Abstract
Purpose
Monitoring the pH of IVF culture media is a good practice, but the required pH levels have been “arbitrarily” set. Assisted reproductive technology centers around the world are spending time and money on pH monitoring without any consensus to date. The objective of this narrative review was to evaluate the importance of pH monitoring during IVF, discover how the oocyte and embryo regulate their intracellular pH and try to determine the optimal pH to be applied.
Methods
A narrative literature review was performed on publications in the PubMed database reporting on the impact of pH on cellular function, oocyte and embryo development, IVF outcomes and pathophysiology, or on physiological pH in the female reproductive tract.
Results
Intracellular pH regulates many cellular processes such as meiotic spindle stability of the oocyte, cell division and differentiation, embryo enzymatic activities, and blastocoel formation. The internal pH of the human embryo is maintained by regulatory mechanisms (mainly Na+/H+ and HCO3−/Cl− exchangers) that can be exceeded, particularly in the oocyte and early-stage embryos. The opinion that the optimal pH for embryo culture is physiological pH is not correct since several physicochemical parameters specific to IVF culture conditions (temperature, medium composition, duration of culture, or implication of CO2) can modify the intracellular pH of the embryo and change its needs and adaptability.
Conclusions
Because correct and stable extracellular pH is essential to embryo health and development, monitoring pH is imperative. However, there is a lack of clinical data on choosing the ideal pH for human IVF culture media.
Keywords: pH, Preimplantation mammalian embryo, CO2, Bicarbonate, Hydrogen ion, IVF, Culture media
Introduction
Despite progress in assisted reproductive technology (ART), the delivery rate per oocyte aspiration during IVF remains low (14.1 to 37.8%) [1]. As well as being affected by problems of aneuploidy linked to the quality of gametes, embryo development also depends on good culture conditions, since the preimplantation embryo is highly sensitive to its environment. Furthermore, there is growing evidence that embryo culture conditions are critical per se not only for pre-implantation embryo development but also for the postimplantation period and long-term health [2–5].
Numerous data suggest the importance of pH control in the IVF culture medium. These data are derived from animal models or from the study of physiology and pH dynamics in the mammalian female genital tract during conception. The preimplantation embryo maintains its intracellular homeostasis but a variation of extracellular pH can be a cause of cellular stress. Maintaining internal pH (pHi) is one of the vital roles of homeostasis. Intracellular pH regulates many cellular processes such as enzymatic reactions, cell division and differentiation, calcium concentration, cytoskeleton establishment, and mitochondrial localization [6, 7]. Therefore, variations in pHi can affect embryonic development. Since most of these data come from animal studies, we need to know which data can be transposed to the human embryo. The human oocyte and embryo appear to have regulation systems, such as membrane ion exchangers, but are these systems effective whatever the preimplantation stage and can they be exceeded? Culture conditions during IVF can be decisive for the good progress of fertilization, then early embryo development and up to the blastocyst stage. It seemed to us essential to present data about the regulatory systems of pHi described at the different stages: oocyte (in particular metaphase II), early embryo, and blastocyst.
Under the conditions of IVF embryo culture, incubator CO2 dissolves in the medium to form carbonic acid. Primarily, the extracellular pH or outside pH (pHo) in a culture medium is the result of a balance between the CO2 concentration in the incubator and the bicarbonate concentration in the culture medium (determined by the medium provider). Therefore, these two settings greatly contribute to the regulation of pHo. But what are the other physicochemical conditions specific to the IVF process that can also affect pHo and how do they influence pHi? In particular, how does the composition of the culture medium used in IVF have an impact on intracellular pH (pHi) regulation? These are the questions we need to answer to understand the importance of extracellular pH management and to choose its optimal pH.
The objective of this work was to examine the importance of controlling the pH of IVF culture media and to determine if there is an optimal pH value to be applied. We aimed to determine the impact of pHi on human oocyte and embryo metabolism and the ways in which pHi is regulated with respect to pHo. Lastly, what are the available data on the physiological pH of the mammalian female genital tract, and the particular physicochemical conditions during IVF that allow us to choose the optimal pH?
Methods
We conducted a narrative review of the relevant literature. The PubMed database was used to retrieve works published between January 1970 and November 2019 using the following search terms: “pH and (preimplantation embryo or cleavage stage embryo or 2-cell embryo or blastocyst or embryo culture or oocyte or IVF)” in all mammalian species. The search was restricted to English-language articles. The titles, abstracts, and reference lists were reviewed and only relevant publications (i.e., those reporting on the impact of intracellular/extracellular pH on cellular function, oocyte and embryo development, IVF outcomes and pathophysiology, or on physiological pH in the female reproductive tract) were selected (Fig. 1). This review examined, compared and discussed study methodologies and results, including experimental data and patients’ characteristics. As this was a narrative review, no review protocol or registration number was required. No specific funding was received.
Fig. 1.
Flow chart for selection of relevant publications
Impact of pHi on mammalian cell function
General data
Maintaining intracellular pH (pHi) is one of the vital roles of cellular homeostasis. For example, it has been shown in various types of somatic cells that ionic membrane permeability into Na+, K+ or Cl− ions, as well as membrane conductance, are modified according to pHi values [7]. Ion channel activity may vary according to pHi values. In addition, the H+ ions bind to the membranes, attract the anions and thus modify the conductance. The mitochondrial redox chain requires a proton gradient across the mitochondrial membrane, so its function is partly related to pHi [7]. The same is true for lysosomal enzymes [7]. At the nuclear level, even small changes in pHi (0.1 pH units) can alter DNA synthesis [8].
Oocyte
The meiotic spindle stability of the mouse oocyte is significantly impacted by pH changes [9]. Using polarized light microscopy, Swearman et al. have shown a reversible increase in the density of the meiotic spindle (actin filament polymerization) after incubation of denuded mouse oocytes in a medium at pH 7.4–7.5 instead of pH 7.33 [9]. Disturbance of the meiotic spindle may be a cause of aneuploidy and maturation arrest. Other authors emphasize the importance of pHi in the occurrence of oocyte aneuploidy: Cheng et al. have shown that mouse oocyte pHi increases with advancing age, which may be a result of decreases in HCO3−/Cl− exchanger activity [10]. In aged oocytes, the increase in pHi may damage the cohesion complex structure, resulting in aneuploidy [10]. Some authors have demonstrated oscillations in pHi during bovine oocyte maturation and in connection with the activity of the M-phase promoting factor (MPF) [11]. In humans, some data suggest that a slight increase in pHo (7.5) may be beneficial for fertilization [12]. These authors found significantly lower fertilization and cleavage rate at pH 7.0 compared with pH 7.5.
