Abstract
Purpose
The aim of this study is to provide data on the practice of Luteal Phase Oocyte Retrieval (LuPOR). The authors assess cell-free DNA levels in follicular fluid (ff cfDNA) from poor responders undergoing natural cycles, and comparing it to respective data originating from follicular phase oocyte retrievals.
Methods
Forty-seven women were eligible for this prospective study. Participants were classified as poor responders based on Bologna criteria while being detected with a second follicular wave. Follicular fluid was collected and prepared for cfDNA extraction. Levels of cfDNA were quantified via Q-PCR employing the ALU115 and ALU247 primers. These primers are associated with apoptotic and necrotic events. Levels of ff cfDNA resulting from follicular phase oocyte retrieval (FoPOR) and LuPOR-performed in a single menstrual cycle were associated with the number and maturation status of yielded oocytes and the number and fertilization status of resulting zygotes. Survival rate following thawing of cryopreserved zygotes, along with the resulting number of cleavage stage and blastocyst stage embryos are provided.
Results
Mean levels of ALU115 were significantly lower during FoPOR when compared to LuPOR (0.79 ± 0.72 vs 1.46 ± 1.59 ng/μl, p = 0.02). Regarding the FoPOR group, a significant positive correlation of serum estradiol and ALU115 concentration (p = 0.04) was revealed. A significant negative correlation between serum estradiol and cfDNA integrity was observed both during FoPOR (p = 0.03) and LuPOR (p = 0.03). A significant lower number of retrieved (1.09 ± 0.28 vs 1.29 ± 0.58, p = 0.02) and MII oocytes (0.77 ± 0.55 vs 1.08 ± 0.61, p = 0.02) was observed when comparing the FoPOR to LuPOR groups respectively. The integrity of cfDNA was observed to be higher in FoPOR originating embryos that arrested either prior to cleavage (0.28 ± 0.13 vs 0.17 ± 0.10, p = 0.006) or prior to blastocyst formation (0.28 ± 0.12 vs 0.13 ± 0.06, p = 0.04). In the case of LuPOR originating embryos, cfDNA integrity was observed to be higher in embryos that arrested only prior to the blastocyst stage (0.27 ± 0.20 vs 0.11 ± 0.07, p = 0.008). Similarly, cfDNA integrity was observed to be lower in top quality blastocysts originating from FoPOR (0.07 ± 0.04 vs 0.17 ± 0.05, p = 0.03) and in top quality cleavage stage embryos (0.09 ± 0.06 vs 0.31 ± 0.22, p = 0.01) and blastocysts (0.06 ± 0.02 vs 0.14 ± 0.06, p = 0.02) originating from LuPOR.
Conclusions
Our results indicate that ff originating from LuPOR presents with higher levels of cfDNA. The higher cfDNA levels are attributed to mainly apoptotic events, as the ALU247 levels and DNA integrity did not differ statistically significantly between FoPOR and LuPOR. The absolute mean level of ALU247 corresponding to necrotic events was higher in LuPOR. Regarding embryological data, cfDNA integrity was correlated with both number and quality of cleavage stage embryos in both FoPOR and LuPOR, along with blastocyst stage embryos in LuPOR. Necrotic events were associated with poorer blastocyst formation rate and blastocyst quality in LuPOR. As the comparison between FoPOR and LuPOR results to similar IVF laboratory data, the practice of LuPOR may stand as a promising approach for poor responders, while it merits further investigation.
Keywords: Cell-free DNA, Follicular fluid, Luteal phase oocyte retrieval, Poor responders, Natural cycles, Apoptosis
Introduction
Poor responders represent a time-sensitive group of patients in Assisted Reproduction Technology (ART), typically accompanied by a compromised oocyte quality and yield [1]. However, the challenge regarding definition, diagnosis, and management still fuels a heated debate, in light of the lack of a universally accepted line of approach [2]. With regard to management, in vitro fertilization (IVF) specialists may opt for a wide pallet of strategies, ranging from numerous proposed ovarian stimulation protocols [3], to natural cycles [1], to the “freeze and collect” [4], even at times recruiting an empirical approach treatment. Recently, employment of the double oocyte retrieval in a single menstrual cycle has enriched the standing options of poor responders’ management [5]. This approach is based on the observation of the “second follicular wave phenomenon”, initially studied on various animal models [6–9] and thenceforth on women [10]. The aforementioned physiological process is characterized by the detection of at least two follicular waves during the same menstrual cycle, leading to the development of more than one privileged follicles [11]. However, the underlying mechanisms of this phenomenon still remains a “black box” [12].
For patients presenting with poor IVF prognosis, the double oocyte retrieval process is predominantly introduced in clinical practice in the context of performing a dual ovarian stimulation-known as “DuoStim” protocol as reported in literature. The DuoStim protocol describes the practice of oocyte retrieval during both the follicular and the subsequent luteal phase in a single menstrual cycle [13]. These prospective and retrospective studies comparing data from the follicular to the luteal phase, equally report statistically significant embryological results with regard to the mean number of oocytes retrieved, mature MII oocytes, fertilization rate, embryonic development to cleavage stage, blastocyst formation, and euploidy rate [14–20]. Moreover, in terms of reporting on clinical pregnancy status and live birth rates, it is clear that data from both phases does not demonstrate any statistically significant difference [14, 15, 18, 20]. Interestingly, a retrospective study indicated that embryos originating from the luteal phase seem to present with a higher implantation rate, in comparison to those corresponding to the follicular phase [21]. From the perspective of management, attempting a comparison between the DuoStim practice and the conventional stimulation protocol, a higher mean number of oocyte yield and subsequent embryo number was indicated, favoring the practice of DuoStim [14–16, 20, 22]. Nonetheless, clinical pregnancy and live birth rates were reported to be similar [14, 20]. The considerable differences among the various studies regarding the population involved, the study design, the ovarian stimulation protocols employed, or even the duration of each protocol, should be taken into account. These differences may serve as confounders rendering conclusions drawn on the practice of DuoStim weak.
What is of importance, is that little is known on the developmental competence and dynamics of oocytes and embryos originating from a dual retrieval during natural IVF cycles [4]. The majority of clinicians opt for the aforementioned approach in cases of oncology patients, who undergo fertility preservation protocols [23, 24]. Similarly, when referring to patients presenting with poor response, the issue of time stands as a considerable motivation for adopting this practice [5, 22, 25]. Our team has introduced the abbreviation of “LuPOR,” in an attempt to describe the practice of luteal phase oocyte retrieval, with the corresponding abbreviation of “FoPOR” for follicular phase. This study retrospectively showcased promising results with regard to the number of mature oocytes and fertilization rate, in case of poor responders employing the LuPOR approach [4]. Albeit morphological observations have been reported, the molecular aspect entailed in the double follicular wave and the LuPOR approach has yet to be adequately investigated. Emerging data on the molecular physiology aspects of the double follicular wave through LuPOR would be of heightened interest and of added value, especially for poor responders during natural IVF cycles.
In the era of personalized and precision medicine, evaluation of cell-free DNA concentration has emerged as an “add-on. This novel tool is coupled with promising diagnostic and prognostic accuracy, especially with regard to several types of gynecological cancers, obstetric disorders, or even fetal abnormalities [26–28]. Interestingly, high levels of cfDNA in maternal plasma were correlated with decreased pregnancy rates [29]. The superiority of it is ascertained by its non-invasive nature, in light of being easily measured in various biological fluids, such as serum or urine [30]. Evaluating cfDNA levels relies on common molecular techniques, ascertaining efficiency on a patient management [26]. Evaluating cfDNA levels in follicular fluid (ff cfDNA) presents as a competent biomarker in the context of assisted reproduction, with respect to assessing the oocyte microenvironment, thus contributing to an enhanced embryo selection procedure. Published studies have examined the concentrations of ff cfDNA following stimulated IVF cycles. The results indicated a positive correlation of increased ff cfDNA levels and poor prognosis for IVF treatment. Increased ff cfDNA levels are associated with a lower number of oocytes retrieved, poor embryo quality, an extensive duration, and heightened dosage of the stimulation protocol employed. Moreover, the aforementioned studies revealed that the origin of ff cfDNA is principally attributed to the apoptotic process [31–33]. This conclusion was enabled assessing the quantification of Arthrobacter luteus (ALU) repeats, namely ALU115 amplifying both short and long fragments of cfDNA originating from apoptosis or necrosis respectively, as well as ALU247 that amplifying only the long fragments corresponding to necrotic events [34]. Short fragments of approximately 160–180 base pairs (bp) are cleaved by endogenous caspase-activated DNAase during apoptotic cell death. Long fragments of approximately 10,000 bp are cleaved during necrosis. Hence, cfDNA in ff could represent a tool that non-invasively evaluates the ambient environment of the oocyte.
