can provide partial (red region indicated by red arrow) haplotype linkage information of derivative Chr5. Paternal and maternal haplotypes of the embryos were separated depending on sperm haplotype (Fig. 2d). Based on the chromosome aneuploidy analysis, we know that Chr5 and Chr14 of E1 and E6 were amphidiploid, as shown in Fig. 2d. For example, also as shown in Fig. 2d, we took part of S29 (red region) as derivative Chr5 proband, and E6 is consistent with S29, which allowed us to deduce that E6 inherited derivative Chr5. E6 is consistent with S21 in the red region linked with derivative Chr14, which allowed us to deduce that E6 inherited derivative Chr14. Thus, only E1 inherited normal Chr5 and Chr14 that were inconsistent with S29 and S21.
Using chromosome haplotyping analysis, we can accurately determine that E1 of case 2 is wild-type, while the other five embryos analyzed above are carriers of the reciprocal translocation.
Diagnosis of single gene disorders
Mutation site detection and SNP linkage analysis were used together to ensure the accuracy of the diagnosis of the NF type II family (case 1). Both NGS sequencing (Fig. 3a) and Sanger sequencing (Fig. 3b) were performed after specific PCR amplification for the NF2 pathogenic variant (NF2, c.1574 + 2 T > C) to determine affected embryos. E2 and E3 of cycle 1 were identified as carrying the paternal pathogenic variant, whereas the other seven embryos of this family were identified as being free of NF type II. To avoid allele drop-out, single sperm SNP linkage analysis was established to verify whether pathogenic variant alleles were present in the embryos. S8 carried pathogenic variant alleles which could be taken as the proband, while S7 inherited wild-type alleles which could be taken as the normal control (Fig. 3c). Eight SNP markers (within 1 Mb around NF2) were selected from NGS linkage analysis and confirmed by Sanger sequencing to illustrate single sperm linkage results (Fig. 3d). In concordance with the mutation site detection analysis, the linkage analysis verified that only E2 and E3 from cycle 1 were affected with NF type II.
Compared with the NF type II family, it is more difficult to diagnose SMA in the embryos of the SMA family (case 2). Because SMN2 is highly homologous to SMN1, direct detection of mutations in SMN1 is difficult, especially in embryos. Therefore, to improve diagnostic accuracy, we used three independent methods to confirm this diagnosis. Table 1 shows Sanger sequencing results at two different loci that vary between SMN1 and SMN2, which can distinguish affected embryos from unaffected embryos. E5 and E6 lost two copies of SMN1 exons 7 and 8, and were thus diagnosed as affected embryos, whereas the other embryos tested had at least one copy of SMN1 exons 7 and 8. Furthermore, we examined the different site 1 between SMN1 and SMN2 of the six embryos. Certain sequences containing different sequences between SMN1 and SMN2 were amplified using specific primers and detected by NGS. Through this detection method, we reconfirmed that E5 and E6 are affected embryos, whereas E1 and E2 are wild-type embryos free of SMN1 mutation (Fig. 4a, Table S1, and Fig. S1C). In addition to analyzing embryos using these two methods, sperm linkage for paternal mutation analysis and pedigree linkage for maternal mutation analysis further confirmed the accuracy of the diagnosis. As shown in Fig. 4b, S29 was determined to be the proband carrying the mutant allele by Sanger sequencing of the different site 2, while S21 carried the normal allele by Sanger sequencing of the different site 1. From Fig. 4b, we can deduce that E1 and E2 inherited the normal allele from the father, while E4 and E6 inherited the mutant allele from the father. The lack of chromosome 5q arm in E5 was from the father. E3 was more complicated, inheriting both a normal allele and a disease-carrying allele from the father. Compared to analyzing the paternal pathogenic variant-carrying allele, the maternal pathogenic variant-carrying allele was much more straightforward. E5 and E6 inherited the pathogenic variant-carrying allele, whereas the other embryos inherited the normal allele from the mother (Fig. 4c). To be more precise, seven SNPs (within 2.4 Mb upstream of SMN1) and six SNPs (within 0.8 Mb downstream of SMN1) listed in the NGS linkage analysis results were confirmed by Sanger sequencing (Fig. 4d). E1 and E2 inherited normal alleles from both parents, whereas E6 inherited pathogenic variant-carrying alleles from both parents. Both haplotyping analysis (Fig. 4b) and SNP analysis (Fig. 4d) revealed that there is paternal chromosome exchange at about 2 Mb upstream of SMN1 of E2. E5 only inherited the pathogenic variant-carrying allele from the mother. It is worth noting that fraternal linkage analysis of E3 is ambiguous, and 4 SNPs indicated that it inherited the normal allele, while 3 SNPs indicated that it inherited the disease-carrying allele. This is likely because E3 carried genetic materials from both chromosome 5 of the paternal allele.
