Abstract
Purpose
Fetal growth restriction (FGR) is a high-risk pregnancy, and placental dysfunction is the main cause of FGR. The upregulation of asymmetric dimethylarginine (ADMA) is linked to FGR pathology, but the mechanism needs to be investigated.
Methods
The levels of ADMA and other related molecules were measured in human biological samples. We further used human umbilical vein endothelial cells (HUVECs) to reveal the mechanism of ADMA-induced FGR in vitro.
Results
Compared with the control group, FGR patients had higher placental resistance, and ADMA levels were increased in the maternal blood, cord blood, and placenta; additionally, nitric oxide (NO) production decreased, accompanied by a decreased expression of endogenous NO synthase (eNOS). The expression of vascular growth factor (VEGF) and placental growth factor (PLGF) in the maternal blood during the third trimester and umbilical cord of the FGR group was lower than the control group. The PLGF levels in the placentas of the FGR group were also reduced, while the expression of soluble fms-like tyrosine kinase-1 (sFlt-1) increased. In in vitro cell experiments, NO production was obviously lower when the cells were exposed to 100 μM of ADMA, with no difference in eNOS expression. There was a dose-dependent decrease in PLGF expression with increasing doses of ADMA, and the levels of sFlt-1 increased. Moreover, we confirmed that tube formation in HUVECs was lower after ADMA treatment compared with the control group.
Conclusion
The accumulation of ADMA during pregnancy has an adverse effect on fetal development via interference with placental endothelial function and angiogenesis.
Keywords: Fetal growth restriction, Endothelial function, Angiogenesis, Asymmetric dimethylarginine, Nitric oxide
Introduction
Fetal growth restriction (FGR) limits the genetic growth potential of the fetus [1]. The diagnostic criterion for FGR is that the estimated fetal weight (EFW) is below the 10th percentile for the normal standard of gestational age [2]. Epidemiological evidence suggests that FGR increases the risk of adverse perinatal outcomes and long-term complications [3–6]. Among the many pathological factors related to FGR, placental dysfunction is the most important [7]. During normal early pregnancy, trophoblasts invade the parental spiral artery, remodeling and destroying its smooth muscle layer. Additionally, uterine decidual natural killer (dNK) cells directly destroy the arterial muscular layer and indirectly guide the interstitial trophoblast to invade the spiral arteries [8]. This process of spiral arterial remodeling significantly increases the diameter of the spiral artery, thus effectively exchanging gases and nutrients to achieve the fetal growth potential. When the endothelial function is impaired, vascular tone and homeostasis are disrupted [9]. This will reduce the trophoblast invasion, resulting in incomplete remodeling of the placental vessels [10] and increasing the uterine spiral arterial wall thickness [11]. Thus, the uteroplacental circulation continues with high resistance and low blood flow, leading to reduced placental perfusion [12]. Placental ischemia and hypoxia compromise fetal growth and development in the uterus. It has been reported that endothelial dysfunction can affect placental development and participate in the occurrence of FGR [13, 14]. However, the molecular mechanism of FGR is still unclear and needs further investigation.
Nitric oxide (NO) is the most important endogenous vasodilator that plays a role in regulating placental circulation [15]. It is involved in the regulation of placental vascular tone and resistance, as well as placental trophoblast invasion [16, 17]. NO is produced by l-arginine under the action of NO synthase (NOS), which has three types, namely, neuronal NOS (nNOS), endothelial NOS (eNOS), and inducible NOS (iNOS). The NO produced in vascular endothelial cells, which mainly results from the activity of eNOS, functions in vasodilation and the regulation of blood perfusion [18]. Asymmetric dimethylarginine (ADMA) is a competitive inhibitor of NOS that inhibits NOS activity and reduces NO production [19]. ADMA is synthesized by the protein arginine methyltransferase (PRMT), which has nine isoforms, and PRMT1 is the main synthetase of human ADMA [20]. ADMA is degraded by dimethylarginine dimethylaminohydrolase (DDAH) [21], which has two isoforms: DDAH1 and DDAH2. DDAH1 is widely expressed in vivo, and its expression location is consistent with the main metabolic sites of ADMA, whereas DDAH2 is mainly expressed in placental tissues. Many studies have shown that elevated ADMA levels reduce vasodilation by inhibiting NOS activity, resulting in endothelial dysfunction and involvement in cardiovascular-related diseases and pre-eclampsia (PE) [22, 23]. However, the role of ADMA in FGR and its regulatory mechanisms remain to be elucidated. Here, we speculate that high levels of ADMA would reduce the NO bioavailability through the inhibition of eNOS, impair endothelium-mediated vasodilation, and affect placental perfusion, ultimately leading to FGR.
Rapid villus angiogenesis is another critical factor in fetal growth [10]. Angiogenic factors, such as the vascular endothelial growth factor (VEGF) and placental growth factor (PLGF), act synergistically, which promote placental angiogenesis and play a key role in placental development and functional maintenance [24, 25]. As a regulator of angiogenesis, NO stimulates endothelial cell proliferation and VEGF expression [26]. Moreover, NO appears to be involved in the modulation of PLGF and the anti-angiogenic factor soluble fms-like tyrosine kinase-1 (sFlt-1) [27]. We hypothesized that by inhibiting NOS activity, ADMA may also result in an imbalance between angiogenic factors and anti-angiogenic factors, affecting the establishment of maternal-fetal blood circulation. This may further reduce the placental blood perfusion, leading to FGR.
Much evidence suggests that NO is a potent factor in the maintenance of placental function, and the level of ADMA increases in FGR patients [28, 29]. However, how the ADMA/NO pathway regulates endothelial function and angiogenesis remains largely unknown. In this study, we investigated the effect of ADMA on placental function and fetal development by examining human blood samples, placental and umbilical cord tissues, and in vitro cell experiments.
Materials and methods
Clinical subjects and recruitment of participants
This study was conducted on the basis of a cohort study to focus on the early-life exposure to air pollutants and adverse pregnancy outcomes in Beijing, China. A total of 2500 pregnant women were recruited from January 1, 2017 to January 1, 2019 at the Beijing Obstetrics and Gynecology Hospital of Capital Medical University during their first trimester, and they were followed until delivery. The protocol for this study was approved by the Medical Ethics Committee of the Beijing Obstetrics and Gynecology Hospital, Capital Medical University. All of the study participants gave written informed consent prior to participating in the study. Maternal blood samples were obtained during the first (7–13 weeks), second (23–25 weeks), and third (28–34 weeks) trimesters of pregnancy and after delivery. Cord blood, placental tissue, and umbilical cord tissue samples were obtained after delivery. Blood specimens were centrifuged at 1000×g for 10 min, and the serum was stored in the specimen bank at − 80 °C. Tissue specimens were washed with ice-cold saline to clear the hemorrhage and stored immediately at − 80 °C. In order to assess the placental circulation during the second (22–24 weeks) and third (28–34 weeks) trimesters of pregnancy, a Doppler ultrasound was used to detect the umbilical artery (UA) pulsatility index (PI), resistance index (RI), and peak systolic velocity/end diastolic velocity (PSV/EDV, S/D). Furthermore, the placenta was examined by a pathologist following delivery to detect placental function and villous angiogenesis.
The EFW was evaluated according to the biometric parameters, including biparietal diameter (BPD), femur length (FL), head circumference (HC), and abdominal circumference (AC), as measured by ultrasound. Seventy-six pregnant women were diagnosed with FGR during pregnancy, with an incidence of 3.04%. In addition to multiple pregnancies, chromosome abnormalities of pregnant women or their husbands, fetal abnormality, pregnant women with chronic or gestational hypertension, gestational diabetes, thyroid disease and other metabolic-related diseases, immune system diseases, and malnutrition, the remaining 20 cases were considered to be placental-derived FGR, as the case group for this study. The birth weight (BW) of all 20 FGR cases was below the 10th percentile for the normal standard of gestational age when they were finally born. The control group was matched with the appropriate gestational age (AGA) for maternal age (± 5 years), body mass index (BMI) (± 1 kg/m2), gestational age (± 1 weeks), delivery mode, husband height (± 5 cm), and fetal gender in a ratio of 1:1.
Cell culture and chemical treatments
The human umbilical vein endothelial cell (HUVEC) cell line was obtained from Molecular Cell Laboratory of Tsinghua University (Beijing, China). The cells were cultured in endothelial cell medium (ECM, ScienCell) supplemented with 5% fetal bovine serum (FBS), 1% endothelial cell growth supplement (ECGS), and 1% penicillin/streptomycin solution (P/S) and incubated at 37 °C with an atmosphere of 5% CO2/95% air. The active 3rd-4th generation cells were used in the experiment. Confluent cells in 6-well plates were treated with ADMA (25, 50 and 100 μM) (Sigma, USA) for 24 h. Untreated HUVECs was served as control group.