The aim of the present study is to uniquely evaluate the levels of cfDNA in ff samples, originated from FoPOR and LuPOR practices, in the same natural ART cycle in poor responders. To what extent is the second follicular wave dependent on necrotic or apoptotic events? In vitro fertilization practitioners may question whether opting for LuPOR is a valid option enabling efficient management of the special cohort of poor responders. In light of that, the rationale fueling design of this study was to provide for first time data aiming to assist in decision making regarding LuPOR employment in routine clinical practice for poor responders’ management. More particularly, the authors set out to compare ff cfDNA levels resulting from FoPOR and LuPOR in a single menstrual cycle, and attempt respective associations with the number and maturation status of corresponding oocytes, the number of subsequent fertilized oocytes, as well as the number and quality of blastocysts. Data from this research project may contribute towards buttressing the validity of the practice of LuPOR when employed as the method of choice, in natural cycles for poor responders.
Methods
Study population
This prospective study recruited a total of 47 infertile women classified as poor responders based on Bologna criteria [35], during the time period from April 2018 to December 2018. Informed consent was obtained from all individual participants. A second follicular recruitment was detected for all patients during the luteal phase of the same menstrual cycle, and thus following FoPOR they were further submitted to LuPOR. Only natural IVF cycles were included in an attempt to exclude the factor of the ovarian stimulation protocol. For the quantification of cfDNA levels, 47 ff samples were collected from FoPOR and served as the control group, while 47 ff samples originated from LuPOR of the same cycle and served as the study group. Baseline levels of follicle stimulating hormone (FSH), luteinizing hormone (LH), anti-müllerian hormone (AMH), prolactin, progesterone, and estradiol (E2) levels were evaluated via the chemiluminescent microparticle immunoassay on a Roche Cobas E-411 Immunoassay analyzer (Roche Diagnostics GmbH, Mannheim, Germany). In order to enable evaluation of the oocyte maturation status, only intracytoplasmic sperm injection (ICSI) cases addressing mild male factor were included. ICSI was performed for all cases on the grounds of oligozoospermia which was diagnosed following a semen analysis, according to the World Health Organization (WHO) criteria. The exclusion criteria of our study referred to polycystic ovary syndrome, inflammatory diseases, such as endometriosis or chronic endometritis, sexually transmitted diseases, and diagnosis of cancer. Finally, cases for which the oocyte retrieval yielded no oocyte, regarding either FoPOR or LuPOR, were excluded as no associations with oocyte characteristics would be possible. The study protocol (133-26/03/2018) was approved by the Hospital Ethics Board in accordance with the Helsinki declaration.
Natural IVF cycle protocol
At the first appointment, patients’ baseline hormonal profile was recorded, including measurements of FSH, LH, AMH, and prolactin on day 2 of the menstrual cycle, along with progesterone levels evaluated on day 21 of the menstrual cycle. E2 was evaluated on the day of hCG administration both in FoPOR and in LuPOR.
On day 8 of the menstrual cycle, follicular growth was monitored via transvaginal ultrasound, coupled with frequent-possibly-daily evaluations on serum LH and E2 levels. Timing of further monitoring was individualized depending on follicular growth and E2 levels. Once the dominant follicle was observed with a diameter of 17 mm or more, and coupled by a serum E2 level > 100 pg/ml, an intramuscular injection of 6500 IU of human Chorionic Gonadotropin (hCG) was administered to trigger ovulation. Following 36 h, a transvaginal ultrasonographically monitored follicular aspiration, under mild anesthesia was performed.
Using a microscopical work chamber ascertaining stable environment for handling gametes, the collected follicular fluid following retrieval was placed into a collection petri dish, in order to detect the presence of oocytes. The collected oocytes were placed in Fert Media (ORIGIO Sequential Media) for a 2-h incubation time. Thereafter, 38 h post hCG, the oocytes were denuded employing enzymatic and mechanical removal of the cumulus-oocyte complexes, using 80 IU/ml hyaluronidase (FertiPro). The oocyte maturation status was recorded as follows: mature, referring to metaphase II (MII) stage oocyte, immature, referring to the germinal vehicle (GV) or metaphase I (MI) stage oocyte, or abnormal, based on identification of several irregular morphological characteristics with regard to oocyte shape, size, ooplasm, structure of perivitelline space, zona pellucida, or polar body morphology [36]. Mature oocytes were placed into dishes with Step-1 culture media (ORIGIO Continuous Culture Media) for 1 h post denudation. ICSI dishes have been prepared with two drops of Quinn’s Advantage Medium with HEPES (SAGE) with 5% human serum albumin (HSA) under mineral oil. The mature oocytes were inseminated employing ICSI 39 h post hCG insemination. Following the ICSI procedure, the oocytes were cultured in culture dishes containing Step-1 culture media (ORIGIO Continuous Culture Media) with 5% HSA under mineral oil into incubator at 37 °C, with 5% O2, 6% CO2, 89% Ν2, and 95% humid atmosphere.
Fertilization evaluation was performed 16 to 18 h post insemination, classifying oocytes as 1PN (pronucleus), 2PN, 3PN, and lysed. Hence, normally fertilized zygotes were identified by two pronuclei and the extrusion of the second polar body, whereas the rest were considered as abnormally fertilized oocytes or lysed. The strategy opted for these patients has been previously described by Sfakianoudis et al. 2019 as “freeze and collect” or “embryo banking.” This approach entails cryopreservation of all zygotes resulting from consecutive natural cycles performed for each patient. Cryopreservation is performed employing the slow freezing technique, using the FreezeKit Cleave (Vitrolife). As a result, the patients gradually ascertain an adequate number of zygotes namely 8–10 zygotes in a storage. Frozen-thaw cycles, including a group of 4 embryos thawed per cycle, are subsequently initiated leading to respective embryo transfers (ETs). This is justified as Greek Legislation allows transfer of up to 4 embryos for women aged 40 years or older. Understandably, the embryos thawed may correspond to oocytes retrieved from different menstrual cycles and different menstrual cycle phases. The embryos are cultured until the embryo transfer procedure which may include FoPOR and LuPOR embryos. Zygotes were thawed and cultured in separate droplets of culture medium till the day of embryo transfer. Embryos of poor quality did not qualify for embryo transfer. Embryo quality on cleavage stage was assessed according to ALPHA/ESHRE 2011 consensus [37], while blastocyst quality was assessed according to Gardner’s grading system [38]. Cleavage stage embryos graded as 7cA or 8cA and blastocysts graded as 4AA, 5AA, or 6AA were regarded as top. All other gradings were regarded as non-top. The number of embryos transferred for patients participating in this study ranged from 2 to 4 according to legislation (article 6 of the Law 3305/2005 “Application of the methods of Medically Assisted Reproduction” that is approved by the Greek Parliament) [39], according to the patient’s age, and according to the number of previous failed IVF attempts. According to Greek legislation, a maximum number of 3 transferred embryos is allowed in female patients below the age of 40, while a maximum number of 4 is allowed for older patients. Only 9 ETs were performed employing embryos originating strictly from FoPOR or LuPOR. Five ETs were performed employing embryos originating solely from the LuPOR stage and 4 ETs were performed employing embryos originating solely from FoPOR stage. In all the aforementioned ETs, single embryo transfer was performed due to either failure of all of the thawed embryos to survive, or the lack of embryos of adequate quality to be considered for ET. The remaining of the ETs were performed employing a combination of both FoPOR and LuPOR originating embryos.
An observation of elevated serum E2 levels during the luteal phase indicated new follicle recruitment. These patients were monitored for follicular growth via transvaginal ultrasonography 7 days post performing FoPOR. Further monitoring led to the detection of the dominant follicle that has reached a diameter of 17 mm or more, and confirmed by a serum E2 level > 100 pg/ml. Following this, an intramuscular injection of 6500 IU of hCG was administered in order to trigger ovulation at the luteal phase. Thirty-six hours following administration of the hCG injection, LuPOR was performed similarly to FoPOR. Identical laboratory protocols were performed as described above, resulting to another round of cryopreserved embryos originating from the same menstrual cycle.