Table 1.
Sanger sequencing results at two different sites varying between SMN1 and SMN2 for six embryos
| Embryo | Different site 1 (SMN1/SMN2) | Different site 2 (SMN1/SMN2) |
|---|---|---|
| E1 | C/− | G/− |
| E2 | C/− | G/− |
| E3 | C/T | G/A |
| E4 | C/T | G/A |
| E5 | −/T | −/A |
| E6 | −/T | −/A |
Summary of the detection and clinical outcome
Chromosome aneuploidy analysis, reciprocal translocation detection, and single gene disease diagnosis revealed that E4 in cycle 2 of case 1, and E1 in case 2 were suitable for embryo transfer because they were unaffected by the monogenic disorders tested, and displayed no significant chromosomal alternations (specifically, E1 in case 2 was free of the reciprocal translocation) (Table 2). All other embryos were unsuitable for transfer due to aneuploidy and/or being affected by single gene disorders. Furthermore, E2 and E3 in cycle 1 of case 1 inherited paternal pathogenic variants, and E5 and E6 in case 2 also carried SMN1 pathogenic variants from both parents (Table 2).
Table 2.
Summary of PGD results for fifteen embryos
| Embryo | CNV | Reciprocal translocation | Diagnosis for monogenic disorder | ||
|---|---|---|---|---|---|
| Case 1 | Between Chr13 and Chr18 | NF type II (paternal mutation) | |||
| Cycle 1 | E1 | Aneuploid | Unbalanced | No | |
| E2 | Aneuploid | Carrier | Yes | ||
| E3 | Euploid | Carrier | Yes | ||
| E4 | Aneuploid | Carrier | No | ||
| E5 | Aneuploid | Unbalanced | No | ||
| Cycle 2 | E1 | Aneuploid | Unbalanced | No | |
| E2 | Aneuploid | Unbalanced | No | ||
| E3 | Aneuploid | Unbalanced | No | ||
| E4 | Euploid | Carrier | No | ||
| Case 2 | Between Chr5 and Chr14 | SMA | |||
| Maternal mutation | Paternal mutation | ||||
| E1 | Euploid | Normal | No | No | |
| E2 | Aneuploid | Unbalanced | No | No | |
| E3 | Aneuploid | Unbalanced | No | Yes | |
| E4 | Aneuploid | Unbalanced | No | Yes | |
| E5 | Aneuploid | Unbalanced | Yes | – | |
| E6 | Aneuploid | Carrier | Yes | Yes | |
E4 in cycle 2 of case 1, and E1 in case 2 were suitable for embryo transfer because they were unaffected by the monogenic disorders tested, and displayed no significant chromosomal alternations
E1 in case 2 was transplanted, but unfortunately, the embryo failed to implant. In case 1 cycle 2, the E4 that inherited paternal wild-type NF2 allele and the chromosome reciprocal translocation was abandoned upon the couple’s request, as they preferred to move on to the next cycle.