ADMA, VEGF, and PLGF assays
ADMA in the serum and tissues, and VEGF and PLGF in the serum were measured by the enzyme-linked immunosorbent assay (ELISA) kit (BlueGene Biotech, China). All samples were tested in duplicate. Serum was measured directly after preparation. Tissues were minced to small pieces and homogenized in PBS. Then, the homogenates were centrifugated for 15 min at 1500×g. Remove the supernate and measure the protein concentration with bicinchoninic acid (BCA) protein assay kit (Beyotime Biotechnology, China) to calculate the ADMA content per unit weight of the protein. In this assay, the test sample and the enzyme-labeled antigen or antibody were reacted with an antigen or antibody bound to a solid phase carrier to form an antigen-antibody complex, and a substrate was added for color development. The optical density (OD) value was measured at 450 nm using a microplate reader (TECAN, Switzerland), and the sample concentration was calculated from a standard curve.
NO production assay
NO was determined by the Total Nitric Oxide Assay Kit (Beyotime Biotechnology, China) and tested in duplicate. NO is extremely unstable and metabolizes rapidly to nitrate and nitrite in vivo. In this method, nitrate reductase is used to reduce nitrate to nitrite, and then, nitrite is detected based on the classical Griess reagent to determine the total NO production. Samples, flavin adenine dinucleotide (FAD), nicotinamide adenine dinucleotide phosphate (NADPH), and nitrate reductase were added in turn, mixed, and incubated at 37 °C for 30 min. Then, lactate dehydrogenase (LDH) was added to remove excess NADPH and Griess reagent was added in a ratio of 1:1. The absorbance at 540 nm was measured using a microplate reader (TECAN, Switzerland). NO production in the samples was calculated according to the standard curve.
Western blot analysis
Proteins from tissues and cells (30 μg per lane) were separated by 12% polyacrylamide gel electrophoresis and then transferred to polyvinylidene fluoride membranes (Merck Millipore, USA). Membranes were probed with primary antibodies which included rabbit anti-human antibodies anti-PRMT1 (1:1000, Cell Signaling Technology, USA), anti-DDAH1 (1:2000, Abcam, UK), anti-DDAH2 (1:1000, Abcam, UK), anti-eNOS (1:1000, Cell Signaling Technology, USA), anti-VEGF (1:1000, Proteintech, USA), anti-PLGF (1:500, Proteintech, USA), anti-sFlt-1 (1:2000, Abcam, UK), anti-glyceraldehyde-3-phosphate dehydrogenase (GAPDH) (1:1000, Cell Signaling Technology, USA), and mouse anti-human antibody anti-β-actin (1:1000, Cell Signaling Technology, USA). The membranes were washed in Tris-buffered saline with Tween 20 (TBST) and then added horseradish peroxidase (HRP)-labeled secondary antibodies anti-rabbit IgG (1:1000, Cell Signaling Technology, USA) or anti-mouse IgG (1:1000, Cell Signaling Technology, USA) and incubated for 1 h at room temperature. The membranes were immersed in the enhanced chemiluminescence for 5 min. Amersham imager 600 (General Electric Company, USA) was used to capture images and analyze the gray value of target strips, and its ratio to the internal reference was used as the relative protein expression.
Matrigel tube formation assay
In vitro Matrigel-based tube formation assay was used to evaluate the angiogenic ability of endothelial cells. In brief, Matrigel (Corning, NY, USA) was thawed at 4 °C overnight, and 50 μL of Matrigel was added to each well of pre-chilled 96-well plate and incubated at 37 °C for 45 min. In this experiment, ADMA (25, 50, and 100 μM) were added to cell culture medium overnight and then again before seeding HUVECs in Matrigel. Cells (1.5 × 104) were seeded into each Matrigel-coated well. After 6 h incubation at 37 °C, five pictures of the tube formation per well were taken using an inverted microscope camera. Total tube length was quantitated using the ImageJ 1.8.0 image analysis system.
Statistical analysis
Statistical analyses were performed using IBM SPSS statistics 23, and preparations of figures were performed using GraphPad prism 7. Experimental test results and measurement data were expressed as mean ± standard deviation (SD). Student’s 2-tailed t test was used for comparison between two independent sample groups. Categorical data was expressed as frequency or percentage and analyzed with chi-square test. Correlation of BW percentile with maternal plasma ADMA was assessed using the Spearman (not normally distributed variables) correlation coefficients. P value < 0.05 was considered statistically significant.
Results
Characteristics of mothers and newborns
The maternal and neonatal characteristics of each group are described in Table 1. We analyzed 20 patients in both the control and FGR groups. Both the groups were comparable in terms of maternal age, BMI, gestational age, delivery mode, husband height, and fetal gender. The mean BW, BW percentile, neonatal body length, ponderal index, and placental weight were significantly lower in the FGR group than in the control group.
Table 1.
Maternal and newborns group characteristics
| Variables | Control (n = 20) | FGR (n = 20) | P values |
|---|---|---|---|
| Maternal age (years) | 31.15 ± 2.23 | 30.25 ± 3.97 | 0.383 |
| Pre-pregnancy BMI | 21.30 ± 2.19 | 21.75 ± 3.20 | 0.612 |
| BMI before delivery | 26.47 ± 2.08 | 27.51 ± 3.47 | 0.259 |
| Gestational age (weeks) | 39.63 ± 1.41 | 39.50 ± 1.15 | 0.809 |
| Cesarean deliveries | 7 (35%) | 5 (25%) | 0.490 |
| Husband height (cm) | 173.95 ± 4.49 | 175.45 ± 4.05 | 0.274 |
| Fetal gender (F/M) | 13/7 | 13/7 | |
| BW (gram) | 3557.5 ± 159.7 | 2741.7 ± 195.6 | < 0.001*** |
| BW percentile | 67.93 ± 12.78 | 4.27 ± 1.24 | < 0.001*** |
| Body length (cm) | 51.20 ± 1.70 | 49.40 ± 1.98 | 0.004** |
| Ponderal index | 26.62 ± 2.35 | 23.02 ± 3.75 | < 0.001*** |
| Placental weight (gram) | 602.5 ± 62.86 | 502.25 ± 67.01 | < 0.001*** |
BMI is defined as pregnant women weight × height−2 (kg/m2). Ponderal index is defined as birth weight × body length−3 (kg/m3). Values are expressed as mean ± SD, frequency, and percentage
FGR fetal growth restriction, BMI body mass index, BW birth weight, F female, M male
**P < 0.01, ***P < 0.001
Placental resistance increases in the FGR group
Table 2 shows the blood flow parameters PI, RI, and S/D of UA as detected by Doppler ultrasonography. Compared with the second trimester, the UA blood flow parameter values were significantly decreased during the third trimester in both the groups. However, the UA-PI, RI, and S/D values in the FGR group were significantly higher than those in the control group at the same time point, which indicated an increase in placental resistance in the FGR patients. Furthermore, placental pathology revealed only scattered calcification and intervillous fibrin deposition in normal pregnant women (Fig. 1a), but reduced villus vessels, larger infarcts, and extensive fibrin deposition were found in most pregnancies complicated by FGR (Fig. 1b). The above results confirmed that the occurrence of FGR is associated with insufficient placental perfusion.
Table 2.
UA blood flow parameters at the second and third trimesters
| Variables | PI | RI | S/D | ||||||
|---|---|---|---|---|---|---|---|---|---|
| 2nd T | 3rd T | P values | 2nd T | 3rd T | P values | 2nd T | 3rd T | P values | |
| Control (n = 20) | 1.06 ± 0.17 | 0.83 ± 0.13 | < 0.001*** | 0.66 ± 0.06 | 0.57 ± 0.06 | < 0.001*** | 3.02 ± 0.48 | 2.40 ± 0.32 | < 0.001*** |
| FGR (n = 20) | 1.23 ± 0.11 | 0.96 ± 0.13 | < 0.001*** | 0.72 ± 0.03 | 0.62 ± 0.06 | < 0.001*** | 3.61 ± 0.41 | 2.68 ± 0.38 | < 0.001*** |
| P values | < 0.001*** | 0.005** | < 0.001*** | 0.026** | < 0.001*** | 0.015** | |||
Values are expressed as mean ± SD
UA umbilical artery, FGR fetal growth restriction, PI pulsatility index, RI resistance index, S/D peak systolic velocity/end diastolic velocity, T trimester
**P < 0.01, ***P < 0.001
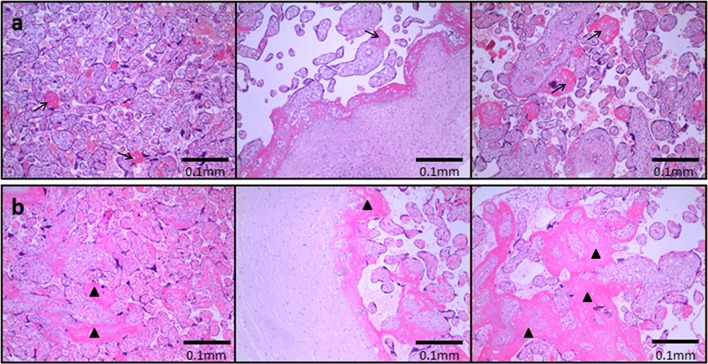
Fig. 1.