Follicular fluid sample preparation and cfDNA extraction
Follicular fluid samples from the same patient were pooled from all respective follicles, during oocyte retrieval procedure, similar to the study protocol employed by Traver and colleagues [40]. Following oocyte retrieval, the ff was placed into round-bottom falcon tubes and centrifuged at 1000g for 15 min, in order to remove any histologic remnant stemming from the oocyte retrieval procedure. Bloodstained and cloudy ff samples were excluded. The supernatants were filtered using a 0.45-μm filter to eliminate cell debris, and immediately stored at − 80 °C. Preparation of the filtered ff samples and the cfDNA extraction procedure were based on the protocol published by Umetani and colleagues [40]. According to this protocol, 50 μl of each ff sample was mixed with 50 μl of a solution buffer, containing 25 ml/l Tween 20, 50 mmol/l Tris, and 1 mmol/l EDTA and then digested with 8 μl of proteinase K (PK) (Macherey-Nagel, Germany) at 70 °C for 20 min followed by heat-inactivation and insolubilization at 95 °C for 5 min. Digestion was performed in Veriti 96-well Thermal Cycler (Applied Biosystems). A final centrifugation was conducted at 10.000g for 5 min and collected supernatants were then stored at − 20 °C until cfDNA quantification.
Quantification of cfDNA
The concentration of cfDNA was quantified via real-time polymerase chain reaction (RT-PCR) for human ALU repeats (Custom DNA Oligos, Eurofins, Genomics, Austria), using two primer sets that generate a 115-bp amplicon (ALU115 primers) and a 247-bp amplicon (ALU247 primers), respectively [40]. DNA integrity was assessed employing the ratio of Q247/Q115. The value corresponding to Q247 reflects cfDNA concentration originating from the ALU247 primer, while the value corresponding to Q115 represents cfDNA concentration originating from the ALU115 primer. When the value of the aforementioned ratio was equal to 1, it was assumed that cfDNA originated principally from necrotic events whereas when the value was equal to 0, it was assumed that the cfDNA concentration could be mainly attributed to apoptotic processes.
For each 96-well PCR plate, a 100× reaction mixture was prepared by adding 800 μl nano-filtration H2O, 1000 μl SYBR Green I Master Mix (Kapa Biosystems), 50 μl of 0.25 μM forward, and 50 μl of 0.25 μM reverse primers (either ALU115 or ALU247). In each well, 1 μl of each PK-digested ff sample was added to 9 μl of reaction mixture (final volume: 10 μl). Thereafter, a film carefully covered the loaded PCR plate. The PCR plate was placed onto the PCR instrument (Bio-Rad iCycler Thermal Cycler IQ5 Multicolor Real-Time PCR Detection System). Real-time (RT-PCR) amplification was performed with precycling heat activation of DNA polymerase at 95 °C for 3 min, followed 35 cycles of denaturation at 95 °C for 3 s, annealing at 63 °C for 30 s, and extension at 55–95 °C for 15 s and 20 °C for hold. A negative control (without template) and 2 intracontrol samples were added in each qPCR plate. All measurements were performed in quadruplicate. Follicular fluid cfDNA concentrations were calculated based on a standard curve prepared with successive dilutions (10 ng to 0.01 pg) of prepared genomic DNA, obtained from peripheral blood of a healthy volunteer. The detection limit of the method was 0.01 pg. Following RT-PCR for quantification of ff cfDNA, electrophoresis was performed employing a 2% agarose gel electrophoresis, to ascertain validity of RT-PCR results.
Statistical analysis
Statistical analysis was conducted employing the R statistical programming language through the RStudio interpreter (Boston, MA, USA). The primary outcome measures of this study are in comparison of the ff FoPOR and ff LuPOR cfDNA levels, as well as the association of those levels with the number and maturation status of oocytes resulting from FoPOR and LuPOR of the same menstrual cycle respectively. The secondary outcome measure reports on the comparison between the FoPOR and LuPOR, with regard to the number of zygotes, abnormally fertilized, and lysed oocytes, and DNA integrity, with the latter being assessed employing the ratio of Q247/Q115 as described above. For the calculation of sample-size, a power analysis was performed, expecting a medium effect size (Cohen’s d = 0.5), and setting the significance level at p = 0.05 and the power at 0.9. The sample size was calculated at n = 44 patients. A total of 47 patients were recruited for the study in order to compensate for possible attrition bias.
Spearman’s correlation coefficient was employed to evaluate possible associations. Normality of the distribution was examined via the Shapiro-Wilk’s test. The distribution of most parameters was not normal and thus the Wilcoxon rank-sum test (Mann-Whitney U test) was preferred to examine potential differences between groups. In cases of distributions of both groups being normal, Student’s t test was preferred.
Results
A total of 47 women classified as poor responders according to the Bologna criteria participated in the present study, as depicted in flow diagram (Fig. 1). All patients underwent 2 natural cycle oocyte retrievals—one in the follicular phase and one in the luteal phase—during the same menstrual cycle.
Fig. 1.
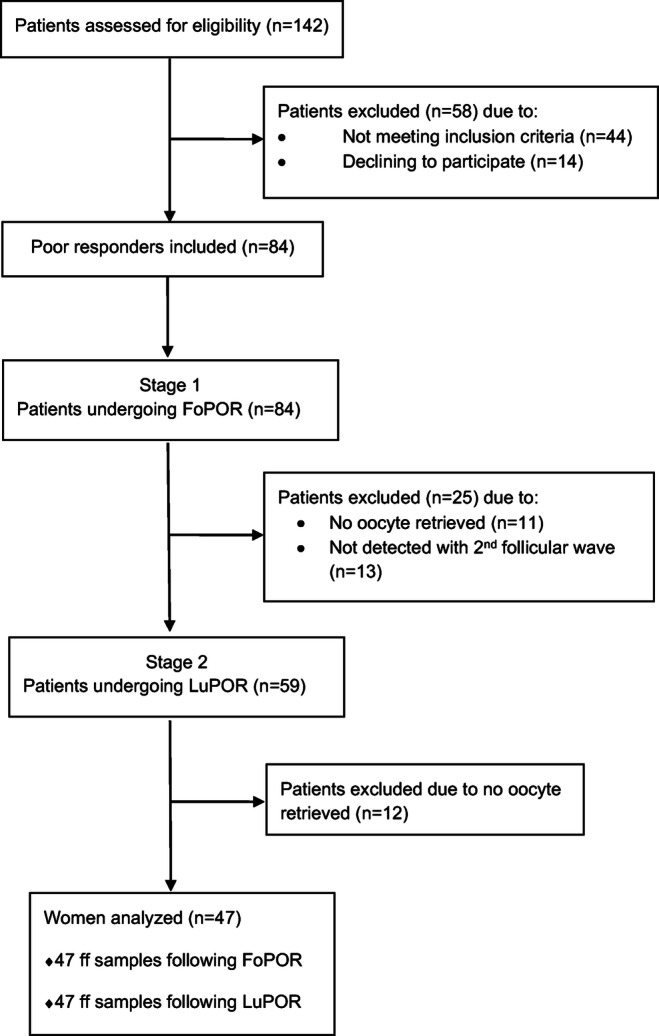
Flow diagram depicting the process of recruitment of this prospective study
The mean age of our patients was 42.91 ± 3.77 years old. The serum baseline hormonal levels of FSH, LH, prolactin, progesterone, and AMH, along with respective ranges are presented in Table 1. Furthermore, the mean levels of serum E2 as were measured prior to both FoPOR and LuPOR procedures, along with their respective ranges, are also presented in Table 1. Mean values regarding Antral follicle count, number of oocytes retrieved, maturation status, and number of pronuclei recorded, as well as lysis of the oocytes are presented in Table 2, while Table 3 provides the total numbers for these parameters respectively.
Table 1.
