Abstract
Purpose
To determine the impact of accelerated telomere shortening on the fertility parameters and treatment outcomes of a woman with dyskeratosis congenita (DKC).
Methods
A case study of the clinical data, blood, discarded oocytes, and arrested embryos of a woman with DKC and donated cryopreserved embryos from unaffected patients. Mean telomere length in blood cells was analyzed by flow cytometry–fluorescence in situ hybridization (flow-FISH) and qPCR. The load of short telomeres in blood cells was measured by universal single telomere length analysis (Universal STELA). The mean telomere length in embryos was analyzed by single-cell amplification of telomere repeats (SCATR) PCR.
Results
Comparison of clinical parameters revealed that the DKC patient had reduced anti-Mullerian hormone (0.3 vs 4.1 ± 5.7 ng/ML), reduced oocytes retrieved (7 vs 18.5 ± 9.5), reduced fertilization rate, and reduced euploidy rate relative to unaffected patients. Additionally, mean telomere length in DKC embryos were shorter than unaffected embryos. However, hormone treatment led to increased leukocyte telomere length, while the load of short telomeres was also shown to decrease during the course of treatment.
Conclusions
We demonstrate for the first time the direct detrimental impacts of short telomeres on female fertility. We further demonstrate positive effects of hormone treatments for people with telomere disorders.
Keywords: Female reproductive aging, Telomeres, Oocytes, Dyskeratosis congenita
Introduction
Telomeres are repetitive DNA sequences, (TTAGGG) n, that work in concert with the protein complex known as shelterin to protect chromosome ends [1]. Telomeres are maintained primarily by the enzyme, telomerase. Telomerase activity is absent in most adult cells in humans, leading to progressive telomere shortening over time in tissues which lack a stem cell compartment [2–4]. Germline stem cells in females are lost by about 20 weeks of fetal development. Thus, the adult female germline lacks appreciable telomerase activity [5, 6]. In contrast, the male maintains a germline stem cell population with robust telomerase activity throughout his lifespan.
Oocyte attrition, embryo developmental arrest, and miscarriage increase with advancing maternal age. Donor oocytes abrogate the effects of aging on reproduction, suggesting that the oocyte must be the locus of reproductive aging in women [7–12]. How maternal aging so profoundly disrupts oocyte function remains poorly understood. The inherent challenges of studying oocyte quality and developmental potential in humans have led to a reliance on cell culture and animal models. Nuclear transfer in senescence-accelerated mice (SAM) demonstrates that the reproductive aging phenotype segregates with the nucleus rather than the mitochondria or cytoplasm [13]. A wealth of evidence suggests that telomeres may contribute to reproductive aging [10, 14]. Telomeres mediate aging not only in dividing cells but also in post mitotic cells [15]. Telomeres are essential during meiosis, and telomere dysfunction impairs synapsis and chiasma formation [16]. Telomere shortening disrupts meiotic spindles, reduces developmental potential, and increases apoptosis in mice [17]. Mice null or haploinsufficient for telomerase phenocopy the profile of reproductive aging in women, with progressive infertility caused by numerous defects in oocytes and embryos [10]. Together, these studies suggest a central role for telomere attrition in reproductive aging. However, limited access to human oocytes and embryos has made testing the telomere theory of reproductive aging in women challenging.
Mutations in genes encoding components of telomerase create a natural experiment to examine the effects of telomeres on function in humans. Dyskeratosis congenita (DKC), the canonical human telomeropathy, presents with epidermal abnormalities and bone marrow failure [18–20]. Telomeropathy is associated with diminished ovarian reserve, as estimated by circulating levels of anti-Mullerian hormone (AMH) [21]. The effects of DKC on meiotic and preimplantation embryo development have yet to be characterized. The grave state of health complicates interpretation of the effects of telomeres on reproductive function in most DKC patients. Here we report studies of ovarian and embryo function in a patient with DKC who pursued assisted reproductive technology for fertility preservation via embryo banking. This patient provides a natural experiment to test the telomere theory of reproductive aging in women and presents the first direct evidence of telomere-mediated infertility in humans. The case also reports the first attempt at fertility preservation in a woman with DKC.
