Abstract
Coccidiosis, a parasitic infection caused by one or more of the numerous species of Eimeria is recognised as the disease that has severe economic impact on poultry production. In an experiment to determine the effect of vaccination on the infection with a Local isolate and Houghton strain of Eimeria tenella (E. tenella). Ninety (90) day-old Dominant black cockerel chicks of D109 strain were randomly divided into nine groups (A–I). Two commercially available anti-coccidial vaccines—Livacox® (Biopharm, Czech Republic) and Immucox® (Vetech, Ontario, Canada) were used to immunize the chicks. Immunization was done at 5 days-old by oral gavage and infection was carried out with 1.7 × 104E. tenella of either Houghton strain or a field (Local) isolate at 4-weeks old. Six (6) days post-challenge, generalized pallor, moderate bloody diarrhea, slight reduction in feed intake and weight gain characterized the infected groups. There is a significant difference (p < 0.05) in packed cell volume of group B, unvaccinated and infected with Houghton strain of E. tenella and group E vaccinated with Livacox® and infected with Houghton strain. The Immunogenicity of Livacox® and Immucox® was determined primarily on the ability of the vaccinated chickens to overcome the effect of the virulent challenge by E. tenella including blood loss, reduction in feed intake and feed conversion and weight loss. The two anticoccidial vaccines used in this experiment were effective in varying degrees especially with the local isolate.
Keywords: Anticoccidial, Vaccination, Clinical, Growth, Parameters, Eimeria tenella
Introduction
Coccidiosis is a ubiquitous intestinal protozoan infection of poultry seriously impairing the growth and feed utilization of infected animals (Dalloul and Lillehoj 2005; Wongi et al. 2004). In chickens, it is caused by a protozoal parasite of the phylum apicomplexa belonging to the genus Eimeria. Intestinal infections, in particular coccidiosis, is becoming increasingly prevalent in commercially-bred chickens and are inflicting severe economic losses on the poultry industry (Yun et al. 2000). Avian coccidiosis is recognised as the parasitic disease that has severe economic impact on poultry production (Allen and Fetterer 2002), which has been estimated to exceeds $3 billion USD globally every year (Blake and Tomley 2014; Liu et al. 2018). Eimeria are present wherever poultry are found (Dalloul and Lillehoj 2005; Crouch et al. 2003). In recent years, intensive livestock and poultry rearing practices have led to an increase in animal stress and incidence of disease. This intensive farming method means that large numbers of birds are kept together in houses. This favours the parasite and the result is that large number of oocysts are present which leads to a disease flock (McDougald and Fuller 2005). Losses are attributed to malabsorption, inefficient feed utilization, impaired growth rate in broilers, and a temporary reduction of egg production in layers (Williams 1998). Chemotherapy has been used extensively to control coccidiosis, but drug resistance in field strains of parasites mandates development of alternative methods to control this disease (Yun et al. 2000). But because infection with one species of Eimeria induces protective immunity in the host that is long lasting and exquisitely specific to that particular parasite, this has been exploited in the development and composition of anticoccidial vaccines.
Two types of anticoccidial vaccines are currently available, non-attenuated and attenuated. Immucox® is an example of a non-attenuated vaccine which comprise wild-type strains of E. of Eimeria acervulina, E. tenella, E. maxima and E. necatrix with or without E.brunetti. The numbers of oocysts in these vaccines are calculated so that, if they are administered at the correct dose, pathogenic effects should not be observed. Livacox T®, Livacox D® are examples of an attenuated vaccine which comprise strains that have been selected so that they have reduced or no pathogenicity (Shirley 1993).
The objective of the study is to therefore immunize chickens with multivalent, live attenuated Livacox®, (Biopharm) and live non-attenuated Immucox® (Vetech) commercially available anticoccidial vaccines in Nigeria and then assess growth parameters, clinical signs, packed cell volume (PCV), red blood cell count and Haemoglobin concentration of these chickens, when challenged with a Local isolate of Eimeria tenella and standard strain (Houghton) Eimeria tenella, homologous to the vaccine strain.
Materials and methods
Chickens
Ninety (90) day-old Dominant Black cockerel chicks of D109 strain were purchased from a commercial hatchery in Jos Plateau State Nigeria. Thereafter, the chicks were separated into nine groups (A–I) of 10 chicks each and provided feed and water ad libitum.