Placental pathological findings in the a control and b FGR groups. Placentas were fixed with formalin, stained with hematoxylin-eosin (HE), and then examined under the microscope. a Only scattered calcification and intervillous fibrin deposition (arrows) could be found in normal pregnant women. b Extensive infarcts (triangles) and reduced villus vessels were found in pregnancies complicated by FGR. Scale bar = 0.1 mm
ADMA is closely related to FGR caused by placental insufficiency
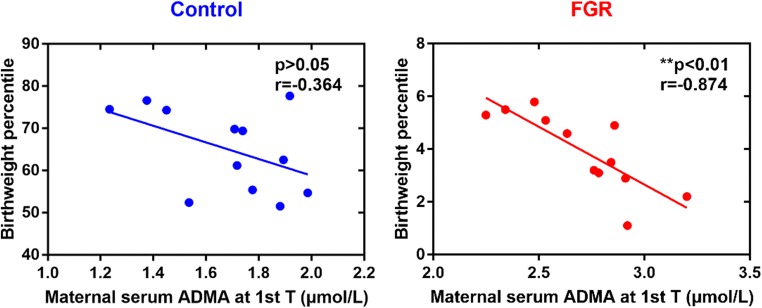
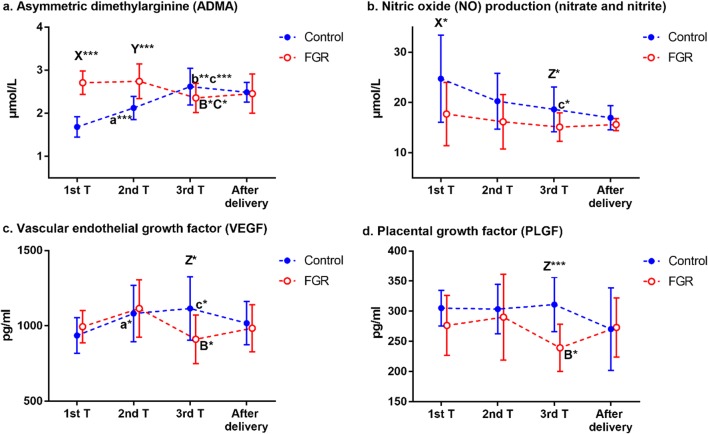
To investigate the relationship between ADMA and FGR, first, we examined ADMA levels in maternal peripheral blood, cord blood, and placental tissues (Fig. 2). Maternal serum ADMA levels showed a rising trend in the control group during the course of pregnancy; however, the ADMA levels in women with FGR decreased during late pregnancy compared with early and mid-pregnancy (Fig. 3a). ADMA levels in early and mid-pregnancy were higher in the FGR group than in the control group, but there was no significant difference during late pregnancy and after delivery (Table 3; Fig. 3a). ADMA levels in the placenta of FGR patients were higher than in normal pregnant women (Table 3). Because considerable remodeling of the placenta takes place toward the end of the first trimester [10], we speculated ADMA could impact placental remodeling, final weight, and its functional capacity and ultimately depress fetal growth. Thus, we tested the correlation between maternal serum ADMA during early pregnancy and neonatal BW percentile. As expected, in the control group, BW percentile did not correlate with maternal serum ADMA during early pregnancy. In contrast, however, BW percentile correlated strongly and inversely with maternal serum ADMA during early pregnancy in the FGR group (Fig. 2). Furthermore, ADMA levels in cord blood increased in the FGR group (Table 3), indicating that ADMA may play a role in FGR by affecting the fetal circulatory system.
Fig. 2.
Correlation between birthweight percentile and maternal serum ADMA concentration during early pregnancy in the control and FGR group. T trimester
Fig. 3.
Sequential changes in a ADMA, b NO, c VEGF, and d PLGF levels during the course of pregnancy in the control and FGR groups. Values are expressed as mean ± SD. a*/a*** represents P < 0.05/P < 0.001 between 1st and 2nd trimesters in control group, b** represents P < 0.01 between 2nd and 3rd trimesters in control group, c*/c*** represents P < 0.05/P < 0.001 between 1st and 3rd trimesters in control group; B* represents P < 0.05 between 2nd and 3rd trimesters in FGR group, C* represents P < 0.05 between 1st and 3rd trimesters in FGR group; X*/X*** represents P < 0.05/P < 0.001 between control and FGR groups at 1st trimester, Y*** represents P < 0.001 between control and FGR groups at 2nd trimester, Z* represents P < 0.05 between control and FGR groups at 3rd trimester
Table 3.
Comparison of ADMA, NO, VEGF, and PLGF levels in the serum or placenta between the two groups
| Concentration | Control | FGR | P values |
|---|---|---|---|
| Maternal peripheral blood | |||
| 1st T | n = 12 | n = 12 | |
| ADMA (μmol/L) | 1.684 ± 0.236 | 2.709 ± 0.272 | < 0.001*** |
| NO (μmol/L) | 24.74 ± 8.69 | 17.72 ± 6.30 | 0.034* |
| VEGF (pg/mL) | 935.97 ± 117.87 | 994.26 ± 108.00 | 0.220 |
| PLGF (pg/mL) | 305.27 ± 29.69 | 276.60 ± 49.87 | 0.101 |
| 2nd T | n = 12 | n = 12 | |
| ADMA (μmol/L) | 2.122 ± 0.269 | 2.742 ± 0.404 | < 0.001*** |
| NO (μmol/L) | 20.27 ± 5.55 | 16.16 ± 5.44 | 0.080 |
| VEGF (pg/mL) | 1082.36 ± 187.74 | 1115.43 ± 190.92 | 0.673 |
| PLGF (pg/mL) | 303.54 ± 41.03 | 290.38 ± 71.27 | 0.583 |
| 3rd T | n = 12 | n = 12 | |
| ADMA (μmol/L) | 2.620 ± 0.428 | 2.358 ± 0.340 | 0.111 |
| NO (μmol/L) | 18.63 ± 4.46 | 15.12 ± 2.83 | 0.031* |
| VEGF (pg/mL) | 1115.17 ± 209.96 | 911.53 ± 162.24 | 0.014* |
| PLGF (pg/mL) | 311.18 ± 45.06 | 239.47 ± 38.90 | < 0.001*** |
| After delivery | n = 12 | n = 12 | |
| ADMA (μmol/L) | 2.489 ± 0.230 | 2.454 ± 0.456 | 0.817 |
| NO (μmol/L) | 16.97 ± 2.40 | 15.62 ± 1.21 | 0.096 |
| VEGF (pg/mL) | 1017.60 ± 143.87 | 984.49 ± 156.06 | 0.594 |
| PLGF (pg/mL) | 270.57 ± 68.68 | 273.15 ± 49.25 | 0.917 |
| Cord blood | n = 20 | n = 20 | |
| ADMA (μmol/L) | 2.448 ± 0.422 | 2.978 ± 1.028 | 0.039* |
| NO (μmol/L) | 19.65 ± 6.30 | 15.70 ± 6.21 | 0.053 |
| VEGF (pg/mL) | 1051.15 ± 146.61 | 1038.93 ± 148.25 | 0.795 |
| PLGF (pg/mL) | 288.46 ± 57.18 | 254.52 ± 53.60 | 0.041* |
| Placental tissue | n = 16 | n = 16 | |
| ADMA (μmol/g) | 3.491 ± 0.678 | 4.108 ± 0.933 | 0.041* |
| NO (μmol/g) | 30.79 ± 7.45 | 25.41 ± 6.89 | 0.025* |
Values are expressed as mean ± SD
FGR fetal growth restriction, ADMA asymmetric dimethylarginine, NO nitric oxide, VEGF vascular endothelial growth factor, PLGF placental growth factor, T trimester
*P < 0.05, ***P < 0.001
The accumulation of ADMA in the FGR group is related to elevated PRMT1 expression in the placenta and decreased DDAH1 expression in the umbilical cord
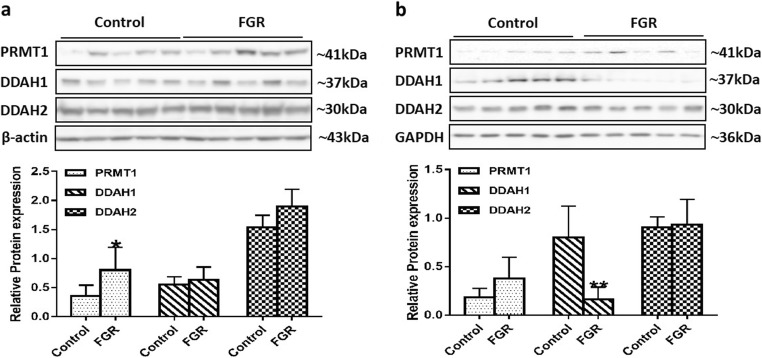
To explore the molecular mechanism underlying elevated ADMA levels in the FGR group, we tested the expression of ADMA metabolism-related proteins PRMT1, DDAH1, and DDAH2 in placental and umbilical cord tissues. Compared with normal pregnant women, the expression level of PRMT1 was significantly increased in FGR placental tissues, while there was no significant difference in the expression of DDAH1 and DDAH2 between the two groups (Fig. 4a). In another respect, Fig. 4b shows that the DDAH1 level was significantly decreased and the PRMT1 level was increased in the umbilical cord tissues of the FGR group, while the DDAH2 level was not significantly different.