Case
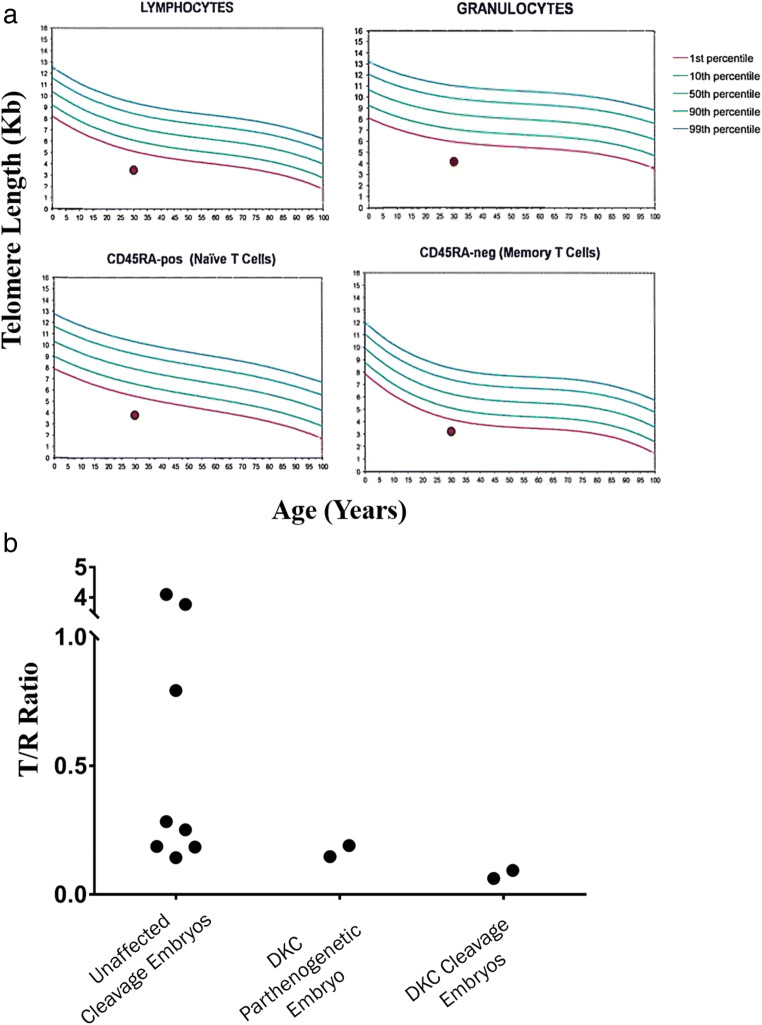
This 30-year-old gravida 1, para 0 had a history of chronic aplastic anemia, attributed to a telomeropathy. She was referred for elective embryo cryopreservation for possible future transfer to a gestational surrogate. She had an elective termination at age 17. Progressive bone marrow failure and telangiectasias appeared by her mid-twenties. Telomere Restriction Fragment (TRF) assay demonstrated the leukocyte telomere length of less than first percentile, which was 3.6 standard deviations below the age-adjusted mean (Fig. 1a). Candidate gene and exon sequencing were unrevealing (data not shown), and no causal mutation could be identified. Based on short telomeres and clinical manifestations, she was diagnosed with idiopathic telomeropathy, hereafter referred to as dyskeratosis congenita (DKC).
Fig. 1.