Discussion
Unique features of these families and comparison of our new PGD strategy with other methods
In the cases of the two families in this study, there were obstacles in paternal linkage analysis due to lacking information from essential family members. Additionally, both reciprocal translocations from the husbands in each couple, as well as the NF2 pathogenic variant in the husband of case 1, are de novo. In the NF type II family, we speculate that the husband carries a de novo pathogenic variant of NF2, which is not found in other living family members, and was not symptomatic in his father during his lifetime. Meanwhile, in the SMA family, it was unknown whether the husband inherited the SMN1 pathogenic variant from his father or mother (his mother passed away), since its high carrier and de novo frequency. Detection of SMN1 copy number in PGD relies on linkage analysis to provide sufficient evidence to eliminate SMN2’s interference, and successfully distinguish normal embryos from carrier embryos [13, 22, 25]. Without this information, the accuracy of the diagnosis would have been compromised. To overcome these difficulties, and to increase the accuracy of diagnosis, additional information about CNVs, pathogenic variants, SNPs, and reciprocal translocation analysis with NGS was necessary. Since invasive procedures were required to retrieve the biopsied trophectoderm (TE) cells, our novel approach allows us to collect as much information as we can without using substantial amounts of starting material, which conventional FISH and PCR-based direct mutation detection were not able to do [14, 17]. The other typical approach used in PGD, array-CGH, cannot provide information on single gene mutations, and fails to detect extra or missing fragments smaller than 6 Mb [14].
To improve the accuracy of diagnosis, we established a new method combining sperm linkage with NGS-based quantitative analysis on the basis of MARSALA technology. CNVs, SNVs, and SNP information were simultaneously obtained from one NGS run [11, 17, 31]. In particular, for single gene mutation detection, single sperm linkage can help distinguish normal alleles from mutant alleles. NGS-based analysis identifies the specific sites different between SMN1 and SMN2; it can also provide copy number information for exons 7 and 8 in SMN1, which is undetectable by karyomapping approaches [27]. NGS followed by two site-specific PCRs can help evaluate copy numbers of SMN1 (exons 7 and 8) using the SMN2 homologous fragment as the reference. This method has never been reported before on embryonic single-cell sequencing analysis. To detect reciprocal translocations in embryonic single cells, we relied on chromosome haplotype analysis to differentiate wild-type embryos from carrier embryos, a method which can achieve high accuracy by deep sequencing [8, 32]. The flexible mutation sites used for PCR design and variable sequencing depth depending on clinical demand make this method more widely applicable than other methods such as array-CGH and karyomapping, especially for complicated clinical cases described here and for other monogenic disorder cases difficult to detect [6, 12, 17, 18].
The new NGS-based quantitative analysis can be used for PGD in other cases beyond SMA
SMA represents a challenging monogenic disorder for PGD because the pathogenic variant cannot be detected directly in embryos, not only due to the mutation type (exon deletion) but also the existence of a highly homologous gene. SMN1 and SMN2 are highly homologous, and this new NGS-based quantitative method could determine the copy numbers of these two homologous genes in one PCR reaction using the same primers. To deduce the copy number of SMN1, the copy number of SMN2 is used as a reference. This approach needs further refinement in the future, as the number of SMN2 copies can be variable [3, 16]. In this case, the copy numbers of SMN1 and SMN2 of family members were identified by MLPA (Multiplex Ligation-dependent Probe Amplification), allowing us to deduce the precise gene copy number in embryos by our new NGS-based quantitative method. Here, we deduced copy number variation and illustrated the allele inheritance using MLPA, sperm analysis, and NGS-based quantitative analysis results (Fig. S1 and Table S1). In Fig. S1A, we illustrated the MLPA results of the family members. MLPA detection, combined with sperm haplotyping analysis, constituted the inheritance origin of the alleles of the embryos (Fig. S1B). In addition, SNP-linkage analysis (Fig. 4b and c) and NGS-based quantitative analysis (Fig. 4a) were used to identify copy numbers of SMN1 and SMN2 and the inheritance map of the six embryos in case 2 (Fig. S1C). This new NGS-based quantitative analysis strategy is a reliable approach for detection of copy number in PGD. It is extraordinarily powerful in the evaluation of monogenic diseases involving copy number in embryos.