Early-stage embryo
In the cytoskeleton, alkalinization, and acidification of pHi alter the actin network (microfilaments). These cytoskeletal disturbances also result in a disruption of mitochondrial distribution in the cytoplasm. In the study of Squirrell et al., changes in the pHi of embryonic hamster cells were induced either by a weak trimethylamine (TMA) base or a weak 5.5-dimethyl-2.4-oxazolinedione (DMO) acid [6]. The normal pHi of these 2-cell embryos is 7.4, and it was modified by these acidic or alkaline treatments to range from 6.85 to 7.68. Both treatments displaced mitochondrial organization from a perinuclear localization to a more homogeneous localization throughout the cytoplasm (with a faster effect after alkalinization by TMA). pHi changes by TMA and DMO also had an effect on the cytoskeleton (on microfilaments but not microtubules). With an alkaline pHi, microfilaments formed aggregates while with an acidic pHi, the microfilament network was established but weakened [6]. These cellular effects of pHi changes were possibly caused by indirect mechanisms via cellular pathways involving Ca2+ signaling. Development up to the hamster morula and blastocyst stage was reduced by both acidic and alkaline treatments in a dose-dependent response. This effect was partially reversible when exposure did not exceed 12 h and was irreversible if it exceeded 12 h. This suggests that the embryo has limited ability to recover from cellular stress induced by pHi variations.
At the metabolic level, the pHi value can have an impact on enzymatic activities: for example, a pHi above 7.2 stimulates phosphofructokinase (PFK) activity, which can be altered even by very moderate pH variations of 0.2. The increase of PFK activity can prematurely activate the glycolysis process and may be responsible for stopping development in culture [13]. The presence of weak acids in the extracellular media can decrease the pHi of the mouse early-stage embryo and cause a reduction in glycolytic activity [13]. This effect is not found at the mouse morula stage, which seems to have more efficient regulation systems.
Blastocyst
In addition to previous data on the importance of pHi in cell metabolism, pH regulation systems (in particular ion exchanger function) are important for mammalian blastocyst development. For example, Na+/H+ exchanger activity contributes to the trans-trophectodermal Na+ flux that is required for blastocoel expansion [14, 15]. Blastocoel formation is driven by an osmotic gradient with Na+ accumulation in the cavity. We will see below how the blastocyst regulates its pHi.
How do the mammalian oocyte and the embryo regulate their pHi?
Data on the pHi values of the human oocyte and embryo are rather sparse: Phillips et al. found a pHi of 6.98 ± 0.02 for the metaphase II oocyte (n = 9) and 7.04 ± 0.07 for the germinal vesicle (n = 4) [16]. Concerning the early-stage human embryo, the same authors found a pHi of 7.12 ± 0.01 in cleavage stage human embryos in the largest published series (n = 199) [16]. In a smaller series (n = 13 to 32), Dale et al. reported pHi values ranging from 7.3 ± 0.3 to 7.4 ± 0.1 from the germinal vesicle stage to the zygote stage [12]. These differences between the findings of the two teams cannot be explained except as a result of different media compositions that may affect pHi. Moreover, we must consider the oocyte source (the woman’s characteristics such as age or ovarian function, immature or failed fertilized oocyte) when discussing pHi regulation. Unfortunately, these data are not detailed in most studies. Another limitation of these data is that pHi was measured in embryos developing outside their natural environment, and it is possible that the incubation conditions change the pHi, especially the ambient bicarbonate-CO2 complex. It is currently impossible to measure the pHi of the embryo in vivo. It has been shown that the pHi of mammalian embryos (mouse and hamster) is not equivalent to pHo. pHi can be maintained for pHo values of 7.0 to 7.4 but beyond that, a change in pHi occurs and impairs the developmental capacities of the embryo [17, 18]. Intracellular cytoplasmic buffer systems exist that are short-term regulatory systems, active in seconds and immediately correcting the pHi. This immediate buffering capacity is measured in mM protons. In hamsters, this fast regulatory buffer system is significantly greater in the oocyte at 51.4 ± 2.4 mM/pH compared with 1-cell embryo post-activation at 17.9 ± 0.5 mM/pH (p < 0.05) [19]. This is probably related to the fact that ion exchangers are not very active in the hamster oocyte.
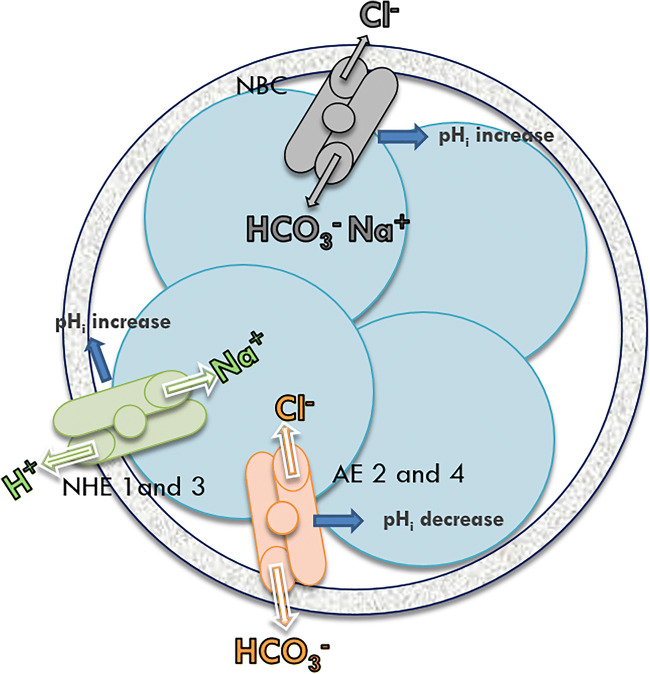
In addition to the short-term regulation systems, which are rapidly exceeded, there are more efficient regulation systems by ion channels. The classic regulatory mechanisms are the Na+/H+ exchanger and the Na+ dependent HCO3−/Cl− exchanger against acidosis and the HCO3−/Cl− exchanger against alkalosis. The Na+/H+ antiporter is active below pH 6.8 in humans and the HCO3−/Cl− exchanger is active at approximately pH 7.2 to 7.3 (again in humans) requiring intracellular HCO3− [16] (Fig. 2). There are several isoforms of Na+/H+ antiporters and NHE (Na+/H+ exchanger) 1 and 3 are the most common isoforms in oocytes and embryos [14]. In mice, the Na+/H+ antiporter appears to be activated at below pHi 7.1 [20]. Also in mice, it seems that NHE1 alone plays an active role in recovery from acidosis [21]. The HCO3−/Cl− exchanger exists as four anion exchanger (AE) isoforms, AE1–AE4, belonging to solute carrier family 4 (SLC4) [22]). Mice embryos express AE2 and AE4 at each stage but not AE3 [23]. AE2 must be produced from both the maternal and embryonic genomes and is the most likely to be responsible for HCO3−/Cl− activity at the one-cell stage [18].