Telomere length in leukocytes and arrested embryos from patient with DKC. a Leukocyte telomere length as measured by flow cytometry–fluorescence in situ hybridization (flow-FISH) of the DKC patient. Results are less than the first percentile in lymphocytes, granulocytes, naïve T cells, and memory T cells. b Analysis of telomere length in blastomeres by SCATR PCR comparing embryos (N = 8) from unaffected patients to DKC patient’s parthenogenetic embryo (N = 2) and fertilized cleavage embryos (N = 2). NK, natural killer; T, telomere target; R, multi-copy reference gene
Past medical history was significant for Coats retinopathy, with blindness in her right eye since age six. She also had mild chronic renal failure with serum creatinine levels 1.2 to 1.3. Her Papanicolaou smear was positive for HPV and high-grade epithelial lesion, treated by loop electrosurgical excision procedure (LEEP) after colposcopic-directed biopsies returned CIN 2–3. She never smoked cigarettes. Family history was notable for a sister with mild aplastic anemia and similarly uninformative genetic evaluation and a brother with Coats retinopathy. Her parents are healthy. Previously, she took continuous oral contraceptive pills, folate, and B-complex vitamins. She was sexually active with her husband and reported normal sexual function. Physical examination showed a pale-appearing, thin Caucasian female. Her BMI was 17.9, normal vital signs, and physical examination unremarkable except for pallor, rare skin telangiectasias, and premature hair graying.
She was counseled against becoming pregnant, given the severity of her medical condition. She agreed to freeze any embryos before transferring into a gestational surrogate. Given that she already had reached the upper life expectancy for patients with DKC, we held extensive discussions about posthumous procreation. Her hematologist and nephrologist provided medical clearance for controlled ovarian stimulation. She underwent in vitro fertilization (IVF) and preimplantation genetic testing for aneuploidy (PGT-A) for embryo banking. An estradiol prime protocol and 600 IU/day of gonadotropin, using recombinant FSH and human menopausal gonadotropin, with GnRH antagonist, were used for ovarian stimulation. Patient agreed to have discarded materials collected for research and was consented. Unaffected patients were consented, and their data and samples were compared with those from the DKC patient.
Results
The patient’s ovarian reserve was markedly diminished, with AMH 0.3 ng/ml (vs. 4.11 ± 5.72 in age-matched controls), antral follicle count (AFC) 8. Controlled ovarian stimulation required nearly twice the duration and dose yet generated half the follicle and oocyte number of age-matched, healthy controls (Table 1). When the lead follicle reached 20 mm, oocyte maturation was triggered by recombinant human chorionic gonadotropin (Ovidrel® 250 μg, Merck Serono). Thirty-six hours later, she underwent ultrasound-guided transvaginal oocyte retrieval under IV sedation. Immediately following retrieval, cumulus corona cells were stripped, revealing seven metaphase II (MII) oocytes. MII oocytes were fertilized by intracytoplasmic sperm injection (ICSI). On day one, five of the seven oocytes cleaved. One parthenogenetic 2-cell embryo was identified. The two embryos which reached blastocyst stage underwent trophectoderm biopsy for comprehensive chromosome screening by next-generation sequencing (NGS), revealing one euploid, and the other had complex aneuploidy.
Table 1.
Clinical characteristics
| Unaffected patient controls (N = 150) | Dyskeratosis congenita patient | |
|---|---|---|
| Age (years) | 30 ± 0.07 | 30 |
| Anti-Müllerian hormone (ng/ml) | 4.1 ± 5.7 | 0.3 |
| Stimulation | 308.8 ± 120.6 | 600 |
| Stimulation duration (days) | 9.8 ± 2.4 | 18 |
| # of oocytes retrieved | 18.5 ± 9.5 | 7 |
| # of oocytes fertilized | 10.7 ± 6.7 | 5 |
| # of euploid embryos | 3.9 ± 3.4 | 1 |
All parameters are presented as mean ± standard deviation where possible
Telomere content in unfertilized oocytes and arrested embryos from the DKC patient and frozen embryos from unaffected patients was measured by single-cell amplification of telomere repeats (SCATR) PCR (previously known as SCT-pqPCR) [22]. Telomere lengths in oocytes and embryos from the patient were generally shorter than in unaffected controls (Fig. 1b). Intriguingly, telomeres forming the DKC patient’s parthenogenetic embryo were longer than those in her cleavage embryos, despite receiving no long telomeres from sperm.
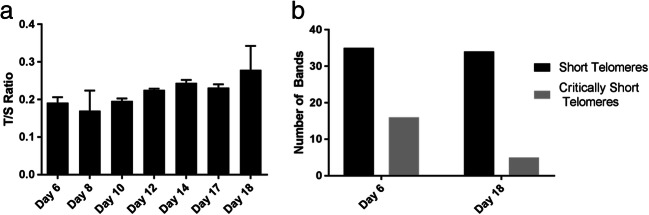
Mean telomere length was measured by qPCR in the DKC patient’s lymphocytes extracted from whole blood collected during ovarian stimulation [23]. QPCR revealed a progressive, linear increase in mean telomere DNA from days 6 to 18 of treatment (Fig. 2a).
