Abstract
Purpose
To identify factors predictive of having supernumerary embryos in a fresh IVF cycle and create a prediction model for clinical counseling.
Methods
We utilized a multivariable Poisson regression to identify predictive factors and then entered these into a logistic regression model, calculating a risk index for each significant variable. The final model was tested using a receiver operating characteristic curve.
Results
A total of 60,616 fresh transfer cycles were reported to the Society for Assisted Reproductive Technology in 2014. Of these, 47.17% produced supernumerary embryos. A multivariate Poisson regression identified factors predictive of having supernumerary embryos, with age and AMH being the most predictive. Clinical prediction models were developed with acceptable and excellent discrimination. 23.5% of our cohort did not achieve a live birth following their fresh transfer and had excess embryos cryopreserved for future attempts.
Conclusion
Our study suggests that in a minority of fresh IVF cycles in the USA, the fresh transfer is not successful, and there are excess embryos cryopreserved for future use. The likelihood of excess embryos beyond those that would be transferred can be predicted with satisfactory precision prior to initiation of the cycle and with improved precision after fresh embryo transfer. Providing patients with a realistic estimate of their chances of having excess embryos at an initial IVF consult especially those with suspected poor prognosis can be beneficial in determining whether to proceed with multiple embryo banking cycles as opposed to proceeding with a fresh transfer, and whether to opt for an enhanced embryo selection technique such as preimplantation genetic testing for aneuploidy (PGT-A).
Electronic supplementary material
The online version of this article (10.1007/s10815-020-01731-8) contains supplementary material, which is available to authorized users.
Keywords: Prediction model, Counseling tool, Embryo selection, Fresh IVF cycle, Preimplantation genetic testing
Introduction
In vitro fertilization (IVF) is a challenging process for patients to undertake, physically, psychologically, and financially [1]. The ASRM Ethics Committee has noted the propensity of patients to overestimate their chance of success with IVF and highlights the importance of counseling patients on their prognosis, as well as the risks and costs of treatment [2]. Live birth is, of course, the most important outcome of IVF, and should be primary in patient counseling [3]. Patients undergoing IVF, in conjunction with their physicians, must make a number of decisions regarding the specifics of an IVF plan, including planning a fresh or cryopreserved embryo transfer, transfer at the cleavage or blastocyst stage, use of preimplantation genetic testing for aneuploidy (PGT-A), and potential utilization of other selection technologies offered by some clinics, such as time lapse embryo selection. The most studied embryo selection technique, PGT-A has been regarded as the optimal method to select embryos with a high implantation potential, while avoiding transfer of embryos destined to result in failed implantation or a miscarriage [4–6]. Studies focusing on implantation rate and live birth per embryo transfer have shown these parameters to be increased with PGT-A [7]. However, a recent randomized controlled trial demonstrated no significant difference in ongoing pregnancy rates when embryos were selected for transfer based on morphology compared to PGT-A [8]. The largest randomized controlled trials of PGT-A have required a minimum of 2 embryos for inclusion [8–10]. Thus, understanding the likelihood that a patient will achieve this milestone is vital in determining whether the results of these trials are applicable to individual patients.
The impact of these decisions on outcome of the IVF cycle may vary based on the patient’s response to stimulation [11]. For an embryo selection technique to increase the chances of live birth in the first embryo transfer, there must be a cohort of embryos from which to select. Moreover, patients desiring more than one child may plan multiple egg retrievals prior to an embryo transfer to increase the chances of future children from cryopreserved embryos. Providing precise information on the likelihood of surrogate outcomes such as excess cryopreserved embryos can aid patients in decision-making and enhance their ability to achieve their reproductive goals in the most timely and cost-effective fashion. At present, we have no counseling tools for women regarding their chances of having excess embryos after a fresh IVF cycle. Therefore, using the Society for Assisted Reproductive Technology (SART) database, our objective is to determine what proportion of IVF cycles in the USA in 2014, in which women underwent a fresh embryo transfer, produced supernumerary embryos for cryopreservation, and assess factors predictive of having supernumerary embryos. We created a prediction model based on identified factors for clinical counseling.
Materials and methods
Study design
This was a study of women who underwent a fresh autologous IVF cycle in 2014. Data were obtained from the SART database. This work was approved in advance by the University of Utah IRB as exempt research and was also approved by the SART Research Committee. Data were collected and verified by SART and reported to the Centers for Disease Control and Prevention in compliance with the Fertility Clinic Success Rate and Certification Act of 1992 (Public Law 102-493). The data in the SART CORS are validated annually with some clinics having on-site visits for chart review based on an algorithm for clinic selection. During each visit, data reported by the clinic were compared with information recorded in patients’ charts. Ten out of 11 data fields selected for validation were found to have discrepancy rates of ≤ 5% (Center for Disease Control and Prevention, American Society for Reproductive Medicine, and Society for Assisted Reproductive Technology, 2012 Assisted Reproductive Technology Success Rates: National Summary and Fertility Clinic Reports, Washington, DC: US Dept. of Health and Human Services, 2014). We included cycles where a fresh transfer was performed. We excluded freeze-all cycles, frozen embryo transfer cycles, and cycles where preimplantation genetic testing (PGT) was performed. We defined supernumerary as the presence of one or more embryos cryopreserved following a fresh IVF transfer.