Our advanced MARSALA method is efficient and accurate for reciprocal translocation diagnosis
For the first time, the advanced MARSALA approach is reported as a promising diagnostic method for complicated PGD cases containing reciprocal translocation and single gene disorder. Compared with array-CGH, this method is more efficient, as we obtained copy number in higher resolution and even at the single gene level, with one DNA library preparation and one next-generation sequencing run. Furthermore, it is more accurate than traditional methods, since it uses several lines of evidence for the two complicated cases.
In case 2, the husband has a reciprocal translocation between Chr5 and Chr14, which is particularly relevant as the SMN1 gene is located on Chr5. We found that this derivative Chr5 serves as a marker for the mutant allele of SMN1 deletion mutation in the husband of case 2. From S21 and S29 CNV analysis and SMN1 specific detection results, it was clearly shown that the SMN1 deletion allele is linked with derivative Chr5, and the SMN1 wild-type allele is linked with normal Chr5 (Fig. S1B). Thus, we deduced that embryos that inherited normal Chr5 inherited the SMN1 wild-type allele (ignoring chromosome exchange). Following this simple ratiocination with combined information from the sperm and embryos, E1, E4, and E6 in case 2 could be diagnosed (Fig. S1C). In conclusion, we have shown through this representative PGD case that with a modified MARSALA and NGS detection strategy combined with haplotype information from the sperm, one can provide accurate diagnoses for couples with complicated chromosomal abnormality changes (such as reciprocal translocation) and monogenetic diseases (such as NF2 and SMA).
Limitations of this novel strategy
There are a few limitations of this novel strategy. Firstly, we need single sperm DNA analysis to assist embryo diagnosis. NGS of multiple single sperm in separate tubes will increase costs. Secondly, this novel strategy is more appropriate for male patients or carriers. For female patient or carriers, polar bodies taken as probands are needed. If there are no sufficient matured oocytes and embryos, it is difficult to perform genetic diagnosis using this approach.
Electronic supplementary material
(DOCX 89.6 kb)
Fig. S1 Inherited patterns of chromosome 5 and identification of SMN1 and SMN2 copy numbers. A: MLPA results of SMN1 and SMN2 copy numbers. (0.8): detection value. We deduce the husband carried 1 copy SMN2 Exon7, and there is an allowable error between actual copy number and detection copy number. B: Inherited patterns of Chr 5 in this family. Inherited patterns are based on the SMN1 gene and ignore chromosome exchange. Nor: normal chromosome around breakpoint; der: derivative chromosome around breakpoint. C: Inherited patterns of Chr 5 in six embryos. We illustrated chromosome exchanges that influenced the diagnosis of SMN1, like E2, and omitted irrelevant exchanges for clarity (TIF 4.21 mb)
Footnotes
Yuqian Wang and Xiaohui Zhu are joint First Authors
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Boycott KM, Hartley T, Biesecker LG, Gibbs RA, Innes AM, Riess O, Belmont J, Dunwoodie SL, Jojic N, Lassmann T, Mackay D, Temple IK, Visel A, Baynam G. A diagnosis for all rare genetic diseases: the horizon and the next frontiers. Cell. 2019;177:32–37. doi: 10.1016/j.cell.2019.02.040. [DOI] [PubMed] [Google Scholar]
