Abstract
Toxoplasmosis is a common zoonotic disease, which is of particular importance in women before and during pregnancy. In this study, the chemiluminescent microparticle immunoassay was used to detect Toxoplasma gondii and the seroepidemiology of Toxoplasma gondii was investigated among childbearing women in Qom, Iran. In this study, 202 blood samples were collected from childbearing women for evaluation of toxoplasmosis. Serum samples were isolated to determine anti-Toxoplasma IgM and IgG antibodies. Based on findings, the total prevalence of anti-Toxoplasma antibodies was 134 (66.3%) [95% CI 59.6–72.5]. In addition, 133 (65.8%) [95% CI 59.1–72.1], 19 (9.4%) [95% CI 6.1–14.2] and 18 (8.9%) [95% CI 5.7–13.6] samples were IgG-positive, IgM-positive and positive for both antibodies, respectively. The highest infection rate was reported over the age of 35 years, and positive lgM antibodies were detected in women below 29 years. T. gondii infection showed a significant correlation with age, pregnancy, contact with cats, exposure to soil, and consumption of raw food (P < 0.05). The serological tests revealed that nearly 33.7% [95% CI 27.5–40.4] of women at childbearing age (49.1% [95% CI 36.4–61.9] of pregnant women versus 27.9% [95% CI 21.3–35.6] of non-pregnant women) had no anti-Toxoplasma antibodies. Therefore, this population is more prone to acute infections with Toxoplasma.
Keywords: Toxoplasma gondii, Detection, Immunoassay, IgM, IgG, Seroepidemiology, Childbearing age
Introduction
Toxoplasma gondii is described as an intracellular protozoan parasite, causing toxoplasmosis in warm-blooded animals and humans. Parasitic contamination has been reported in humans and other hosts around the world. Several factors including age, geographic location, and eating patterns can influence the prevalence of infection (M’bondoukwé et al. 2018; Shapiro et al. 2019). Generally, toxoplasmosis in immunocompromised patients can lead to severe encephalitis through reactivation of latent or acute infection (Wang et al. 2017). Toxoplasmosis occurs in humans via multiple routes, such as handling and ingestion of unwashed vegetables, fruits, or water contaminated with oocysts from cats and other felids and/or handling and use of raw/undercooked meat with cysts consisting of viable bradyzoites (Pereira et al. 2010). Congenital acquisition (i.e., transplacental transfer of tachyzoites, particularly in women who are infected for the first time during pregnancy), blood transfusion, and organ transplant are among other routes of acquisition (Singh 2016). Infection during pregnancy can be transmitted to the fetus, resulting in congenital toxoplasmosis and fetal complications, such as hydrocephalus, microcephaly, jaundice, abortion, brain calcification, mental retardation, blindness, encephalitis, chorioretinitis, pneumonia, and even fetal death (Austeng et al. 2010). However, maternal infection before pregnancy cannot be transferred to the fetus, and the mother becomes immunized against future Toxoplasma infections. In general, diagnosis of toxoplasmosis is of particular importance in newborns with congenital infections, immunocompromised individuals, pregnant women infected during pregnancy, and patients with chorioretinitis (Maldonado et al. 2017). The prevalence of this disease varies widely between countries from lowest seroprevalence (1%) found in some countries in the Far East to the highest (90%) in some parts of European and South American countries (Fallahi et al. 2018). Generally, The prevalence of T. gondii infection depends mostly on the eating habits, climate, and culture of a region (Wilking et al. 2016). For instance, in France, use of undercooked meat is quite common, leading to the high seroprevalence of this infection. In addition, the tropical climate of Latin America and sub-Saharan Africa promotes the survival of oocytes, resulting in high prevalence rates in these regions. A meta-analysis by Rostami et al. (2019) demonstrated that the overall prevalence of acute toxoplasma infection in pregnant women globally is 1.1% while the prevalence is 2.8% in Iran. The prevalence of toxoplasmosis is high in Iran, nevertheless, there are differences in the seroprevalence rates reported in different regions of Iran, which could be attributed to environmental, socioeconomic, and cultural diversities (Gharavi et al. 2018). The seroepidemiology of toxoplasmosis has been examined in several studies from Iran and other countries. In the majority of conducted studies, identification of T. gondii-specific IgG and IgM antibodies could contribute to the diagnosis of acute and chronic toxoplasmosis. In Iran, multiple studies have examined the prevalence of these antibodies among women. The seropositivity of T. gondii is estimated at 48–74.6%, 33–44%, 22–37%, and 27–54% in Northern, Northwest, Southern, and Central regions of Iran, respectively (Akhlaghi et al. 2014). In addition, in some rather limited epidemiological studies, the prevalence of immunity to toxoplasmosis has been examined among women of childbearing age in some provinces of Iran. According to the literature, the prevalence of immunity to toxoplasmosis varies from 4.6 to 74.6%; this might be due to variations in the sampling method, target population, laboratory tools, and cut-off points for positive test results (Borna et al. 2013). Therefore, comprehensive epidemiological information is necessary to assess the health significance of this common parasitic infection and to identify the prevalence, severity, and risk factors among women of childbearing age.