Fig. 2.
Intracellular pH (pHi) regulation by ion channels in mammalian cleavage stage embryos. The ion channels are the Na+/H+ antiporter (active at a pH below 6.8 in humans) and the Na+ dependent HCO3−/Cl− exchanger against acidosis, and HCO3−/Cl− exchanger against alkalosis (activated at a pH higher than 7.2/7.3 in humans). NHE: Na+/H+ exchanger, NHE 1 and 3 isoforms (described by Barr et al. 1997 (mRNA) in mice [14]), Lane et al. 1998 in hamsters [20], and Phillips et al. 2000 in humans [16]). AE HCO3−/Cl−: anion exchanger (solute carrier family 4, SLC4) AE2 and AE4 isoforms (described by Dagilgan et al. 2015 in mice [23] and Romero et al. 2013 in humans [22]). NBC: Na+-driven HCO3−/Cl− exchanger: in mouse cleavage-stage embryos, NBC is less active than NHE against acidosis (Erdogan et al. 2011 [30]), Siyanov and Baltz, 2013 [21]). The outer grey circle is the zona pellucida. The circles inside the zona pellucida are blastomeres of an early-stage embryo
Thus, in mammalian embryos, pHi is maintained between 7 and 7.3 and this requires the presence of HCO3−, Na+, Cl−, and H+. The function of the HCO3−/Cl− exchanger and the Na+/H+ antiporter has been demonstrated in mice, cows, hamsters, and humans [11].
The regulatory mechanisms do not activate above or below a specific pHi threshold, so there may be a lag time during which pHi follows pHo [24]. Moreover, if pHo variations are too abrupt or too marked, the regulation mechanisms may be exceeded. Moreover, in humans, Phillips et al. have shown that oocytes and early embryos are unable to regulate mild acidosis (pH 7.0) at any stage of development up to the human blastocyst, but can regulate against alkalosis (pH 8.0) [16]. We detail below the activity of these regulation systems according to the mammalian oocyte and embryo stages.
Regulation systems: specific data on mammalian oocytes
Activation of the pH regulation mechanisms (Na+/H+ and HCO3−/Cl− exchangers) was shown in mice at the end of oocyte growth (when the oocyte reaches 80% of its maximum size), leading to a pHi rise of 0.25 units and coinciding with the acquisition of meiotic skills [25]. Until the mouse oocyte has acquired pHi regulation systems, it has been shown that in preantral follicles, granulosa cells regulate oocyte pHi against acidosis via gap junctions and mechanisms including the Na+/H+ exchanger (NHE) isoforms NHE1 and NHE3 [26]. These regulatory mechanisms are no longer active in the mature oocyte blocked in metaphase II and they become active again after fertilization in hamsters and mice [19, 27]. Some data show that mammalian mature oocytes and zygotes do not have an effective pHi regulation system by exchangers until 6–10 h after fertilization [11]. These exchangers appear to be active in the mature bovine oocyte [17]. Activation of these exchangers is not related to protein synthesis or cytoskeletal movements but rather is mediated by calcium oscillations and via the protein kinase C pathway [19]. In hamsters, their activity is greatest 8 to 10 h after oocyte activation [19].
In the mouse oocyte, HCO3−/Cl− exchanger activity is high in the germinal vesicle stage and is inhibited in the oocyte during the meiotic maturation process [25, 28]. Inactivation of the HCO3−/Cl− exchanger between the metaphase I (MI) and metaphase II (MII) stages and its activation after fertilization are related to the MEK/MAPK pathway (negative regulation) [27, 29]. Na+/H+ exchanger activity during oocyte maturation varies in a very similar way to that of the HCO3−/Cl exchanger: present in the GV oocyte, inactivation during oocyte maturation, inactive in the MII stage and active in the 1-cell embryo [19, 25, 30]. Conversely, Na+ dependent HCO3−/Cl− exchanger activity is higher in the MII and zygote stages than in the germinal vesicle [30]. Some authors suggest that the inactivation of Na+/H+ during meiotic maturation allows glycine accumulation that maintains cell volume [31].
Data on human pHi regulation systems are very sparse. Some authors have found a pHi of 7.4 in the mature oocyte and during fertilization in women [12]. These authors have shown that fertilization stages (mature oocyte, young zygote) are sensitive to pH variations [12], suggesting ineffective or absent pHi regulation systems. These data, therefore, suggest that pHi regulation systems are not active in all stages of human oocyte and embryo development and are sometimes limited.
Regulation systems: specific data on mammalian early-stage embryos
The early-stage human embryo has active regulation systems: the HCO3−/Cl− exchanger counteracts alkalosis, while acidosis is compensated by the Na+/H+ antiporter [16]. During the human cleavage stage, pHi appears to be stable [16], but the embryo is less equipped to regulate its pHi than the blastocyst [32–34]. The Na+/H+ antiporter against acid overload is not fully functional and HCO3−/Cl− exchanger expression also appears to be reduced [33]. The latter findings have been demonstrated in animal studies; Na+/H+ antiporter activity in the early stage embryo varies according to the species and may vary within the same species depending on the strain, as has been demonstrated in mice [35].
