Abstract
Objectives
To compare progestin ovarian stimulation protocols with gonadotropin-releasing hormone analogue (agonists and antagonists) protocols on newborn outcomes.
Methods
The PubMed, Embase, Cochrane Central Register of Controlled Trials, and BioMed Central databases were searched for studies comparing progestin prime ovarian stimulation (PPOS) protocols with gonadotropin-releasing hormone analogues. Data were pooled by meta-analysis using a random effects model.
Main outcome measures
Primary endpoint was the risk of newborn congenital malformations.
Results
A total of 4 studies involving 9274 live-born infants were included. No important harm was observed with PPOS in terms of congenital malformations (OR 0.92; 95% CI 0.63–1.34; p = 0.65) (very low quality of evidence (QOE)) and low birth weight (OR 1.06; 95% CI 0.95–1.18; p = 0.29) (very low QOE) as compared with GnRH-a short protocols. In addition, a trend to a lower risk of preterm birth (OR 0.90; 95% CI 0.80–1.02; p = 0.10) (very low QOE) was found among patients treated with a PPOS protocol.
Conclusions
PPOS protocols, compared with GnRH-a protocols, are associated with a similar congenital malformation risk profile. Therefore, PPOS might represent a safe and appealing treatment option for infertile patients.
Electronic supplementary material
The online version of this article (10.1007/s10815-020-01755-0) contains supplementary material, which is available to authorized users.
Keywords: Progesterone, Congenital malformations, In vitro fertilization, Progestin-primed ovarian stimulation
Introduction
Assisted reproductive technologies (ART) have impressively evolved in the last few years in terms of number of cycles performed, number of newborns, and development of new strategies.
The most widespread protocol variants for controlled ovarian hyperstimulation (COH) in ART are based on gonadotropin-releasing hormone agonists (GnRH-a) and antagonists. GnRH analogues are used to block the luteinizing hormone (LH) surge associated with the increase of estrogens.
Progestin prime ovarian stimulation (PPOS) became a reality in ART techniques when, in the latest 2000, the feasibility of COH during luteal phase stimulation was shown [1]. Investigators noticed that no spontaneous LH surge occurred during luteal phase stimulation. It was then postulated that endogenous progesterone could block the rise of this gonadotropic hormone [2]. Progesterone administration from the early follicular phase is able to inhibit the follicular growth and the LH surge by blocking the estradiol signal and slowing the LH pulse frequency, increasing its amplitude and decreasing its plasma content [3, 4].
PPOS protocols have recently gained popularity due to economic and clinical convenience. The ease of oral administration of progesterone in addition to the improvement of freezing oocytes and embryo techniques has allowed the development of PPOS protocols. However, some concerns regarding long-term safety and the impact on newborns have been raised [5, 6].
Data comparing the impact of PPOS with GnRH-a and antagonist protocols on children are scarce [5–8], and individual studies might not provide adequately powered analysis. This raises the need for a systematic appraisal of treatment effects and quality of evidence. Therefore, the aim of this investigation was to provide a comprehensive and quantitative assessment of evidence about the safety on the offspring of PPOS protocols compared with GnRH-a and antagonist protocols.
Methods
Data sources and search strategy
A systematic review was performed according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses 2009 guidelines [9]. Three reviewers (IZ, GAF, JJHM) independently identified the relevant studies by an electronic search of the MEDLINE, Embase, Cochrane Central Register of Controlled Trials, and BioMed Central (from inception to September 2019). The following search terms and keywords were used: “progestin,” “progesterone,” “medroxyprogesterone,” “ovarian stimulation,” “malformation,” and “defects” (Supplementary appendix). No language, publication date, study design, or publication status restrictions were imposed. This study is registered with PROSPERO.
Study selection
Three reviewers (IZ, GAF, JJHM) independently assessed trial eligibility on the basis of titles, abstracts, and full-text reports. Discrepancies in study selection were discussed and resolved with another investigator (AC). Eligible studies had to satisfy the following prespecified criteria: (1) studies including patients for in vitro infertility treatment; (2) investigations comparing the use of progestin with GnRH-a or antagonists for ovarian stimulation; (3) availability of newborn clinical outcome data. Exclusion criteria were as follows: (1) studies administrating progestin in luteal phase; (2) lack of any newborn clinical outcome data.
Studies with more than two arms for which a subset of interventions satisfied the inclusion criteria were kept in the analysis after having discarded the arms that did not satisfy the inclusion criteria.
Data extraction and quality assessment
Two investigators (IZ, JJHM) independently extracted data (baseline characteristics, definition of outcomes, and number of events) using a standardized data abstraction form. The same investigators independently and systematically assessed the studies’ methodological quality using the Risk of Bias In Non-randomized Studies of Interventions assessment Tool from the Cochrane handbook (ROBINS-I) [10], assessing seven domains of bias for each outcome: (1) confounding, (2) selection of participants, (3) classification of interventions, (4) deviations from intended interventions, (5) missing data, (6) measurement of outcomes, and (7) selection of the reported result. Disagreements were resolved with another investigator (AC). Quality of evidence (QOE) was evaluated according to the approach proposed by the Grading of Recommendations, Assessment, Development and Evaluation (GRADE) Working Group [11].