The Architect Toxo IgG, IgM, and IgG avidity assay, a chemiluminescent microparticle immunoassay (CMIA), is described as a fully automated tool for evaluating the patient’s immune status and excluding acute infection. In our facility, the Architect IgG and IgM avidity assay (Abbott) is commonly used for prenatal screening of toxoplasmosis; accordingly, it was applied as the gold standard in our study (Sickinger et al. 2008). The present study aimed to identify Toxoplasma antibodies in pregnant women from Qom, Iran, using the CMIA method, considering its cost-effectiveness, simple technique, and high specificity and sensitivity for screening toxoplasmosis. In this study, the CMIA method was used to diagnose toxoplasmosis for the first time in Iran.
Methodology
Study design and population
Out of 300 eligible samples, 202 women, aged 16 to 50 years, consented to participate in this cross-sectional study. Samples were collected randomly from non-pregnant and pregnant women of childbearing age, referred to Qom Laboratory Clinic for routine tests in 2017–2018. A questionnaire, including marital status, demographic information, and educational level of the participants, was completed and coded by trained interviewers during blood sampling. As part of our routine clinical practice, the Architect Toxo IgM and IgG assays were used to assess the sera prospectively. Generally, the Architect Toxo IgG assay is a two-step, fully automated CMIA method, used to identify T. gondii IgG antibodies quantitatively.
According to the literature, T. gondii-specific antibodies bind to microparticles, coated with T. gondii recombinant antigens P30 (SAG1) and P35 (GRA8), and form an antigen–antibody compound (Sickinger et al. 2009) In the second step, the murine anti-human IgG conjugate (acridinium-labeled) was added after washing to form a reaction mixture, containing T. gondii IgG antibodies bound to the microparticles. The final chemiluminescent reaction was calculated as relative light units (RLUs) after another cycle of washing and adding the pretrigger and trigger solutions. The RLUs identified by the Architect optical system had a direct association with the level of Toxo IgG antibodies. Using an established calibration curve, the results were automatically analyzed. The specificity and the sensitivity of this method are 99.6% and 99.7%, respectively.
The specimens were graded as follows according to the IgG concentration: reactive, ≥ 3.0 IU/mL; gray zone, 1.6–2.9 IU/mL; and nonreactive, < 1.6 IU/mL. Moreover, the Architect Toxo IgM assay, with a μ-capture format, was used in this study. The assay included IgM antibodies bound to paramagnetic microparticles, which were coated with anti-human IgM mouse monoclonal antibodies. For the detection of IgM antibodies, incubation was carried out with a native T. gondii lysate, complexed to an anti-Toxo P30 mouse monoclonal F(ab′)2 fragment (acridinium-labeled). The specimens were graded as follows regarding IgM concentration: reactive, ≥ 0.6 IU/mL; gray zone, 0.5–0.6 IU/mL; and nonreactive; < 0.5 IU/mL (Heidari et al. 2019; Khomarlou et al. 2018; Sani et al. 2014; Sickinger et al. 2009; Taherian et al. 2018).
Statistical analysis
We used SPSS version 20 to analyze the data. Accordingly, quantitative variables were reported by mean and standard deviation of qualitative variables by number and percent. In addition, Chi-square and Fisher’s tests were carried out to compare the between groups. Also, to assess for a relation between the prevalence of immune markers (IgG and IgM) and predictors, logistic regression analyses were performed, the odds ratios (ORs) (95% CI) were also calculated. All tests were three-tailed, and P < 0.05 was defined as statistically significant.
Results
Samples
Out of 300 eligible samples, 202 women, aged 16 to 50 years, consented to participate in this study. The mean age of the participants was 31.85 ± 7.49 years, and most of them were below 29 years. The majority of women were housewives (90.6%) and had a high school diploma (53%). In addition, most of the participants lived in urban areas (99%). Two (1%) out of 202 women reported blood transfusion, and 27.2% were pregnant during the study. From 202 participants, only 31.7% had contact with cats at home. In addition, the frequency of contact with soil was 39.6% among the participants. Regarding dairy, meat, and vegetable consumption, only 6.9% of the participants consumed disinfected vegetables, while 83.7% used raw vegetables. The frequencies of raw milk/egg and meat consumption were 54% and 9.9%, respectively (Table 1).
Table 1.