Fig. 2.
Ovarian stimulation increases leukocyte telomere length and reduced the load of short telomeres. a qPCR assay shows telomere length in leukocytes collected from the DKC patient days 6 to 18 of ovarian stimulation. Error bars indicate mean ± SD among replicates. b Quantification of short telomeres by STELA from days 6 to 18 of stimulation. The y-axis represents the total number of bands present in the 12 lanes of the STELA blot for each sample
Universal STELA was used to assess the load of short telomeres through stimulation [24]. Telomeres below 5 kb were designated as short, while telomeres below 2 kb as critically sort. The assay demonstrated that the number of short telomeres (≤ 5 kb) did not change between day 6 and day 18, but the number of critically short telomeres (≤ 2 kb) (Fig. 2b) decreased with ovarian stimulation.
Discussion
This young woman with dyskeratosis congenita, who presented for fertility preservation, had markedly reduced ovarian reserve and impaired response to ovarian stimulation, poor embryo development, and telomere attrition in oocytes and embryos. Even with an ovarian stimulation regimen using twice the dose of gonadotropin, for nearly twice the duration, this patient produced half the expected number of oocytes of age- and BMI-matched controls. Just two of her embryos reached the blastocyst stage of development, and only embryo was euploid by NGS. Together, these data support a model in which short telomeres impair reproductive function in women, regardless of chronological age. These findings are consistent with effects of experimental telomere attrition on reproductive function in mice [13]. That even a single blastocyst was found to be euploid by PGS exemplifies the promise of ART for fertility preservation in women with telomeropathies, whose telomere attrition predisposes them to precocious reproductive aging.
Telomeres in this patient’s oocytes and embryos were shorter than those from age-matched, unaffected controls. However, it is worth noting that the developmental potential of unaffected control embryos is unknown as they were never cultured after thawing. Therefore, while telomere lengths in her embryos were not dramatically shorter than some of the unaffected samples, this does not suggest similar levels of developmental potential. Still, two of her embryos reached blastocyst stage, and one of these was euploid, implying these embryos somehow had reconstituted their very short telomeres. How telomeres reset during early human development, in the absence of appreciable telomerase activity, remains an enigma. In mice telomeres elongate during early development via sister chromatid exchange and alterative lengthening of telomeres (ALT) [25]. Telomere elongation takes place even in telomerase null and parthenogenetic mouse embryos. If this is also true in humans, this would suggest that successful embryos have access to a telomere lengthening mechanism that can permit survival to blastocyst stage. The fact that telomeres in the DKC patient’s parthenogenetic cells appear to be longer than those in cleavage embryos suggest continued attrition with development and thus a failure in these embryos to activate any potential telomere lengthening mechanism.