Descriptive characteristics on mean values of patients’ age and hormonal levels
| Mean ± SD | Range | |
|---|---|---|
| Age (years) | 42.91 ± 3.77 | 32–48 |
| FSH on day 3 (mIU/ml) | 11.22 ± 2.81 | 4.56–15.6 |
| LH on day3 (mIU/ml) | 8.58 ± 4.54 | 2.5–22.1 |
| Prolactin on day 3 (ng/ml) | 16.67 ± 7.90 | 4.7–40 |
| Progesterone on day 21*(ng/ml) | 15.83 ± 5.01 | 11.1–30.2 |
| E2 on trigger day prior to FoPOR (pg/ml) | 197.51 ± 87.94 | 138–520 |
| E2 on trigger day prior to LuPOR (pg/ml) | 286.21 ± 102.29 | 133–490 |
| AMH day 3 (ng/ml) | 0.55 ± 0.41 | 0.01–1.9 |
*Of the previous menstrual cycle
FSH, follicle-stimulating hormone; LH, luteinizing hormone; E2, estradiol; AMH, anti-müllerian hormone; FoPOR, follicular phase oocyte retrieval; LuPOR, luteal phase oocyte retrieval
Table 2.
Oocytes’ characteristics, embryological data, and molecular results for FoPOR and LuPOR
| FoPOR | LuPOR | p value | ||||
|---|---|---|---|---|---|---|
| Mean ± SD | Range | Mean ± SD | Range | |||
| U/S follicle count | 1.77 ± 0.90 | 1–4 | 1.77 ± 0.90 | 1–5 | NS | |
| Oocytes retrieved | 1.09 ± 0.28 | 1–2 | 1.29 ± 0.58 | 1–4 | 0.02 | |
| Maturation status of oocytes retrieved | MII | 0.77 ± 0.55 | 0–2 | 1.08 ± 0.61 | 0–3 | 0.02 |
| MI | 0.02 ± 0.14 | 0–1 | 0.08 ± 0.28 | 0–1 | NS | |
| GV | 0.15 ± 0.36 | 0–1 | 0.06 ± 0.24 | 0–1 | NS | |
| Abnormal | 0.11 ± 0.31 | 0–1 | 0.04 ± 0.20 | 0–1 | NS | |
| Fertilization status following insemination | 2PN | 0.49 ± 0.58 | 0–2 | 0.68 ± 0.62 | 0–2 | NS |
| 1PN | 0.13 ± 0.33 | 0–1 | 0.11 ± 0.37 | 0–2 | NS | |
| 3PN | 0.06 ± 0.24 | 0–1 | 0.09 ± 0.28 | 0–1 | NS | |
| Lysed | 0.04 ± 0.20 | 0–1 | 0.13 ± 0.33 | 0–1 | NS | |
| Zygotes survived | 0.40 ± 0.49 | 0–1 | 0.60 ± 0.61 | 0–2 | NS | |
| Cleavage stage embryos | 0.30 ± 0.46 | 0–1 | 0.47 ± 0.58 | 0–2 | NS | |
| Blastocysts | 0.19 ± 0.39 | 0–1 | 0.29 ± 0.55 | 0–2 | NS | |
| Molecular results | ALU 115 (ng/μl) | 0.79 ± 0.72 | 0.078–2.475 | 1.46 ± 1.59 | 0.11–6.5 | 0.02 |
| ALU 247 (ng/μl) | 0.15 ± 0.14 | 0.0353–0.53 | 0.55 ± 0.88 | 0.007–2.83 | NS | |
| cfDNA integrity | 0.24 ± 0.12 | 0.04–0.951 | 0.23 ± 0.19 | 0.03–0.71 | NS | |
FoPOR, follicular phase oocyte retrieval; LuPOR, luteal phase oocyte retrieval; U/S, ultrasound; PN, pronuleus/i; MI, metaphase I; MII, metaphase II; GV, germinal vehicle; cfDNA, cell-free DNA; NS, not significant; SD, standard deviation
Table 3.
Total number of examined parameters, from follicle to blastocyst, regarding FoPOR and LuPOR
| FoPOR | LuPOR | ||
|---|---|---|---|
| U/S follicle count | 83 | 83 | |
| Oocytes retrieved | 51 | 61 | |
| Maturation status of oocytes retrieved | MII (%) | 35 (68.63) | 51 (83.61) |
| MI (%) | 6 (11.76) | 4 (6.56) | |
| GV (%) | 7 (13.72) | 3 (5.88) | |
| Abnormal (%) | 5 (9.8) | 3 (5.88) | |
| Fertilization status following insemination | 2PN (%) | 22 (62.86) | 32 (62.74) |
| 1PN (%) | 6 (17.14) | 5 (9.8) | |
| 3PN (%) | 3 (8.57) | 4 (7.84) | |
| Lysed (%) | 2 (5.71) | 6 (11.76) | |
| Not fertilized (%) | 2 (5.71) | 4 (7.84) | |
| Zygotes survived following thaw (%) | 19 (86.36) | 28 (87.5) | |
| Cleavage stage embryos (%) | 14 (63.63) | 22 (68.75) | |
| Single cleavage stage ET | 4 | 5 | |
| Blastocysts (%) | 8 (44.44) | 12 (42.86) | |
| Blastocysts employed for ET | 8 | 12 | |
FoPOR, follicular phase oocyte retrieval; LuPOR, luteal phase oocyte retrieval; U/S, ultrasound; PN, pronuleus/i; MI, metaphase I; MII, metaphase II; GV, germinal vehicle; ET, embryo transfer; NS, not significant. Oocyte maturity is calculated per oocyte retrieved; normal or abnormal fertilization is calculated per MII oocyte; cleavage rate is per 2PN zygote; blastocyst formation rate is calculated per 2PN zygote, excluding embryos transferred on day 3
The mean levels of ALU115 present as statistically significantly lower during FoPOR when compared to LuPOR (0.79 ± 0.72 ng/μl vs 1.46 ± 1.59 ng/μl, p value = 0.02). No statistically significant difference was observed between the two groups neither regarding the concentration of ALU247 (0.07 ± 0.14 ng/μl vs 0.22 ± 0.47 ng/μl) nor the cfDNA integrity (0.15 ± 0.14 vs 0.55 ± 0.87 ng/μl). For both FoPOR and LuPOR, the cfDNA integrity on average was measured to be < 0.5 ng/μl, corresponding to mainly apoptotic events.
In the FoPOR group, the results revealed a statistically significant positive correlation of serum E2 levels and ALU115 concentration (p value = 0.04). ALU247 was not associated with any of the examined parameters, namely the number and maturation status of oocytes, the number of zygotes, as well as the number of abnormally fertilized and lysed oocytes. The cfDNA integrity was negatively correlated with serum E2 levels (p value = 0.03). This negative correlation was observed in the LuPOR group as well (p value = 0.03). No other statistically significant difference was observed between ALU115, ALU247, and cfDNA integrity for any of the examined parameters, with regard to the number and maturation status of oocytes, the number of zygotes, as well as the number of abnormally fertilized and lysed oocytes.
A lower number of oocytes was retrieved during FoPOR when compared to LuPOR (1.09 ± 0.28 vs 1.29 ± 0.58, p 00 = 0.02). Number of MII oocytes collected in FoPOR was also decreased when compared to LuPOR (0.77 ± 0.55 vs 1.08 ± 0.61, p value = 0.02). No statistically significant difference was observed regarding the number of 2PN zygotes, or the number of abnormally fertilized and lysed oocytes.
Clinical outcomes following thawing
No statistically significant difference was observed between FoPOR and LuPOR regarding number of zygotes survived, survival rate, number of cleavage stage embryos, cleavage rate, number of blastocysts, and blastocyst formation rate.
A total of 22 zygotes originating from FoPOR corresponding to 22 retrievals were cryopreserved. Nineteen out of 22 were successfully thawed (survival rate 86.37%), and 14 cleaved (cleavage rate 63.64%). Four cleavage stage embryos were transferred at that stage, employing single embryo transfer, and the remaining were cultured till blastocyst stage, leading to the development of 8 blastocysts (blastocyst formation rate 44.44%).
A total of 32 zygotes originating from LuPOR corresponding to 28 retrievals were cryopreserved. Twenty-eight out of 32 were successfully thawed (survival rate 87.50%), and 22 cleaved (cleavage rate 68.75%). Four cleavage stage embryos were transferred at that stage, employing single embryo transfers, and the remaining were cultured to the blastocyst stage, leading to the development of 12blastocysts (blastocyst formation rate 44.44%). All the blastocyst stage embryo transfers performed included 2–4 embryos; hence, clinical outcome data corresponding to blastocyst transfers is not presented herein as no result could not reflect data related strictly to the studied FoPOR or LuPOR cycles. The total number of zygotes that survived following thaw, cleavage stage embryos, and blastocysts are presented in Table 3.