General characteristics of study participants
| Variable | Participants (n = 202) |
|---|---|
| Age | |
| Mean ± sd | 31.85 ± 7.49 (range 16–50) |
| ≤ 29 | 77 (38.1%) |
| 30–34 | 67 (33.2%) |
| ≥ 35 | 58 (28.7%) |
| Level of education | |
| Less than diploma | 42 (20.8%) |
| Diploma | 107 (53%) |
| Academic | 53 (26.2%) |
| Job | |
| Home wife | 183 (90.6%) |
| Other | 19 (9.4%) |
| Residential place | |
| Urban | 200 (99%) |
| Rural | 2 (1%) |
| Blood transfusion | |
| Yes | 2 (1%) |
| Pregnant | |
| Yes | 55 (27.2%) |
| Contact with cats | |
| Yes | 64 (31.7%) |
| Contact with soil | |
| Yes | 80 (39.6%) |
| Consumed raw vegetable | |
| Yes | 169 (83.7%) |
| Disinfected vegetables | |
| Yes | 14 (6.9%) |
| Consumed raw milk/egg | |
| Yes | 109 (54%) |
| Consumed raw meat | |
| Yes | 20 (9.9%) |
Prevalence of anti-Toxoplasma antibodies
Table 2 presents the combined seroprevalence of anti-Toxoplasma antibodies in terms of age and pregnancy. The overall lgG seropositivity was reported to be 133/202 (65.8%) [95% CI 59.1–72.1]. Based on the findings, IgG seropositivity was more frequent in non-pregnant women (106/147; 72.1%) [95% CI 64.4–78.7], compared to pregnant women (27/55; 49.1%) [95% CI 36.4–61.9] (P = 0.002). In addition, IgG seropositivity was more common in older age groups, with the highest IgG seropositivity reported above 35 years of age (43/58; 74.1%) [95% CI 61.6–83.7] (Table 2). On the other hand, the overall prevalence of IgM seropositivity was reported to be 19/202 (9.4%) [95% CI 6.1–14.2]. IgM seropositivity was more common in non-pregnant women (17/147; 11.5%) [95% CI 7.3–17.7], compared to pregnant women (2/55; 3.6%) [95% CI 1–12.3] (P = 0.086). Based on the findings, IgM seropositivity was more common in younger age groups, with the highest IgM seropositivity reported below 29 years of age (11/77; 14.3%) [95% CI 8.2–23.8] (Table 2).
Table 2.
Seroprevalence of antibodies to Toxoplasma gondii according to pregnancy and age
| Status | Age | Pregnant | Total | ||||
|---|---|---|---|---|---|---|---|
| ≤ 29 (n (%)) | 30–34 (n (%)) | ≥ 35 (n (%)) | No (n = 147) | Yes (n = 55) | P value | ||
| IgG+ | 46(59.7%) [48.6–69.9] | 44(65.7%) [53.7–75.9] | 43(74.1%) [61.6–83.7] | 106(72.1%) [64.4–78.7] | 27(49.1%) [36.4–61.9] | 0.002a | 133(65.8%) [59.1–72.1] |
| IgM+ | 11(14.3%) [8.2–23.8] | 4(5.9%) [2.3–14.4] | 4(6.9%) [2.7–16.4] | 17(11.5%) [7.3–17.7] | 2(3.6%) [1–12.3] | 0.086a | 19(9.4%) [6.1–14.2] |
| IgG+ and IgM+ | 10(12.9%) [7.2–22.3] | 4(5.9%) [2.3–14.4] | 4(6.9%) [2.7–16.4] | 17(11.5%) [7.3–17.7] | 1(1.8%) [0.3–9.6] | 0.028b | 18(8.9%) [5.7–13.6] |
| IgG+ or IgM+ | 47(61.04%) [49.9–71.7] | 44(65.7%) [53.7–75.9] | 43(74.1%) [61.6–83.7] | 106(72.1%) [64.4–78.7] | 28(50.9%) [38.1–63.6] | 0.005a | 134(66.3%) [59.6–72.5] |
| IgG- and IgM- | 30(38.9%) [29.8–50.1] | 23(34.3%) [24.1–46.3] | 15(25.9%) [16.3–38.4] | 41(27.9%) [21.3–35.6] | 27(49.1%) [36.4–61.9] | 0.005a | 68(33.7%) [27.5–40.4] |
| IgG- and IgM + | 1(1.3%) [0.2–7] | 0(0%) [0–5.4] | 0(0%) [0–6.2] | 0(0%) [0–2.5] | 1(1.8%) [0.3–9.6] | 0.272b | 1(0.5%) [0.1–2.8] |
Prevalence is presented as number (%) and [CI 95%] that calculated by Newcombe method
aChi2 test
bFisher exact test
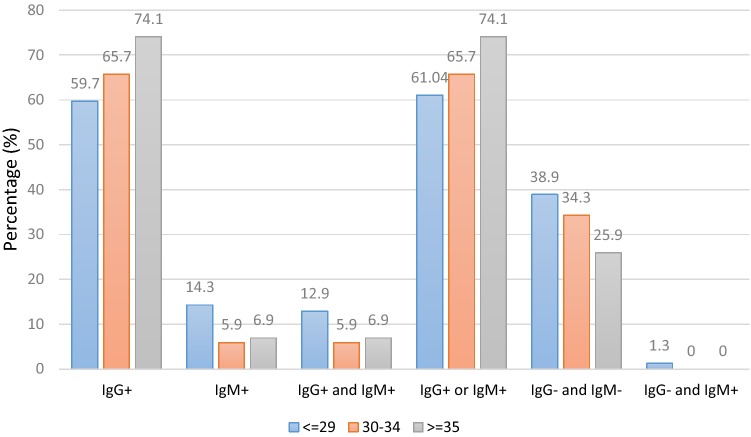
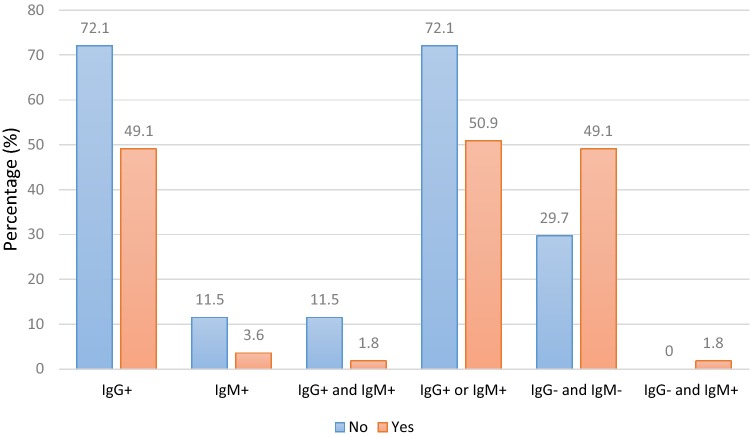