Na+-dependent HCO3−/Cl− exchangers are less active in one-cell stage mouse embryos than in GV and MII oocytes [30]. pHi in mouse embryos is regulated against acidosis mainly by the Na+/H+ exchanger NHE1 isoform [21].
In addition, the HCO3−/Cl− exchanger, which is active in mice and hamsters at pH > 7.2, appears to be inactive in bovine embryos (or above pH 7.9) [17]. Bovine embryos appear unlikely to correct a pH rise above 7.2/7.3 [17]. The same authors have shown in bovine embryos that inhibition of these regulation systems, even during very moderate pHi changes (+ 0.13 or − 0.21), significantly impairs development to the morula and blastocyst stage, as well as blastocyst expansion [17].
Moreover, in mouse embryos, Zander-Fox et al. achieved artificial reduction of the pHi of the embryo through exposure to a weak acid (DMO) during the preimplantation period, either through “chronic” exposure (from zygote to blastocyst) or through “acute” exposure (2-cell embryo stage or from 2 to 8-cell stage) [34]. They observed consequences on the development of the mouse blastocyst (decrease in the number of cells, increase in apoptosis, decrease in the expression of genes related to cellular stress and involved in apoptosis), irrespective of the periods of exposure. Moreover, exposure to DMO during early embryo cell divisions decreased fetal weight and cranial perimeter (p < 0.05) [34].
Regulation systems: specific data on the mammalian blastocyst stage
Mammalian embryos at the morula and blastocyst stage appear to be more efficient in regulating their pHi, particularly because of the presence of tight junctions [32, 36]. This is physiologically explained by adaptation of the embryo to the uterine environment which is more acidic than that of the tubes. The impact of embryo cell compaction on the embryo’s ability to regulate its pHi has been demonstrated by Edwards et al. [32]. These authors showed greater embryo sensitivity to lower pHi by inducing decompaction of the mouse morula by cytochalasin B and a decrease in this sensitivity by prematurely inducing compaction with wheat germ agglutinin (WGA) [32]. The same team has shown that pHi increases slightly in mouse blastocysts [32], while others found stable pHi throughout murine embryonic development [37].
However, it was noteworthy that HCO3−/Cl− exchanger activity was lowest at the blastocyst stage. According to Dagilgan et al., the change is an adaptation to their normal in vivo environment, where the morula and blastocyst are in the acidic uterus (compared with the alkaline oviduct for the early embryo) [23]. However, the same authors showed that despite low HCO3−/Cl− activity at the mouse morula and blastocyst stage, embryos at these stages succeed in complete recovery from alkalosis, probably by other types of mechanism (against ammonium accumulation) [23].
In view of these data on pH regulation systems in mammalian oocytes and embryos, it appears that oocytes and embryos at the early stages of development have limited pHi regulation mechanisms and thus are highly sensitive to variations in pHo. The blastocyst seems more competent at regulating its pHi with more cellular membranes that are less permeable to H+ ions [12, 34]. However, there is evidence to suggest that the blastocyst remains sensitive to alkaline environments, which highlights the importance of pH control in IVF culture media during the handling and culture of the in vitro blastocyst.
What is the optimal external pH?
Data on the mammalian female genital tract
The above data highlight not only the importance of pHi in oocyte and embryo metabolism and development but also the limits of the pHi regulation systems and therefore the importance of regulating extracellular pH. The question that arises is that of the ideal pH value. To try to answer this question, it is essential to be familiar with the physiology of the female genital tract.
In the female genital tract, the principal pH regulation mechanisms are the HCO3−/Cl− exchanger, which decreases alkalosis, and the Na+/H+ and Na+, HCO3−/Cl− channels, which decrease acidosis [38]. Data on the pH of mammalian tubal and uterine secretions are very sparse and almost non-existent in humans (Table 1). Moreover, these data should be interpreted with caution because of the technical difficulty of pH measurement, and because some authors did not specify the cycle period.
Table 1.
Physiological pH values in tubal and uterine secretions of different species
Tubes
Human data are very sparse, but data from other mammals (cows, swine, rabbits) suggest that the pH is more alkaline in tubal than in uterine secretions [38–40]. In rabbits, the pH was found to be around 7.94 [41]. This alkalinity is associated with high carbonic anhydrase activity. Moreover, the pH in tubal secretions seems to have some variations during the cycle in some species. Nichol et al. showed pH fluctuations in pigs, with peaks of high amplitude at low frequency and peaks of low amplitude at high frequency (in the peri-ovulatory period) [40]. In women, follicular fluid pH ranged between 7.22 and 7.7 [42, 43] and the pH of tubal secretions between 7.3 and 7.7 [42] (Table 1).
Uterus
Some authors have reported a slightly higher pH in the bovine uterus just after ovulation (7.35) than before ovulation (7.22) [44], while others have reported a pH ranging from 7.47 to 7.9 in rabbits [38, 45] (Table 1).
Particular physicochemical conditions during the IVF process
The opinion that the optimal pHo for embryo culture is the physiological pH is probably not correct. Several physicochemical parameters specific to culture conditions during IVF can modify the intracellular pH of the embryo, and can probably change its needs and adaptability. The pH in the fallopian tubes or the uterus is therefore probably not the optimal pH in a culture medium. The recommendations of the manufacturers of culture media are to maintain a pHo slightly higher than the pHi to compensate for the acidification mechanisms related to cell metabolism (and therefore generally between 7.2 and 7.4). However, as the pH scale is logarithmic, a pH variation of 7.2 to 7.4 corresponds to a decrease in the concentration of H+ ions by a factor of 1.6.
Several embryo culture conditions have been identified as being able to affect pHo and pHi, such as the temperature of the incubator, the altitude of the laboratory (CO2 pressure), the composition of the medium and the duration of culture.
Temperature
This can affect pH in two ways: (1) by slightly modifying the conductance of hydrogen ions [7], and (2) by introducing a slight measurement error via a temperature effect on the electrode measuring pH (0.01 to 0.02 pH units/°C) (some devices have a calculation formula to correct this) [46].