Statistical analysis
Data were analyzed using STATA (version 15). We computed the incidence proportion of supernumerary embryos in our cohort. To identify factors predictive of having supernumerary embryos, we first fitted a univariable Poisson regression model with a robust variance estimate. We then combined all variables with p value < .20 into a multivariable Poisson regression model and eliminated non-significant variables using an interactive backwards variable selection to arrive at the final model.
Prediction model derivation
After identifying all patient-level and cycle-level variables predictive of supernumerary embryos, we first entered patient-level variables into a logistic regression model using an inclusion criterion of 1% to ensure model stability. We chose to initially separate patient-level predictors from cycle-level predictors so as to be able to provide a predicted probability of having supernumerary embryos whenever a patient presents for their initial IVF consultation. Subsequently, we created a second model that also incorporated cycle-level predictors such as number of oocytes retrieved and stage and number of embryos transferred. Any variable that did not alter the area under the curve for the receiver operating characteristic curve (ROC) was removed from the prediction model. We then utilized methods described by Sullivan et al. [12] to modify the final logistic model into a risk index. The number of points assigned to each significant covariate equaled its regression coefficient divided by the parameter estimated in the model with the smallest absolute value rounded to the nearest whole number. The accuracy of the derived model was tested with an ROC curve. A subsequent final model was designed using the same methodology but included the cycle-level predictive variables. A general rule of thumb with interpreting area under the ROC curve (AUC) follows this principle: AUC = 0.5 suggests no discrimination and the model is no better than flipping a coin, 0.7 > AUC < 0.8 is considered acceptable discrimination, 0.8 > AUC < 0.9 is considered excellent discrimination while AUC > 0.9 is considered outstanding discrimination. In clinical practice, an AUC > 0.7 is an acceptable tool and it is extremely unusual to observe an AUC > 0.9 [13, 14]
Results
Incidence of supernumerary embryos
There were 141,020 cycles reported to SART in 2014. Of these, 42,526 were excluded as frozen embryo transfer cycles, 467 cycles were excluded because cycles started as a fresh transfer but ultimately thawed embryos were transferred, 5850 cycles were excluded because PGT was performed, and 31,541 cycles were excluded because all embryos were cryopreserved (Fig. 1). Subsequently, 60,616 cycles with a fresh embryo transfer in 2014 were included in our analysis. Of these, 28,590 (47.17%, 95% CI 46.77–47.56%) produced supernumerary embryos. These cycles had an average of 4 embryos cryopreserved after a fresh transfer. Table 1 depicts the number of excess embryos cryopreserved by age and embryo stage of development as utilized by the American Society for Reproductive Medicine, with means and standard deviations (SD) [15].
Fig. 1.

Flow chart of study subjects
Table 1.
Mean embryos cryopreserved by age and embryo stage at transfer in cycles where supernumerary embryos were produced
| Age (years) | < 35 | 35–37 | 38–40 | 41, 42 | > 42 |
|---|---|---|---|---|---|
| Mean (SD) embryos cryopreserved after cleavage stage transfer | 3 (3) | 3 (3) | 3 (2) | 2 (2) | 3 (2) |
| Mean (SD) embryos cryopreserved after blastocyst stage transfer | 4 (3) | 4 (3) | 3 (2) | 2 (2) | 2 (2) |
Relationship between supernumerary embryos and live birth
Of 28,590 cycles with supernumerary embryos following fresh transfers in 2014, 14,347 (50.18%, 95% CI 49.60–50.76%) achieved a live birth following the fresh transfer. There were 30,026 cycles where supernumerary embryos were not produced following a fresh transfer in 2014. Of these, only 8538 (26.66%, 95% CI 26.18–27.15%) achieved a live birth following their fresh transfer. Live birth rates, following the initial fresh transfer, were significantly different between these two groups (p < .001). Of the cohort, 23.5% did not achieve a live birth and had supernumerary embryos cryopreserved for future attempts. This is the proportion of cycles in which an enhanced selection technique could increase the chance of live birth.
Factors predictive of having supernumerary embryos
We included 29,496 cycles in our multivariate analyses after excluding subjects with missing anti-mullerian hormone (AMH) levels, BMI, and gravidity status. Characteristics predictive of having supernumerary embryos are presented in Table 2 with the referent category in parentheses, univariable risk ratios, adjusted risk ratios, and p values provided.
Table 2.