The frequency distributions of positive and negative seroprevalence of anti-Toxoplasma antibodies followed the same trend, with separate anti-Toxoplasma antibody distributions. The significant differences between non-pregnant and pregnant women for positive lgG and lgM (8.9%; 95% CI 5.7–13.6), positive lgG or lgM (66.3%; 95% CI 59.6–72.5), negative lgG and lgM (33.7%; 95% CI 27.5–40.4), and negative lgG and positive lgM (0.5%; 95% CI 0.1–2.8) were 0.028, 0.005, 0.005, and 0.272, respectively (Table 2). The seroprevalence of anti-Toxoplasma antibodies according to age is presented in Fig. 1.
Fig. 1.
Toxoplasma gondii IgG and IgM seroprevalence in childbearing women according to the age category
The highest seroprevalence of lgG antibodies was reported in women above 35 years of age. The highest seroprevalence rates in terms of age are as follows: positive lgM, < 29 years; positive lgM and lgG, < 29 years; positive lgG or lgM, > 35 years; negative lgG and lgM, < 29 years; and negative lgG and positive lgM, < 29 years (Fig. 1). Additionally, the seroprevalence of antibodies according to pregnancy status is demonstrated in Fig. 2. Except for two categories, i.e., negative lgG/negative lgM and negative lgG/positive lgM, the highest seroprevalence was reported in non-pregnant women (Fig. 2).
Fig. 2.
Toxoplasma gondii IgG and IgM seroprevalence in childbearing women according to the pregnant status
Predictors of T. gondii in childbearing women
Tables 3 and 4 indicate the predictors of toxoplasmosis seropositivity regarding the seroprevalence of anti-Toxoplasma lgG and lgM antibodies in childbearing women. As presented in the logistic regression analysis (Table 3), predictors of toxoplasmosis seropositivity included age (P = 0.02), having a high school diploma (P = 0.01), being a housewife (P = 0.008), pregnancy (P = 0.003), having cats at home (P < 0.001), contact with soil (P = 0.002), and consumption of raw vegetables (P < 0.001), raw milk/eggs (P < 0.001), and raw meat (P = 0.01).
Table 3.
Predictors of Toxoplasma gondii IgG in women of childbearing age
| Variable | Category | Seroprevalence IgG+ | Logistic regression | |
|---|---|---|---|---|
| N (%) | Odds ratio (95% CI) | P value | ||
| Age | Year | 1.05 (1.008–1.1) | 0.02 | |
| Level of education | Academic | 27 (50.9%) | Reference | |
| Diploma | 77 (72%) | 2.47 (1.25–4.9) | 0.01 | |
| Less than diploma | 29 (69%) | 2.15 (0.92–5.01) | 0.07 | |
| Job | Other | 7 (36.8%) | Reference | |
| Home wife | 126 (68.9%) | 3.79 (1.41–10.1) | 0.008 | |
| Pregnant | No | 106 (72.1%) | Reference | |
| Yes | 27 (49.1%) | 0.37 (0.19–0.71) | 0.003 | |
| Contact with cats | No | 74 (53.6%) | Reference | |
| Yes | 59 (92.2%) | 10.2 (3.86–26.9) | 0.000 | |
| Contact with soil | No | 70 (57.4%) | Reference | |
| Yes | 63 (78.8%) | 2.75 (1.44–5.25) | 0.002 | |
| Consumed raw vegetable | No | 6 (18.2%) | Reference | |
| Yes | 127 (75.1%) | 13.6 (5.3–35.2) | 0.000 | |
| Disinfected vegetables | No | 126 (67%) | Reference | |
| Yes | 7 (50%) | .49 (.16–1.46) | 0.203 | |
| Consumed raw milk/egg | No | 46 (49.5%) | Reference | |
| Yes | 87 (79.8%) | 4.04 (2.17–7.5) | 0.000 | |
| Consumed raw meat | No | 113 (62.1%) | Reference | |
| Yes | 20 (100%) | 11.6a (1.52–88.8) | 0.01 | |
Prevalence is presented as number (%) and [CI 95%] that calculated by Newcombe method
aChi2 test
Table 4.