Test vaccines
Two commercially available anti-coccidial vaccines—LIVACOX® (Biopharm, Czech Republic) and IMMUCOX® (Vetech, Ontario, Canada) were used to immunize the chickens according to the manufacturers’ recommendation.
LIVACOX®
LIVACOX® (Biopharm) developed by Dr Petre’ Bedrnik of the Research Institute of Biopharmacy and Veterinary Drugs, 254 49 Jilove’u Prahy, Czech Republic and marketed by Biopharm. Livacox® Q (consisting of E. acervulina, E. tenella, E. maxima and E. necatrix) are attenuated vaccines. All coccidial strains in this vaccine are drug-sensitive. Livacox® Q, 1000 doses (10 ml); 1 ml vaccine contains 30,000–50,000 oocysts of each E acervulina, E tenella and E maxima and 10,000 oocysts of E necatrix.
IMMUCOX®
IMMUCOX® (Vetech) Developed by Dr Eng-Hong Lee and Marketed by Vetech Laboratories Inc. Guelph, Ontario Canada. Marketed first in Canada, and then other countries. IMMUCOX® (consisting of E. acervulina, E. tenella, E. maxima and E. necatrix with or without E. brunetti) is each composed of several virulent species. IMMUCOX®, 200 doses.
Experimental design
The experiment comprises of two phases: 1st phase—vaccination of birds and 2nd phase—subsequent challenge with coccidia. The vaccination phase included two treatments: birds given the test vaccines in floor-pens and unvaccinated birds from the same hatch maintained coccidia-free in suspended wire mesh cages. The following are the nine groups and their respective treatment regimens presented in Table 1.
Table 1.
Experimental design and regimen
| Groups | Vaccination status | Vaccine | Challenge status | Challenge E. tenella |
|---|---|---|---|---|
| A | Unvaccinated | Nil | Unchallenged | Nil (absolute control) |
| B | Unvaccinated | Nil | Challenged | Houghton |
| C | Unvaccinated | Nil | Challenged | Local isolate |
| D | Vaccinated | LIVACOX® | Challenged | Houghton |
| E | Vaccinated | LIVACOX® | Challenged | Local isolate |
| F | Vaccinated | IMMUCOX® | Challenged | Local isolate |
| G | Vaccinated | IMMUCOX® | Challenged | Houghton |
| H | Vaccinated | LIVACOX® | Unchallenged | Nil (vaccine control) |
| I | Vaccinated | IMMUCOX® | Unchallenged | Nil (vaccine control) |
Vaccination
Immunisation against infectious bursal disease
Livacox® Q and Immucox® vaccines were used to vaccinate both the E. tenella-infected groups and the uninfected group (A: control). These vaccines were administered according to manufacturers’ instructions. Livacox® and Immucox® were administered on day 5 by oral gavage: directly delivering the reconstituted vaccines into the crop of the chicks, so as to ensure each chick is adequately vaccinated.
Newcastle disease
Other vaccines administered to the chickens include:
NVRI, Vom Nigeria Infectious Bursal Disease (Gumboro) vaccine in 200 doses was reconstituted in 2 l of drinking water and administered to the birds in calculated volume for 90 birds at day 11.
NVRI, Vom Nigeria Newcastle disease (LaSota strain) vaccine in ampoules of 200 hundred doses was reconstituted in 2 l of chlorine-free drinking water and administered to the birds in calculated volume for 90 birds at day 21.
Challenge parasites (Eimeria tenella)
Local isolate of Eimeria tenella
A field (Local) isolate of Eimeria tenella (Akanbi and Taiwo 2009; Ogedengbe et al. 2009) recovered from a case of caecal coccidiosis that occurred in a poultry flock whose carcasses were submitted for post mortem examination was used for the challenge of chickens in the groups C, E and F. These parasites were used for the challenge infection after they were passaged once in 3-week-old coccidia-free chicks in strict isolation. Caecal coccidiosis was reproduced and the oocysts were collected from litter and faeces as described by Long and Rowell (1975) and Hodgson (1970), respectively. Briefly, oocysts for experimental inocula (local E. tenella) was obtained from the cecal fecal material of an infected chicken in isolated laboratory condition and purified accordingly before been used for inoculation as previously described (McDougald and Fuller 2005).