- 2.Boycott KM, Lau LP, Cutillo CM, Austin CP (2019b) International collaborative actions and transparency to understand, diagnose, and develop therapies for rare diseases. EMBO Mol Med. e10486. [DOI] [PMC free article] [PubMed]
- 3.Calucho M, Bernal S, Alías L, March F, Venceslá A, Rodríguez-Álvarez FJ, Aller E, Fernández RM, Borrego S, Millán JM, Hernández-Chico C, Cuscó I, Fuentes-Prior P, Tizzano EF. Correlation between SMA type and SMN2 copy number revisited: an analysis of 625 unrelated Spanish patients and a compilation of 2834 reported cases. Neuromuscul Disord. 2018;28:208–215. doi: 10.1016/j.nmd.2018.01.003. [DOI] [PubMed] [Google Scholar]
- 4.d’Ydewalle C, Sumner CJ. Spinal muscular atrophy therapeutics: where do we stand? Neurotherapeutics. 2015;12:303–316. doi: 10.1007/s13311-015-0337-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.D'Amico A, Mercuri E, Tiziano FD, Bertini E. Spinal muscular atrophy. Orphanet J Rare Dis. 2011;6:71. doi: 10.1186/1750-1172-6-71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Deleye L, De Coninck D, Deforce D, Van Nieuwerburgh F. Genome-wide copy number alteration detection in preimplantation genetic diagnosis. Methods Mol Biol. 2018;1712:27–42. doi: 10.1007/978-1-4939-7514-3_3. [DOI] [PubMed] [Google Scholar]
- 7.Fiorentino F, Spizzichino L, Bono S, Biricik A, Kokkali G, Rienzi L, Ubaldi FM, Iammarrone E, Gordon A, Pantos K. PGD for reciprocal and Robertsonian translocations using array comparative genomic hybridization. Hum Reprod. 2011;26:1925–1935. doi: 10.1093/humrep/der082. [DOI] [PubMed] [Google Scholar]
- 8.Fiorentino F, Bono S, Biricik A, Nuccitelli A, Cotroneo E, Cottone G, Kokocinski F, Michel C, Minasi MG, Greco E. Application of next-generation sequencing technology for comprehensive aneuploidy screening of blastocysts in clinical preimplantation genetic screening cycles. Hum Reprod. 2014;29:2802–2813. doi: 10.1093/humrep/deu277. [DOI] [PubMed] [Google Scholar]
- 9.Fischer J, Colls P, Escudero T, Munné S. Preimplantation genetic diagnosis (PGD) improves pregnancy outcome for translocation carriers with a history of recurrent losses. Fertil Steril. 2010;94:283–289. doi: 10.1016/j.fertnstert.2009.02.060. [DOI] [PubMed] [Google Scholar]
- 10.Halliday D, Emmanouil B, Vassallo G, Lascelles K, Nicholson J, Chandratre S, Anand G, Wasik M, Pretorius P, Evans DG, Parry A (2019) Trends in phenotype in the English paediatric neurofibromatosis type 2 cohort stratified by genetic severity. Clin Genet. [DOI] [PubMed]
- 11.Handyside AH, Harton GL, Mariani B, Thornhill AR, Affara N, Shaw MA, Griffin DK. Karyomapping: a universal method for genome wide analysis of genetic disease based on mapping crossovers between parental haplotypes. J Med Genet. 2010;47:651–658. doi: 10.1136/jmg.2009.069971. [DOI] [PubMed] [Google Scholar]
- 12.Huang L, Ma F, Chapman A, Lu S, Xie XS. Single-cell whole-genome amplification and sequencing: methodology and applications. Annu Rev Genomics Hum Genet. 2015;16:79–102. doi: 10.1146/annurev-genom-090413-025352. [DOI] [PubMed] [Google Scholar]