Predictors of Toxoplasma gondii IgM in women of childbearing age
| Variable | Category | Seroprevalence IgM+ | Logistic regression | |
|---|---|---|---|---|
| N (%) | Odds ratio (95% CI) | P value | ||
| Age | Year | 0.97 (0.91–1.04) | 0.40 | |
| Level of education | Academic | 6 (14.3%) | Reference | |
| Diploma | 7 (6.5%) | 1.31 (0.39–4.39) | 0.67 | |
| Less than diploma | 6 (11.3%) | 0.55 (0.17–1.72) | 0.30 | |
| Job | Other | 1 (5.3%) | Reference | |
| Home wife | 18 (9.8%) | 1.96 (0.25–15.58) | 0.52 | |
| Pregnant | No | 17 (11.6%) | Reference | |
| Yes | 2 (3.6%) | 0.29 (0.06–1.29) | 0.10 | |
| Contact with cats | No | 11 (8%) | Reference | |
| Yes | 8 (12.5%) | 1.56 (0.63–4.32) | 0.31 | |
| Contact with soil | No | 8 (6.6%) | Reference | |
| Yes | 11 (13.8%) | 2.27 (0.87–5.92) | 0.09 | |
| Consumed raw vegetable | No | 1 (3%) | Reference | |
| Yes | 18 (10.7%) | 3.81 (0.49–29.6) | 0.20 | |
| Disinfected vegetables | No | 16 (8.5%) | Reference | |
| Yes | 3 (21.4%) | 2.93 (0.74–11.60) | 0.12 | |
| Consumed raw milk/egg | No | 4 (4.3%) | Reference | |
| Yes | 15 (13.8%) | 3.55 (1.13–11.11) | 0.03 | |
| Consumed raw meat | No | 17 (9.3%) | Reference | |
| Yes | 2 (10%) | 1.08 (0.23–5.05) | 0.92 | |
The strong predictors of toxoplasmosis seropositivity were consumption of raw vegetables (OR = 13.3), having cats at home (OR = 10.2), and consumption of raw milk/eggs (OR = 4.04) (Table 3). In addition, among all these predictors, the only variable reducing the risk of toxoplasmosis seropositivity was pregnancy (OR = 0.37), whereas other variables increased the risk of toxoplasmosis seropositivity (Table 3). The results of logistic regression analysis revealed that only consumption of raw milk/eggs (OR = 3.55; P = 0.03) was a significant predictor of toxoplasmosis seropositivity according to the anti-Toxoplasma lgM antibodies (Table 4).
Discussion
Toxoplasmosis is recognized as a treatable, but potentially lethal infection. Unfortunately, primary infection during pregnancy can result in irreversible fetal outcomes. This opportunistic infection can occur in immunocompromised people. The prevalence of this infection varies in different regions of Iran, where the risk of premature delivery, congenital malformations, miscarriage, and level of exposure to infection (especially in women) is high. According to previous findings, variations in infection sources (i.e., tissue cysts from the flesh of animals and oocysts from soil contaminated by cat feces) can explain the geographical differences in the prevalence rates (Alavi and Alavi 2016; Dong et al. 2018; Ebrahimzade et al. 2019). There are no reports from Iran regarding the prevalence of Toxoplasma infections, based on the CMIA method. Therefore, we investigated Toxoplasma infections by examining anti-Toxoplasma antibodies in the serum samples of women at childbearing age in an urban locality of Qom, Iran. The prevalence of anti-Toxoplasma antibodies was 66.3% (134/202) in the samples. Overall, 65.8% (133/202) of the samples were IgG-positive, 9.4% (19/202) were IgM-positive, and 8.9% (18/202) were positive for both antibodies (Table 2). In non-pregnant women, the seroprevalence of anti-T. gondii antibodies was higher than that of pregnant women (52.48%, 106/202 versus 13.86%, 28/202). The higher prevalence of these antibodies in non-pregnant women could be related to the large density of oocystes in the soil, higher consumption of raw vegetables (127/202) and milk/eggs (87/202), and greater cat population (59/202; Table 3) in our study area, compared to other regions. T. gondii infection had a significant correlation with age, educational level (high school diploma), pregnancy, contact with cats, exposure to soil, being a housewife, and consumption of raw vegetables, raw milk/eggs, and raw meat (P ≤ 0.05). The strongest predictors of toxoplasmosis seropositivity were consumption of raw vegetables (OR = 13.3), having cats at home (OR = 10.2), and consumption of raw milk/eggs (OR = 4.04) (Table 3). The results showed that only consumption of raw milk/eggs (OR = 3.55, P = 0.03) was a significant predictor of seropositivity according to the anti-Toxoplasma lgM concentration.