Local isolate of Eimeria tenella molecular identification
Genomic DNA was extracted from this local Eimeria tenella using a random amplified polymorphic DNA-sequence characterized amplified regions (RAPD-SCAR) marker based multiplex-polymerase chain reaction (PCR) as previously described by our team (Ogedengbe et al. 2009). The isolate used in this experiment was therefore confirmed to be E. tenella.
Houghton strain of Eimeria tenella
The Houghton strain of Eimeria tenella used was generously donated by the former Director of the Institute for Animal Health (IAH), Compton UK, Professor M.E Shirley and Dr Blake, formerly of the Molecular Parasitology laboratory, IAH. The Houghton (H) strain of E. tenella was derived from an isolate from a field case of caecal coccidiosis in 1949 (Chapman and Shirley 2003).
Challenge procedures
Sporulated local isolate of Eimeria tenella oocysts and Houghton strain were used to challenged the birds at 4 weeks of age. Inoculation (infection with the oocysts) in each case was given orally by gavage and dosage given was 1.7 × 104 sporulated oocysts per bird at 4 weeks post-anti-coccidial vaccination as described earlier (Chapman et al. 2005).
Clinical signs
Following challenge, the chickens in the vaccinated and challenged groups were monitored daily. Also, the absolute control group was monitored in a separate enclosure to prevent accidental infection.
Oocyst output
Collection of oocysts from litter and fecal material
The litter oocysts sampling was carried out by picking up small samples of surface litter from random locations within the floored, wood shavings-board pens for vaccinated birds and under the suspended wire meshed cages for control birds, these were placed in labeled cellophane bags that were tightly secured and immediately taken to the diagnostic Parasitology laboratory. One method of oocysts counting was routinely used; the McMaster method was used for counting oocysts from litter samples and faeces.
Feed intake, feed conversion and weight gain
Total feed intake
The chicks were fed ad libitum and daily feed intake per group was measured using a Salter® measuring scale and recorded. This was summed up for each group at the end of the experiment and the Total feed intake for each group was calculated and recorded.
Feed conversion ratio
At the end of the experiment, the feed conversion ratio for each experimental group was calculated as total amount of feed consumed in kilogram divided by total weight gain of birds (in kg) and the values were recorded.
Total weight gain
Mean daily weight gains were taken and recorded per experimental group using a Salter® measuring scale.
Haematology
Blood samples were taken from the birds in all the nine groups just before the experimental challenge and 6 days post-challenge for packed cell volume (PCV), Red cell count and Haemoglobin concentration determination. 2 ml of blood was taken in EDTA bottles at each time from the chickens.
Packed cell volume measurement
For PCV determination, the blood samples in each group was mixed gently by repeated inversion and allowed to flow into a capillary tube by capillary action to about ¾ full, and then sealed with plastacin. The capillary tube was then centrifuged at 1000 rmp for 5 min using the haematocrit centrifuge. The PCV was read using the Hawksley microhaematocrit reader (Haemocytometer), 1869 England, the results were then tabulated.
Haemoglobin concentration measurement
For haemoglobin concentration (Hb Conc.), the spectrophotometer was put on and three test tubes were labeled: Blank, Standard, and Test. 5 ml Drabkin’s solution was pipetted into the blank and test tubes, then the blood samples in each group was mixed thoroughly and 0.02 ml of distilled water and blood was pipetted into the labeled tubes. The Blank and Test, test tubes were shaken by inversion several times and incubated at room temperature for complete conversion. Absorbance was measured and recorded against the reagent blank at 540 nm wave length. The standard solution of cyanomaethmoglobin was measured, tested and calculated using the Haemoglobin concentration formulae.
Red blood cell count
Red Blood Cell count was determined by thoroughly mixing 0.02 ml of blood from each group, which was pipetted into 3.98 ml of normal saline to make 1:20 dilution and then mixed by repeated inversion. Cover slip was fixed on the Neubauer improved counting chamber, ensuring that the Newton ring is seen. The chamber was filled by means of Pasteur pipette and allowed to settle for 2 min. The number of cells in the 80 small (5) squares at the central ruled areas were observed under low power (× 40) objective lens of the microscope.
Statistics
The measurements of feed intake, and weight gain were analyzed in Microsoft Excel spreadsheet® 2011 (Microsoft Inc., Seattle-WA, USA) to generate the histograms and percentages at 95% confidence intervals and p values calculated using MedCalc (SAS Institute Inc., Cary-NC, USA).