Fig. 4.
Expression of ADMA metabolism-related protein in the placental and umbilical cord tissues. Protein bands of PRMT1, DDAH1 and DDAH2 in the placental (a) and umbilical cord (b) tissues were examined by Western blot analysis (n = 5 per group). β-Actin and GAPDH were used as internal references. Values are expressed as mean ± SD. *P < 0.05, **P < 0.01 compared with “control”
NO production in mother and fetus is reduced in the FGR group
To determine whether elevated ADMA levels in FGR patients would reduce NO bioavailability, we evaluated NO generation by measuring nitrate and nitrite levels in maternal peripheral blood, cord blood, and placental tissues in both groups. Total NO production decreased significantly during late pregnancy as compared with early pregnancy in the control group (Fig. 3b). During the entire pregnancy, NO levels in the FGR group also showed a downward trend, but there was no significant difference (Fig. 3b). NO levels were significantly lower in the FGR group than in the control group, even in early pregnancy, and remained low throughout the course of pregnancy, but there was no difference after delivery (Table 3; Fig. 3b). NO levels in FGR patients were lower than that in normal pregnant women in placental tissues (Table 3). In addition, in the cord blood of the FGR group, NO levels were slightly lower than in the control group (Table 3). The above results showed that the NO production in the mother and fetus was reduced in the FGR group, indicating that the increased ADMA would reduce NO bioavailability and affect the maternal and fetoplacental circulation.
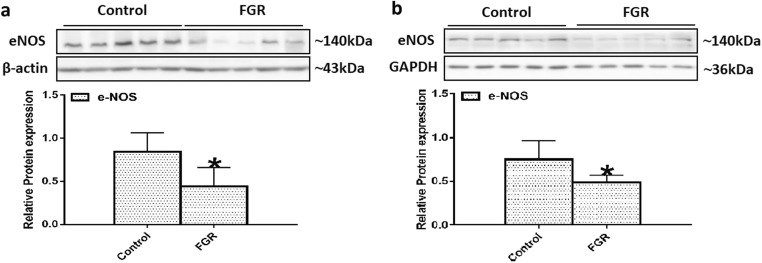
Difference in eNOS expression between the two groups
During pregnancy, eNOS is mainly synthesized by placental and umbilical vascular endothelial cells [30]. To confirm whether the reduced NO bioavailability in the mother and fetus was related to the expression of eNOS, we further assessed the eNOS level in the two groups. Our results showed that the expression level of eNOS was decreased in the FGR group as compared with the control group in both the placental (Fig. 5a) and umbilical cord (Fig. 5b) tissues.
Fig. 5.
Expression of eNOS in the placental and umbilical cord tissues. Protein bands of eNOS in the placental (a) and umbilical cord (b) tissues were examined by Western blot analysis (n = 5 per group). β-Actin and GAPDH were used as internal references. Values are expressed as mean ± SD. *P < 0.05 compared with “control”
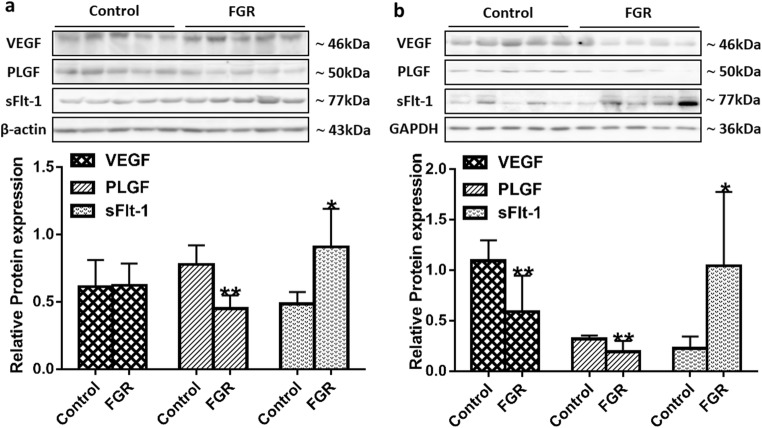
The reduced NO bioavailability suppresses the expression of VEGF and PLGF but increases the expression of sFlt-1
Pro-angiogenic growth factors, such as VEGF and PLGF, can promote trophoblast cell proliferation and invasion during early pregnancy and facilitate angiogenesis and remodeling [31, 32]. SFlt-1 is the splice variant of VEGF receptor-1 (Flt-l), which can form a heterodimer with cell-surface receptor Flt-l and block the biological effects of VEGF and PLGF [33]. While NO is the main mediator in angiogenesis [27], increased ADMA can impair angiogenesis by inhibiting eNOS activity. Therefore, we hypothesized that the expression of VEGF and PLGF would be reduced and the expression of sFlt-1 would be increased in the FGR patients. To test this hypothesis, we examined VEGF, PLGF, and sFlt-1 levels in the maternal peripheral blood, cord blood, or placental and umbilical cord tissues.
Maternal serum VEGF levels increased significantly from the first to the third trimester of pregnancy in the control group (Fig. 3c), and the PLGF levels did not change significantly across the three trimesters (Fig. 3d). However, in women with FGR, VEGF and PLGF levels showed a downward trend from the second to the third trimester of pregnancy (Fig. 3c and d), and the levels of both were lower in the FGR group than in the control group at 28–34 weeks of gestation (Table 3; Fig. 3c and d). The expression level of PLGF in the placenta and umbilical cord of the FGR group was lower than that in the control group (Fig. 6a and b). The expression level of VEGF was also significantly decreased in the umbilical cord of the FGR group (Fig. 6b), and there was no significant difference in the placenta between the two groups (Fig. 6a). The expression level of sFlt-1 in the FGR placenta and umbilical cord was higher than in the control group (Fig. 6a and b). In addition, PLGF levels showed a significant reduction in the cord blood of the FGR group (Table 3).
Fig. 6.