Medium composition
High lactate concentrations (5 mM) significantly decrease the pHi of zygotes by approximately 0.1 units [13]. Lactate acts as a weak acid (as well as pyruvate) in the cytosol of the zygote but this effect is not found in the morula stage which seems insensitive to these variations, probably due to compaction. In addition, the proportions of certain non-essential amino acids such as taurine or glycine have an impact on pHi by behaving like zwitterions (molecules with a negative and positive electrical charge that can depending on pH, attract anions or protons). Generally, in IVF culture media, they can attach protons and thus buffer the medium and avoid a drop in pHi [32]. Furthermore, inter-batch pH variations can exist. Tarahomi et al. found that three out of the five media they studied displayed inter-batch pH variations from 0.20 to 0.23 (p < 0.05) [47]. This suggests the necessity of CO2 adjustment by systematic pH measurement each time the batch of the medium is changed.
Culture time
During 4 days of culture, the pH increases significantly between the first and the fourth day with variations of 0.04 to 0.08 (p < 0.05) depending on the media [47], but still remaining within the tolerated thresholds for clinical practice (7.2 to 7.4). According to these authors, this variation in pH may be due to (1) a change in amino acid protein composition during culture by degradation (during embryo metabolism), (2) ammonium ion release by protein degradation (embryo metabolism), or (3) related to the evaporation of the medium [47].
Differential culture (intentional change in pHo during culture)
The need to change the pHo during embryo culture is very little documented. One study has shown a benefit of increasing CO2 (thus lowering the pH) between fertilization and cleavage time in bovine embryos, but we must note that in this study the fertilization and cleavage media were different and pHo values were not precisely measured [48]. Hentemann et al. showed in a mouse embryo assay (MEA) model an increase in the rate of good morphology embryos when applying different pHo: 7.3 to the zygote stage then 7.15 for cleavage stage embryos [49]. The limitation of this study was that pH was modified by altering the concentration of bicarbonates in the medium. But bicarbonates themselves influence pH-independent cell metabolism as they are involved in many cellular transport mechanisms [50].
CO2
The pH variations induced by different CO2 levels may need to take into account the impact of CO2 itself (apart from its effect on pHo) on embryo metabolism and its action as a weak acid on the embryo’s ability to regulate its pHi. In hamsters, Carney and Bavister found a benefit of 10% CO2 content vs 5% on development at the blastocyst stage, but no effect of pHo or bicarbonate content [51].
Altitude of the laboratory
This can modify the effective CO2 content in an inversely proportional way. Thus, the higher the altitude, the higher the CO2 content in the incubator required to obtain a target pH value. Increasing the CO2 concentration as a percentage of gas composition at a higher altitude merely restores the number of CO2 molecules to the same number as achieved by lower percentages at a lower elevation.
To conclude this section, we must say that embryologists in IVF laboratories face a real difficulty in choosing the ideal pHo value or the specific range of values, for the simple reason that we do not have any clinical data (in particular from randomized clinical trials) enabling us to determine the optimal pH value (or range of values) to be applied during embryo culture.
pH measurement methods
Beyond the clinical interest of controlling the pH of the culture medium, we must consider the analytical difficulty of reliably measuring pH in such media. The probes are not always very precise, the frequency of calibration is important (often before each use) and even short exposure of the medium to air must be avoided. From a general point of view, the reference method for measuring pH is hydrogen potentiometry. The silver chloride electrode is considered as a reference electrode in pH meters, although the calomel electrode is an older and robust reference electrode (recommended for solutions with proteins or organic buffers). One constraint of these measurement systems is the necessity of calibration before each use because the electrode does not give reproducible potentials over longer periods of time.
Due to these limitations and because of the need to measure pH in low volumes of the medium, pH measurements by a blood gas apparatus (fixed or portable) after sampling with a needle and syringe can be useful. This method reliably measures pH inside microdroplets of embryo culture medium. A portable blood gas apparatus has been developed and represents a good alternative, avoiding transport of the syringe outside the IVF laboratory.
Continuous pH measurement systems by an optical fluorescence measurement device (suitable for some IVF incubators) are under development or already marketed (OCTAX Log & Guard™ or SAFE Sens™).
Conclusion
Good knowledge of the mechanisms of cellular ion homeostasis is essential to develop cell culture systems that minimize cell stress and improve embryo viability. The mammalian oocyte and embryo stages for which pHo control is most critical are those whose pHi regulatory mechanisms are weak, namely the denuded oocyte and the early stages of development. The blastocyst seems more competent at regulating its pHi with more cellular membranes that are less permeable to H+ ions. The importance of pHi homeostasis for cell metabolism and embryo viability in mammalian embryos has been demonstrated. Because correct and stable pH is essential to embryo health and development, monitoring the pH values of IVF culture media is imperative. However, to date, there are no clinical data enabling us to determine the ideal pH for all specific IVF culture media. Randomized trials are needed to answer this question, although they could only be comparative and not controlled since there is no reference value.