Ethical approval
Adequate measures were taken to minimize pain or discomfort and the procedures were carried out in accordance with the Guidelines laid down by the International Animal Ethics Committee, Faculty of Veterinary Medicine, University of Ilorin ethical committee laws and regulations and Animal Use and Care Committee guidelines for Care and Use of Animals at the National Veterinary Research Institute, Nigeria.
Results
Challenge outcome
Also, the challenge with 1.7 × 104E. tenella of either H-strain or L-isolate at 4-weeks old was successful as evident by the establishment of cecal coccidiosis resulting in the varying degrees of clinical signs of generalized pallor, slight reduction in weight gain and moderate bloody diarrhea in the infected groups D–G.
Clinical signs
Six (6) days post-challenge, no signs of coccidiosis were seen in groups A, H and I birds. Group B chicken, unimmunized and challenged with Houghton strain of E. tenella, showed moderate pallor, slight reduction in weight gain and moderate bloody diarrhea. Group C, unimmunized and challenged with Local isolate of E. tenella showed severe pallor, slight reduction in weight gain and marked bloody diarrhea. Group D, immunized with Livacox® and challenged with Houghton strain of E. tenella had slight pallor, slight reduction in weight gain and slight bloody diarrhoea. Group E, immunized with Livacox® and challenged with Local isolate of E. tenella showed moderate pallor, slight reduction in weight gain and moderate bloody diarrhea. Group F, immunized with Immucox and challenged with Local isolate of E. tenella showed moderate pallor, slight reduction in weight gain with severe bloody diarrhoea. Group G, immunized with Immucox and challenged with Houghton strain of E. tenella showed slight pallor, slight reduction in weight gain and slight bloody diarrhoea.
Oocyst output
Oocyst output was observed in only the infected control groups B and C. Other groups had no oocysts excretion (Fig. 1).
Fig. 1.
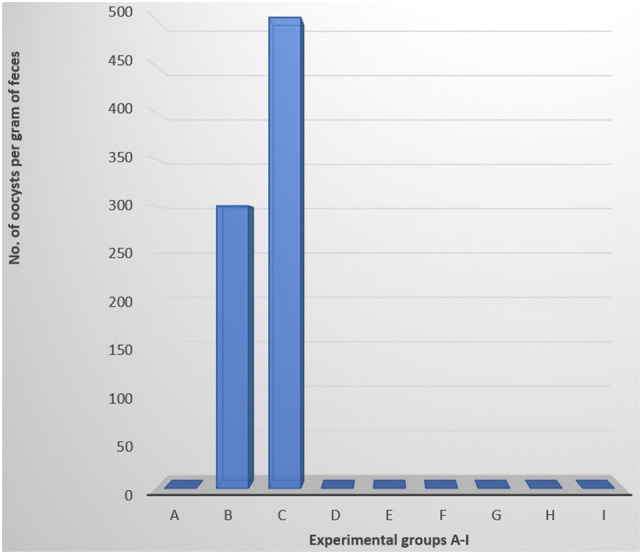
Oocysts count per gram of faeces of groups A–I, 6 days post-challenge
Feed intake, feed conversion and weight gain
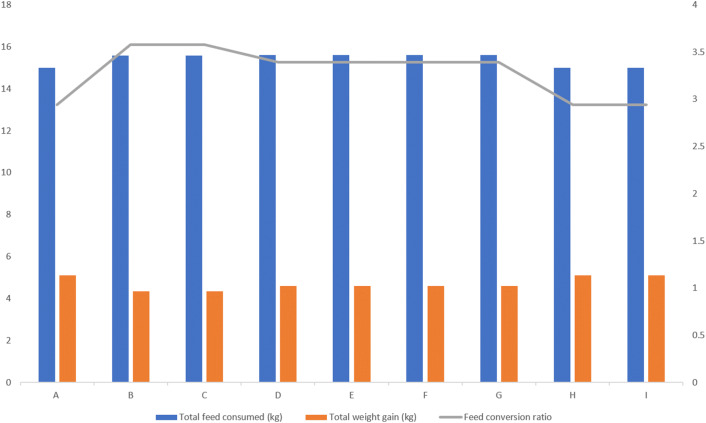
The feed intake, feed conversion and weight gain was taken and represented graphically on a three axes graph (Fig. 2). Total feed consumed was highest in groups D, E, F, and G with 15.603 kg per group (see Fig. 2), lower by 0.014 kg (14 g) in groups B and C with 15.589 kg per group and lowest by 0.603 kg (603 g) in groups A, H and I with 15.000 kg. Total weight gain was highest in groups A, H and I with 5.1 kg (average of 0.51 kg per bird), followed by groups D, E, F, and G with 4.6 kg (average of 0.46 kg per bird) and lowest in groups B and C with 4.35 kg (average 0.435 kg per bird) while the total feed conversion ratio was highest in groups A, H and I with 2.94, followed by groups D, E, F, and G with 3.39 and lowest in groups B and C with 3.58 (Fig. 2).