Data synthesis and data analysis
Outcome measures
The primary endpoint was the risk of newborn congenital malformations. Secondary endpoints included the risk of low birth weight and the risk of preterm birth. Endpoints were attributed according to the definition used in each study.
Statistical analysis
For dichotomous outcomes, the odds ratios (ORs) with 95% confidence intervals (CIs) were calculated from the available data and trial-specific ORs were combined with the DerSimonian and Laird random effects model with the estimate of heterogeneity being taken from the Mantel-Haenszel model [12]. The number of patients needed to treat for an additional harmful outcome (NNH) was calculated from weighted estimates of pooled ORs from the random effects meta-analytic model. The presence of heterogeneity among studies was evaluated with the Cochran Q chi-square test with p ≤ 0.1 considered to be of statistical significance, estimating the between-studies variance tau-square, and using the I2 test to evaluate inconsistency. The I2 statistic is derived from the Q statistic (100% × (Q − df)/Q) and describes the percentage of total variation across studies that is due to heterogeneity; a value of 0% indicates no observed heterogeneity, and larger values show increasing heterogeneity. I2 values of 25%, 50%, and 75% have been assigned adjectives of low, moderate, and high heterogeneity, respectively [13]. The presence of publication bias was not assessed as less than 10 studies were included in the present investigation.
Sensitive analysis and meta-regression
The effects of progestin and GnRH-a on outcomes were also assessed by calculating ORs with 95% CI using a fixed effects model with the Mantel and Haenszel method. Subgroup analyses were performed to assess the influence of progestin type (progestin vs progestin derivates) and number of infants at birth (singleton vs twins) on primary endpoint. A random effects meta-regression was performed to assess the impact on treatment effect of maternal age, progestin dose, and percentage of male-factor infertility.
The statistical level of significance was 2-tailed p < 0.05. Analyses were performed using Stata version 13.1 (Stata Corp., College Station, TX).
Results
Search results
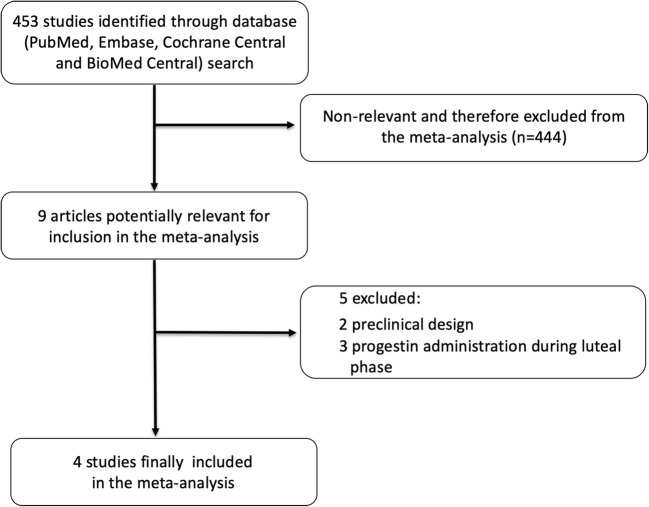
Figure 1 displays the preferred reporting items for Systematic Reviews and Meta-Analyses flow diagram for study search and selection. Of the 453 citations screened, 444 were excluded as they were considered non-relevant, 2 were excluded because of a preclinical design, and 3 because progestin administration was performed during luteal phase.
Fig. 1.
The Preferred Reporting Items for Systematic Reviews and Meta-Analyses flow diagram for study search and selection. Flow diagram of the search for studies included in the meta-analysis according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses statement
Therefore, a total of 4 studies including 10,121 cycles and 9274 live-born infants, after a progestin ovarian stimulation for in vitro fertilization/intracytoplasmic sperm injection (IVF/ICSI) as compared with a GnRH-a short protocol, were selected and included in the meta-analysis.
Study characteristics and bias assessment
The main trial and patient characteristics of the included studies are reported in Table 1. All studies had a retrospective cohort design. Two studies used micronized progesterone Utrogestan® (Laboratories Besins International, Paris, France) [14, 15], one medroxyprogesterone acetate (MPA) [8], and another dydrogesterone [16] for the progestin ovarian stimulation protocol (Table 2).
Table 1.