Intriguingly, this patient’s leukocyte telomere length increased during ovarian stimulation. Presumably, the supraphysiologic levels of estrogen resulting from controlled ovarian hyperstimulation activate telomerase activity. This finding is consistent with prior studies reporting an estrogen response element in the promoter of the TERT gene and studies of dyskeratosis congenita patients, whose leukocyte telomere length increased during treatment with a synthetic steroid [26]. Notably, the telomere response to elevated estrogen levels in our patient was apparent after a relatively brief exposure, just 18 days, to elevated estrogen. Presumably, measuring the load of short telomeres (by Universal STELA) provided a more sensitive measure of telomere response compared to mean telomere length. Future studies should examine whether women with telomeropathies may benefit from estrogen supplementation. Finally, this is the first reported case of fertility preservation in a woman with DKC, which otherwise causes progressive follicular depletion. This study has also for the first time produced direct evidence of the telomere theory of reproductive aging in women, though further studies are required to further characterize the results presented here.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.de Lange T. Shelterin: the protein complex that shapes and safeguards human telomeres. Genes Dev. 2005;19(18):2100–2110. doi: 10.1101/gad.1346005. [DOI] [PubMed] [Google Scholar]
- 2.Feng J, Funk WD, Wang SS, Weinrich SL, Avilion AA, Chiu CP, Adams RR, Chang E, Allsopp RC, Yu J, Le S, West MD, Harley CB, Andrews WH, Greider CW, Villeponteau B. The RNA Component of Human Telomerase. Science. 1995;269(5228):1236–1241. doi: 10.1126/science.7544491. [DOI] [PubMed] [Google Scholar]
- 3.Blackburn EH. Telomeres and telomerase: their mechanisms of action and the effects of altering their functions. FEBS Lett. 2005;579(4):859–862. doi: 10.1016/j.febslet.2004.11.036. [DOI] [PubMed] [Google Scholar]
- 4.Britt-Compton B, Capper R, Rowson J, Baird DM. Short telomeres are preferentially elongated by telomerase in human cells. FEBS Lett. 2009;583(18):3076–3080. doi: 10.1016/j.febslet.2009.08.029. [DOI] [PubMed] [Google Scholar]
- 5.Baker TG. Primordial germ cells. In: Austin CR, Short RV, editors. Reproduction in Mammals, vol. 1:: Germ Cells and Fertilization. Cambridge: Cambridge University Press; 1970. p. 1–13.
- 6.Wright DL, Jones EL, Mayer JF, Oehninger S, Gibbons WE, Lanzendorf SE. Characterization of telomerase activity in the human oocyte and preimplantation embryo. Mol Hum Reprod. 2001;7(10):947–955. doi: 10.1093/molehr/7.10.947. [DOI] [PubMed] [Google Scholar]
- 7.Navot D, Bergh PA, Williams MA, Garrisi GJ, Guzman I, Sandler B, Grunfeld L. Poor oocyte quality rather than implantation failure as a cause of age-related decline in female fertility. Lancet. 1991;337(8754):1375–1377. doi: 10.1016/0140-6736(91)93060-M. [DOI] [PubMed] [Google Scholar]
- 8.Hassold T, Chiu D. Maternal age-specific rates of numerical chromosome abnormalities with special reference to trisomy. Hum Genet. 1985;70(1):11–17. doi: 10.1007/BF00389450. [DOI] [PubMed] [Google Scholar]
- 9.Plachot M, Veiga A, Montagut J, de Grouchy J, Calderon G, Lepretre S, Junca AM, Santalo J, Carles E, Mandelbaum J. Are clinical and biological IVF parameters correlated with chromosomal disorders in early life: a multicentric study. Hum Reprod. 1988;3(5):627–635. doi: 10.1093/oxfordjournals.humrep.a136758. [DOI] [PubMed] [Google Scholar]
- 10.Kalmbach KH, Fontes Antunes DM, Dracxler RC, Knier TW, Seth-Smith ML, Wang F, Liu L, Keefe DL. Telomeres and human reproduction. Fertil Steril. 2013;99(1):23–29. doi: 10.1016/j.fertnstert.2012.11.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Keefe DL, Liu L, Marquard K. Telomeres and aging-related meiotic dysfunction in women. Cell Mol Life Sci. 2007;64:139–43. [DOI] [PMC free article] [PubMed]