Poisson regression model identifying factors predictive of having supernumerary embryos
| Variable (referent) | Risk ratio (95% CI) | p value | Adjusted risk ratio (95% CI) | p value | |
|---|---|---|---|---|---|
| Age (< 35) | 35–37 | 0.80 (0.78–0.81) | < .001 | 0.98 (0.96–1.01) | .20 |
| 38–40 | 0.54 (0.53–0.56) | < .001 | 0.88 (0.85–0.91) | < .001 | |
| 41–42 | 0.28 (0.26–0.29) | < .001 | 0.60 (0.55–0.66) | < .001 | |
| > 42 | 0.11 (0.10–0.13) | < .001 | 0.30 (0.25–0.36) | < .001 | |
| BMI (18.5–24.9) | < 18.5 | 0.99 (0.93–1.05) | .634 | 0.99 (0.93–1.04) | .614 |
| 25.0–29.9 | 0.96 (0.94–0.98) | < .001 | 0.98 (0.96–1.00) | .081 | |
| > 30 | 0.95 (0.92–0.97) | < .001 | 0.96 (0.94–0.99) | .007 | |
| AMH (1.0–3.0) | < 1.0 | 0.48 (0.46–0.50) | < .001 | 0.88 (0.85–0.92) | < .001 |
| > 3.0 | 1.41 (1.38–1.44) | < .001 | 1.00 (0.98–1.03) | .737 | |
| Nulligravida | Gravida 1+ | 0.87 (0.85–0.88) | < .001 | 1.00 (0.98–1.02) | .831 |
| Prior fresh transfer (0) | 1 | 0.77 (0.75–0.78) | < .001 | 0.86 (0.84–0.89) | < .001 |
| 2 | 0.60 (0.58–0.63) | < .001 | 0.76 (0.72–0.81) | < .001 | |
| 3 | 0.49 (0.46–0.52) | < .001 | 0.75 (0.69–0.81) | < .001 | |
| 4+ | 0.35 (0.54–0.55) | < .001 | 0.72 (0.65–0.81) | < .001 | |
| Prior frozen transfer (0) | 1 | 1.05 (1.02–1.09) | < .001 | 1.23 (1.17–1.29) | < .001 |
| 2 | 1.22 (1.16–1.28) | < .001 | 1.35 (1.26–1.44) | < .001 | |
| 3 | 1.27 (1.17–1.38) | < .001 | 1.35 (1.22–1.50) | < .001 | |
| 4+ | 1.28 (1.15–1.42) | < .001 | 1.44 (1.25–1.65) | < .001 | |
| # eggs retrieved (1–6) | 7–9 | 2.92 (2.78–3.07) | < .001 | 2.35 (2.19–2.53) | < .001 |
| 10–13 | 4.25 (4.06–4.46) | < .001 | 2.93 (2.73–3.15) | < .001 | |
| 14–18 | 5.46 (5.21–5.71) | < .001 | 3.35 (3.12–3.60) | < .001 | |
| 19–35+ | 6.43 (6.15–6.72) | < .001 | 3.65 (3.39–3.93) | < .001 | |
| Sperm source (partner) | Donor | 0.95 (0.92–0.99) | .012 | 1.05 (1.01–1.10) | .022 |
| ICSI (no ICSI) | All mature | 0.93 (0.92–0.95) | < .001 | 0.97 (0.95–0.99) | .023 |
| Some mature | 1.18 (1.14–1.22) | < .001 | 0.94 (0.90–0.98) | .008 | |
| Day of transfer (5) | 2 | 0.24 (0.21–0.26) | < .001 | 0.84 (0.77–0.93) | .001 |
| 3 | 0.36 (0.35–0.37) | < .001 | 0.70 (0.66–0.74) | < .001 | |
| 4 | 0.45 (0.41–0.50) | < .001 | 0.71 (0.61–0.82) | < .001 | |
| 6 | 0.89 (0.86–0.92) | < .001 | 0.88 (0.84–0.92) | < .001 | |
| Blastocyst transfer | Cleavage transfer | 0.41 (0.40–0.42) | < .001 | 0.81 (0.78–0.85) | < .001 |
| Single embryo transfer | 2 | 0.86 (0.85–0.88) | < .001 | 0.89 (0.87–0.91) | < .001 |
| 3 | 0.35 (0.34–0.37) | < .001 | 0.59 (0.55–0.63) | < .001 | |
| 4 | 0.20 (0.18–0.23) | < .001 | 0.41 (0.33–0.51) | < .001 | |
| 5 | 0.18 (0.15–0.23) | < .001 | 0.52 (0.36–0.74) | < .001 | |
*all adjusted risk ratios with a p-value < .05 are provided in italics
AMH was missing in 44% of cycles reported to SART where a fresh transfer occurred in 2014. In order to evaluate for selection bias, we performed a comparison of significant predictors in the model between cycles with missing AMH and those with AMH provided. The results are summarized in supplemental table 1.