Baseline trial and patient characteristics included in the meta-analysis
| Huang et al. | Wang et al. | Zhang et al. | Zhu et al. | |||||
|---|---|---|---|---|---|---|---|---|
| Progestin | GnRH agonist | Progestin | GnRH agonist | Progestin | GnRH agonist | Progestin | GnRH agonist | |
| Type | DYG | Triptorelin | Micronized progesterone | Triptorelin | MPA | Triptorelin | Micronized progesterone | Triptorelin |
| Maternal age (years) | 31.0 ± 4.1 | 31.5 ± 3.9 | 32.9 ± 3.9 | 34.9 ± 3.7 | 31.3 ± 3.9 | 31.6 ± 3.7 | 31.9 ± 4.1 | 32.1 ± 3.6 |
| Maternal BMI (kg/m2) | 21.6 ± 2.9 | 21.5 ± 2.8 | 21.3 ± 2.9 | 21.4 ± 2.8 | 21.7 ± 3.0 | 21.6 ± 2.8 | 21.8 ± 3.2 | 21.6 ± 2.9 |
| Cause of infertility, n (%) | ||||||||
| Tubal factor | 625 (54.3) | 970 (56.3) | 459 (69.7) | 387 (64.6) | 189 (12.5) | 181 (13.7) | 212 (56.4) | 230 (60.4) |
| Male factor | 147 (12.8) | 237 (13.7) | 78 (11.8) | 84 (14.0) | 700 (46.2) | 643 (48.7) | 79 (21.0) | 90 (23.6) |
| Others | 379 (32.9) | 517 (30.0) | 122 (18.5) | 128 (21.4) | 625 (41.2) | 496 (37.6) | 85 (22.6) | 61 (16.0) |
| Sperm origin, n (%) | ||||||||
| Ejaculated | 1124 (97.7) | 1667 (96.7) | 641 (97.3) | 582 (97.2) | NA | NA | NA | NA |
| Testicular | 21 (1.8) | 53 (3.1) | 17 (2.6) | 16 (2.7) | NA | NA | NA | NA |
| Epididymal | 6 (0.5) | 4 (0.2) | 1 (0.2) | 1 (0.2) | NA | NA | NA | NA |
| No. of embryos transferred, n (%) | ||||||||
| Single | 186 (16.2) | 193 (11.2) | 50 (7.6) | 77 (14.6) | 164 (10.8) | 113 (8.6) | NA | NA |
| Double | 965 (83.8) | 1531 (88.8) | 609 (92.4) | 449 (85.4) | 1350 (89.2) | 1207 (91.4) | NA | NA |
| Embryo stage at transfer, n (%) | ||||||||
| Cleavage stage | 997 (86.6) | 1469 (85.2) | 1168 (92.1) | 955 (85.2) | 1307 (86.3) | 1143 (86.6) | 891 (91.6) | 873 (89.8) |
| Blastocyst stage | 154 (13.4) | 255 (14.8) | 100 (7.9) | 166 (14.8) | 207 (13.7) | 177 (13.4) | 81 (8.3) | 99 (10.2) |
BMI, body mass index; DYG, dydrogesterone; GnRH, gonadotropin-releasing hormone agonist; MPA, medroxyprogesterone acetate
Table 2.
Study key features
| Study | Year of publication | Design | N of patients | Progesterone | Progesterone daily dosage (mg) | Multicentric | Follow-up (months) | ||
|---|---|---|---|---|---|---|---|---|---|
| Overall | Progestin | GnRH agonist | |||||||
| Zhu et al. | 2017 | Retrospective cohort study | 546 | 293 | 253 | Micronized progesterone | 200 | No | 12 |
| Zhang et al. | 2017 | Retrospective cohort study | 3589 | 1931 | 1658 | MPA | 10 | No | 30 |
| Wang et al. | 2018 | Retrospective cohort study | 1589 | 855 | 734 | Micronized progesterone | 100 | No | 32 |
| Huang et al. | 2019 | Retrospective cohort study | 3556 | 1429 | 2127 | DYG | 20 | No | 48 |
DYG, dydrogesterone; MPA, medroxyprogesterone.
Table 3 summarizes the systematic bias assessment of the included studies. There was a serious risk of overall bias for most studies [8, 14, 16], except for one study that showed a critical risk of overall bias [15].
Table 3.
