Abstract
Purpose
To report our experience on homologous intrauterine insemination (IUI) with gonadotropin controlled ovarian stimulation (COS) cycles and to examine different variables which could predict IUI success.
Materials and methods
This is a retrospective analysis of IUIs performed between January 1997 and December 2017. A total of 7359 COS IUI’s procedures (2901 couples) were reviewed. Clinical pregnancy, live birth rate and age, body mass index (BMI), smoking habit, duration of infertility, sperm characteristics before and after treatment (total motile count, morphology, and vitality), day 3 FSH, total gonadotropin dose, and number of follicles were assessed by multivariate logistic regression analysis, and data were expressed as odds ratio (OR).
Results
The mean female age at the time of COS was 35.10 ± 3.93 years. The most common single infertility diagnoses were unexplained infertility (53.55%), mild male factor (19.69%), and anovulation (10.95%). The total progressive motile sperm count (TPMC) was > 1 × 106/ml (mean 1.34 ± 1.08 × 106/ml). The clinical pregnancy rate was 9.38%, and the live birth rate was 7.19% per cycle. Twin pregnancies were 12.17%. Cumulative pregnancy was 21.89% and cumulative live birth rate was 17.58% per couple. Clinical pregnancy and live birth rates were significantly associated with female age [OR 0.97 (95% CI 0.95–0.99) and 0.95 (95% CI 0.93–0.97), respectively] and day 3 FSH [OR 0.91 (95% CI 0.87–0.94) e 0.90 (95% CI 0.87–0.94), respectively].
Conclusions
Clinical pregnancy rate and live birth rates after COS-IUIs were significantly influenced by female age and FSH levels.
Trial registration
Clinical trial registration number: NCT03836118
Electronic supplementary material
The online version of this article (10.1007/s10815-020-01752-3) contains supplementary material, which is available to authorized users.
Keywords: Intrauterine Insemination, Infertility, Maternal Age, Ovarian Reserve
Introduction
Infertility affects one in seven couples [1], and it has been recognized as a public health issue by the World Health Organization [2]. Intrauterine insemination (IUI) is a noninvasive and more affordable infertility treatment that, in selected cases, can provide a reasonable success rate, up to three or four attempts. The treatment is safe, easy to perform, and patients are generally compliant (low dropout rates) since little monitoring is needed, and the risk for complications such as ovarian hyperstimulation syndrome (OHSS) is very low [3].
In general, cycles of homologous IUI, with or without controlled ovarian stimulation (COS), are offered as a first-line treatment to couples with subfertility due to ejaculatory disorders, ovulatory dysfunction, mild/minimal endometriosis, mild/moderate male factor, and unexplained infertility [4]. However, given the many variables potentially impacting the success rate, the role of IUIs as initial step for assisted reproduction treatments remains controversial. In 2009, the ESHRE (European Society of Human Reproduction and Embryology) noted that in the absence of proper trials and data on live birth rate [5], IUIs should be considered poor substitute for IVF (in vitro fertilization) and a cause of high-order multiple births. The 2013 NICE (National Institute for Health and Care Excellence) guidelines [6], based on few studies [7–9], recommended that in cases of unexplained infertility failing expectant management for up to 2 years, patients should proceed directly to IVF. Since then, randomized trials have been published supporting the value of IUI [10, 11]. In a recent multicenter randomized non-inferiority trial, the effectiveness of IVF with single embryo transfer or IVF in a modified natural cycle was compared with IUIs with COS, considering a single healthy live birth as the main outcome. In that study, IUI with COS was found to be non-inferior compared with the two IVF strategies, with a reasonably low multiple birth rate [12]. Concerning healthcare costs, IUI was the most cost-effective strategy for cases of mild male factor or unexplained infertility [13]. A randomized controlled trial by Farquhar et al. reported that in women with unexplained infertility, three cycles of IUI with COS were associated to a three-fold improved live birth rate compared with 3 months of expectant management [10].
Several prognostic factors have been linked to the outcome of IUIs. These factors are related to the type of ovarian stimulation and to specific patient’s characteristics (female patient age, type and duration of infertility, number of mature follicles recruited, endometrial thickness, number of spermatozoa with progressive motility, sperm morphology, number of sperm used in insemination, BMI, smoking) [14]. However, the predictive value of these parameters remains highly contradictory [15].
The aim of the present study was to report the vast experience of an academic tertiary ART center on homologous IUIs after exclusive use of gonadotropins for COS and to determine prognostic factors that could be associated with higher likelihood of pregnancy and live birth.
Materials and methods
Patients and study design
This is a retrospective observational study including all the IUIs performed between January 1997 and December 2017. The study received ethical committee approval, and patients provided written consent for using their anonymized medical records. All couples had been diagnosed with infertility according to the WHO definition (failure to achieve a clinical pregnancy after 12 months or more of regular unprotected sexual intercourse) [16] and had a complete infertility diagnostic work-up. The data collected included medical history, physical examination, transvaginal ultrasound, serum hormone assays between day 2 and 4 of the menstrual cycle, semen analysis, uterine cavity and tubal patency evaluation with hysterosalpingography (HSG) or sonohysterogram (SHG), and/or hysteroscopy and laparoscopy. Cervico-vaginal cultures for Chlamydia and Mycoplasma were carried out and serology tests were used to rule out viral infections (Hepatitis B, C, VDRL, HIV).
Demographic characteristics and outcomes description
The characteristics recorded were female and male age (years), current or previous female smoking (smoking/nonsmoking), female body mass index (BMI) (kg/m2), infertility duration, type (primary or secondary) and etiology, number of intercourses per week, day 3 serum hormones (follicle-stimulating hormone (FSH), luteinizing hormone (LH), and estradiol (E2) levels). The variables of the controlled ovarian stimulation protocol were the number of dominant follicles, the endometrial thickness at the time of human chorionic gonadotropin (hCG) injection, and the total doses of either human menopausal gonadotrophin (HMG) or recombinant FSH (rFSH)].