Prediction model derivation
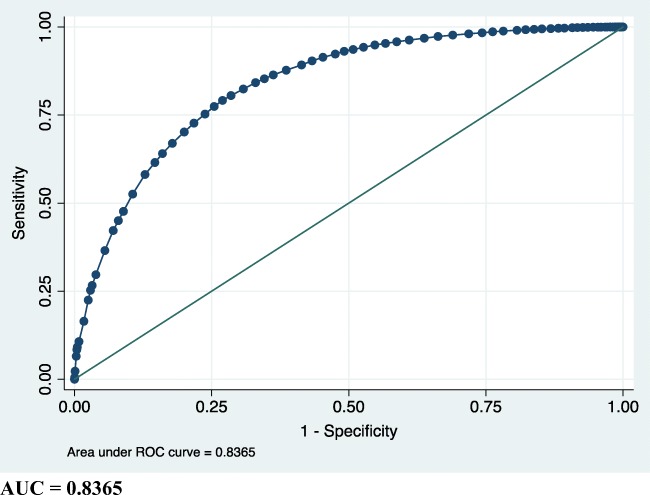
Age, AMH, and number of prior fresh/frozen cycles were necessary predictors included in the model with age and AMH being the most significant covariates (supplemental table 2). BMI and gravidity were excluded because they did not alter the area under the curve for the ROC curve. Therefore, we included a total of 34,191 cycles into the final logistic regression model excluding only those with missing AMH (Fig. 2). Points assigned to these covariates are included in Table 3. Using the summative score of the model, we can predict the probability of the outcome of supernumerary embryos (Table 4). Including the cycle-level covariates increased the accuracy of the prediction model by 10% (Fig. 3).
Fig. 2.
ROC of prediction model utilizing only patient-level variables
Table 3.
Points assigned to patient-level variables
| Variable (referent)* | Points | |
|---|---|---|
| Age (< 35) | 35–37 | − 1 |
| 38–40 | − 3 | |
| 41–42 | − 6 | |
| > 42 | − 10 | |
| AMH (1.0–3.0) | < 1.0 | − 3 |
| > 3.0 | + 2 | |
| Prior fresh cycles (0) | 1 | − 2 |
| 2 | − 3 | |
| 3 | − 4 | |
| 4+ | − 4 | |
| Prior frozen cycles (0) | 1 | + 3 |
| 2 | + 4 | |
| 3 | + 4 | |
| 4+ | + 6 | |
*Referent category is assigned zero points
Table 4.
Predicted probability of the outcome of supernumerary embryos by patient-level score
| Score | Probability supernumerary (%) | Binary decision cut-point for success | ROC area (%) | Sensitivity (%) 95% CI | Specificity (%) 95% CI |
|---|---|---|---|---|---|
| 0 | 1 | ≥ 0 | 50 | 100 | 0 |
| 1 | 2 | ≥ 1 | 51 | 100 (99.9–100) | .94 (.81–1.1) |
| 2 | 2 | ≥ 2 | 51 | 100 (99.9–100) | 1.61 (1.43–1.81) |
| 3 | 3 | ≥ 3 | 51 | 99.9 (99.9–100) | 2.72 (2.49–2.97) |
| 4 | 4 | ≥ 4 | 52 | 99.9 (99.8–99.9) | 3.22 (2.97–3.49) |
| 5 | 5 | ≥ 5 | 53 | 99.8 (99.7–99.9) | 6.11 (5.76–6.47) |
| 6 | 7 | ≥ 6 | 54 | 99.6 (99.5–99.7) | 7.72 (7.34–8.12) |
| 7 | 9 | ≥ 7 | 55 | 99.4 (99.3–99.5) | 9.50 (9.08–9.94) |
| 8 | 12 | ≥ 8 | 56 | 99.0 (98.9–99.2) | 12.6 (12.1–13.1) |
| 9 | 15 | ≥ 9 | 58 | 98.2 (98.0–98.4) | 17.7 (17.1–18.2) |
| 10 | 19 | ≥ 10 | 60 | 97.2 (96.9–97.4) | 22.5 (21.9–23.1) |
| 11 | 24 | ≥ 11 | 61 | 96.3 (96.0–96.5) | 24.8 (24.2–25.5) |
| 12 | 30 | ≥ 12 | 64 | 92.9 (92.5–93.3) | 35.7 (35.0–36.5) |
| 13 | 36 | ≥ 13 | 66 | 89.8 (89.3–90.2) | 41.4 (40.6–42.1) |
| 14 | 43 | ≥ 14 | 67 | 86.0 (85.5–86.5) | 48.1 (47.3–48.8) |
| 15 | 50 | ≥ 15 | 69 | 75.6 (74.9–76.2) | 61.6 (60.9–62.3) |
| 16 | 58 | ≥ 16 | 68 | 67.3 (66.5–68.0) | 68.5 (67.8–69.2) |
| 17 | 64 | ≥ 17 | 67 | 56.4 (55.6–57.1) | 76.6 (76.0–77.2) |
| 18 | 71 | ≥ 18 | 60 | 31.3 (30.6–32.0) | 89.6 (89.1–90.0) |
| 19 | 76 | ≥ 19 | 51 | 2.78 (2.54–3.05) | 99.3 (99.2–99.4) |
| 20 | 81 | ≥ 20 | 50 | .79 (.66–.94) | 99.8 (99.7–99.9) |
| 21 | 85 | ≥ 21 | 50 | .16 (.10–.23) | 100 (99.9–100) |
| 22 | 88 | ≥ 22 | 50 | .05 (.02–.10) | 100 (100–100) |
*Score = summative points (Table 3) + 17 (automatically sets the minimum score to zero)
Fig. 3.