Judgment about each risk of bias item for the included studies
| Study | Bias due to confounding | Bias in selection of patients | Bias in classification of interventions | Bias due to deviations from intended interventions | Bias due to missing data | Bias in measurement of outcomes | Bias in selection of the reported result | Overall bias |
|---|---|---|---|---|---|---|---|---|
| Huang et al. | Serious | Moderate | Low | Serious | NI | Serious | Moderate | Serious |
| Wang et al. | Serious | Critical | Low | Critical | Moderate | Serious | Low | Critical |
| Zhang et al. | Serious | Serious | Low | Critical | NI | Serious | Moderate | Serious |
| Zhu et al. | Serious | Moderate | Low | Serious | NI | Moderate | Moderate | Serious |
NI, no information
Outcomes
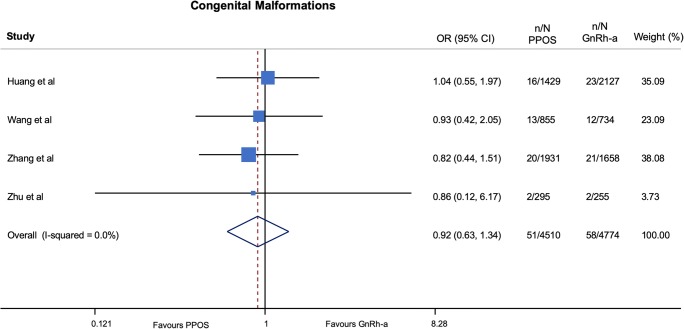
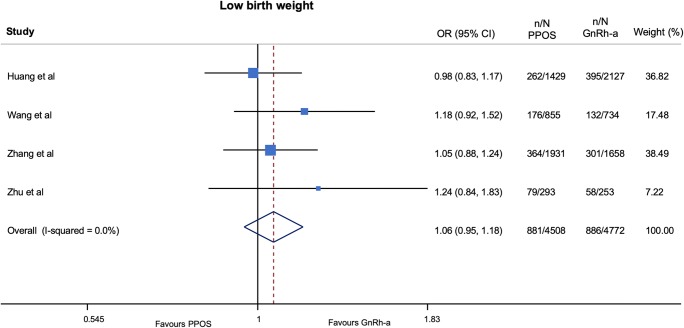
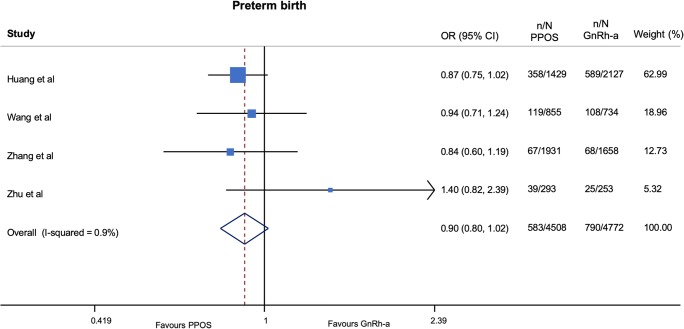
No important harm was observed with progestin ovarian stimulation in terms of congenital malformations (OR 0.92; 95% CI 0.63–1.34; p = 0.65, I2 = 0%, NNH = 1118) (very low QOE) (Fig. 2 and Table 4) and low birth weight (OR 1.06; 95% CI 0.95–1.18; p = 0.29, I2 = 0%, NNH = 107) (very low QOE) (Fig. 3 and Table 5) as compared with GnRH-a short protocols. In addition, a trend to a lower risk of preterm birth (OR 0.90; 95% CI 0.80–1.02; p = 0.10, I2 = 1%, NNH = 221) (very low QOE) (Fig. 4 and Table 4) was found among patients treated with progestin ovarian stimulation protocols.
Fig. 2.
Pooled analysis of studies comparing PPOS vs GnRH-a for congenital malformations. Forest plot reporting study-specific and summary odds ratios (ORs) with 95% confidence intervals (CIs) for the endpoint of congenital malformations. GnRH-a, gonadotropin-releasing hormone agonist; PPOS, progestin prime ovarian stimulation
Table 4.
Pooled analysis of studies comparing PPOS vs GnRH-a short protocols
| Endpoint | Number of events/number of patients, absolute event rate (%) | OR | 95% CI | p | |
|---|---|---|---|---|---|
| PPOS | GnRH-a | ||||
| Congenital malformations | 51/4510 (1.13) | 58/4774 (1.21) | 0.92 | 0.62 to 1.35 | 0.66 |
| Low birth weight | 881/4508 (19.5) | 886/4772 (18.5) | 1.06 | 0.95 to 1.18 | 0.29 |
| Preterm birth | 177/3742 (4.73) | 264/3917 (6.74) | 0.90 | 0.8 to 1.02 | 0.10 |
CI, confidence interval; GnRH-a, gonadotropin-releasing hormone agonist; OR, odds ratio; PPOS, progestin prime ovarian stimulation
Fig. 3.
Pooled analysis of studies comparing PPOS vs GnRH-a for low birth weight. Forest plot reporting study-specific and summary odds ratios (ORs) with 95% confidence intervals (CIs) for the endpoint of low birth weight. GnRH-a, gonadotropin-releasing hormone agonist; PPOS, progestin prime ovarian stimulation
Table 5.
Pooled analysis of studies comparing PPOS vs GnRH-a according to fixed effects model
| Endpoint | OR | 95% CI | p |
|---|---|---|---|
| Congenital malformation | 0.91 | 0.63 to 1.34 | 0.65 |
| Low birth weight | 1.06 | 0.95 to 1.18 | 0.29 |
| Preterm birth | 0.90 | 0.8 to 1.02 | 0.10 |
CI, confidence interval; OR, odds ratio
Fig. 4.