- 13.Kolb SJ, Kissel JT. Spinal muscular atrophy. Neurol Clin. 2015;33:831–846. doi: 10.1016/j.ncl.2015.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Liss J, Chromik I, Szczyglińska J, Jagiełło M, Łukaszuk A, Łukaszuk K. Current methods for preimplantation genetic diagnosis. Ginekol Pol. 2016;87:522–526. doi: 10.5603/GP.2016.0037. [DOI] [PubMed] [Google Scholar]
- 15.Long C, Amoasii L, Bassel-Duby R, Olson EN. Genome editing of monogenic neuromuscular diseases. JAMA Neurol. 2016;73:1349. doi: 10.1001/jamaneurol.2016.3388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lorson CL, Hahnen E, Androphy EJ, Wirth B. A single nucleotide in the SMN gene regulates splicing and is responsible for spinal muscular atrophy. Proc Natl Acad Sci U S A. 1999;96:6307–6311. doi: 10.1073/pnas.96.11.6307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lu L, Lv B, Huang K, Xue Z, Zhu X, Fan G. Recent advances in preimplantation genetic diagnosis and screening. J Assist Reprod Genet. 2016;33:1129–1134. doi: 10.1007/s10815-016-0750-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Łukaszuk K, Pukszta S, Wells D, Cybulska C, Liss J, Płóciennik Ł, Kuczyński W, Zabielska J. Routine use of next-generation sequencing for preimplantation genetic diagnosis of blastomeres obtained from embryos on day 3 in fresh in vitro fertilization cycles. Fertil Steril. 2015;103:1031–1036. doi: 10.1016/j.fertnstert.2014.12.123. [DOI] [PubMed] [Google Scholar]
- 19.Malcov M, Schwartz T, Mei-Raz N, Yosef DB, Amit A, Lessing JB, Shomrat R, Orr-Urtreger A, Yaron Y. Multiplex nested PCR for preimplantation genetic diagnosis of spinal muscular atrophy. Fetal Diagn Ther. 2004;19:199–206. doi: 10.1159/000075151. [DOI] [PubMed] [Google Scholar]
- 20.Messina S. New directions for SMA therapy. J Clin Med. 2018;7:251. doi: 10.3390/jcm7090251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Moutou C, Gardes N, Rongieres C, Ohl J, Bettahar-Lebugle K, Wittemer C, Gerlinger P, Viville S. Allele-specific amplification for preimplantation genetic diagnosis (PGD) of spinal muscular atrophy. Prenat Diagn. 2001;21:498–503. doi: 10.1002/pd.110. [DOI] [PubMed] [Google Scholar]
- 22.Moutou C, Machev N, Gardes N, Viville S. Case report: birth after preimplantation genetic diagnosis of a subtle mutation in SMN1 gene. Prenat Diagn. 2006;26:1037–1041. doi: 10.1002/pd.1551. [DOI] [PubMed] [Google Scholar]
- 23.Munne S. Analysis of chromosome segregation during preimplantation genetic diagnosis in both male and female translocation heterozygotes. Cytogenet Genome Res. 2005;111:305–309. doi: 10.1159/000086904. [DOI] [PubMed] [Google Scholar]
- 24.Ogino S, Wilson R. Genetic testing and risk assessment for spinal muscular atrophy (SMA) Hum Genet. 2002;111:477–500. doi: 10.1007/s00439-002-0828-x. [DOI] [PubMed] [Google Scholar]
- 25.Ren Y, Zhi X, Zhu X, Huang J, Lian Y, Li R, Jin H, Zhang Y, Zhang W, Nie Y, Wei Y, Liu Z, Song D, Liu P, Qiao J, Yan L. Clinical applications of MARSALA for preimplantation genetic diagnosis of spinal muscular atrophy. J Genet Genomics. 2016;43:541–547. doi: 10.1016/j.jgg.2016.03.011. [DOI] [PubMed] [Google Scholar]