Embryo cleavage in FoPOR was associated with cfDNA integrity, as lower levels of cfDNA integrity were observed in cycles with cleavage stage embryos, compared to cycles with embryos that failed to cleave (0.17 ± 0.10 vs 0.28 ± 0.13, p = 0.006). No statistically significantly difference was observed regarding the levels of ALU-115 or ALU-247. No similar association was observed in LuPOR. No association was observed regarding cleavage stage embryo quality and levels of ALU 115 both in FoPOR and in LuPOR. Top quality cleavage stage embryos, compared to non-top, presented with lower ALU 247 levels (0.07 ± 0.06 vs 0.58 ± 0.88, p = 0.04) and cfDNA integrity levels (0.09 ± 0.06 vs 0.31 ± 0.22, p = 0.01) in LuPOR. No similar association was observed in FoPOR.
Blastocyst formation was also associated with cfDNA integrity as lower levels of cfDNA integrity were observed in cycles with blastocysts, compared to cycles that failed to lead to blastocyst formation, both in FoPOR (0.13 ± 0.06 vs 0.28 ± 0.12 p = 0.04) and in LuPOR (0.11 ± 0.07 vs 0.27 ± 0.20, p = 0.008). In LuPOR originating data, blastocyst formation was also associated with ALU-247, as lower levels of cfDNA integrity were observed in cycles with blastocysts, compared to cycles with no blastocyst formation, in LuPOR (0.12 ± 0.18 vs 0.74 ± 1.01, p = 0.03). The levels of ALU 247 were statistically significantly lower in top-quality blastocysts compared to non-top in LuPOR (0.02 ± 0.02 vs 0.23 ± 0.19, p = 0.02). No similar association was observed in FoPOR. Statistically significant lower levels of cfDNA integrity were observed, both in FoPOR and in LuPOR, in blastocysts of top-quality compared to blastocysts of non-top quality (FoPOR: 0.07 ± 0.04 vs 0.17 ± 0.05, p = 0.03; LuPOR: 0.06 ± 0.02 vs 0.14 ± 0.06, p = 0.02).
Only nine frozen cycles included embryos originating strictly from either FoPOR or LuPOR. Out of the 4 single embryo transfers employing embryos originating from FoPOR only 1 led to a clinical pregnancy and a subsequent live birth. Out of the 5 single embryo transfers employing embryos from LuPOR, 2 of them led to a clinical pregnancy and 1 led to a subsequent live-birth. Mean levels of cfDNA concentration and integrity for both FoPOR and LuPOR groups, according to the developmental stage, are presented in Table 4.
Table 4.
Association of cfDNA concentrations and integrity with embryological data regarding FoPOR and LuPOR
| FoPOR | LuPOR | |||
|---|---|---|---|---|
| Cleaved zygote | Arrested zygote | Cleaved zygote | Arrested zygote | |
| ALU115 (ng/μl) | 0.96 ± 0.74 | 0.71 ± 0.71 | 0.98 ± 1.14 | 1.82 ± 1.77 |
| ALU247 (ng/μl) | 0.15 ± 0.15 | 0.15 ± 0.13 | 0.32 ± 0.65 | 0.72 ± 0.99 |
| cfDNA integrity | 0.17 ± 0.10* | 0.28 ± 0.13 | 0.21 ± 0.18 | 0.26 ± 0.20 |
| Blastocyst | Arrested prior to blastocyst stage | Blastocyst | Arrested prior to blastocyst stage | |
| ALU115 (ng/μl) | 1.03 ± 0.79 | 0.74 ± 0.71 | 0.75 ± 0.56 | 1.79 ± 1.80 |
| ALU247 (ng/μl) | 0.12 ± 0.10 | 0.17 ± 0.14 | 0.12 ± 0.18 | 0.74 ± 1.01 |
| cfDNA integrity | 0.13 ± 0.06* | 0.28 ± 0.12 | 0.11 ± 0.07* | 0.27 ± 0.20 |
FoPOR, follicular fluid oocyte retrieval; LuPOR, luteal phase oocyte retrieval; cfDNA, cell-free DNA, *statistically significant
Discussion
The time sensitive nature of poor responders related to the commonly anticipated advanced maternal age is undoubtable. Increased likelihood of IVF cycle cancellation, due to reasons such as failure of oocyte retrieval, or response to stimulation protocol, or even gametes’ fertilization failure has been increasingly documented [42]. Providing data delineating on the effectiveness and safe practice of LuPOR is of importance especially in light of the fact that LuPOR may in fact address the time issue for poor responders. This has been suggested to be achieved by ascertaining a higher yield of oocytes of an equally developmental capacity, in a shorter amount of time [4].
The current study included only natural ART cycles in an effort to limit potential detrimental effects in ff microenvironment, stemming from ovarian stimulation protocols [1]. It has been reported both in animal models [43] and in human studies [44] that COS protocols increase apoptosis in granulosa cells through the examined mechanism of caspase-8, -9, and -3, along with poly-(ADP-ribose)-polymerase cleavage. Furthermore, COS has been associated with preterm birth and low birth weight [45]. Nonetheless, the recent update by the working group of European Society of Human Reproduction and Embryology (ESHRE) in October 2019, on the eminent and highly anticipated review report publication on guidelines on ovarian stimulation, indicates how “young” the field of ART still is, and that fine and perhaps coarse tuning may be required towards establishing optimal practice. Concerning the efficiency of the natural cycles approach on this group, favorable pregnancy results have been documented [46]. Conclusively, this may appear to be a more cost-effective and patient-friendly line of management compared to stimulation protocols, especially in light of the promising IVF outcomes [47]. Based on the aforementioned, combining the natural cycle approach employing both FoPOR and LuPOR to serve the “freeze and collect” approach appears to be a valid option for this distinct cohort of patients. This may allow for the combination of both the mild approach of natural cycles, along with enabling a more time-efficient collection of cryopreserved embryos in storage.
This prospective study focuses on examining the second follicular wave phenomenon employing a combination of observations regarding the oocyte, and the subsequent embryos, in line with molecular approaches, during natural ICSI cycles for poor responders. A comparison of ff cfDNA levels resulting from FoPOR and LuPOR in a single menstrual cycle was performed, and respective associations were attempted. These included the number and maturation status of corresponding oocytes, as well as the number of subsequent normally or abnormally fertilized oocytes, the zygote survival rate, along with the cleavage stage embryo and number of blastocysts. In addition to the evaluation of specific morphological parameters regarding oocyte maturation and subsequent fertilization, molecular analysis of cfDNA in ff was conducted measuring ALU specific repeats, in order to assess the microenvironment of the oocyte employing a non-invasive tool. As aforementioned, ALU115 amplifies both short cfDNA fragments stemming from apoptosis, and long fragments stemming from necrosis, whereas ALU247 amplifies only long cfDNA fragments.
Our results indicate that the mean levels of ALU115 during FoPOR were statistically significantly lower, compared to LuPOR data. This may be attributed to the successive follicular recruitment during luteal phase. This entails the apoptosis of antral and preantral follicles during the developmental process leading to the formation of the dominant follicle [48]. It is widely known that atresia of antral follicles is mainly induced by apoptosis of surrounding granulosa cells. A recent study confirmed the appearance of receptors of cleaved caspase 3 (cCASP3)—a marker that intervenes in the pathway of apoptosis’ induction—in atretic antral follicles [49]. The latter process, in combination with the second follicular recruitment in a single menstrual cycle, may contribute to observing higher ff cfDNA levels during the luteal phase of the patients examined in the present study. Particularly, these patients present with a high level of FSH, even following FoPOR, in conjunction with increased E2 levels, leading to the development of a second follicular wave [10]. Moreover, the comparison between FoPOR and LuPOR data revealed no statistically significant difference, regarding the mean levels of ALU247 and the DNA integrity. According to our results, the value of the respective ratio was < 0.5 ng/μl, with regard to both FoPOR and LuPOR groups. This undoubtedly entails the prevalence of the apoptotic origin of cfDNA in both follicular and luteal phases of the same menstrual cycle. All the aforementioned observations indicate that necrosis may not represent the dominant event entailed during either the follicular or luteal phase. This may serve as reassuring data with regard to the peril of sudden cell death ultimately triggered by necrosis [50]. The latter is in accordance with available published data, indicating that cfDNA in human ff mainly originates from apoptotic events rather than necrotic ones [33, 40].