Pooled analysis of studies comparing PPOS vs GnRH-a for preterm birth. Forest plot reporting study-specific and summary odds ratios (ORs) with 95% confidence intervals (CIs) for the endpoint of preterm birth. GnRH-a, gonadotropin-releasing hormone agonist; PPOS, progestin prime ovarian stimulation
Sensitivity analysis
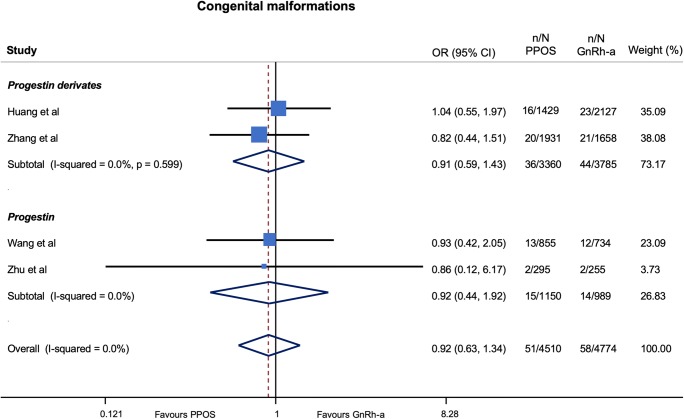
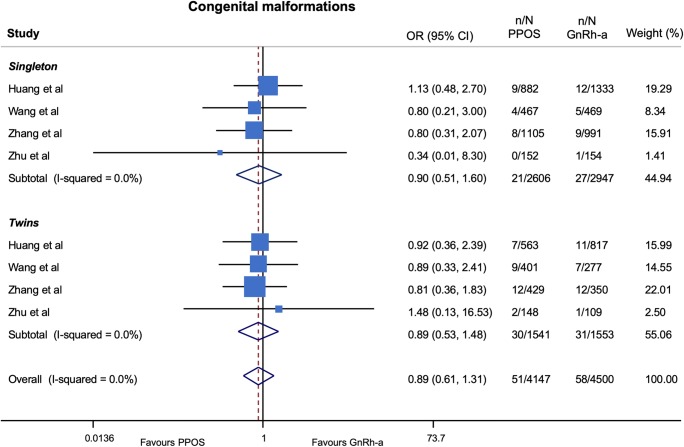
With respect to the risk of congenital malformations, the results were consistent, compared with those obtained in the main analysis, when stratifying by progestin type (Fig. 5) and number of infants at birth (singleton vs twins) (Fig. 6) as well as after calculation of ORs using a fixed effects model (Table 5).
Fig. 5.
Risk of congenital malformations stratified for type of progestin used in PPOS protocols. GnRH-a, gonadotropin-releasing hormone agonist; OR, odds ratios; PPOS, progestin prime ovarian stimulation
Fig. 6.
Risk of congenital malformations stratified for number of infants at birth GnRH-a, gonadotropin-releasing hormone agonist; OR, odds ratios; PPOS, progestin prime ovarian stimulation
Random effects meta-regression did not show a significant impact on the risk of congenital malformations neither of progestin dose (p = 0.94), maternal age (p = 0.99) nor of male-factor infertility (p = 0.79).
Discussion
The recent incorporation of progestins into the therapeutic arsenal used to block the LH peak during COH conforms a new and innovative option [17, 18]. As for every novelty, safety needs to be well settled. Our study provides a comprehensive and updated quantitative analysis of available evidence on newborn outcomes. After analyzing the data on 9274 live-born infants, the main finding of our study has been that a similar odds of congenital malformations and low birth weight were identified in patients treated with PPOS protocols as compared with those treated with GnRH-a short protocols. Additionally, a trend to a lower risk of preterm birth was observed among patients treated with PPOS protocols.
The effects of PPOS protocols on the risk of congenital malformations were not affected by the maternal age, progestin dose, or percentage of male-factor infertility, as assessed by meta-regression analysis.
During the latter years, little novelty has arisen in what refers to COH protocols. After years dominated by GnRH analogues or antagonists, the improvements in cryopreservation programs and the introduction of the “freeze-all” concept [19] have allowed the incorporation of progestins for the treatment of infertile patients, not just in the luteal phase but also during the follicular stage.
The first PPOS protocol was reported by Kuang et al. in 2015 [2]. The effect of hMG and MPA was compared with a short GnRH-a protocol in a prospective controlled cohort. Ovulation was induced with a GnRH-a or co-triggered by a GnRH-a and human chorionic gonadotropin (hCG) [2]. This new concept, which has been revealed useful in patients treated with ART for oocyte preservation, in donors and in patients who need a preimplantation genetic testing, seemed an appealing alternative not only to prevent the ovarian hyperstimulation syndrome (OHSS) but also as a standard approach during IVF/ICSI cycles.
Contradictory effects of progesterone on oocyte maturation and embryo development have been reported both in vitro and in vivo. Salehnia et al. showed a decreased maturation rate in mouse germinal vesicle (GV) oocytes after adding different progesterone concentrations to the in vitro oocyte maturation culture medium [20]. Silva et al. also found a decrease of up to 40% in the number of bovine blastocysts after adding progesterone to cumulus-oocyte complexes [21]. By contrast, Carter et al. reported that the addition of progesterone to culture medium did not affect the number of embryos developing to the blastocyst stage [22]. This adds to previous work, showing better results in terms of fertilization and cleavage rates after progesterone exposition in both animal models and humans [14, 23–25].