On the other hand, the seroprevalence of antibodies had no significant association with education below high school diploma, occupation, or use of disinfected vegetables. However, contact with cats, soil exposure, and consumption of raw or half-cocked meat, vegetables, and milk/eggs were major risk factors for T. gondii seropositivity. In line with previous studies, both routes of infection, i.e., ingestion of oocysts and tissue cysts in meat (animal–human and food-borne transmission, respectively), were found among women of childbearing age (Narouie et al. 2012). Based on our findings, seropositivity increased with advancing age due to the higher risk of exposure; this finding is consistent with the results of previous studies. One of the reasons for the difference in the incidence of Toxoplasma infection may be variations in the diagnostic techniques. In addition, contact with animals, low socioeconomic status, and low hygienic level may result in this significant difference, as described in the literature (Narouie et al. 2012). Similar to previous studies, the seropositivity of T. gondii was associated with the consumption of raw/half-cocked food (meat, vegetables, and milk/eggs), exposure to soil, and contact with cats (potential risk factors for toxoplasmosis) (Sadooghian et al. 2017).
Salehi-Moghaddam et al., Sharif et al., and Shemirani et al. have reported similar findings to our study. They estimated the prevalence of Toxoplasma infection to be 68.3%, 55.5%, and 55%, respectively in the general population and among women admitted to gynecology clinics in South of Tehran, Iran (Sharif et al. 2016). Likewise, Assmar et al. (1997) by studying the general population of Iran, estimated the prevalence of Toxoplasma infection to be 51.8% in 1997. In addition, Ghorbani et al. (1981) reported prevalence rates of 17.7% and 55.7% in Khuzestan (Southwest of Iran) and rural Northern regions of Iran in 1981, respectively. In another study, the prevalence of Toxoplasma infection was 42.7–44.5% in South of Iran. Overall, the prevalence of T. gondii infection is widely distributed, ranging from 0% among Eskimos to 94% in Guatemala and Costa Rica (Gibson and Coleman 1958).
Based on several studies, the seroprevalence of Toxoplasma antibodies is widely distributed around the world: India (36.8%), Croatia (36.4%), Turkey (49.4%), Ireland (12.8%), India (22.40%), Thailand (21.7%), Paris, France (83%), Brazil (65.8%), Maracaibo, Venezuela (33%), Ethiopia (81.4%), and USA (31.7%) (Fouladvand et al. 2010). According to some other reports, the prevalence of Toxoplasma antibodies is high in some countries, including Brazil (1), Tunisia (2), Cameroon (Njinikom), Central Ethiopia, Romania (Timis), and Switzerland (Zurich) (65.8%, 58.4%, 54.5%, 81.4%, 57.6%, and 40%, respectively) (Wam et al. 2016).
According to our findings, toxoplasmosis is prevalent in Qom due to extensive contact between potential T. gondii sources and the general population. Since the level of anti-Toxoplasma IgG antibodies may remain high for an extended period, an increase in the level of IgG antibodies may represent chronic infection reactivation, persistent immune responses to a dormant infection, or an active primary infection (Brown et al. 2005). In addition, severity and duration of infection had significant correlations with IgG titer. In our population, domestic and stray cats, warm weather, humidity, and frequent arrival of immigrants in the city were potential factors for the high seroprevalence of IgG antibodies. As noted earlier, the seroprevalence of anti-T. gondii antibodies varies in different parts of Iran and can be attributed to climatic, geographic, nutritional, and behavioral factors. In addition, contact with cats, socioeconomic status, sampling methods, transmission routes, type of laboratory tools, and cut-off points for positive test results are influential factors (Alvarado-Esquivel et al. 2007; Azad et al. 2017).
Differences in rural/urban settings are also effective, as the main sources of exposure appear to be similar; therefore, differences in risk factors for Toxoplasma seropositivity can be explained by environmental differences, besides climatic variations. The population in our study was selected from an urban region. To evaluate the effects of different factors, further research should be conducted at public health level considering individual characteristics, personal habits, and geographic/regional conditions. Also, serological methods should be compared to evaluate Toxoplasma antibodies. Overall, the observed differences may be related to variations in nutritional habits, socioeconomic status, and exposure to animals (contact with cats) between different regions. Moreover, these differences could be attributed to the diversity of assays and sampling methods, as well as geographic and climatic factors.
Conclusion
According to the present results, more than one-third of women at childbearing age (33.7%) were at risk of infection during pregnancy. Therefore, by applying appropriate practices, the living conditions of these women can be improved and infection can be eradicated; in addition, raising awareness and control of pathogens are suggested. The present findings showed that 57% of pregnant women and 67% of women at childbearing age were seronegative for Toxoplasma and susceptible to acute infection. These women are at high risk of congenital toxoplasmosis, and monitoring is essential for them. Therefore, awareness should be raised to increase the safety of women, who are susceptible to toxoplasmosis at the age of marriage.
Acknowledgements
The author would like to thanks from colleagues especially Mohamad Ashrafi for their kind and generous assistance.