Interestingly, results demonstrated a statistically significant positive correlation of serum E2 levels and ALU115 levels originating from FoPOR, along with a statistically significant negative correlation of serum E2 levels and ff cfDNA integrity both in FoPOR and in LuPOR. The levels of cfDNA have been negatively associated with follicular levels of E2 in literature [33], indicating a possible indirect association with serum E2 levels, as follicular and serum levels of E2 are both correlated [51]. The aforementioned data suggests the involvement of E2 in the apoptosis process, as previously indicated by studies performed on animal models. Particularly, a potential mechanism that has been proposed refers to the impact of tumor necrosis factor alpha (TNF-α) on ovarian stereoidogenesis through the inhibition of E2 production. The above factor presents with a contributing role during the necrosis process [52], and thus it is assumed that high E2 levels go hand-in hand with apoptotic events. Similarly, when referring to LuPOR data, only a statistically significant negative correlation of serum E2 levels with cfDNA integrity was observed. This is in accordance with the abovementioned explanations. The levels of ff serum E2 have also been correlated. Finally, our results showed no statistically significant correlation of either ALU115, and ALU247 originating from FoPOR or LuPOR with the number of abnormally fertilized oocytes, or lysed oocytes, which confirms the equal developmental capacity of oocytes resulting from either follicular waves of the menstrual cycle. It should be mentioned that no correlation analysis was performed between follicle size and ALU levels, as all follicles aspirated were larger than 17 mm, as our study was performed on natural cycles.
Regarding IVF laboratory data, when assessing the oocytes’ parameters recorded for FoPOR and LuPOR, it was observed that a statistically significant lower number of oocytes was retrieved, in conjunction with fewer MII oocytes originating from FoPOR. This may render a biological paradox, even though observation from prospective and retrospective studies similarly recorded a higher number of oocytes retrieved, along with MII oocytes at LuPOR [5, 15, 19, 21]. Nonetheless, it should be noted that the above studies reported on the practice of a double ovarian stimulation during the follicular and luteal phase prior to performing FoPOR and LuPOR respectively. Interestingly, our results report no statistically significant differences regarding the number of 2PN zygotes, and abnormally fertilized or lysed oocytes post-insemination, cleavage stage embryos or blastocysts between FoPOR and LuPOR groups. This leads to the conclusion that both FoPOR and LuPOR appear equally efficient with regard to these outcome measures.
The levels of ff cfDNA integrity were associated with both cleavage and blastocyst formation rate in FoPOR cycles, as well as blastocyst formation rates in LuPOR cycles. In LuPOR cycles, a statistically significant correlation between ALU247 and lower blastocyst formation rate was observed. It is possible that the decreased blastocyst formation rate is attributed mainly to necrotic events. In LuPOR cycles, higher ALU 247 levels were associated with poorer quality embryos both referring to both cleavage and blastocyst stage. The integrity of cfDNA was associated with cleavage stage embryo quality in LuPOR cycles and with blastocyst quality both in FoPOR and in LuPOR cycles. The lack of statistically significant association between the ALU 115 levels, representing the total cfDNA concentration and cleavage rate and quality, contradicts current literature [33, 53]. This may be attributed to the smaller sample size of the present study, or the different study designs, as in the present study strictly natural cycles were employed. Employing natural cycles has possibly resulted to lower cfDNA levels, and thus a statistically significant difference may not be observed. However, in the present study, cfDNA integrity was observed to be associated with both embryo quality and cleavage and blastocyst formation rate. During LuPOR, this difference was observed to be depended on the necrotic events, represented by levels of ALU 247. The correlation between ff cfDNA integrity and cleavage or blastocyst formation rate has not been hitherto reported in literature. Further studies are required to ascertain that the validity of this association is not related to a type I statistical error, especially in the FoPOR group in our study. Necrosis and apoptosis—reflected by ALU in ff—are a result of granulosa cell death. However, the apoptotic granulosa cells continue to produce steroid hormones until the membrane is disrupted [54]. Steroid hormones have been positively associated with fertilization outcomes [55]. On the other hand, during necrosis, steroid hormone synthesis is disrupted. It may be possible that the difference in cf. DNA integrity between the developmentally arrested embryos and those reaching the blastocyst stage is attributed to increased necrosis and subsequent decreased steroid hormones levels in ff.
The present study includes both young poor responders, along with those aged over 40 years. This ascertains that the study is not weakened by being exposed to a risk of selection bias with respect to cfDNA integrity. Limitations of our study refer to the limited size of the studied population, as well as lack of data referring to the all-inclusive patients’ history, namely body max index, exercise activity or smoking, which may be identified as possible sources affecting cfDNA levels [50]. Another limitation of the present study is that embryo transfers did not include embryos originating solely from FoPOR or LuPOR cycles. Single embryo transfers would be the ideal design for the study. However, due to the fact that this design may challenge optimal clinical practice, the authors decided to proceed, abiding by the Greek legislation and patients’ desire, with at least double embryo transfers for the group of poor responders. It should be noted that in a small population, as the one employed in the current study, non-statistically significant differences may be of importance regarding interpretation of the results. Thus, the small sample size is a limitation and may be a source of type 2 statistical error. Although, no statistical trends were observed during the analysis, it is impressive that, nearly all of the parameters examined in the FoPOR group namely MII, 2PN, ALU115, and ALU247 are lower compared to LuPOR. The power analysis regarding the sample size of the present study was performed considering the levels of cfDNA, and expecting a medium effect size, as observed in studies with stimulation cycles. Furthermore, the issue of the half-life of cfDNA in the biological fluid of ff remains under investigation by literature. Indeed, performing FoPOR and LuPOR in the same menstrual cycle entails a double oocyte retrieval, a procedure that may lead to minor vaginal or even intraperitoneal bleeding. Nonetheless, the aforementioned are reported to be rarely observed during clinical practice by previous studies, [55]. The possibility of complications related to LuPOR should be thoroughly investigated, albeit they were not observed in this study.
In light of the prevalence of apoptotic events rather than necrotic ones, LuPOR appears to be a valid option. Our results shed light to the specific questions posed and examined herein. “How does LuPOR compared with FoPOR in regard to necrotic and apoptotic events?. LuPOR, being closer to menstruation—a mainly apoptotic process—presents with higher ALU 115. However, moving from the molecular to the clinical perspective, FoPOR failed to identify as superior to LuPOR, since LuPOR cycles presented with a higher number of oocytes retrieved and MII oocytes, while no difference was observed regarding the resulting number of cleavage stage embryos or blastocysts. These results render LuPOR as a promising approach for the management of poor responders.
Nonetheless, little is still known regarding optimal practice, while practitioners are faced with the lack of a widely accepted consensus. This adds another level of complexity regarding definition, diagnosis, and management of this distinct, time-sensitive group of poor ovarian reserve patients [56]. One of the focal points of this work concentrated on reporting back to the IVF practitioner regarding clinical practice and decision-making when considering the employment of LuPOR. Our study contributes data that may be interpreted by clinicals as pro LuPOR. In our view and according to hitherto collected and published data, the implementation of the combinatorial “FoPOR-LuPOR” approach during the same natural IVF cycle, may be a valid option for clinicians, providing a solution for time-efficient management of this category of patients. Conducting additional, well designed, randomized controlled trials to strengthen this evidence is essential. Employing a wider pallet of biomarkers, namely cytokines, in an attempt to investigate this more in depth, and concur on an efficient treatment for poor responders may be the way forward.
Aknowledgments
The present study was conducted as part of the M.Sc. program “Research in Female Reproduction” of Medical School, National and Kapodistrian University of Athens.
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest.
Footnotes
Sfakianoudis Konstantinos and Tsioulou Petroula are joint first authors.
In this article, the first names of the authors are in the position of the surnames, and vice versa. Meaning the author's given name is recognized as a surname.