Expression of angiogenic and anti-angiogenic factors in the placental and umbilical cord tissues. Protein bands of VEGF, PLGF, and sFlt-1 in the placental (a) and umbilical cord (b) tissues were examined by Western blot analysis (n = 5 per group). β-Actin and GAPDH were used as internal references. Values are expressed as mean ± SD. *P < 0.05, **P < 0.01 compared with “control”
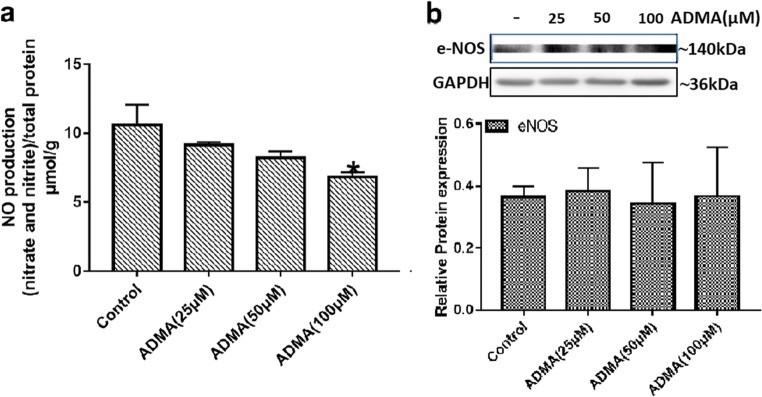
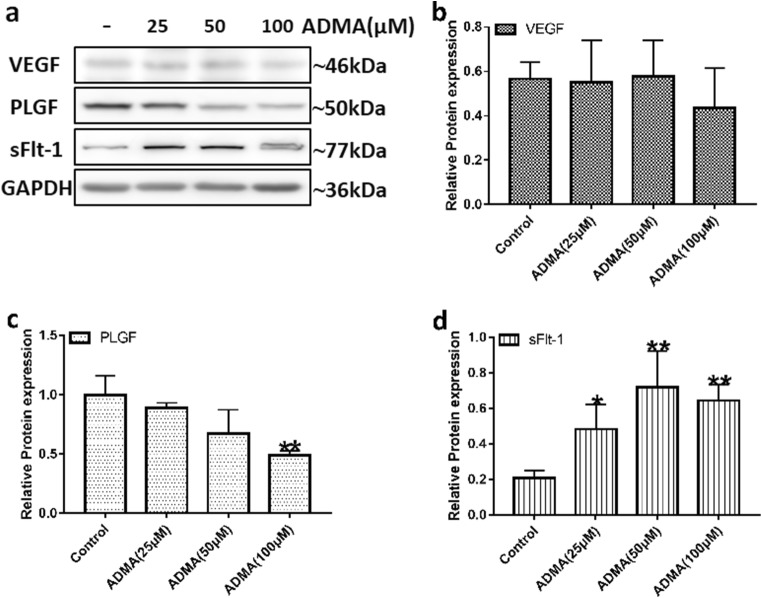
Effects of ADMA on HUVEC cell line
To further elucidate the mechanism of ADMA-induced FGR, we performed in vitro cell experiments. NO production in cells exposed to 100 μM of ADMA was significantly lower compared with the control group (Fig. 7a). The mean levels of NO were 10.61, 9.17, 8.23, and 6.85 μmol/g for control, 25 μM, 50 μM, and 100 μM ADMA, respectively. However, there was no difference in eNOS expression levels between the groups (Fig. 7b). ADMA, at a concentration of 100 μM, could slightly reduce the expression of angiogenic factor VEGF in HUVECs, and with increasing doses of ADMA, there was a significant dose-dependent decrease in PLGF expression (Fig. 8a–c). Furthermore, compared with the untreated group, the levels of the anti-angiogenic factor sFlt-1 in HUVECs treated with different concentrations of ADMA increased significantly (Fig. 8a and d). HUVECs spontaneously differentiate to form tubules when cultured on extracellular matrix components, such as Matrigel, so we further performed a tube formation assay to explore the effect of ADMA on angiogenesis. As depicted in Fig. 9a, after 6 h of incubation on Matrigel, HUVECs formed a tubular network containing many junctions. By measuring the total tube length, we could evaluate and quantify the ability of different test compounds to disrupt tube formation. Treatment with ADMA caused a reduction in branched capillary-like structures, with inhibition rates of 18.8% and 40.2% at concentrations of 50 and 100 μM, respectively (Fig. 9b).
Fig. 7.
HUVECs treated with ADMA resulted in reduced NO production with no significant change in eNOS protein levels. Confluent HUVECs were treated with ADMA (25, 50, and 100 μM) for 24 h. a NO production was represented by nitrite and nitrate using Griess colorimetric assay and normalized to protein concentration (n = 3 per group). b Representative images and densitometry analysis of eNOS protein were detected by western blot, and the independent experiment was repeated for three times. GAPDH was used as internal references. Values are expressed as mean ± SD. *P < 0.05 compared with “control”
Fig. 8.
HUVECs treated with ADMA resulted in an imbalance in the expression of angiogenic and anti-angiogenic factors. a Representative images of VEGF, PLGF, and sFlt-1 protein detected by western blot, and the independent experiment was repeated for three times. b–d Densitometry analysis of the western blot results. GAPDH was used as internal references. Values are expressed as mean ± SD. *P < 0.05, **P < 0.01 compared with “control”
Fig. 9.
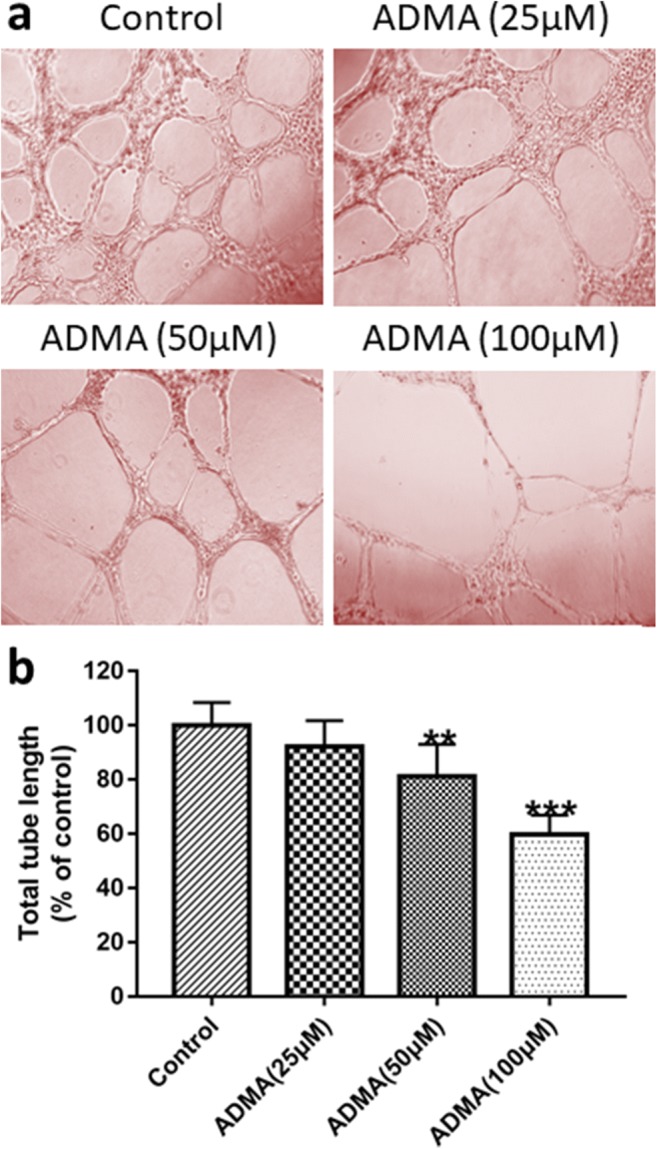
ADMA inhibited tube formation on HUVECs. a Representative images show the morphological features of HUVECs on Matrigel which were treated with different concentrations of ADMA. The tube length decreased after 24 h incubation with ADMA. b The tube length was quantified in each treatment conditions. Percentage of inhibition was expressed using control wells at 100%. **P < 0.01, ***P < 0.001 compared with “control”
Discussion
Pathological changes in the placenta are the focus of studies regarding FGR. At present, many studies support the theory that FGR is caused by a placental circulation disorder. The placenta of FGR patients often has vascular lesions and high circulatory resistance [12]. UA Doppler has been widely used for the screening of placenta-mediated pregnancy complications, such as PE [34], indicating a poor prognosis in a fetus with FGR [35]. This study detected UA blood flow parameters during the second and third trimester, and the values decreased as the gestational age increased. As the placenta gradually matures, the villus blood vessels increase and the UA resistance decreases. Additionally, the UA-PI, RI, and S/D values of FGR patients were higher than those of normal pregnant women during the middle and late stages of pregnancy, indicating that patients with FGR have high UA blood flow resistance and low UA blood flow, which was due to the maternal and fetal circulation disorders caused by the increased placental resistance. The resultant hypoperfusion induces cell stress in the placental tissues, leading to selective inhibition of protein synthesis and reduced cell proliferation, and ultimately increases placental infarction and fibrin deposition [10].