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Akhlaghi L, Ghasemi A, Hadighi R, Tabatabaie F. Study of seroprevalence and risk factors for Toxoplasma gondii among pregnant women in Karaj township of Alborz province, 2013. J Entomol Zool Stud. 2014;2:217–219. [Google Scholar]
- Alavi SM, Alavi L. Toxoplasmosis in Iran: a guide for general physicians working in the Iranian health network setting: a systematic review. Casp J Intern Med. 2016;7:233. [PMC free article] [PubMed] [Google Scholar]
- Alvarado-Esquivel C, et al. Seroepidemiology of infection with Toxoplasma gondii in healthy blood donors of Durango. Mexico. BMC Infect Dis. 2007;7:75. doi: 10.1186/1471-2334-7-75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Assmar M, Amirkhani A, Piazak N, Hovanesian A, Kooloobandi A, Etessami R. Toxoplasmosis in Iran. Results of a seroepidemiological study. Bull Soc Pathol Exot. 1997;90:19–21. [PubMed] [Google Scholar]
- Austeng ME, Eskild A, Jacobsen M, Jenum PA, Whitelaw A, Engdahl B. Maternal infection with Toxoplasma gondii in pregnancy and the risk of hearing loss in the offspring. Int J Audiol. 2010;49:65–68. doi: 10.3109/14992020903214053. [DOI] [PubMed] [Google Scholar]
- Azad ZM, Moravej H, Fasihi-Ramandi M, Masjedian F, Nazari R, Mirnejad R, Moghaddam MM. In vitro synergistic effects of a short cationic peptide and clinically used antibiotics against drug-resistant isolates of Brucella melitensis. J Med Microbiol. 2017;66:919–926. doi: 10.1099/jmm.0.000524. [DOI] [PubMed] [Google Scholar]
- Borna S, Shariat M, Fallahi M, Janani L. Prevalence of immunity to toxoplasmosis among Iranian childbearing age women: systematic review and meta-analysis. Iran J Reprod Med. 2013;11:861. [PMC free article] [PubMed] [Google Scholar]
- Brown AS, Schaefer CA, Quesenberry CP, Jr, Liu L, Babulas VP, Susser ES. Maternal exposure to toxoplasmosis and risk of schizophrenia in adult offspring. Am J Psychiatry. 2005;162:767–773. doi: 10.1176/appi.ajp.162.4.767. [DOI] [PubMed] [Google Scholar]
- Dong H, Su R, Lu Y, Wang M, Liu J, Jian F, Yang Y. Prevalence, risk factors, and genotypes of Toxoplasma gondii in food animals and humans (2000–2017) from China. Front Microbiol. 2018;9:2108. doi: 10.3389/fmicb.2018.02108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebrahimzade E, et al. Investigation of the antimicrobial activity of a short cationic peptide against promastigote and amastigote forms of Leishmania major (MHRO/IR/75/ER): an in vitro study. Exp Parasitol. 2019;196:48–54. doi: 10.1016/j.exppara.2018.11.006. [DOI] [PubMed] [Google Scholar]
- Fallahi S, Rostami A, Shiadeh MN, Behniafar H, Paktinat S. An updated literature review on maternal-fetal and reproductive disorders of Toxoplasma gondii infection. J Gynecol Obstet Hum Reprod. 2018;47:133–140. doi: 10.1016/j.jogoh.2017.12.003. [DOI] [PubMed] [Google Scholar]
- Fouladvand M, Barazesh A, Zandi K, Naeimi B, Tajbakhsh S. Seroepidemiological study of toxoplasmosis in childbearing age women in Bushehr City, south west of Iran in 2009. Afr J Biotechnol. 2010;9:5809–5812. [Google Scholar]
- Gharavi MJ, et al. Prevalence of anti-Toxoplasma gondii antibodies in young Iranians: the CASPIAN III study. Arch Pediatr Infect Dis. 2018;6(1):1–6. [Google Scholar]
- Ghorbani M, Edrissian GH, Afshar A. Serological survey of human toxoplasmosis in mountainous regions of the north-west and south-west parts of Iran (1976–1977) Trans R Soc Trop Med Hyg. 1981;75:38–40. doi: 10.1016/0035-9203(81)90011-0. [DOI] [PubMed] [Google Scholar]
- Gibson CL, Coleman N. The prevalence of Toxoplasma antibodies in Guatemala and Costa Rica. Am J Trop Med Hyg. 1958;7:334–338. doi: 10.4269/ajtmh.1958.7.334. [DOI] [PubMed] [Google Scholar]
- Heidari MF, Arab SS, Noroozi-Aghideh A, Tebyanian H, Latifi AM. Evaluation of the substitutions in 212, 342 and 215 amino acid positions in binding site of organophosphorus acid anhydrolase using the molecular docking and laboratory analysis. Bratisl Lek Listy. 2019;120:139–143. doi: 10.4149/bll_2019_022. [DOI] [PubMed] [Google Scholar]
- Khomarlou N, Aberoomand-Azar P, Lashgari AP, Tebyanian H, Hakakian A, Ranjbar R, Ayatollahi SA. Essential oil composition and in vitro antibacterial activity of Chenopodium album subsp. striatum. Acta Biol Hung. 2018;69:144–155. doi: 10.1556/018.69.2018.2.4. [DOI] [PubMed] [Google Scholar]