Fig. 2.
Comparison of growth parameters indices for all the experimental groups. Groups: A. Unimmunized, unchallenged group, B. Unimmunized and challenged group (Houghton strain), C. Unimmunized and challenged group (Local isolate), D. Immunized (Livacox®) and challenged group (Houghton strain), E. Immunized (Livacox®) and challenged group (Local isolate), F. Immunized (Immucox®) and challenged group (Local isolate), G. Immunized (Immucox®) and challenged group (Houghton strain), H. Immunized (Livacox®) unchallenged group, I. Immunized (Immucox®) unchallenged group
Hematology
Blood loss evaluation showed that, Groups A, H and I, had an increase in packed cell volume (PCV) and RBC count was highest in groups H and I, 6 days post-challenge evaluation, though they were not challenged in the experiment. Group A chickens showed a reduction in PCV, during the pre-challenge evaluation and had a 1.2% increase in packed cell volume (PCV) during the post-challenge evaluation. Groups B and C had the highest reduction in PCV and RBC count, 6th days post-challenge. Groups D, E, F, and G had reduction in PCV and RBC count 6th day post-challenge. Group C chickens had the highest reduction in PCV and RBC count (Table 2), followed by group B. In the Livacox® immunized groups D and E, group D (Houghton strain challenged) had the least reduction in PCV and group E (Local isolate challenged) was next with a reduction of 3.4% in PCV. In Immucox® immunized groups F and G, group F challenged with local isolate had a reduction of 4.4% in PCV while group G challenged with Houghton strain had a reduction of 2.6% in PCV. Excerpt for groups A and E, there was an increase in the mean corpuscular volume (MCV), 6 days-post challenge while the MCHC value increased in groups B, C, D, E, F and G. The MCHC remained normal to near normal in groups A, H and I.
Table 2.
Haematological indices during E.tenella infection in vaccinated and unvaccinated chickens
| Values | Normal | Grp A | Grp B | Grp C | Grp D | Grp E | Grp F | Grp G | Grp H | Grp I |
|---|---|---|---|---|---|---|---|---|---|---|
| PVC% Pre-I | 27–42% | 24.8 | 30.4 | 28.2 | 28.2 | 31 | 29.8 | 30 | 29.6 | 29.9 |
| Po-I | 26 | 23 | 20.4 | 25.8 | 27.6 | 25.4 | 27.4 | 30.2 | 30.3 | |
| RBC × 1012PRE | 2.18–4.12 | 2.1 | 2.9 | 2.8 | 2.7 | 2.76 | 2.84 | 2.94 | 2.73 | 2.87 |
| POST | 2.24 | 2.06 | 1.9 | 2.4 | 2.6 | 2.32 | 2.26 | 2.73 | 2.88 | |
| Hb (g/dI) | 6.83 | 9.7 | 10.04 | 10.06 | 10.08 | 9.54 | 10.32 | 9.98 | 9.81 | 10.15 |
| MCV (fI) Pre | 118.09 | 104.82 | 100.71 | 104.44 | 112.31 | 104.92 | 102.04 | 108.42 | 104.18 | |
| Post | 116.071 | 111.65 | 107.36 | 107.5 | 106.15 | 109.48 | 121.23 | 110.62 | 105.20 | |
| MCHC (gdI) Pre | 39.11 | 33.02 | 35.67 | 35.74 | 30.77 | 34.63 | 33.26 | 33.14 | 33.95 | |
| Post | 37.30 | 43.65 | 49.31 | 39.06 | 34.56 | 40.63 | 36.42 | 32.48 | 33.49 |
Statistics
No significant differences (p > 0.05) was seen in weight gain and feed conversion ratios (FCR) in the vaccinated groups, immucox® or livacox® whether infected with Houghton or local isolate of Eimeria tenella, 6 days post challenge when compared with the unimmunised unchallenged control (group A) and the unimmunised challenge controls (groups B and C). There is a significant difference (p < 0.05) in packed cell volume (PCV) of group B, unvaccinated infected with Houghton strain of Eimeria tenella and group E vaccinated with Livacox and infected with Houghton strain. p < 0.05 in PCV of group C, unvaccinated infected with local isolate of E. tenella and group D, vaccinated with Livacox® and infected with local isolate of E. tenella. p < 0.05 in PCV of group B, unvaccinated, infected with Houghton strain and group G, vaccinated with immucox® and infected with Houghton strain of E. tenella. Also p < 0.05 in PCV of group C, unvaccinated infected with local isolate of E. tenella and group F, vaccinated with immucox® and infected with local isolate of E. tenella. The pre and post-challenge packed cell volumes were significant (p < 0.05) for all the infected groups (B–G), challenged with local isolate or Houghton strain of E. tenella whether vaccinated with immucox® or livacox® or without vaccination at all.