ROC of prediction model utilizing both patient-level and cycle-level characteristics
Obtaining the score for a particular patient allows the clinician to look up the probability of the patient having supernumerary embryos in a fresh IVF cycle (Table 4, column 2). That is informative, but having a binary test that provides a simple yes or no determination is still desirable. Such a test can be constructed as (yes, or positive = that score or higher; no, or negative = a smaller score). The accuracy of such a test is measured by the test characteristics: sensitivity, specificity, and area under the receiver operating characteristic curve (ROC area). The ROC area for a binary test is the average, (sensitivity + specificity)/2. To illustrate, suppose the patient’s score is 15. If the clinician chose to define the binary test as positive if a score of 15 or higher is observed, the sensitivity of such a test would be 75.6% and the specificity would be 61.6%. If we needed sensitivity to be higher, we could use a smaller score for the cutoff value, as long as we were willing to accept an accompanying decrease in specificity (as sensitivity goes up, specificity always goes down). For a clinician who desires to avoid thinking about sensitivity and specificity and simply wants them both to be high, the score that maximizes the ROC area is the cutoff of choice. Table 4, column 4, shows that a score of 15 has the highest ROC area and reasonably high values of sensitivity and specificity. A score of 15 is also the cutoff that has a supernumerary embryos probability of 50% (the cutoff that begins to beat the probability of a coin flip decision).
Discussion
One of the key findings of this study is the strong association between supernumerary embryos and live birth in a fresh cycle, with cycles resulting in live birth having a nearly 2-fold higher chance of having embryos remaining for cryopreservation after a fresh transfer. This is not surprising, as many of the factors predictive of supernumerary embryos are also well-established predictors of ongoing pregnancy or live birth in IVF, including age, AMH, number of eggs retrieved, and blastocyst transfer [16–18]. Because of this strong association, in fewer than one-fourth of the included cycles, the fresh embryo transfer failed, and there were excess embryos available for cryopreservation. These are the cycles in which enhanced embryo selection might have led to a live birth in the first embryo transfer. This suggests that for the majority of couples, opting for enhanced selection is unlikely to improve the chances of a live birth in the first embryo transfer cycle—for those with a good prognosis, live birth from a fresh embryo transfer is likely, while for those with poorer prognoses, there are unlikely to be excess embryo from which to choose.
Preimplantation genetic testing for aneuploidy is a selection technique that is widely utilized in IVF cycles worldwide [19]. Randomized controlled trials that have demonstrated benefits of preimplantation genetic testing have excluded patients with a low likelihood of excess embryos for selection [9, 10, 20–22]. When patients are offered such a technology, it is important that they understand the applicability of these studies to their individual patient factors. Patients should be counseled that it is for a minority of patients that enhanced selection could increase the chances of a live birth. This is important, as PGT-A and other selection techniques add cost and complexity to an IVF cycle. Also of importance is that planning PGT-A generally prevents a fresh transfer, thus delaying potential pregnancy. Perhaps, fresh transfer of the embryo with the best morphology and PGT-A of remaining embryos that meet criteria for cryopreservation may be a better strategy to allow for a chance of pregnancy in the shortest possible time, while optimizing chances of success for future transfers in those patients who do have supernumerary embryos. It is important to note that patients may opt for selection techniques such as PGT-A for other reasons, including a decreased risk of miscarriage in some, but not all studies, and fewer transfers to achieve a live birth [9, 20]. Furthermore, we know that age is a significant risk factor for aneuploidy and there are counseling tools available for embryo aneuploidy rates after PGT-A by age [23]. Utilizing this information in addition to the prediction model derived from this study will provide patients with a more realistic understanding of their chances not only of live birth with an IVF cycle, but also of having an embryo available to transfer.
Using the SART database, we have identified factors predictive of having excess embryos available for cryopreservation after a fresh embryo transfer. Using prognostic variables known prior to initiation of an IVF cycle, we were able to create a model with satisfactory performance in discriminating between those who do and do not produce excess embryos for cryopreservation. However, the addition of cycle-specific factors including number of oocytes retrieved enhanced the discriminatory ability of our model. The initial model is useful in counseling patients, but should be recalculated as additional information becomes available. For example, a 35-year-old patient with an AMH of 1.2 without a history of any prior IVF cycles has a 58% chance of having supernumerary embryos at the initial consult based on calculations from Tables 3 and 4. Using this information, she might plan PGT-A or another selection technique. If the same patient at the time of IVF had only 6 eggs retrieved with ICSI fertilization of all mature eggs using partner sperm followed by a day 3 cleavage stage single embryo transfer, her chances of having excess embryos cryopreserved decrease to 11–20% based on calculations from Tables 5 and 6. The patient might at this point opt out of additional selection techniques. These types of hypothetical situations can be provided to patients at initial counseling in order for them to better understand their chances of success and to plan accordingly.