- 12.Treff NR, Su J, Taylor D, Scott RT., Jr Telomere DNA deficiency is associated with development of human embryonic aneuploidy. PLoS Genet. 2011;7(6):e1002161. doi: 10.1371/journal.pgen.1002161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Liu L, Keefe DL. Nuclear origin of aging-associated meiotic defects in senescence-accelerated mice. Biol Reprod. 2004;71(5):1724–1729. doi: 10.1095/biolreprod.104.028985. [DOI] [PubMed] [Google Scholar]
- 14.Keefe DL, et al. Telomere length predicts embryo fragmentation after in vitro fertilization in women—toward a telomere theory of reproductive aging in women. Am J Obstet Gynecol. 2005;192(4):1256–1260. doi: 10.1016/j.ajog.2005.01.036. [DOI] [PubMed] [Google Scholar]
- 15.Butlera MG, Tilburt J, DeVriesa A, Muralidhara B, Auea G, Hedgesa L, Atkinson J, Schwartz H. Comparison of chromosome telomere integrity in multiple tissues from subjects at different ages. Cancer Genet Cytogenet. 1998;105(2):138–144. 10.1016/S0165-4608(98)00029-6. [DOI] [PMC free article] [PubMed]
- 16.Liu L, Franco S, Spyropoulos B, Moens PB, Blasco MA, Keefe DL. Irregular telomeres impair meiotic synapsis and recombination in mice. Proc Natl Acad Sci U S A. 2004;101(17):6496–6501. doi: 10.1073/pnas.0400755101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Keefe DL, Liu L, Marquard K. Telomeres and aging-related meiotic dysfunc-tion in women. Cell Mol Life Sci 2007;64:139–43. [DOI] [PMC free article] [PubMed]
- 18.Agarwal S, Loh YH, McLoughlin E, Huang J, Park IH, Miller JD, Huo H, Okuka M, Dos Reis RM, Loewer S, Ng HH, Keefe DL, Goldman FD, Klingelhutz AJ, Liu L, Daley GQ. Telomere elongation in induced pluripotent stem cells from dyskeratosis congenita patients. Nature. 2010;464(7286):292–296. doi: 10.1038/nature08792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Batista LF, Pech MF, Zhong FL, Nguyen HN, Xie KT, Zaug AJ, Crary SM, Choi J, Sebastiano V, Cherry A, Giri N, Wernig M, Alter BP, Cech TR, Savage SA, Reijo Pera RA, Artandi SE. Telomere shortening and loss of self-renewal in dyskeratosis congenita induced pluripotent stem cells. Nature. 2011;474(7351):399–402. doi: 10.1038/nature10084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shay J, Wright W. Telomeres in dyskeratosis congenita. Nat Genet. 2004;36:437–438. 10.1038/ng0504-437 [DOI] [PubMed]
- 21.Sklavos MM, Stratton P, Giri N, Alter BP, Savage SA, Pinto LA. Reduced serum levels of anti-Mullerian hormone in females with inherited bone marrow failure syndromes. J Clin Endocrinol Metab. 2015;100(2):E197–E203. doi: 10.1210/jc.2014-2838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wang F, Pan X, Kalmbach K, Seth-Smith ML, Ye X, Antumes DM, Yin Y, Liu L, Keefe DL, Weissman SM. Robust measurement of telomere length in single cells. Proc Natl Acad Sci U S A. 2013;110(21):E1906–E1912. 10.1073/pnas.1306639110. [DOI] [PMC free article] [PubMed]
- 23.Cawthon RM. Telomere Measurement By Quantiative PCR. Nucleic Acids Res. 2002;30(10):e47. 10.1093/nar/30.10.e47. [DOI] [PMC free article] [PubMed]
- 24.Bendix L, Horn PB, Jensen UB, Rubelj I, Kolvraa S. The load of short telomeres, estimated by a new method, universal STELA, correlates with number of senescent cells. Aging Cell. 2010;9(3):383–397. doi: 10.1111/j.1474-9726.2010.00568.x. [DOI] [PubMed] [Google Scholar]
- 25.Liu L, Bailey SM, Okuka M, Muñoz P, Li C, Zhou L, Wu C, Czerwiec E, Sandler L, Seyfang A, Blasco MA, Keefe DL. Telomere lengthening early in development. Nat Cell Biol. 2007;9(12):1436–1441. doi: 10.1038/ncb1664. [DOI] [PubMed] [Google Scholar]
- 26.Townsley DM, Dumitriu B, Liu D, Biancotto A, Weinstein B, Chen C, Hardy N, Mihalek AD, Lingala S, Kim YJ, Yao J, Jones E, Gochuico BR, Heller T, Wu CO, Calado RT, Scheinberg P, Young NS. Danazol treatment for Telomere diseases. N Engl J Med. 2016;374(20):1922–1931. doi: 10.1056/NEJMoa1515319. [DOI] [PMC free article] [PubMed] [Google Scholar]