Apart from the investigations included in the present meta-analysis, the impact of progesterone on the newborn has been investigated only in protocols in which progesterone was administrated before pregnancy (for luteal deprivation or as a contraceptive method) or during pregnancy (for luteal support or in order to prevent a preterm birth). Carmicheal et al. described that progesterone administration between 4 weeks before and 14 weeks after conception led to a 2-fold increased risk of hypospadias as compared with mothers who had not taken progesterone [26]. In addition, Giorlandino et al. found a positive correlation between the use of exogenous progesterone during early pregnancy and the increase of nuchal translucency thickness with no differences regarding the type of progesterone, the route of administration, and the dosage [27]. However, no negative outcomes were found in babies born from mothers who carried out a pregnancy after a failure of the levonorgestrel administration as an emergency contraception [28].
Progesterone has extensively been studied in the prevention of preterm labor with conflicting results. During the first trimester of pregnancy, progesterone decrease due to the removal of the corpus luteum from the ovary has been shown to increase the risk of abortion [29]. However, during subsequent trimesters, available data is controversial [30–32]. Our finding regarding a trend to a slightly lower risk of preterm birth is in line with previous studies carried out which have shown that progesterone decrease causes cervix shortness and in more severe cases increases the risk of preterm birth [33, 34]. However, the confidence interval emerging from our investigation is wide and considerable uncertainty still exists.
The use of progesterone as a LH peak inhibitor might offer some advantages. Its oral administration reduces the mental and physical stress of patients who are no longer obliged to multiple daily injections. This does benefit not only patients but also egg donors because the decrease in the number of injections could increase this altruistic practice. In addition, ART treatments are costly not only at a psychological, but also at an economical level. The price of oral progesterone could significantly reduce the costs of IVF/ICSI cycles.
Based on the results of the present meta-analysis, PPOS represents an appealing treatment option for infertile patients due to its similar risk profile on newborns as compared with GnRH-a protocols. In addition, its low incidence of OHSS, the similar rates of oocyte retrieval and ongoing pregnancies [35, 36], the reduced economic costs, and the easier administration [7] might convert PPOS as the default strategy in the near future.
Limitations
This study should be interpreted in light of some limitations. First, this is a study-level meta-analysis providing average treatment effects. The lack of patient-level data prevents us from assessing the impact of baseline clinical characteristics on treatment effects. In addition, the limited number of studies, the lack of randomization, and the small event rate may reduce the power for detecting smaller significant differences between groups. As assessed by the GRADE framework, QOE emerging from the included studies is very low; therefore, uncertainty about the impact of PPOS on newborn outcomes still exists.
For all above mentioned, a randomized trial of head-to-head comparison of PPOS with a GnRH-a short protocol in patients with in vitro infertility is required to increase the strength of our hypothesis-generating findings.
Conclusions
This meta-analysis indicates that the use of PPOS protocols as compared with that of GnRH-a protocols is safe in terms of newborn outcomes. Therefore, progestin ovarian stimulation might represent an appealing treatment option for infertile patients.
Electronic supplementary material
(DOCX 13 kb)
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.von Wolff M, Thaler CJ, Frambach T, Zeeb C, Lawrenz B, Popovici RM, et al. Ovarian stimulation to cryopreserve fertilized oocytes in cancer patients can be started in the luteal phase. Fertil Steril. 2009;92:1360–1365. doi: 10.1016/j.fertnstert.2008.08.011. [DOI] [PubMed] [Google Scholar]
- 2.Kuang Y, Chen Q, Fu Y, Wang Y, Hong Q, Lyu Q, et al. Medroxyprogesterone acetate is an effective oral alternative for preventing premature luteinizing hormone surges in women undergoing controlled ovarian hyperstimulation for in vitro fertilization. Fertil Steril. 2015;104:62–70.e3. doi: 10.1016/j.fertnstert.2015.03.022. [DOI] [PubMed] [Google Scholar]
- 3.Soules MR, Steiner RA, Clifton DK, Cohen NL, Aksel S, Bremner WJ. Progesterone modulation of pulsatile luteinizing hormone secretion in normal women. J Clin Endocrinol Metab. 1984;58:378–383. doi: 10.1210/jcem-58-2-378. [DOI] [PubMed] [Google Scholar]