Table 5.
Points assigned to all variables
| Variable (referent)* | Points | |
|---|---|---|
| Age (< 35) | 35–37 | − 1 |
| 38–40 | − 4 | |
| 41–42 | − 9 | |
| > 42 | − 18 | |
| AMH (1.0–3.0) | < 1.0 | − 2 |
| > 3.0 | + 1 | |
| Prior fresh cycles (0) | 1 | − 4 |
| 2 | − 6 | |
| 3 | − 7 | |
| 4+ | − 7 | |
| Prior frozen cycle (0) | 1 | + 5 |
| 2 | + 8 | |
| 3 | + 8 | |
| 4+ | + 12 | |
| # eggs retrieved (1–6) | 7–9 | + 12 |
| 10–13 | + 16 | |
| 14–18 | + 20 | |
| 19–35+ | + 25 | |
| Sperm source (partner) | Donor | + 2 |
| ICSI (no ICSI) | All mature eggs | − 1 |
| Some mature eggs | − 1 | |
| Day of transfer (5) | 2 | − 5 |
| 3 | − 6 | |
| 4 | − 7 | |
| 6 | − 5 | |
| Blastocyst transfer | Cleavage transfer | − 5 |
| Single embryo transfer | 2 | − 4 |
| 3 | − 11 | |
| 4 | − 13 | |
| 5 | − 12 | |
*Referent category is assigned zero points
Table 6.
Predicted probability of the outcome by all patient- and cycle-level points
| Score* | Probability supernumerary (%) | Binary decision cut-point for success | ROC area (%), 95% CI | Sensitivity (%) 95% CI | Specificity (%) 95% CI |
|---|---|---|---|---|---|
| 0–34 | 0–10 | ≥ 0 | 50 | 100 | 0 |
| 35–42 | 11–20 | ≥ 35 | 63 | 98 (97.8–98.3) | 28.1 (27.5–28.8) |
| 43–47 | 21–30 | ≥ 43 | 71 | 94.2 (93.9–94.6) | 47.3 (46.6–48.1) |
| 48–52 | 31–40 | ≥ 48 | 74 | 90.4 (89.9–90.8) | 56.7 (56.0–57.4) |
| 53–56 | 41–50 | ≥ 53 | 76 | 84.2 (83.6–84.8) | 67.0 (66.3–67.7) |
| 57–60 | 51–60 | ≥ 57 | 76 | 77.4 (76.8–78.1) | 74.5 (73.9–75.2) |
| 61–64 | 61–70 | ≥ 61 | 75 | 67.0 (66.2–67.7) | 82.2 (81.6–82.7) |
| 65–70 | 71–80 | ≥ 65 | 71 | 52.6 (51.8–53.3) | 89.4 (88.9–89.8) |
| 71–78 | 81–90 | ≥ 71 | 62 | 26.6 (25.9–27.3) | 96.8 (96.5–97.0) |
| 78–max | > 90 | ≥ 78 | 51 | 2.28 (2.06–2.52) | 99.9 (99.8–99.9) |
Score = summative points (Table 5) + 53 (automatically sets the minimum score to zero)
This study has several strengths. We utilized data reported to SART, which captures more than 90% of cycles that occur within the USA [24]. Therefore, the results of this study with a large sample size are generalizable to a heterogeneous group of women who undergo in vitro fertilization within the USA. We also had numerous variables to start with for our analysis before excluding non-significant variables. In addition, the points system utilized for our model is an effective approach for making complex statistical models useful to practitioners especially for simplifying the assessment of an outcome affected by multiple factors [12]. Finally, both models achieved an AUC considered satisfactory for use in clinical practice. There are also important limitations to this study. SART data is entered by personnel at individual clinics, and completeness and accuracy may vary by clinic. Additionally, clinic practices on number and stage of embryos transfer vary, as do criteria for embryo cryopreservation. Our findings remained strongly statistically significant despite this variability, indicating their applicability across a variety of practice patterns. However, our findings may not be applicable to patients outside the USA. Approximately 44% of cycles reported to SART in 2014 where a fresh transfer occurred were missing AMH. Because AMH was a necessary variable, we excluded all of those cycles from the prediction model. While there were small differences in multiple variables between those with and without an AMH value, these were not clinically significant. Finally, the outcomes of cryopreserved embryo transfers from those cycles not resulting in live birth are not known.