ADMA is a natural amino acid synthesized and released by vascular endothelial cells [36]. Holden et al. [37] reported that blood ADMA levels during normal early pregnancy were lower than in non-pregnancy and gradually increased with the increase in gestational weeks. In this study, the trend of ADMA in normal pregnant women during pregnancy was consistent with previous studies. During early pregnancy, reduced ADMA and increased NO can relax the uterine spiral arteries and promote extravillous trophoblasts (EVTs) to migrate retrogradely into the uterine spiral arteries, resulting in a loss of smooth muscle cells in the middle layer and enlargement of the lumen. During late pregnancy, increased ADMA improves uterine contractility by antagonizing NO-induced uterine relaxation and prepares for delivery [38]. However, the abnormal increase of blood ADMA concentration can lead to endothelial dysfunction, which has become a biomarker for the risk assessment of PE, hypercholesterolemia, stroke, and cardiovascular diseases [39, 40]. In this study, BW percentile was found to be inversely correlated with maternal serum ADMA concentration, which suggested that maternal serum ADMA concentration was also an important indicator of FGR in women with impaired placental perfusion. ADMA levels in the peripheral blood of FGR patients were significantly increased in early and mid-pregnancy and accompanied by a decrease in NO bioavailability. In in vitro cell experiments, the addition of exogenous ADMA resulted in a decreased NO production, which confirmed that high levels of ADMA were related to placental vascular endothelial dysfunction, thus participating in the pathogenesis of FGR. However, with increasing doses of exogenous ADMA, there was no difference in eNOS protein levels between the groups, which showed that ADMA did not directly inhibit the expression of eNOS. Another important result is that ADMA levels in fetal cord blood were also increased in the FGR group. It has been pointed out that ADMA could depress fetal growth by inhibition of insulin-like growth factor-1 (IGF-1) and growth hormone (GH) factors in fetal circulation [41].
Placental nutritional transport depends on vascular development, in which NO plays a crucial role. The regulation of eNOS expression and activity and NO bioavailability is considered to be an important mechanism involved in FGR and PE-related placental vascular dysfunction [17]. It was found that the eNOS expression in placenta increased with the continuation of pregnancy [42]. However, in this study, we have shown that eNOS expression significantly decreased in the FGR placenta and umbilical cord. ENOS is mainly synthesized in the placenta during pregnancy, and massive remodeling of the placenta occurs at the end of the first trimester/start of the second trimester [10]. We believe that in FGR patients the increased ADMA levels and decreased NO production in early and mid-pregnancy lead to poorly developed villus trees and a decrease in total eNOS protein. Therefore, at the third trimester, there was no concordant change in NO production as ADMA levels decreased.
Previous studies have confirmed that the endothelial function can be improved by regulating the PRMT/ADMA/DDAH pathway [43]. During pregnancy, the placenta is an important source of ADMA [44], and the present study found that PRMT1 increased significantly in the FGR placenta, while there was no difference in DDAH expression. Our results showed that the increased ADMA in women with FGR was associated with increased synthesis in placenta. However, our study found that DDAH1 expression in the FGR umbilical cord decreased significantly. DDAH1 can be expressed in HUVECs [29], so we speculated that the accumulation of ADMA in fetal circulation originated from ADMA metabolic disorders caused by diminished DDAH1. In another respect, impaired placental circulation can lead to fetal endothelial dysfunction [45], and ADMA in cord blood is mainly generated by the placenta [46]. In the present study, ADMA levels in the placenta of FGR patients increased significantly, so elevated levels of ADMA in fetal cord blood may have partly originated from enhanced accumulation and transport of ADMA in the placenta.
Adverse uteroplacental circulation can affect the development and function of placental villus vessels. Many pro-angiogenic factors are involved in FGR by affecting placental vascular development, and VEGF and PLGF are the current hotspots [47]. In the developing placenta, VEGF and PLGF, which are mainly expressed in syncytiotrophoblast and endothelial cells, bind to the VEGF receptor (VEGFR) and play a pivotal role in neo-angiogenesis and vascular growth [25, 48, 49]. Furthermore, PLGF can affect endothelial function by regulating the effects of VEGF on endothelial cell proliferation and migration [48]. SFlt-1 is a poly-glycosylated protein mainly produced and secreted by the placenta, which has an anti-angiogenic effect and can cause reduced angiogenesis in the placenta and endothelial damage in the mother. In the present study, VEGF and PLGF concentrations were lower in women with FGR during the third trimester of pregnancy and were accompanied by decreased NO bioavailability. The establishment of placental circulation is a complex process, including vasculogenesis and angiogenesis. During the third trimester of pregnancy, vascular growth at the maternal-fetal interface increases exponentially, at a time when angiogenic factors play an important role, essentially keeping pace with the rate of the growing fetus [50]. This may be why VEGF and PLGF decreased during the third trimester in FGR patients, and the association with ADMA was weaker during the first and second trimesters, which was consistent with the changes of the PLGF concentration in women with PE, as shown by an earlier report [51]. Given that VEGF and PLGF are important factors in regulating endothelial cell proliferation and vascular endothelium is the main target of ADMA, we suggest that ADMA can affect placental blood circulation by modulating angiogenic factors, which has been confirmed in subsequent cell experiments. In the placenta, PLGF expression decreased significantly, but there was no difference in VEGF expression, and the in vitro cell experiments showed that ADMA mainly inhibited the expression of PLGF rather than VEGF, indicating that the reduction of PLGF probably better reflects pregnancies associated with a significant placental pathology. In addition, the levels of sFlt-1 in the placenta and umbilical cord of FGR patients, as well as HUVECs treated with exogenous ADMA, were increased. Angiogenesis is regulated by the local balance between angiogenesis stimulators and inhibitors, and an in vitro tube formation assay further demonstrated that ADMA had adverse effects on vascularization by disrupting this balance. Studies have found that the sFlt-1/PLGF ratio is of great value for the prediction of PE and FGR [52, 53]. The decreased expression of VEGF and PLGF in the umbilical cord and PLGF in cord blood suggested that the expression of pro-angiogenic factors in fetal circulation was also inhibited. There is no evidence that angiogenic factors can be transported to the fetus through the placenta, and we speculate that the decrease in fetal angiogenic factor levels may be related to endothelial dysfunction caused by elevated ADMA levels. Decreased levels of fetal VEGF and PLGF will have a negative impact on the growth of fetal multiple organs or tissues [54], resulting in impaired intrauterine development, which is ultimately reflected in BW loss.
To summarize, sufficient vasodilatation of fetoplacental circulation together with rapid villus angiogenesis are the necessary factors for adequate placental development and subsequent fetal growth. This study demonstrated that maternal serum ADMA concentration is an important indicator of FGR. In pregnant women with FGR, elevated PRMT1 expression in the placenta caused the accumulation of ADMA and increased ADMA can impair endothelial function by decreasing NO bioavailability. Moreover, reduced NO bioavailability can result in an imbalance between angiogenic and anti-angiogenic factors mainly by inhibiting the expression of PLGF and increasing the level of sFlt-1, thus suppressing the villus angiogenesis, further affecting placental maturation and functional maintenance and ultimately leading to the occurrence of FGR. Although the study was conducted within a single ethnic region, we believe that the findings of this study can be generalized to other populations because we have excluded environmental/genetic confounders as much as possible and selected for FGR caused by placental dysfunction. By investigating the mechanism of ADMA in FGR, our study provided novel evidence for the etiology of FGR, which is of great significance for FGR prediction, early diagnosis, and the discovery of new therapeutic targets.
Acknowledgments
We would like to thank all subjects who have agreed to participate in the study, as well as the staff at the Beijing Obstetrics and Gynecology Hospital, Capital Medical University and Beijing Maternal and Child Health Care Hospital who participated in the establishment of the birth cohort.
Author contributions
YD: project development, experimental performation, data collection or management, data analysis, manuscript writing. JZ and RL: data collection or management, data analysis, manuscript writing. NX and SBY: data collection or management, data analysis, manuscript editing. YC and THL: project development, experimental design, data collection or management, data analysis, manuscript writing or editing.
Funding information
This work was supported by Grant No 81571130090 from the China’s National Natural Science Foundation Program.
Compliance with ethical standards
The protocol for this study has been approved by the Medical Ethics Committee of the Beijing Obstetrics and Gynecology Hospital, Capital Medical University (ethics number 2016-KY-059-01).
Informed consent
Informed consent was obtained from all individual participants included in the study.
Conflict of interest
The authors declare that they have no conflict of interest.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Yi Chen, Email: bjfcyycy@sina.com.
Tian-He Li, Email: litianhe19890216@163.com.