- 4.Harris TG, Dye S, Robinson JE, Skinner DC, Evans NP. Progesterone can block transmission of the estradiol-induced signal for luteinizing hormone surge generation during a specific period of time immediately after activation of the gonadotropin-releasing hormone surge-generating system. Endocrinology. 1999;140:827–834. doi: 10.1210/endo.140.2.6490. [DOI] [PubMed] [Google Scholar]
- 5.Zhu X, Ye H, Fu Y. Use of Utrogestan during controlled ovarian hyperstimulation in normally ovulating women undergoing in vitro fertilization or intracytoplasmic sperm injection treatments in combination with a “freeze all” strategy: a randomized controlled dose-finding study of 100 mg versus 200 mg. Fertil Steril. 2017;107:379–386.e4. doi: 10.1016/j.fertnstert.2016.10.030. [DOI] [PubMed] [Google Scholar]
- 6.Wang Y, Chen Q, Wang N, Chen H, Lyu Q, Kuang Y. Controlled ovarian stimulation using medroxyprogesterone acetate and hMG in patients with polycystic ovary syndrome treated for IVF. Medicine (Baltimore) 2016;95:e2939. doi: 10.1097/MD.0000000000002939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Huang C-Y, Chen G-Y, Shieh M-L, Li H-Y. An extremely patient-friendly and efficient stimulation protocol for assisted reproductive technology in normal and high responders. Reprod Biol Endocrinol. 2018;16:18. doi: 10.1186/s12958-018-0335-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhang J, Mao X, Wang Y, Chen Q, Lu X, Hong Q, Kuang Y. Neonatal outcomes and congenital malformations in children born after human menopausal gonadotropin and medroxyprogesterone acetate treatment cycles. Arch Gynecol Obstet. 2017;296:1207–1217. doi: 10.1007/s00404-017-4537-z. [DOI] [PubMed] [Google Scholar]
- 9.Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gøtzsche PC, Ioannidis JPA, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: explanation and elaboration. BMJ. 2009;339:b2700. doi: 10.1136/bmj.b2700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sterne JA, Hernán MA, Reeves BC, Savović J, Berkman ND, Viswanathan M, et al. ROBINS-I: a tool for assessing risk of bias in non-randomised studies of interventions. BMJ. 2016;355:i4919. doi: 10.1136/bmj.i4919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Iorio A, Spencer FA, Falavigna M, Alba C, Lang E, Burnand B, et al. Use of GRADE for assessment of evidence about prognosis: rating confidence in estimates of event rates in broad categories of patients. BMJ Br Med J Publishing Group. 2015;350. [DOI] [PubMed]
- 12.Borenstein M, Hedges LV, Higgins JPT, Rothstein HR. A basic introduction to fixed-effect and random-effects models for meta-analysis. Res Synth Methods. 2010;1:97–111. doi: 10.1002/jrsm.12. [DOI] [PubMed] [Google Scholar]
- 13.Higgins JPT, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327:557–560. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhu X, Ye H, Fu Y. Comparison of neonatal outcomes following progesterone use during ovarian stimulation with frozen-thawed embryo transfer. Sci Rep. 2017;7:7835. doi: 10.1038/s41598-017-08472-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang N, Lin J, Zhu Q, Fan Y, Wang Y, Fu Y, et al. Comparison of neonatal outcomes and live-birth defects after progestin-primed ovarian stimulation versus conventional ovarian stimulation for in vitro fertilization. Medicine (Baltimore) 2018;97:e11906. doi: 10.1097/MD.0000000000011906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Huang J, Xie Q, Lin J, Lu X, Wang N, Gao H, et al. Neonatal outcomes and congenital malformations in children born after dydrogesterone application in progestin-primed ovarian stimulation protocol for IVF: a retrospective cohort study. Drug Des Devel Ther. 2019;13:2553–2563. doi: 10.2147/DDDT.S210228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.La Marca A, Capuzzo M. Use of progestins to inhibit spontaneous ovulation during ovarian stimulation: the beginning of a new era? Reprod BioMed Online. 2019;39:321–331. doi: 10.1016/j.rbmo.2019.03.212. [DOI] [PubMed] [Google Scholar]
- 18.Massin N. New stimulation regimens: endogenous and exogenous progesterone use to block the LH surge during ovarian stimulation for IVF. Hum Reprod Update. 2017;23:211–220. doi: 10.1093/humupd/dmw047. [DOI] [PubMed] [Google Scholar]
- 19.Wang A, Santistevan A, Hunter Cohn K, Copperman A, Nulsen J, Miller BT, et al. Freeze-only versus fresh embryo transfer in a multicenter matched cohort study: contribution of progesterone and maternal age to success rates. Fertil Steril. 2017;108:254–261.e4. doi: 10.1016/j.fertnstert.2017.05.007. [DOI] [PubMed] [Google Scholar]
- 20.Jin XY, Zhao LJ, Luo DH, Liu L, Dai YD, Hu XX, Wang YY, Lin X, Hong F, Li TC, Zhang SY. Pinopode score around the time of implantation is predictive of successful implantation following frozen embryo transfer in hormone replacement cycles. Hum Reprod. 2017;32:2394–2403. doi: 10.1093/humrep/dex312. [DOI] [PubMed] [Google Scholar]