In conclusion, we found that in a minority of fresh IVF cycles in the USA, the fresh transfer is not successful, and there are excess embryos cryopreserved for future use. The likelihood of excess embryos beyond those that would be transferred can be predicted with satisfactory precision prior to initiation of the cycle and with improved precision after fresh embryo transfer. Providing patients with suspected poor prognosis with a realistic estimate of their chances of having excess embryos at an initial IVF consult can be beneficial in determining whether to proceed with multiple embryo banking cycles as opposed to proceeding with a fresh transfer after the first cycle. This information can be useful to both patients and providers in planning IVF cycles, especially for older patients who might want more than one child. This information may also help in determining whether utilizing an enhanced embryo selection technique such as PGT-A, which may add cost and complexity to an IVF cycle, might lead to a live birth more quickly. This is consistent with a recent study that demonstrated that both patient age and number of blastocysts impact the cost-effectiveness of PGT-A [25]. As cost is a primary factor in discontinuing IVF treatment [26, 27], patients with limited financial resources and chances of supernumerary embryos might derive greater benefit from an additional attempt at IVF rather than devoting resources to a selection technology that may not increase chances of live birth. Further studies are needed to hone these prediction models and to assess the cost-effectiveness of selection techniques in both good and poor prognosis patient groups.
Acknowledgments
SART wishes to thank all of its members for providing clinical information to the SART CORS database for use by patients and researchers. Without the efforts of our members, this research would not have been possible.
Electronic suppl
ementary material
(DOCX 19 kb)
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Domar AD, Rooney K, Hacker MR, Sakkas D, Dodge LE. Burden of care is the primary reason why insured women terminate in vitro fertilization treatment. Fertil Steril. 2018;109(6):1121–1126. doi: 10.1016/j.fertnstert.2018.02.130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ethics Committee of the American Society for Reproductive Medicine Electronic address, a.a.o. and M. Ethics Committee of the American Society for Reproductive, Fertility treatment when the prognosis is very poor or futile: an Ethics Committee opinion. Fertil Steril. 2019;111(4):659–663. doi: 10.1016/j.fertnstert.2019.01.033. [DOI] [PubMed] [Google Scholar]
- 3.Maheshwari A, McLernon D, Bhattacharya S. Cumulative live birth rate: time for a consensus? Hum Reprod. 2015;30(12):2703–2707. doi: 10.1093/humrep/dev263. [DOI] [PubMed] [Google Scholar]
- 4.Gorodeckaja J, et al. High implantation and clinical pregnancy rates with single vitrified-warmed blastocyst transfer and optional aneuploidy testing for all patients. Hum Fertil (Camb). 2019:1–12. [DOI] [PubMed]
- 5.Maxwell SM, Grifo JA. Should every embryo undergo preimplantation genetic testing for aneuploidy? A review of the modern approach to in vitro fertilization. Best Pract Res Clin Obstet Gynaecol. 2018;53:38–47. doi: 10.1016/j.bpobgyn.2018.07.005. [DOI] [PubMed] [Google Scholar]
- 6.Neal SA, Morin SJ, Franasiak JM, Goodman LR, Juneau CR, Forman EJ, Werner MD, Scott RT Jr Preimplantation genetic testing for aneuploidy is cost-effective, shortens treatment time, and reduces the risk of failed embryo transfer and clinical miscarriage. Fertil Steril. 2018;110(5):896–904. doi: 10.1016/j.fertnstert.2018.06.021. [DOI] [PubMed] [Google Scholar]
- 7.Lee HL, McCulloh D, Hodes-Wertz B, Adler A, McCaffrey C, Grifo JA. In vitro fertilization with preimplantation genetic screening improves implantation and live birth in women age 40 through 43. J Assist Reprod Genet. 2015;32(3):435–444. doi: 10.1007/s10815-014-0417-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Munne S, et al. Preimplantation genetic testing for aneuploidy versus morphology as selection criteria for single frozen-thawed embryo transfer in good-prognosis patients: a multicenter randomized clinical trial. Fertil Steril. 2019;112(6):1071–1079. doi: 10.1016/j.fertnstert.2019.07.1346. [DOI] [PubMed] [Google Scholar]
- 9.Scott RT, Jr, et al. Blastocyst biopsy with comprehensive chromosome screening and fresh embryo transfer significantly increases in vitro fertilization implantation and delivery rates: a randomized controlled trial. Fertil Steril. 2013;100(3):697–703. doi: 10.1016/j.fertnstert.2013.04.035. [DOI] [PubMed] [Google Scholar]
- 10.Ozgur K, Berkkanoglu M, Bulut H, Yoruk GDA, Candurmaz NN, Coetzee K. Single best euploid versus single best unknown-ploidy blastocyst frozen embryo transfers: a randomized controlled trial. J Assist Reprod Genet. 2019;36(4):629–636. doi: 10.1007/s10815-018-01399-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Drakopoulos P, Blockeel C, Stoop D, Camus M, de Vos M, Tournaye H, Polyzos NP. Conventional ovarian stimulation and single embryo transfer for IVF/ICSI. How many oocytes do we need to maximize cumulative live birth rates after utilization of all fresh and frozen embryos? Hum Reprod. 2016;31(2):370–376. doi: 10.1093/humrep/dev316. [DOI] [PubMed] [Google Scholar]
- 12.Sullivan LM, Massaro JM, D'Agostino RB., Sr Presentation of multivariate data for clinical use: the Framingham Study risk score functions. Stat Med. 2004;23(10):1631–1660. doi: 10.1002/sim.1742. [DOI] [PubMed] [Google Scholar]
- 13.Hosmer DW, Lemeshow S. Applied logistic regression. 2. New York: Wiley; 2000. Wiley series in probability and statistics Texts and references section; p. xii. [Google Scholar]
- 14.Harrell, F.E., Regression modeling strategies : with applications to linear models, logistic regression, and survival analysis.