It is clear that the issue of optimal embryo culture conditions is broader than the question of pH alone: the composition of the culture medium (in particular amino acids, ammonium, carbohydrates) [52, 53], the temperature (with the need to maintain 37 °C) [54, 55], the effect of reduced oxygen concentration in the incubator [56, 57], group or single culture [58, 59], the osmolarity and volume of medium [60, 61], incubator performance and oil quality must be taken into account [53]. To date, unfortunately, although we have important data on the impact of pH on embryo development (cf “Early stage embryo” and “Blastocyst”), a limitation is that there are no data demonstrating any influence of pH value (among the various factors relating to culture conditions) on IVF outcomes. Once these culture conditions are optimized, special attention should be paid to selecting the best embryo.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.De Geyter C, Calhaz-Jorge C, Kupka MS, Wyns C, Mocanu E, Motrenko T, et al. ART in Europe, 2014: results generated from European registries by ESHRE: The European IVF-monitoring consortium (EIM) for the European Society of Human Reproduction and Embryology (ESHRE) Hum Reprod. 2018;33(9):1586–1601. doi: 10.1093/humrep/dey242. [DOI] [PubMed] [Google Scholar]
- 2.Dumoulin JC, Land JA, Van Montfoort AP, Nelissen EC, Coonen E, Derhaag JG, et al. Effect of in vitro culture of human embryos on birthweight of newborns. Hum Reprod. 2010;25(3):605–612. doi: 10.1093/humrep/dep456. [DOI] [PubMed] [Google Scholar]
- 3.Nelissen EC, Van Montfoort AP, Coonen E, Derhaag JG, Geraedts JP, Smits LJ, et al. Further evidence that culture media affect perinatal outcome: findings after transfer of fresh and cryopreserved embryos. Hum Reprod. 2012;27(7):1966–1976. doi: 10.1093/humrep/des145. [DOI] [PubMed] [Google Scholar]
- 4.El Hajj N, Haaf T. Epigenetic disturbances in in vitro cultured gametes and embryos: implications for human assisted reproduction. Fertil Steril. 2013;99(3):632–641. doi: 10.1016/j.fertnstert.2012.12.044. [DOI] [PubMed] [Google Scholar]
- 5.Bouillon C, Leandri R, Desch L, Ernst A, Bruno C, Cerf C et al. Does embryo culture medium influence the health and development of children born after in vitro fertilization? PLoS One 2016;11(3):e0150857. doi:10.1371/journal.pone.0150857. [DOI] [PMC free article] [PubMed]
- 6.Squirrell JM, Lane M, Bavister BD. Altering intracellular pH disrupts development and cellular organization in preimplantation hamster embryos. Biol Reprod. 2001;64(6):1845–1854. doi: 10.1095/biolreprod64.6.1845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Roos A, Boron WF. Intracellular pH. Physiol Rev. 1981;61(2):296–434. doi: 10.1152/physrev.1981.61.2.296. [DOI] [PubMed] [Google Scholar]
- 8.Busa WB. Mechanisms and consequences of pH-mediated cell regulation. Annu Rev Physiol. 1986;48:389–402. doi: 10.1146/annurev.ph.48.030186.002133. [DOI] [PubMed] [Google Scholar]
- 9.Swearman H, Koustas G, Knight E, Liperis G, Grupen C, Sjoblom C. pH: the silent variable significantly impacting meiotic spindle assembly in mouse oocytes. Reprod BioMed Online. 2018;37(3):279–290. doi: 10.1016/j.rbmo.2018.06.022. [DOI] [PubMed] [Google Scholar]
- 10.Cheng JM, Li J, Tang JX, Chen SR, Deng SL, Jin C, Zhang Y, Wang XX, Zhou CX, Liu YX. Elevated intracellular pH appears in aged oocytes and causes oocyte aneuploidy associated with the loss of cohesion in mice. Cell Cycle. 2016;15(18):2454–2463. doi: 10.1080/15384101.2016.1201255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lane M, Gardner DK. Regulation of ionic homeostasis by mammalian embryos. Semin Reprod Med. 2000;18(2):195–204. doi: 10.1055/s-2000-12558. [DOI] [PubMed] [Google Scholar]
- 12.Dale B, Menezo Y, Cohen J, DiMatteo L, Wilding M. Intracellular pH regulation in the human oocyte. Hum Reprod. 1998;13(4):964–970. doi: 10.1093/humrep/13.4.964. [DOI] [PubMed] [Google Scholar]
- 13.Edwards LJ, Williams DA, Gardner DK. Intracellular pH of the preimplantation mouse embryo: effects of extracellular pH and weak acids. Mol Reprod Dev. 1998;50(4):434–442. doi: 10.1002/(SICI)1098-2795(199808)50:4<434::AID-MRD7>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- 14.Barr KJ, Garrill A, Jones DH, Orlowski J, Kidder GM. Contributions of Na+/H+ exchanger isoforms to preimplantation development of the mouse. Mol Reprod Dev. 1998;50(2):146–153. doi: 10.1002/(SICI)1098-2795(199806)50:2<146::AID-MRD4>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- 15.Watson AJ, Barcroft LC. Regulation of blastocyst formation. Front Biosci. 2001;6:D708–D730. doi: 10.2741/watson. [DOI] [PubMed] [Google Scholar]
- 16.Phillips KP, Leveille MC, Claman P, Baltz JM. Intracellular pH regulation in human preimplantation embryos. Hum Reprod. 2000;15(4):896–904. doi: 10.1093/humrep/15.4.896. [DOI] [PubMed] [Google Scholar]
- 17.Lane M, Bavister BD. Regulation of intracellular pH in bovine oocytes and cleavage stage embryos. Mol Reprod Dev. 1999;54(4):396–401. doi: 10.1002/(SICI)1098-2795(199912)54:4<396::AID-MRD10>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- 18.Zhao Y, Chauvet PJ, Alper SL, Baltz JM. Expression and function of bicarbonate/chloride exchangers in the preimplantation mouse embryo. J Biol Chem. 1995;270(41):24428–24434. doi: 10.1074/jbc.270.41.24428. [DOI] [PubMed] [Google Scholar]