References
- 1.Sharma D, Shastri S, Sharma P. Intrauterine growth restriction: antenatal and postnatal aspects. Clin Med Insights Pediatr. 2019;10:67–83. doi: 10.4137/CMPed.S40070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.American College of Obstetricians and Gynecologists ACOG Practice bulletin no. 134: fetal growth restriction. Obstet Gynecol. 2013;121(5):1122–1133. doi: 10.1097/01.AOG.0000429658.85846.f9. [DOI] [PubMed] [Google Scholar]
- 3.Hoffman ML, Reed SA, Pillai SM, Jones AK, McFadden KK, Zinn SA, Govoni KE. PHYSIOLOGY AND ENDOCRINOLOGY SYMPOSIUM: the effects of poor maternal nutrition during gestation on offspring postnatal growth and metabolism. J Anim Sci. 2017;95(5):2222–2232. doi: 10.2527/jas.2016.1229. [DOI] [PubMed] [Google Scholar]
- 4.Kramer MS, Zhang X, Dahhou M, Yang S, Martin RM, Oken E, Platt RW. Does fetal growth restriction cause later obesity? Pitfalls in analyzing causal mediators as confounders. Am J Epidemiol. 2017;185(7):585–590. doi: 10.1093/aje/kww109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Martino F, Magenta A, Pannarale G, Martino E, Zanoni C, Perla FM, Puddu PE, Barillà F. Epigenetics and cardiovascular risk in childhood. J Cardiovasc Med. 2016;17(8):539–546. doi: 10.2459/JCM.0000000000000334. [DOI] [PubMed] [Google Scholar]
- 6.Barker DJ. Adult consequences of fetal growth restriction. Clin Obstet Gynecol. 2006;49(2):270–283. doi: 10.1097/00003081-200606000-00009. [DOI] [PubMed] [Google Scholar]
- 7.Groom KM, David AL. The role of aspirin, heparin, and other interventions in the prevention and treatment of fetal growth restriction. Am J Obstet Gynecol. 2018;218(2S):S829–S840. doi: 10.1016/j.ajog.2017.11.565. [DOI] [PubMed] [Google Scholar]
- 8.Sato Y. Endovascular trophoblast and spiral artery remodeling. Mol Cell Endocrinol. 2019;110699. [DOI] [PubMed]
- 9.Su JB. Vascular endothelial dysfunction and pharmacological treatment. World J Cardiol. 2015;7(11):719–741. doi: 10.4330/wjc.v7.i11.719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Burton GJ, Jauniaux E. Pathophysiology of placental-derived fetal growth restriction. Am J Obstet Gynecol. 2017;218(2S):S745–S761. doi: 10.1016/j.ajog.2017.11.577. [DOI] [PubMed] [Google Scholar]
- 11.Zanardo V, Visentin S, Trevisanuto D, Bertin M, Cavallin F, Cosmi E. Fetal aortic wall thickness: a marker of hypertension in IUGR children? Hypertens Res. 2013;36(5):440–443. doi: 10.1038/hr.2012.219. [DOI] [PubMed] [Google Scholar]
- 12.Nardozza LM, Caetano AC, Zamarian AC, Mazzola JB, Silva CP, Marçal VM, Lobo TF, Peixoto AB, Araujo Júnior E, Araujo Júnior E. Fetal growth restriction: current knowledge. Arch Gynecol Obstet. 2017;295(5):1061–1077. doi: 10.1007/s00404-017-4341-9. [DOI] [PubMed] [Google Scholar]
- 13.Yzydorczyk C, Armengaud JB, Peyter AC, Chehade H, Cachat F, Juvet C, Siddeek B, Simoncini S, Sabatier F, Dignat-George F, Mitanchez D, Simeoni U. Endothelial dysfunction in individuals born after fetal growth restriction: cardiovascular and renal consequences and preventive approaches. J Dev Orig Health Dis. 2017;8(4):448–464. doi: 10.1017/S2040174417000265. [DOI] [PubMed] [Google Scholar]
- 14.Brosens I, Pijnenborg R, Vercruysse L, Romero R. The “great obstetrical syndromes” are associated with disorders of deep placentation. Am J Obstet Gynecol. 2011;204(3):193–201. doi: 10.1016/j.ajog.2010.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Johal T, Lees CC, Everett TR, Wilkinson IB. The nitric oxide pathway and possible therapeutic options in pre-eclampsia. Br J Clin Pharmacol. 2014;78(2):244–257. doi: 10.1111/bcp.12301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Reynolds LP, Borowicz PP, Caton JS, Vonnahme KA, Luther JS, Buchanan DS, Hafez SA, Grazul-Bilska AT, Redmer DA. Uteroplacental vascular development and placental function: an update. Int J Dev Biol. 2010;54(2–3):355–366. doi: 10.1387/ijdb.082799lr. [DOI] [PubMed] [Google Scholar]
- 17.Krause BJ, Hanson MA, Casanello P. Role of nitric oxide in placental vascular development and function. Placenta. 2011;32(11):797–805. doi: 10.1016/j.placenta.2011.06.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ikenouchi-Sugita A, Yoshimura R, Kishi T, Umene-Nakano W, Hori H, Hayashi K, Katsuki A, Ueda N, Iwata N, Nakamura J. Three polymorphisms of the eNOS gene and plasma levels of metabolites of nitric oxide in depressed Japanese patients: a preliminary report. Hum Psychopharmacol. 2011;26(7):531–534. doi: 10.1002/hup.1239. [DOI] [PubMed] [Google Scholar]
- 19.Huang LT, Hsieh CS, Chang KA, Tain YL. Roles of nitric oxide and asymmetric dimethylarginine in pregnancy and fetal programming. Int J Mol Sci. 2012;13(11):14606–14622. doi: 10.3390/ijms131114606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Adamopoulos PG, Mavrogiannis AV, Kontos CK, Scorilas A. Novel alternative splice variants of the human protein arginine methyltransferase 1 (PRMT1) gene, discovered using next-generation sequencing. Gene. 2019;699:135–144. doi: 10.1016/j.gene.2019.02.072. [DOI] [PubMed] [Google Scholar]
- 21.You-Lin T, Li-Tung H. Restoration of asymmetric dimethylarginine-nitric oxide balance to prevent the development of hypertension. Int J Mol Sci. 2014;15(7):11773–11782. doi: 10.3390/ijms150711773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Böger RH. Asymmetric dimethylarginine, an endogenous inhibitor of nitric oxide synthase, explains the “l-arginine paradox” and acts as a novel cardiovascular risk factor. J Nutr. 2004;134(10 Suppl):2842–2847. doi: 10.1093/jn/134.10.2842S. [DOI] [PubMed] [Google Scholar]
- 23.Ehsanipoor RM, Fortson W, Fitzmaurice LE, Liao WX, Wing DA, Chen DB, Chan K. Nitric oxide and carbon monoxide production and metabolism in preeclampsia. Reprod Sci. 2013;20(5):542–548. doi: 10.1177/1933719112459231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Travaglino A, Raffone A, Saccone G, Migliorini S, Maruotti GM, Esposito G, et al. Placental morphology, apoptosis, angiogenesis and epithelial mechanisms in early-onset preeclampsia. Eur J Obstet Gynecol Reprod Biol. 2019;234:200–206. doi: 10.1016/j.ejogrb.2018.12.039. [DOI] [PubMed] [Google Scholar]
- 25.De Falco S. The discovery of placenta growth factor and its biological activity. Exp Mol Med. 2012;44(1):1–9. doi: 10.3858/emm.2012.44.1.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Vanella L, Di Giacomo C, Acquaviva R. The DDAH/NOS pathway in human prostatic cancer cell lines: antiangiogenic effect of LNAME. Int J Oncol. 2011;39(5):1303–1310. doi: 10.3892/ijo.2011.1107. [DOI] [PubMed] [Google Scholar]
- 27.Groesch KA, Torry RJ, Wilber AC, Abrams R, Bieniarz A, Guilbert LJ, Torry DS. Nitric oxide generation affects pro- and anti-angiogenic growth factor expression in primary human trophoblast. Placenta. 2011;32(12):926–931. doi: 10.1016/j.placenta.2011.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.He Q, Liu X, Zhong Y, Xu SS, Zhang ZM, Tang LL, Zhang LY, du LZ. Arginine bioavailability and endothelin-1 system in the regulation of vascular function of umbilical vein endothelial cells from intrauterine growth restricted newborns. Nutr Metab Cardiovasc Dis. 2018;28(12):1285–1295. doi: 10.1016/j.numecd.2018.09.002. [DOI] [PubMed] [Google Scholar]