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Change history
6/6/2023
A Correction to this paper has been published: 10.1007/s10815-023-02841-9
References
- 1.Jirge PR. Poor ovarian reserve. J Hum Reprod Sci. 2016;9:63–69. doi: 10.4103/0974-1208.183514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Polyzos NP, Devroey P. A systematic review of randomized trials for the treatment of poor ovarian responders: is there any light at the end of the tunnel? Fertil Steril. 2011;96:1058–1061. doi: 10.1016/j.fertnstert.2011.09.048. [DOI] [PubMed] [Google Scholar]
- 3.Ubaldi F, Vaiarelli A, D’Anna R, Rienzi L. Management of poor responders in IVF: is there anything new? BioMed Res Int. 2014;2014. [DOI] [PMC free article] [PubMed]
- 4.Sfakianoudis K, Simopoulou M, Maziotis E, Giannelou P, Tsioulou P, Rapani A, et al. Evaluation of the Second Follicular Wave Phenomenon in Natural Cycle Assisted Reproduction: A Key Option for Poor Responders through Luteal Phase Oocyte Retrieval. Med Kaunas Lith. 2019;55. [DOI] [PMC free article] [PubMed]
- 5.Kuang Y, Chen Q, Hong Q, Lyu Q, Ai A, Fu Y, et al. Double stimulations during the follicular and luteal phases of poor responders in IVF/ICSI programmes (Shanghai protocol) Reprod Biomed Online. 2014;29:684–691. doi: 10.1016/j.rbmo.2014.08.009. [DOI] [PubMed] [Google Scholar]
- 6.Adams G. Comparative patterns of follicle development and selection in ruminants. J Reprod Fertil Suppl. 1999;54:17–32. [PubMed] [Google Scholar]
- 7.Adams GP. Matteri R, Kastelic J, Ko J, Ginther Oj. Association between surges of follicle-stimulating hormone and the emergence of follicular waves in heifers. Reproduction. 1992;94:177–188. doi: 10.1530/jrf.0.0940177. [DOI] [PubMed] [Google Scholar]
- 8.Ginther O. Major and minor follicular waves during the equine estrous cycle. J Equine Vet Sci. 1993;13:18–25. doi: 10.1016/S0737-0806(07)80012-8. [DOI] [Google Scholar]
- 9.Ginther O, Kastelic J, Knopf L. Composition and characteristics of follicular waves during the bovine estrous cycle. Anim Reprod Sci. 1989;20:187–200. doi: 10.1016/0378-4320(89)90084-5. [DOI] [Google Scholar]
- 10.Baerwald AR, Adams GP, Pierson RA. A new model for ovarian follicular development during the human menstrual cycle. Fertil Steril. 2003;80:116–122. doi: 10.1016/S0015-0282(03)00544-2. [DOI] [PubMed] [Google Scholar]
- 11.Baerwald AR, Adams GP, Pierson RA. Ovarian antral folliculogenesis during the human menstrual cycle: a review. Hum Reprod Update. 2012;18:73–91. doi: 10.1093/humupd/dmr039. [DOI] [PubMed] [Google Scholar]
- 12.Vaiarelli A, Venturella R, Vizziello D, Bulletti F, Ubaldi FM. Dual ovarian stimulation and random start in assisted reproductive technologies: from ovarian biology to clinical application. Curr Opin Obstet Gynecol. 2017;29:153–159. doi: 10.1097/GCO.0000000000000365. [DOI] [PubMed] [Google Scholar]
- 13.Kuang Y, Hong Q, Chen Q, Lyu Q, Ai A, Fu Y, et al. Luteal-phase ovarian stimulation is feasible for producing competent oocytes in women undergoing in vitro fertilization/intracytoplasmic sperm injection treatment, with optimal pregnancy outcomes in frozen-thawed embryo transfer cycles. Fertil Steril. 2014;101:105–111. doi: 10.1016/j.fertnstert.2013.09.007. [DOI] [PubMed] [Google Scholar]
- 14.Jin B, Niu Z, Xu B, Chen Q, Zhang A. Comparison of clinical outcomes among dual ovarian stimulation, mild stimulation and luteal phase stimulation protocols in women with poor ovarian response. Gynecol Endocrinol Off J Int Soc Gynecol Endocrinol. 2018;34:694–697. doi: 10.1080/09513590.2018.1435636. [DOI] [PubMed] [Google Scholar]
- 15.Liu C, Jiang H, Zhang W, Yin H. Double ovarian stimulation during the follicular and luteal phase in women >/=38 years: a retrospective case-control study. Reprod Biomed Online. 2017;35:678–684. doi: 10.1016/j.rbmo.2017.08.019. [DOI] [PubMed] [Google Scholar]
- 16.Madani T, Hemat M, Arabipoor A, Khodabakhshi SH, Zolfaghari Z. Double mild stimulation and egg collection in the same cycle for management of poor ovarian responders. J Gynecol Obstet Hum Reprod. 2018. [DOI] [PubMed]
- 17.Rashtian J, Zhang J. Luteal-phase ovarian stimulation increases the number of mature oocytes in older women with severe diminished ovarian reserve. Syst Biol Reprod Med. 2018;64:216–219. doi: 10.1080/19396368.2018.1448902. [DOI] [PubMed] [Google Scholar]
- 18.Ubaldi FM, Capalbo A, Vaiarelli A, Cimadomo D, Colamaria S, Alviggi C, et al. Follicular versus luteal phase ovarian stimulation during the same menstrual cycle (DuoStim) in a reduced ovarian reserve population results in a similar euploid blastocyst formation rate: new insight in ovarian reserve exploitation. Fertil Steril. 2016;105:1488–1495. doi: 10.1016/j.fertnstert.2016.03.002. [DOI] [PubMed] [Google Scholar]
- 19.Vaiarelli A, Cimadomo D, Trabucco E, Vallefuoco R, Buffo L, Dusi L, et al. Double Stimulation in the Same Ovarian Cycle (DuoStim) to Maximize the Number of Oocytes Retrieved From Poor Prognosis Patients: A Multicenter Experience and SWOT Analysis. Front Endocrinol. 2018;9:317. doi: 10.3389/fendo.2018.00317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhang W, Wang M, Wang S, Bao H, Qu Q, Zhang N, et al. Luteal phase ovarian stimulation for poor ovarian responders. JBRA Assist Reprod. 2018;22:193–198. doi: 10.5935/1518-0557.20180045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhang Q, Guo XM, Li Y. Implantation rates subsequent to the transfer of embryos produced at different phases during double stimulation of poor ovarian responders. Reprod Fertil Dev. 2017;29:1178–1183. doi: 10.1071/RD16020. [DOI] [PubMed] [Google Scholar]
- 22.Cardoso MC de A, Evangelista A, Sartorio C, Vaz G, Werneck CLV, Guimaraes FM, et al. Can ovarian double-stimulation in the same menstrual cycle improve IVF outcomes? JBRA Assist Reprod. 2017;21:217–21. [DOI] [PMC free article] [PubMed]
- 23.Creux H, Monnier P, Son W-Y, Tulandi T, Buckett W. Immature oocyte retrieval and in vitro oocyte maturation at different phases of the menstrual cycle in women with cancer who require urgent gonadotoxic treatment. Fertil Steril. 2017;107:198–204. doi: 10.1016/j.fertnstert.2016.09.041. [DOI] [PubMed] [Google Scholar]
- 24.Maman E, Meirow D, Brengauz M, Raanani H, Dor J, Hourvitz A. Luteal phase oocyte retrieval and in vitro maturation is an optional procedure for urgent fertility preservation. Fertil Steril. 2011;95:64–67. doi: 10.1016/j.fertnstert.2010.06.064. [DOI] [PubMed] [Google Scholar]
- 25.Xu B, Li Y. Flexible ovarian stimulation in a poor responder: a case report and literature review. Reprod Biomed Online. 2013;26:378–383. doi: 10.1016/j.rbmo.2012.11.020. [DOI] [PubMed] [Google Scholar]
- 26.Dyr B, Boomer T, Almasri EA, Wardrop JL, Rafalko J, Chibuk J, et al. A new era in aneuploidy screening: cfDNA testing in> 30,000 multifetal gestations: Experience at one clinical laboratory. PloS One. 2019;14 (8). [DOI] [PMC free article] [PubMed]
- 27.Li B, Pu K, Ge L, Wu X. Diagnostic significance assessment of the circulating cell-free DNA in ovarian cancer: An updated meta-analysis. Gene. 2019;143993. [DOI] [PubMed]