- 19.Lane M, Baltz JM, Bavister BD. Na+/H+ antiporter activity in hamster embryos is activated during fertilization. Dev Biol. 1999;208(1):244–252. doi: 10.1006/dbio.1999.9198. [DOI] [PubMed] [Google Scholar]
- 20.Lane M, Baltz JM, Bavister BD. Regulation of intracellular pH in hamster preimplantation embryos by the sodium hydrogen (Na+/H+) antiporter. Biol Reprod. 1998;59(6):1483–1490. doi: 10.1095/biolreprod59.6.1483. [DOI] [PubMed] [Google Scholar]
- 21.Siyanov V, Baltz JM. NHE1 is the sodium-hydrogen exchanger active in acute intracellular pH regulation in preimplantation mouse embryos. Biol Reprod. 2013;88(6):157. doi: 10.1095/biolreprod.113.109033. [DOI] [PubMed] [Google Scholar]
- 22.Romero MF, Chen AP, Parker MD, Boron WF. The SLC4 family of bicarbonate (HCO(3)(−)) transporters. Mol Asp Med. 2013;34(2–3):159–182. doi: 10.1016/j.mam.2012.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dagilgan S, Dundar-Yenilmez E, Tuli A, Urunsak IF, Erdogan S. Evaluation of intracellular pH regulation and alkalosis defense mechanisms in preimplantation embryos. Theriogenology. 2015;83(6):1075–1084. doi: 10.1016/j.theriogenology.2014.12.011. [DOI] [PubMed] [Google Scholar]
- 24.Swain JE. Is there an optimal pH for culture media used in clinical IVF? Hum Reprod Update. 2012;18(3):333–339. doi: 10.1093/humupd/dmr053. [DOI] [PubMed] [Google Scholar]
- 25.Erdogan S, FitzHarris G, Tartia AP, Baltz JM. Mechanisms regulating intracellular pH are activated during growth of the mouse oocyte coincident with acquisition of meiotic competence. Dev Biol. 2005;286(1):352–360. doi: 10.1016/j.ydbio.2005.08.009. [DOI] [PubMed] [Google Scholar]
- 26.FitzHarris G, Siyanov V, Baltz JM. Granulosa cells regulate oocyte intracellular pH against acidosis in preantral follicles by multiple mechanisms. Development. 2007;134(23):4283–4295. doi: 10.1242/dev.005272. [DOI] [PubMed] [Google Scholar]
- 27.Phillips KP, Petrunewich MA, Collins JL, Baltz JM. The intracellular pH-regulatory HCO3-/cl- exchanger in the mouse oocyte is inactivated during first meiotic metaphase and reactivated after egg activation via the MAP kinase pathway. Mol Biol Cell. 2002;13(11):3800–3810. doi: 10.1091/mbc.e02-04-0242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Phillips KP, Baltz JM. Intracellular pH regulation by HCO3-/Cl- exchange is activated during early mouse zygote development. Dev Biol. 1999;208(2):392–405. doi: 10.1006/dbio.1999.9199. [DOI] [PubMed] [Google Scholar]
- 29.Zhou C, Tiberi M, Liang B, Alper SL, Baltz JM. HCO3(−)/Cl(−) exchange inactivation and reactivation during mouse oocyte meiosis correlates with MEK/MAPK-regulated Ae2 plasma membrane localization. PLoS One. 2009;4(10):e7417. doi: 10.1371/journal.pone.0007417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Erdogan S, Cetinkaya A, Tuli A, Yilmaz ED, Dogan A. Changes in the activity of defense mechanisms against induced acidosis during meiotic maturation in mouse oocytes. Theriogenology. 2011;75(6):1057–1066. doi: 10.1016/j.theriogenology.2010.11.014. [DOI] [PubMed] [Google Scholar]
- 31.Zhou C, Fitzharris G, Alper SL, Baltz JM. Na+/H+ exchange is inactivated during mouse oocyte meiosis, facilitating glycine accumulation that maintains embryo cell volume. J Cell Physiol. 2013;228(10):2042–2053. doi: 10.1002/jcp.24370. [DOI] [PubMed] [Google Scholar]
- 32.Edwards LJ, Williams DA, Gardner DK. Intracellular pH of the mouse preimplantation embryo: amino acids act as buffers of intracellular pH. Hum Reprod. 1998;13(12):3441–3448. doi: 10.1093/humrep/13.12.3441. [DOI] [PubMed] [Google Scholar]
- 33.Lane M, Gardner DK. Understanding cellular disruptions during early embryo development that perturb viability and fetal development. Reprod Fertil Dev. 2005;17(3):371–378. doi: 10.1071/RD04102. [DOI] [PubMed] [Google Scholar]
- 34.Zander-Fox DL, Mitchell M, Thompson JG, Lane M. Alterations in mouse embryo intracellular pH by DMO during culture impair implantation and fetal growth. Reprod BioMed Online. 2010;21(2):219–229. doi: 10.1016/j.rbmo.2010.05.001. [DOI] [PubMed] [Google Scholar]
- 35.Steeves CL, Lane M, Bavister BD, Phillips KP, Baltz JM. Differences in intracellular pH regulation by Na(+)/H(+) antiporter among two-cell mouse embryos derived from females of different strains. Biol Reprod. 2001;65(1):14–22. doi: 10.1095/biolreprod65.1.14. [DOI] [PubMed] [Google Scholar]
- 36.Swain JE. Optimizing the culture environment in the IVF laboratory: impact of pH and buffer capacity on gamete and embryo quality. Reprod BioMed Online. 2010;21(1):6–16. doi: 10.1016/j.rbmo.2010.03.012. [DOI] [PubMed] [Google Scholar]
- 37.Zhao Y, Baltz JM. Bicarbonate/chloride exchange and intracellular pH throughout preimplantation mouse embryo development. Am J Phys. 1996;271(5 Pt 1):C1512–C1520. doi: 10.1152/ajpcell.1996.271.5.C1512. [DOI] [PubMed] [Google Scholar]
- 38.Ng KYB, Mingels R, Morgan H, Macklon N, Cheong Y. In vivo oxygen, temperature and pH dynamics in the female reproductive tract and their importance in human conception: a systematic review. Hum Reprod Update. 2018;24(1):15–34. doi: 10.1093/humupd/dmx028. [DOI] [PubMed] [Google Scholar]
- 39.Hugentobler S, Morris DG, Kane MT, Sreenan JM. In situ oviduct and uterine pH in cattle. Theriogenology. 2004;61(7–8):1419–1427. doi: 10.1016/j.theriogenology.2003.08.008. [DOI] [PubMed] [Google Scholar]