Discussion
The immunization of these experimental chickens at 5 days-old by oral gavage of a non-attenuated (immucox®) and an attenuated (livacox®) was successful as evident by no clinical signs seen six (6) days post-challenge in the absolute control group A (unvaccinated, unchallenged), and in the vaccinated control groups H (Livacox®) and I (Immucox®) chickens. Also, the challenge with 1.7 × 104E. tenella of either H-strain or L-isolate at 4-weeks old was successful as evident by the establishment of cecal coccidiosis resulting in the varying degrees of clinical signs of generalized pallor, slight reduction in weight gain and moderate bloody diarrhea in the infected groups D–G. Eimeria tenella infection is known to cause acute intestinal disorders (Bussière et al. 2018), including cecal bleeding and epithelia damage responsible for important economic losses in poultry farming worldwide. The intestinal parasitism by this experimental infection with E. tenella, was a major stress factor in the chickens in the infected groups D–G and led to inadequate nutrition, depression of feed conversion and its attendant reduction in weight in the challenged and unvaccinated groups, this finding is in line with previous reports (Dalloul and Lillehoj 2005; Wongi et al. 2004; McDougald and Fuller 2005). Also, this has severe economic implications on the production of poultry as previously observed (Allen and Fetterer 2002). In the group C chickens, (unimmunized and challenged with Local isolate of E. tenella), these clinical signs were more severe than in the group B chickens (unimmunized and challenged with Houghton strain of E. tenella). Invariably this shows that the field isolate despite its passage in coccidia-free chicken before being used for this challenge experiment, still retain high virulence when compared with the H-strain, hence the severity of clinical signs. In the immunized and challenged groups D–G, the clinical signs of coccidiosis were less severe. Though there was a slight increase in feed consumption, and a reduction in weight gained with its consequence reduction in feed efficiency in vaccinated and infected groups D, E, F, and G, the weight gain and FCRs in these groups vaccinated with Immucox® and Livacox® whether infected with Houghton or local isolate of Eimeria tenella 6 days-post-challenge, shows that the differences seen in total weight gain and FCRs of the groups were not significant (p value > 0.05) at 6 days post-challenge (42 days old). There is a significant difference (p < 0.05) in packed cell volume (PCV) of group B, unvaccinated infected with Houghton strain of E. tenella and group E vaccinated with Livacox® and infected with Houghton strain. p < 0.05 in PCV of group C, unvaccinated infected with local isolate of E. tenella and group D, vaccinated with Livacox® and infected with local isolate of E. tenella. p < 0.05 in PCV of group B, unvaccinated, infected with Houghton strain and group G, vaccinated with immucox® and infected with Houghton strain of E. tenella. The lack of oocysts in the faeces of all the experimental groups up till the time of vaccination and throughout the studies for group A (unimmunized, unchallenged) birds, and the absence of signs of clinical coccidiosis in groups A, H and I demonstrates the success of the procedures adopted to prevent contamination by extraneous coccidia in accordance with earlier findings (Crouch et al. 2003). It has been established that experimental inoculation with 1 × 105 sporulated oocysts of E. tenella can cause morbidity, mortality, and greatly reduced weight gain, making this one of the most pathogenic species in chickens (McDougald, 2003). Also, the inoculation of chickens with 1 × 103–3 × 103 oocysts is sufficient to cause bloody droppings and other signs of infection but severity of infection may vary with the