- 29.Laskowska M, Laskowska K, Oleszczuk J. The relation of maternal serum eNOS, NOSTRIN and ADMA levels with aetiopathogenesis of preeclampsia and/or intrauterine fetal growth restriction. J Matern Fetal Neonatal Med. 2015;28(1):26–32. doi: 10.3109/14767058.2014.900036. [DOI] [PubMed] [Google Scholar]
- 30.Wikström AK, Haglund B, Olovsson M, Lindeberg SN. The risk of maternal ischaemic heart disease after gestational hypertensive disease. BJOG. 2005;121(11):1486–1491. doi: 10.1111/j.1471-0528.2005.00733.x. [DOI] [PubMed] [Google Scholar]
- 31.Lyall F, Robson SC, Bulmer JN. Spiral artery remodeling and trophoblast invasion in preeclampsia and fetal growth restriction: relationship to clinical outcome. Hypertension. 2013;62(6):1046–1054. doi: 10.1161/HYPERTENSIONAHA.113.01892. [DOI] [PubMed] [Google Scholar]
- 32.Duhig KE, Chappell LC, Shennan AH. How placental growth factor detection might improve diagnosis and management of pre-eclampsia. Expert Rev Mol Diagn. 2014;14(4):403–406. doi: 10.1586/14737159.2014.908121. [DOI] [PubMed] [Google Scholar]
- 33.Al-Ani B, Hewett PW, Cudmore MJ, Fujisawa T, Saifeddine M, Williams H, et al. Activation of proteinase-activated receptor 2 stimulates soluble vascular endothelial growth factor receptor 1 release via epidermal growth factor receptor transactivation in endothelial cells. Hypertension. 2010;55(3):689–697. doi: 10.1161/HYPERTENSIONAHA.109.136333. [DOI] [PubMed] [Google Scholar]
- 34.Velauthar L, Plana MN, Kalidindi M, Zamora J, Thilaganathan B, Illanes SE. First-trimester uterine artery Doppler and adverse pregnancy outcome: a meta-analysis involving 55,974 women. Ultrasound Obstet Gynecol. 2014;43(5):500–507. doi: 10.1002/uog.13275. [DOI] [PubMed] [Google Scholar]
- 35.Alfirevic Z, Stampalija T, Dowswell T. Fetal and umbilical Doppler ultrasound in high-risk pregnancies. Cochrane Database Syst Rev. 2017;6(6):CD007529. doi: 10.1002/14651858.CD007529.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tsukahara H, Ohta N, Tokuriki S, Nishijima K, Kotsuji F, Kawakami H, Ohta N, Sekine K, Nagasaka H, Mayumi M. Determination of asymmetric dimethylarginine, an endogenous nitric oxide synthase inhibitor, in umbilical blood. Metabolism. 2008;57(2):215–220. doi: 10.1016/j.metabol.2007.09.003. [DOI] [PubMed] [Google Scholar]
- 37.Holden DP, Fickling SA, Whitley GS, Nussey SS. Plasma concentrations of asymmetric dimethylarginine, a natural inhibitor of nitric oxide synthase, in normal pregnancy and preeclampsia. Am J Obstet Gynecol. 1998;178(3):551–556. doi: 10.1016/s0002-9378(98)70437-5. [DOI] [PubMed] [Google Scholar]
- 38.Vida G, Sulyok E, Ertl T, Martens-Lobenhoffer J, Bode-Böger SM. Birth by cesarean section is associated with elevated neonatal plasma levels of dimethylarginines. Pediatr Int. 2012;54(4):476–479. doi: 10.1111/j.1442-200X.2012.03605.x. [DOI] [PubMed] [Google Scholar]
- 39.Vallance P, Leiper J. Cardiovascular biology of the asymmetric dimethylarginine: dimethylarginine dimethylaminohydrolase pathway. Arterioscler Thromb Vasc Biol. 2004;24(6):1023–1030. doi: 10.1161/01.ATV.0000128897.54893.26. [DOI] [PubMed] [Google Scholar]
- 40.Masoura S, Kalogiannidis IA, Gitas G, Goutsioulis A, Koiou E, Athanasiadis A, Vavatsi N. Biomarkers in preeclampsia: a novel approach to early detection of the disease. J Obstet Gynaecol. 2012;32(7):609–616. doi: 10.3109/01443615.2012.709290. [DOI] [PubMed] [Google Scholar]
- 41.Tsikas D, Bollenbach A, Savvidou MD. Inverse correlation between maternal plasma asymmetric dimethylarginine (ADMA) and birthweight percentile in women with impaired placental perfusion: circulating ADMA as an NO-independent indicator of fetal growth restriction. Amino Acids. 2017;50(2):341–351. doi: 10.1007/s00726-017-2522-2. [DOI] [PubMed] [Google Scholar]
- 42.Rossmanith WG, Hoffmeister U, Wolfahrt S, Kleine B, McLean M, Jacobs RA, Grossman AB. Expression and functional analysis of endothelial nitric oxide synthase (eNOS) in human placenta. Mol Hum Reprod. 1999;5(5):487–494. doi: 10.1093/molehr/5.5.487. [DOI] [PubMed] [Google Scholar]
- 43.Xiao HB, Liu ZK, Lu XY, Deng CN, Luo ZF. Icariin regulates PRMT/ADMA/DDAH pathway to improve endothelial function. Pharmacol Rep. 2015;67(6):1147–1154. doi: 10.1016/j.pharep.2015.04.017. [DOI] [PubMed] [Google Scholar]
- 44.Braekke K, Ueland PM, Harsem NK, Staff AC Asymmetric dimethylarginine in the maternal and fetal circulation in preeclampsia. Pediatr Res. 2009;66(4):411–415. doi: 10.1203/PDR.0b013e3181b33392. [DOI] [PubMed] [Google Scholar]
- 45.Poston L. Endothelial dysfunction in pre-eclampsia. Pharmacol Rep. 2006;58Suppl(Suppl):69–74. [PubMed] [Google Scholar]
- 46.Vida G, Sulyok E, Ertl T, Martens-Lobenhoffer J, Bode-Boger SM. Plasma asymmetric dimethylarginine concentration during the perinatal period. Neonatology. 2007;92(1):8–13. doi: 10.1159/000098411. [DOI] [PubMed] [Google Scholar]
- 47.Mullins E, Prior T, Roberts I, Kumar S. Changes in the fetal and neonatal cytokine profile in pregnancies complicated by fetal growth restriction. Am J Reprod Immunol. 2013;69(5):441–448. doi: 10.1111/aji.12052. [DOI] [PubMed] [Google Scholar]
- 48.Garg P, Jaryal AK, Kachhawa G, Deepak KK, Kriplani A. Estimation of asymmetric dimethylarginine (ADMA), placental growth factor (PLGF) and pentraxin 3 (PTX 3) in women with preeclampsia. Pregnancy Hypertens. 2018:14245–51. [DOI] [PubMed]
- 49.Kajal K, Panda AK, Bhat J, Chakraborty D, Bose S, Bhattacharjee P, Sarkar T, Chatterjee S, Kar SK, Sa G. Andrographolide binds to ATP-binding pocket of VEGFR2 to impede VEGFA-mediated tumor-angiogenesis. Sci Rep. 2019;9(1):4073. doi: 10.1038/s41598-019-40626-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Chen DB, Zheng J. Regulation of placental angiogenesis. Microcirculation. 2014;2(1):15–25. doi: 10.1111/micc.12093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Algeri P, Ornaghi S, Bernasconi DP, Cappellini F, Signorini S, Brambilla P, Urban G, Vergani P. Feto-maternal correlation of PTX3, sFlt-1 and PlGF in physiological and pre-eclamptic pregnancies. Hypertens Pregnancy. 2014;33(3):360–370. doi: 10.3109/10641955.2014.903962. [DOI] [PubMed] [Google Scholar]
- 52.Herraiz I, Simón E, Gómez-Arriaga PI, Quezada MS, García-Burguillo A, López-Jiménez EA, Galindo A. Clinical implementation of the sFlt-1/PlGF ratio to identify preeclampsia and fetal growth restriction: a prospective cohort study. Pregnancy Hypertens. 2018;13:279–285. doi: 10.1016/j.preghy.2018.06.017. [DOI] [PubMed] [Google Scholar]
- 53.Dröge LA, Höller A, Ehrlich L, Verlohren S, Henrich W, Perschel FH. Diagnosis of preeclampsia and fetal growth restriction with the sFlt-1/PlGF ratio: diagnostic accuracy of the automated immunoassay Kryptor? Pregnancy Hypertens. 2017;8:31–36. doi: 10.1016/j.preghy.2017.02.005. [DOI] [PubMed] [Google Scholar]
- 54.Krishnan T, David AL. Placenta-directed gene therapy for fetal growth restriction. Semin Fetal Neonatal Med. 2017;22(6):415–422. doi: 10.1016/j.siny.2017.04.005. [DOI] [PubMed] [Google Scholar]