- 26.Scriven PN, Handyside AH, Ogilvie CM. Chromosome translocations: segregation modes and strategies for preimplantation genetic diagnosis. Prenat Diagn. 1998;18:1437–1449. doi: 10.1002/(SICI)1097-0223(199812)18:13<1437::AID-PD497>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- 27.Thornhill AR, Handyside AH, Ottolini C, Natesan SA, Taylor J, Sage K, Harton G, Cliffe K, Affara N, Konstantinidis M, Wells D, Griffin DK. Karyomapping—a comprehensive means of simultaneous monogenic and cytogenetic PGD: comparison with standard approaches in real time for Marfan syndrome. J Assist Reprod Genet. 2015;32:347–356. doi: 10.1007/s10815-014-0405-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tisdale S, Pellizzoni L. Disease mechanisms and therapeutic approaches in spinal muscular atrophy. J Neurosci. 2015;35:8691–8700. doi: 10.1523/JNEUROSCI.0417-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wirth B. An update of the mutation spectrum of the survival motor neuron gene (SMN1) in autosomal recessive spinal muscular atrophy (SMA) Hum Mutat. 2000;15:228–237. doi: 10.1002/(SICI)1098-1004(200003)15:3<228::AID-HUMU3>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- 30.Xu J, Zhang Z, Niu W, Yang Q, Yao G, Shi S, Jin H, Song W, Chen L, Zhang X, Guo Y, Su Y, Hu L, Zhai J, Zhang Y, Dong F, Gao Y, Li W, Bo S, Hu M, Ren J, Huang L, Lu S, Xie XS, Sun Y. Mapping allele with resolved carrier status of Robertsonian and reciprocal translocation in human preimplantation embryos. Proc Natl Acad Sci U S A. 2017;114:E8695–E8702. doi: 10.1073/pnas.1715053114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yan L, Huang L, Xu L, Huang J, Ma F, Zhu X, Tang Y, Liu M, Lian Y, Liu P, Li R, Lu S, Tang F, Qiao J, Xie XS. Live births after simultaneous avoidance of monogenic diseases and chromosome abnormality by next-generation sequencing with linkage analyses. Proc Natl Acad Sci. 2015;112:15964–15969. doi: 10.1073/pnas.1523297113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yan Z, Wang Y, Nie Y, Zhi X, Zhu X, Qin M, Guan S, Ren Y, Kuo Y, Chang D, Chen W, Yuan P, Yan L, Qiao J. Identifying normal embryos from reciprocal translocation carriers by whole chromosome haplotyping. J Genet Genomics. 2018;45:505–508. doi: 10.1016/j.jgg.2018.05.006. [DOI] [PubMed] [Google Scholar]
- 33.Yury V, Svetlana R, Oleg V, Anna C, Tatyana S, Christina M, Michael L, Brian K, Kevin L, Anver K. Preimplantation diagnosis for neurofibromatosis. Reprod BioMed. 2002;4(3):218–222. doi: 10.1016/S1472-6483(10)61809-3. [DOI] [PubMed] [Google Scholar]
- 34.Zhang W, Liu Y, Wang L, Wang H, Ma M, Xu M, Xu X, Gao Z, Duan J, Cram DS, Yao Y. Clinical application of next-generation sequencing in preimplantation genetic diagnosis cycles for Robertsonian and reciprocal translocations. J Assist Reprod Genet. 2016;33:899–906. doi: 10.1007/s10815-016-0724-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX 89.6 kb)
Fig. S1 Inherited patterns of chromosome 5 and identification of SMN1 and SMN2 copy numbers. A: MLPA results of SMN1 and SMN2 copy numbers. (0.8): detection value. We deduce the husband carried 1 copy SMN2 Exon7, and there is an allowable error between actual copy number and detection copy number. B: Inherited patterns of Chr 5 in this family. Inherited patterns are based on the SMN1 gene and ignore chromosome exchange. Nor: normal chromosome around breakpoint; der: derivative chromosome around breakpoint. C: Inherited patterns of Chr 5 in six embryos. We illustrated chromosome exchanges that influenced the diagnosis of SMN1, like E2, and omitted irrelevant exchanges for clarity (TIF 4.21 mb)