isolate, the number of oocysts ingested and immune status of the birds (McDougald, 2003). For the assessment of weight gain after challenge in immunized chickens the size of the inoculum to be given must be established before the actual experiment (Chapman et al. 2005), and this was carried out in the coccidia-free chicken. A dose is required that will depress weight gain and cause moderate to severe lesions without killing the birds (Chapman et al. 2005) as is the case with this experiment, where a challenge dose of 1.7 × 104 sporulated E. tenella oocysts of the Houghton strain and local isolate were used to challenge the chickens after a previous passage in 3 weeks old coccidia-free chickens was done to determine the pathogenicity of the isolates. While the differences in PCV, seen in the Livacox® vaccinated, Houghton strain (group E) and local isolate (group D) challenged, may be due to geographical variations in the antigenicity of coccidia strains (Fitz-coy 1992). The increase in the calculated values of the MCV (groups B, C, D, E, F and G) and MCHC (groups B, C, D, E, F and G), 6 days post-challenge showed that there was a regenerative anaemia (Macrocytic hypochromic), while MCV (A, H and I) MCHC (A, H and I) remained normal to near normal. The results reported herein confirm the conclusion of Lee (1987) with observation of signs of clinical coccidiosis with live non-attenuated vaccine (Immucox®); and with Livacox®, by Bedrnik et al. (1989) that vaccination against coccidiosis can be a viable alternative to the used of anticoccidial drugs. These results confirmed the hypothesis that vaccinated birds kept on litter produce significant numbers of oocysts initially but produce hardly any further oocysts if they are given a second homologous vaccination (Williams and Catchpole 2000).
Conclusion
These results goes to show that the two anticoccidial vaccines used in this experiment reduced blood loss in the birds irrespective of the pathogenicity of the strain or isolate of E. tenella used, though lesser blood loss was seen in the Houghton strain. This result establishes the fact that the local isolate of E. tenella caused more reduction in PCV than the Houghton strain of E. tenella, hence it is more pathogenic than the Houghton strain. Regardless of the mild signs of clinical coccidiosis in the Immucox® and Livacox®, the vaccines could be said to be protective in the vaccinated and infected groups (D, E, F and G).
Acknowledgements
The authors sincerely thank Dr. Sanusi, Mr. Nanbol and Rev.d Ajibade of NVRI for their technical assistance. Also Prof. Shirley and Dr Blake for generously providing ≈ 6million E. tenella oocysts of the Houghton strain, and Prof. L.R. McDougald, R.B. Williams, H.D. Chapman and Drs Dalloul and Lillehoj E.P for providing reprints of their work on coccidiosis. We are grateful to Dr. Ola-Fadunsin for helping to read through the manuscript and for his suggestions.
Compliance with ethical standards
Conflict of interest
The authors declare that there is no conflict of interest.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Akanbi BO, Taiwo V. Comparative pathology of the caeca of Anticoccidial immunized chicks infected with a Nigerian isolate and Houghton strain of Eimeria tenella. J Comp Pathol. 2009;141:278. doi: 10.1016/j.jcpa.2009.08.003. [DOI] [Google Scholar]
- Allen PC, Fetterer RH. Recent advances in biology and immunobiology of Eimeria species and in diagnosis and control of infection with these coccidian parasites of poultry. Clin Microbiol Rev. 2002;15:58–65. doi: 10.1128/CMR.15.1.58-65.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bedrnik P, Kucera J, Firmanova A, Jurkovic P. Field vaccination of broilers against coccidiosis. Avian Pathol. 1989;18:255–264. doi: 10.1080/03079458908418600. [DOI] [PubMed] [Google Scholar]