- 28.Gil M, Galeva S, Jani J, Konstantinidou L, Akolekar R, Plana MN, et al. Screening for trisomies by cfDNA testing of maternal blood in twin pregnancy: update of The Fetal Medicine Foundation results and meta‐analysis. Ultrasound Obstet Gynecol. 2019 53(6):734-742. [DOI] [PubMed]
- 29.Czamanski-Cohen J, Sarid O, Cwikel J, Lunenfeld E, Douvdevani A, Levitas E, et al. Increased plasma cell-free DNA is associated with low pregnancy rates among women undergoing IVF–embryo transfer. Reprod Biomed Online. 2013;26:36–41. doi: 10.1016/j.rbmo.2012.09.018. [DOI] [PubMed] [Google Scholar]
- 30.Streleckiene G, Reid HM, Arnold N, Bauerschlag D, Forster M. Quantifying cell free DNA in urine: comparison between commercial kits, impact of gender and inter-individual variation. BioTechniques. 2018;64:225–230. doi: 10.2144/btn-2018-0003. [DOI] [PubMed] [Google Scholar]
- 31.Dimopoulou M, Anifandis G, Messini CI, Dafopoulos K, Kouris S, Sotiriou S, et al. Follicular fluid oocyte/cumulus-free DNA concentrations as a potential biomolecular marker of embryo quality and IVF outcome. BioMed Res Int. 2014;2014. [DOI] [PMC free article] [PubMed]
- 32.Guan Y, Zhang W, Wang X, Cai P, Jia Q, Zhao W. Cell-free DNA induced apoptosis of granulosa cells by oxidative stress. Clin Chim Acta Int J Clin Chem. 2017;473:213–217. doi: 10.1016/j.cca.2016.11.023. [DOI] [PubMed] [Google Scholar]
- 33.Scalici E, Traver S, Molinari N, Mullet T, Monforte M, Vintejoux E, et al. Cell-free DNA in human follicular fluid as a biomarker of embryo quality. Hum Reprod. 2014;29:2661–2669. doi: 10.1093/humrep/deu238. [DOI] [PubMed] [Google Scholar]
- 34.Hussein NA, Mohamed SN, Ahmed MA. Plasma ALU-247, ALU-115, and cfDNA Integrity as Diagnostic and Prognostic Biomarkers for Breast Cancer. Appl Biochem Biotechnol. 2019;187:1028–1045. doi: 10.1007/s12010-018-2858-4. [DOI] [PubMed] [Google Scholar]
- 35.Ferraretti AP, La Marca A, Fauser BCJM, Tarlatzis B, Nargund G, Gianaroli L, et al. ESHRE consensus on the definition of “poor response” to ovarian stimulation for in vitro fertilization: the Bologna criteria. Hum Reprod. 2011;26:1616–1624. doi: 10.1093/humrep/der092. [DOI] [PubMed] [Google Scholar]
- 36.Lazzaroni-Tealdi E, Barad DH, Albertini DF, Yu Y, Kushnir VA, Russell H, et al. In: Kim S, editor. Oocyte Scoring Enhances Embryo-Scoring in Predicting Pregnancy Chances with IVF Where It Counts Most, vol. 10: PLOS ONE; 2015; 10(12). [DOI] [PMC free article] [PubMed]
- 37.ALPHA Scientists In Reproductive Medicine, ESHRE Special Interest Group Embryology. Istanbul consensus workshop on embryo assessment: proceedings of an expert meeting. Reprod Biomed Online. 2011;22:632–646. doi: 10.1016/j.rbmo.2011.02.001. [DOI] [PubMed] [Google Scholar]
- 38.Gardner DK, Schoolcraft WB. Culture and transfer of human blastocysts. Curr Opin Obstet Gynecol. 1999;11:307–311. doi: 10.1097/00001703-199906000-00013. [DOI] [PubMed] [Google Scholar]
- 39.Leon G, Papetta A, Spiliopoulou C. Overview of the Greek legislation regarding assisted reproduction and comparison with the EU legal framework. Reprod Biomed Online. 2011;23:820–823. doi: 10.1016/j.rbmo.2011.07.024. [DOI] [PubMed] [Google Scholar]
- 40.Traver S, Scalici E, Mullet T, Molinari N, Vincens C, Anahory T, et al. Cell-free DNA in human follicular microenvironment: New prognostic biomarker to predict in vitro fertilization outcomes. PLoS ONE. 2015;10:1–18. [DOI] [PMC free article] [PubMed]
- 41.Umetani N. Increased Integrity of Free Circulating DNA in Sera of Patients with Colorectal or Periampullary Cancer: Direct Quantitative PCR for ALU Repeats. Clin Chem. 2006;52:1062–9.. [DOI] [PubMed]
- 42.Gonda KJ, Domar AD, Gleicher N, Marrs RP. Insights from clinical experience in treating IVF poor responders. Reprod Biomed Online. 2018;36:12–9. [DOI] [PubMed]
- 43.Park EJ, Shin JjW, Seo YS, Kim DW, Hong SY, Park WI, et al. Gonadotropin-releasing hormone-agonist induces apoptosis of human granulosa-luteal cells via caspase-8,-9 and-3, and poly-(ADP-ribose)-polymerase cleavage. Biosci Trends. 2011;5:120–8. [DOI] [PubMed]
- 44.Liu S, Feng HL, Marchesi D, Chen Z-J, Hershlag A. Dose-dependent effects of gonadotropin on oocyte developmental competence and apoptosis. Reprod Fertil Dev. 2011;23:990–6. [DOI] [PubMed]
- 45.Kamath MS, Kirubakaran R, Mascarenhas M, Sunkara SK. Perinatal outcomes after stimulated versus natural cycle IVF: a systematic review and meta-analysis. Reprod Biomed Online. 2018;36:94–101. [DOI] [PubMed]
- 46.Schimberni M, Morgia F, Colabianchi J, Giallonardo A, Piscitelli C, Giannini P, et al. Natural-cycle in vitro fertilization in poor responder patients: a survey of 500 consecutive cycles. Fertil Steril. 2009;92:1297–301. [DOI] [PubMed]
- 47.Practice Committee of the American Society for Reproductive Medicine. Comparison of pregnancy rates for poor responders using IVF with mild ovarian stimulation versus conventional IVF: a guideline. Fertil Steril. 2018;109:993–9. [DOI] [PubMed]
- 48.Matsuda F, Inoue N, Manabe N, Ohkura S. Follicular Growth and Atresia in Mammalian Ovaries: Regulation by Survival and Death of Granulosa Cells. J Reprod Dev. 2012;58:44–50. [DOI] [PubMed]
- 49.Matsuda F, Inoue N, Manabe N, Ohkura S. Follicular Growth and Atresia in Mammalian Ovaries: Regulation by Survival and Death of Granulosa Cells. J Reprod Dev. 2012;58:44–50. [DOI] [PubMed]
- 50.Aucamp J, Bronkhorst AJ, Badenhorst CPS, Pretorius PJ. The diverse origins of circulating cell-free DNA in the human body: a critical reevaluation of the literature: The diverse origins of circulating cell-free DNA. Biol Rev. 2018;93:1649–83. [DOI] [PubMed]
- 51.von Wolff M, Stute P, Eisenhut M, Marti U, Bitterlich N, Bersinger NA. Serum and follicular fluid testosterone concentrations do not correlate, questioning the impact of androgen supplementation on the follicular endocrine milieu. Reprod Biomed Online. 2017;35:616–23. [DOI] [PubMed]
- 52.Sakumoto R, Shibaya M, Okuda K. Tumor necrosis factor-alpha (TNF alpha) inhibits progesterone and estradiol-17beta production from cultured granulosa cells: presence of TNFalpha receptors in bovine granulosa and theca cells. J Reprod Dev. 2003;49:441–9. [DOI] [PubMed]
- 53.Regan SL, Knight PG, Yovich JL, Leung Y, Arfuso F, Dharmarajan A. Granulosa cell apoptosis in the ovarian follicle—a changing view. Front Endocrinol. 2018;9:61. [DOI] [PMC free article] [PubMed]
- 54.Lamb JD, Zamah AM, Shen S, McCulloch C, Cedars MI, Rosen MP. Follicular fluid steroid hormone levels are associated with fertilization outcome after intracytoplasmic sperm injection. Fertil Steril. 2010;94:952–7. [DOI] [PMC free article] [PubMed]
- 55.Gameiro S, Boivin J, Peronace L, Verhaak CM. Why do patients discontinue fertility treatment? A systematic review of reasons and predictors of discontinuation in fertility treatment. Hum Reprod Update. 2012;18:652–69. [DOI] [PMC free article] [PubMed]
- 56.Polyzos NP, Devroey P. A systematic review of randomized trials for the treatment of poor ovarian responders: is there any light at the end of the tunnel? Fertil Steril. 2011;96:1058–1061.e7. doi: 10.1016/j.fertnstert.2011.09.048. [DOI] [PubMed] [Google Scholar]