- M’bondoukwé NP, et al. Prevalence of and risk factors for malaria, filariasis, and intestinal parasites as single infections or co-infections in different settlements of Gabon. Central Africa. Infect Dis Poverty. 2018;7:6. doi: 10.1186/s40249-017-0381-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maldonado YA, Read JS, CoI D. Diagnosis, treatment, and prevention of congenital toxoplasmosis in the United States. Pediatrics. 2017;139:e20163860. doi: 10.1542/peds.2016-3860. [DOI] [PubMed] [Google Scholar]
- Narouie B, Modrak MJ, Miri A. Seroepidemiology evaluation of Toxoplasma IgG values in women at their marriage age and pathogenesis factors. Life Sci J. 2012;9:185–190. [Google Scholar]
- Pereira KS, Franco RM, Leal DA. Transmission of toxoplasmosis (Toxoplasma gondii) by foods. In: Taylor S, editor. Advances in food and nutrition research. Amsterdam: Elsevier; 2010. pp. 1–19. [DOI] [PubMed] [Google Scholar]
- Rostami A, et al. Acute Toxoplasma infection in pregnant women worldwide: a systematic review and meta-analysis. PLoS Negl Trop Dis. 2019;13:e0007807. doi: 10.1371/journal.pntd.0007807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadooghian S, Mahmoudvand H, Mohammadi MA, Sarcheshmeh NN, Kareshk AT, Kamiabi H, Zia-Ali N. Prevalence of Toxoplasma gondii Infection among Healthy Blood Donors in Northeast of Iran. Iran J Parasitol. 2017;12:554. [PMC free article] [PubMed] [Google Scholar]
- Sani MRM, Moghaddam MM, Aghamollaei H, Hassanpour K, Taheri RA, Farnoosh G. Investigation of caspase-1 activity and interleukin-1β production in murine macrophage cell lines infected with Leishmania major. Asian Pac J Trop Med. 2014;7:S70–S73. doi: 10.1016/S1995-7645(14)60205-4. [DOI] [PubMed] [Google Scholar]
- Shapiro K, Bahia-Oliveira L, Dixon B, Dumètre A, de Wit LA, VanWormer E, Villena I. Environmental transmission of Toxoplasma gondii: oocysts in water, soil and food. Food Waterborne Parasitol. 2019;15:e00049. doi: 10.1016/j.fawpar.2019.e00049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharif M, Daryani A, Ebrahimnejad Z, Gholami S, Ahmadpour E, Borhani S, Lamsechi N. Seroprevalence of anti-Toxoplasma IgG and IgM among individuals who were referred to medical laboratories in Mazandaran province, northern Iran. J Infect Public Health. 2016;9:75–80. doi: 10.1016/j.jiph.2015.06.006. [DOI] [PubMed] [Google Scholar]
- Sickinger E, et al. Performance characteristics of the new ARCHITECT Toxo IgG and Toxo IgG avidity assays. Diagn Microbiol Infect Dis. 2008;62:235–244. doi: 10.1016/j.diagmicrobio.2008.07.005. [DOI] [PubMed] [Google Scholar]
- Sickinger E, et al. Evaluation of the Abbott ARCHITECT Toxo IgM assay. Diagn Microbiol Infect Dis. 2009;64:275–282. doi: 10.1016/j.diagmicrobio.2009.03.016. [DOI] [PubMed] [Google Scholar]
- Singh S. Congenital toxoplasmosis: clinical features, outcomes, treatment, and prevention. Trop Parasitol. 2016;6:113. doi: 10.4103/2229-5070.190813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taherian A, Fazilati M, Moghadam AT, Tebyanian H. Optimization of purification procedure for horse F(ab)2 antivenom against Androctonus crassicauda (Scorpion) venom. Trop J Pharm Res. 2018;17:409–414. doi: 10.4314/tjpr.v17i3.4. [DOI] [Google Scholar]
- Wam EC, Sama LF, Ali IM, Ebile WA, Aghangu LA, Tume CB. Seroprevalence of Toxoplasma gondii IgG and IgM antibodies and associated risk factors in women of child-bearing age in Njinikom. NW Cameroon. BMC Res Notes. 2016;9:406. doi: 10.1186/s13104-016-2206-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z-D, et al. Toxoplasma gondii infection in immunocompromised patients: a systematic review and meta-analysis. Front Microbiol. 2017;8:389. doi: 10.3389/fmicb.2017.00389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilking H, Thamm M, Stark K, Aebischer T, Seeber F. Prevalence, incidence estimations, and risk factors of Toxoplasma gondii infection in Germany: a representative, cross-sectional, serological study. Sci Rep. 2016;6:22551. doi: 10.1038/srep22551. [DOI] [PMC free article] [PubMed] [Google Scholar]