- Blake DP, Tomley FM. Securing poultry production from the ever-present Eimeria challenge. Trends Parasitol. 2014;30:12–19. doi: 10.1016/j.pt.2013.10.003. [DOI] [PubMed] [Google Scholar]
- Bussière FI, Niepceron A, Sausset A, Esnault E, Silvestre A, Walker RA, Smith NC, Quéré P, Laurent F. Establishment of an in vitro chicken epithelial cell line model to investigate Eimeria tenella gamete development. Parasites Vectors. 2018;11:1–8. doi: 10.1186/s13071-018-2622-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapman HD, Shirley MW. The Houghton strain of Eimeria tenella: a review of the type strain selected for genome sequencing. Avian Pathol. 2003;32:115–127. doi: 10.1080/0307945021000071588. [DOI] [PubMed] [Google Scholar]
- Chapman HD, Roberts B, Shirley MW, Williams RB. Guidelines for evaluating the efficacy and safety of live anticoccidial vaccines, and obtaining approval for their use in chickens and turkeys. Avian Pathol. 2005;34:279–290. doi: 10.1080/03079450500178378. [DOI] [PubMed] [Google Scholar]
- Crouch CF, Andrew SJ, Ward RG, Francis MJ. Protective efficacy of a live attenuated anticoccidial vaccine administered to 1-day-old chickens. Avian Pathol. 2003;32:297–304. doi: 10.1080/10307945031000097912. [DOI] [PubMed] [Google Scholar]
- Dalloul R, Lillehoj EP. Recent advances in immunomodulation and vaccination strategies against coccidiosis. Avian Dis. 2005;49:1–8. doi: 10.1637/7306-11150R. [DOI] [PubMed] [Google Scholar]
- Fitz-coy SH. Antigenic variation among strains of Eimeria maxima and E. tenella of the chicken. Avian Dis. 1992;36:40–43. doi: 10.2307/1591712. [DOI] [PubMed] [Google Scholar]
- Hodgson JN. Coccidiosis: oocyst counting technique for coccidiostat evaluation. Exp Parasitol. 1970;28:99–102. doi: 10.1016/0014-4894(70)90073-1. [DOI] [PubMed] [Google Scholar]
- Lee EH. Vaccination against coccidoisis in commercial roaster chickens. Can Vet J. 1987;28:434–436. [PMC free article] [PubMed] [Google Scholar]
- Liu J, Liu L, Li L, Tian D, Li W, Xu L, Yan R, Li X, Song X. Protective immunity induced by Eimeria common antigen 14–3-3 against Eimeria tenella, Eimeria acervulina and Eimeria maxima. BMC Vet Res. 2018;14:1–11. doi: 10.1186/s12917-017-1323-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long PL, Rowell JG. Sampling broiler house litter for coccidial oocysts. Br Poult Sci. 1975;16:583–592. doi: 10.1080/00071667508416233. [DOI] [PubMed] [Google Scholar]
- McDougald LR. Protozoal infections. In: McDougald LR, editor. Diseases of poultry. 11. Oxford: Blackwell; 2003. p. 979. [Google Scholar]
- McDougald LR, Fuller L (2005) United States Patent: 6,908,620
- Ogedengbe ME, Barta JR, Ogedengbe JD, Akanbi BO, Ogo IN. Use of a sequence characterized amplified regions (SCARS)-multiplex PCR method to identify Eimeria species of chickens from widely distributed geographic areas. Trop Vet. 2009;27:36–44. [Google Scholar]
- Shirley MW (1993) Live vaccines for the control of Coccidiosis. In: Proceedings of the 6th international coccidiosis conference, held in Guelph, Ontario, Canada, 1993, pp 61–72
- Williams RB. Epidemiological aspects of the use of live anticoccidial vaccines for chickens. Int J Parasitol. 1998;28:1089–1098. doi: 10.1016/S0020-7519(98)00066-6. [DOI] [PubMed] [Google Scholar]
- Williams RB, Catchpole J. A new protocol for a challenge test to assess the efficacy of live anticoccidial vaccines for chickens. Vaccine. 2000;18:1178–1185. doi: 10.1016/S0264-410X(99)00387-4. [DOI] [PubMed] [Google Scholar]
- Wongi M, Rami DA, Lillehoj HS. Application of biotechnological tools for coccidia vaccine development. J Vet Sci. 2004;5:279–288. doi: 10.4142/jvs.2004.5.4.279. [DOI] [PubMed] [Google Scholar]
- Yun CH, Lillehoj HS, Lillehoj EP. Intestinal immune responses to coccidiosis. Dev Comp Immunol. 2000;24:303–324. doi: 10.1016/S0145-305X(99)00080-4. [DOI] [PubMed] [Google Scholar]