- 40.Nichol R, Hunter RH, Cooke GM. Oviduct fluid pH in intact and unilaterally ovariectomized pigs. Can J Physiol Pharmacol. 1997;75(9):1069–1074. doi: 10.1139/y97-115. [DOI] [PubMed] [Google Scholar]
- 41.Maas DH, Stein B, Metzger H. PO2 and pH measurements within the rabbit oviduct following tubal microsurgery: reanastomosis of previously dissected tubes. Adv Exp Med Biol. 1984;169:561–570. doi: 10.1007/978-1-4684-1188-1_50. [DOI] [PubMed] [Google Scholar]
- 42.David A, Serr DM, Czernobilsky B. Chemical composition of human oviduct fluid. Fertil Steril. 1973;24(6):435–439. doi: 10.1016/S0015-0282(16)39731-X. [DOI] [PubMed] [Google Scholar]
- 43.Imoedemhe DA, Chan RC, Ramadan IA, Sigue AB. Changes in follicular fluid gas and pH during carbon dioxide pneumoperitoneum for laparoscopic aspiration and their effect on human oocyte fertilizability. Fertil Steril. 1993;59(1):177–182. doi: 10.1016/S0015-0282(16)55635-0. [DOI] [PubMed] [Google Scholar]
- 44.Mather EC. “In vivo” uterine lumen pH values of the bovine. Theriogenology. 1975;3(3):113–119. doi: 10.1016/0093-691X(75)90160-0. [DOI] [PubMed] [Google Scholar]
- 45.Iritani A, Nishikawa Y, Gomes WR, VanDemark NL. Secretion rates and chemical composition of oviduct and uterine fluids in rabbits. J Anim Sci. 1971;33(4):829–835. doi: 10.2527/jas1971.334829x. [DOI] [PubMed] [Google Scholar]
- 46.Bates RG, Covington AK. Behavior of the glass electrode and other pH-responsive electrodes in biological media. Ann N Y Acad Sci. 1968;148(1):67–80. doi: 10.1111/j.1749-6632.1968.tb20341.x. [DOI] [PubMed] [Google Scholar]
- 47.Tarahomi M, de Melker AA, van Wely M, Hamer G, Repping S, Mastenbroek S. pH stability of human preimplantation embryo culture media: effects of culture and batches. Reprod BioMed Online. 2018;37(4):409–414. doi: 10.1016/j.rbmo.2018.08.011. [DOI] [PubMed] [Google Scholar]
- 48.Higdon HL, 3rd, Blackhurst DW, Boone WR. Incubator management in an assisted reproductive technology laboratory. Fertil Steril. 2008;89(3):703–710. doi: 10.1016/j.fertnstert.2007.03.040. [DOI] [PubMed] [Google Scholar]
- 49.Hentemann M, Mousavi K, Bertheussen K. Differential pH in embryo culture. Fertil Steril. 2011;95(4):1291–1294. doi: 10.1016/j.fertnstert.2010.10.018. [DOI] [PubMed] [Google Scholar]
- 50.Swain J. Embryo culture and pH. Fertil Steril. 2011;95(8):e67; author reply e8. doi:10.1016/j.fertnstert.2011.04.024. [DOI] [PubMed]
- 51.Carney EW, Bavister BD. Regulation of hamster embryo development in vitro by carbon dioxide. Biol Reprod. 1987;36(5):1155–1163. doi: 10.1095/biolreprod36.5.1155. [DOI] [PubMed] [Google Scholar]
- 52.Lane M, Gardner DK. Embryo culture medium: which is the best? Best Pract Res Clin Obstet Gynaecol. 2007;21(1):83–100. doi: 10.1016/j.bpobgyn.2006.09.009. [DOI] [PubMed] [Google Scholar]
- 53.Gardner DK, Kelley RL. Impact of the IVF laboratory environment on human preimplantation embryo phenotype. J Dev Orig Health Dis. 2017;8(4):418–435. doi: 10.1017/S2040174417000368. [DOI] [PubMed] [Google Scholar]
- 54.Hong KH, Lee H, Forman EJ, Upham KM, Scott RT., Jr Examining the temperature of embryo culture in in vitro fertilization: a randomized controlled trial comparing traditional core temperature (37 degrees C) to a more physiologic, cooler temperature (36 degrees C) Fertil Steril. 2014;102(3):767–773. doi: 10.1016/j.fertnstert.2014.06.009. [DOI] [PubMed] [Google Scholar]
- 55.Fujiwara M, Takahashi K, Izuno M, Duan YR, Kazono M, Kimura F, Noda Y. Effect of micro-environment maintenance on embryo culture after in-vitro fertilization: comparison of top-load mini incubator and conventional front-load incubator. J Assist Reprod Genet. 2007;24(1):5–9. doi: 10.1007/s10815-006-9088-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Meintjes M, Chantilis SJ, Douglas JD, Rodriguez AJ, Guerami AR, Bookout DM, Barnett BD, Madden JD. A controlled randomized trial evaluating the effect of lowered incubator oxygen tension on live births in a predominantly blastocyst transfer program. Hum Reprod. 2009;24(2):300–307. doi: 10.1093/humrep/den368. [DOI] [PubMed] [Google Scholar]
- 57.Gomes Sobrinho DB, Oliveira JB, Petersen CG, Mauri AL, Silva LF, Massaro FC, Baruffi RL, Cavagna M, Franco JG Jr IVF/ICSI outcomes after culture of human embryos at low oxygen tension: a meta-analysis. Reprod Biol Endocrinol. 2011;9:143. doi: 10.1186/1477-7827-9-143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Moessner J, Dodson WC. The quality of human embryo growth is improved when embryos are cultured in groups rather than separately. Fertil Steril. 1995;64(5):1034–1035. doi: 10.1016/s0015-0282(16)57925-4. [DOI] [PubMed] [Google Scholar]
- 59.Ebner T, Shebl O, Moser M, Mayer RB, Arzt W, Tews G. Group culture of human zygotes is superior to individual culture in terms of blastulation, implantation and life birth. Reprod BioMed Online. 2010;21(6):762–768. doi: 10.1016/j.rbmo.2010.06.038. [DOI] [PubMed] [Google Scholar]
- 60.Swain JE, Cabrera L, Xu X, Smith GD. Microdrop preparation factors influence culture-media osmolality, which can impair mouse embryo preimplantation development. Reprod BioMed Online. 2012;24(2):142–147. doi: 10.1016/j.rbmo.2011.10.008. [DOI] [PubMed] [Google Scholar]
- 61.Swain JE. Controversies in ART: considerations and risks for uninterrupted embryo culture. Reprod BioMed Online. 2019;39(1):19–26. doi: 10.1016/j.rbmo.2019.02.009. [DOI] [PubMed] [Google Scholar]