- 15.Practice Committee of the American Society for Reproductive Medicine. Electronic address, A.a.o. and T. Practice Committee of the Society for Assisted Reproductive Guidance on the limits to the number of embryos to transfer: a committee opinion. Fertil Steril. 2017;107(4):901–903. doi: 10.1016/j.fertnstert.2017.02.107. [DOI] [PubMed] [Google Scholar]
- 16.McLernon DJ, et al. Predicting the chances of a live birth after one or more complete cycles of in vitro fertilisation: population based study of linked cycle data from 113 873 women. BMJ. 2016;355:i5735. doi: 10.1136/bmj.i5735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.van Loendersloot LL, et al. Individualized decision-making in IVF: calculating the chances of pregnancy. Hum Reprod. 2013;28(11):2972–2980. doi: 10.1093/humrep/det315. [DOI] [PubMed] [Google Scholar]
- 18.Nelson SM, Fleming R, Gaudoin M, Choi B, Santo-Domingo K, Yao M. Antimullerian hormone levels and antral follicle count as prognostic indicators in a personalized prediction model of live birth. Fertil Steril. 2015;104(2):325–332. doi: 10.1016/j.fertnstert.2015.04.032. [DOI] [PubMed] [Google Scholar]
- 19.Weissman A, Shoham G, Shoham Z, Fishel S, Leong M, Yaron Y. Preimplantation genetic screening: results of a worldwide web-based survey. Reprod BioMed Online. 2017;35(6):693–700. doi: 10.1016/j.rbmo.2017.09.001. [DOI] [PubMed] [Google Scholar]
- 20.Rubio C, Bellver J, Rodrigo L, Castillón G, Guillén A, Vidal C, Giles J, Ferrando M, Cabanillas S, Remohí J, Pellicer A, Simón C. In vitro fertilization with preimplantation genetic diagnosis for aneuploidies in advanced maternal age: a randomized, controlled study. Fertil Steril. 2017;107(5):1122–1129. doi: 10.1016/j.fertnstert.2017.03.011. [DOI] [PubMed] [Google Scholar]
- 21.Forman EJ, Hong KH, Ferry KM, Tao X, Taylor D, Levy B, Treff NR, Scott RT Jr In vitro fertilization with single euploid blastocyst transfer: a randomized controlled trial. Fertil Steril. 2013;100(1):100–107. doi: 10.1016/j.fertnstert.2013.02.056. [DOI] [PubMed] [Google Scholar]
- 22.Yang YS, et al. Preimplantation genetic screening of blastocysts by multiplex qPCR followed by fresh embryo transfer: validation and verification. Mol Cytogenet. 2015;8:49. doi: 10.1186/s13039-015-0140-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Franasiak JM, Forman EJ, Hong KH, Werner MD, Upham KM, Treff NR, Scott RT Jr The nature of aneuploidy with increasing age of the female partner: a review of 15,169 consecutive trophectoderm biopsies evaluated with comprehensive chromosomal screening. Fertil Steril. 2014;101(3):656–663. doi: 10.1016/j.fertnstert.2013.11.004. [DOI] [PubMed] [Google Scholar]
- 24.Sunderam S, et al. Assisted reproductive technology surveillance - United States, 2015. MMWR Surveill Summ. 2018;67(3):1–28. doi: 10.15585/mmwr.ss6703a1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Somigliana E, Busnelli A, Paffoni A, Vigano P, Riccaboni A, Rubio C, Capalbo A. Cost-effectiveness of preimplantation genetic testing for aneuploidies. Fertil Steril. 2019;111(6):1169–1176. doi: 10.1016/j.fertnstert.2019.01.025. [DOI] [PubMed] [Google Scholar]
- 26.Dawson AA, Diedrich K, Felberbaum RE. Why do couples refuse or discontinue ART? Arch Gynecol Obstet. 2005;273(1):3–11. doi: 10.1007/s00404-005-0010-5. [DOI] [PubMed] [Google Scholar]
- 27.Goldfarb J, Austin C, Lisbona H, Loret de Mola R, Peskin B, Stewart S. Factors influencing patients’ decision not to repeat IVF. J Assist Reprod Genet. 1997;14(7):381–384. doi: 10.1007/BF02766144. [DOI] [PMC free article] [PubMed] [Google Scholar]