- 21.Silva CC, Knight PG. Effects of androgens, progesterone and their antagonists on the developmental competence of in vitro matured bovine oocytes. J Reprod Fertil. 2000;119:261–269. doi: 10.1530/reprod/119.2.261. [DOI] [PubMed] [Google Scholar]
- 22.Carter F, Rings F, Mamo S, Holker M, Kuzmany A, Besenfelder U, Havlicek V, Mehta JP, Tesfaye D, Schellander K, Lonergan P. Effect of elevated circulating progesterone concentration on bovine blastocyst development and global transcriptome following endoscopic transfer of in vitro produced embryos to the bovine oviduct. Biol Reprod. 2010;83:707–719. doi: 10.1095/biolreprod.109.082354. [DOI] [PubMed] [Google Scholar]
- 23.Willingham-Rocky LA, Hinrichs K, Westhusin ME, Kraemer DC. Effects of stage of oestrous cycle and progesterone supplementation during culture on maturation of canine oocytes in vitro. Reproduction. 2003;126:501–508. doi: 10.1530/rep.0.1260501. [DOI] [PubMed] [Google Scholar]
- 24.Vannucchi CI, de Oliveira CM, Marques MG, Assumpção MEOÁ, Visintin JA. In vitro canine oocyte nuclear maturation in homologous oviductal cell co-culture with hormone-supplemented media. Theriogenology. 2006;66:1677–1681. doi: 10.1016/j.theriogenology.2006.01.008. [DOI] [PubMed] [Google Scholar]
- 25.Zhang X, Armstrong DT. Effects of follicle-stimulating hormone and ovarian steroids during vitro meiotic maturation on fertilization of rat oocytes. Gamete Res. 1989;23:267–277. doi: 10.1002/mrd.1120230304. [DOI] [PubMed] [Google Scholar]
- 26.Carmichael SL, Shaw GM, Laurent C, Croughan MS, Olney RS, Lammer EJ. Maternal progestin intake and risk of hypospadias. Arch Pediatr Adolesc Med. 2005;159:957. doi: 10.1001/archpedi.159.10.957. [DOI] [PubMed] [Google Scholar]
- 27.Giorlandino C, Cignini P, Padula F, Giannarelli D, d’Emidio L, Aloisi A, et al. Effects of exogenous progesterone on fetal nuchal translucency: an observational prospective study. Am J Obstet Gynecol. 2015;212:335.e1–335.e7. doi: 10.1016/j.ajog.2014.10.003. [DOI] [PubMed] [Google Scholar]
- 28.De Santis M, Cavaliere AF, Straface G, Carducci B, Caruso A. Failure of the emergency contraceptive levonorgestrel and the risk of adverse effects in pregnancy and on fetal development: an observational cohort study. Fertil Steril. 2005;84:296–299. doi: 10.1016/j.fertnstert.2005.01.136. [DOI] [PubMed] [Google Scholar]
- 29.Murthy YS, Arronet GH, Parekh MC. Luteal phase inadequacy. Its significance in infertility. Obstet Gynecol. 1970;36:758–761. [PubMed] [Google Scholar]
- 30.Norman JE, Marlow N, Messow C-M, Shennan A, Bennett PR, Thornton S, Robson SC, McConnachie A, Petrou S, Sebire NJ, Lavender T, Whyte S, Norrie J, OPPTIMUM study group Vaginal progesterone prophylaxis for preterm birth (the OPPTIMUM study): a multicentre, randomised, double-blind trial. Lancet. 2016;387:2106–2116. doi: 10.1016/S0140-6736(16)00350-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Romero R, Conde-Agudelo A, Da Fonseca E, O’Brien JM, Cetingoz E, Creasy GW, et al. Vaginal progesterone for preventing preterm birth and adverse perinatal outcomes in singleton gestations with a short cervix: a meta-analysis of individual patient data. Am J Obstet Gynecol. 2018;218:161–180. doi: 10.1016/j.ajog.2017.11.576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Romero R, Nicolaides KH, Conde-Agudelo A, O’Brien JM, Cetingoz E, Da Fonseca E, et al. Vaginal progesterone decreases preterm birth ≤ 34 weeks of gestation in women with a singleton pregnancy and a short cervix: an updated meta-analysis including data from the OPPTIMUM study. Ultrasound Obstet Gynecol. 2016;48:308–317. doi: 10.1002/uog.15953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Word R, Li X-H, Hnat M, Carrick K. Dynamics of cervical remodeling during pregnancy and parturition: mechanisms and current concepts. Semin Reprod Med. 2007;25:069–079. doi: 10.1055/s-2006-956777. [DOI] [PubMed] [Google Scholar]
- 34.Giacalone PL, Daurés JP, Faure JM, Boulot P, Hedon B, Laffargue F. The effects of mifepristone on uterine sensitivity to oxytocin and on fetal heart rate patterns. Eur J Obstet Gynecol Reprod Biol. 2001;97:30–34. doi: 10.1016/S0301-2115(00)00506-6. [DOI] [PubMed] [Google Scholar]
- 35.Xiao Z, Peng J, Yang J, Xu W. Flexible GnRH antagonist protocol versus progestin-primed ovarian stimulation (PPOS) protocol in patients with polycystic ovary syndrome: comparison of clinical outcomes and ovarian response. Curr Med Sci. 2019;39:431–436. doi: 10.1007/s11596-019-2055-x. [DOI] [PubMed] [Google Scholar]
- 36.Yildiz S, Turkgeldi E, Angun B, Eraslan A, Urman B, Ata B. Comparison of a novel flexible progestin primed ovarian stimulation protocol and the flexible gonadotropin-releasing hormone antagonist protocol for assisted reproductive technology. Fertil Steril. 2019;112:677–683. doi: 10.1016/j.fertnstert.2019.06.009. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX 13 kb)