We did not include AMH since it was not offered before 2011.
Sperm quality parameters assessed, before and after preparation, included total motile count (TMC), total progressive motile count (TPMC), morphology and vitality (evaluated with E&N test), and the difference (delta) between before and after preparation for each parameter.
The main clinical outcome measures were clinical pregnancy (PR) and live birth rates (LBR) per cycle and per couple. Clinical PR was defined as the presence of a gestational sac and fetal heartbeat on ultrasonography 7–8 weeks after IUI and LBR was considered as the birth of at least one live born after 24 weeks of gestation [17].
Minimal criteria for entering the IUI program were at least one patent fallopian tube and a total progressive motile sperm concentration (TPMSC) post-preparation of at least 1 × 106/mL.
Ovarian stimulation
Patients were treated with HMG/rFSH according to standard protocols and monitoring (75 IU/d starting from day 3 for a various duration depending on the ovarian response) [17]. An injection of uHCG 5000 IU/rHCG 250 mg was given to trigger ovulation when the dominant follicle had an average diameter of 18 mm or more. If three or more follicles of at least 15 mm were present, the cycle was canceled, and patients were instructed to refrain from unprotected sexual intercourse. Anovulatory and polycystic ovarian syndrome (PCO) patients were included in IUI COS cycles after failing clomiphene citrate for 3–6 cycles. The use of letrozole for ovulation induction is not allowed in our country.
Sperm preparation
On the day of IUI, a semen sample was obtained by masturbation after 2–5 days of abstinence and collected in a sterile cup. TPMSC was determined by multiplying grade A or grade A + B sperm motility percentages by sperm volume and concentration. Sperm capacitation was performed using density gradient centrifugation or swim-up procedure to remove seminal fluid and enhance sperm quality for IUI.
Intrauterine insemination
A single IUI was performed 36-h post-HCG. The washed motile sperm population was concentrated in 0.5 mL and loaded in a soft catheter for insemination (Wallace® Intrauterine Insemination catheter, Smiths Medical International, Australia), and the patient remained supine for 10 min after the procedure. Daily treatment with micronized progesterone was prescribed for 14 days starting on the same night of IUI in all patients. Serum ß-HCG was determined 14 days later, and clinical pregnancy was confirmed by the presence of a gestational sac and fetal heartbeat on ultrasonography 7–8 weeks after IUI.
Statistical analysis
Results were expressed as mean ± standard deviation or percentage. All considered variables were analyzed by univariable logistic regression, and variables with a p value less than 0.25 were then submitted to multivariable logistic regression analysis, to identify factors associated to prognosis. Results of the logistic regression analysis were expressed as odds ratio (OR) and 95% confidence interval (CI). The ROC curve was used to assess the discriminative performance of the fitted logistic model. An AUC equal to 0.5 indicates no discriminative power whereas an AUC of 1.0 shows a perfect discrimination.
The time for the first pregnancy was reported with a Kaplan-Meier graph; couples with no live birth were considered as censored with the number of performed IUI cycles as time.
All analyses were made with Stata15 (2013, Stata Corp, TX, USA). Statistical significance was established at p < 0.05.
Results
During the study period, a total of 7359 cycles were started, and 6323 IUI procedures were performed in 2901 couples. The main characteristics of the study population are provided in supplementary Table 1. The cancelation rate was 14.08%: 1000 cycles were stopped because of either excessive (n = 658) or no ovarian response (n = 342). Fifteen IUIs were not completed because of ejaculation failure on the day of IUI and 21 for couple decision. The clinical PR was 9.38% for cycle, while the LBR was 7.19%. Twin pregnancies were 12.17%.
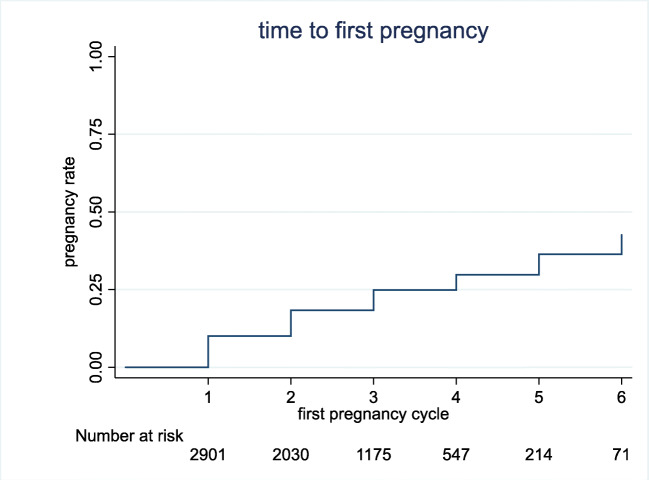
Cumulative pregnancy rate was 21.89% for couple, and the cumulative delivery rate was 17.58%. The median number of IUI cycles for couple was 2 cycles (range 1–13), and 615 cycles were performed in women who were > 40 years (8.35%). A total of 236 couples (8.14%) obtained a live birth after their first IUI cycle. The relation between number of procedures and PR is shown in Fig. 1.
Fig. 1.
Relation between number of procedures and pregnancy rate (Kaplan-Meier’s curve)
Table 1 shows univariable and multivariable results for PR and LBR. Female and male ages were significantly lower in the pregnant group. Pregnancy rate was not related to sperm count. There were no significant differences in TPMSC between pregnant and not pregnant patients, while the percentage of normal morphology pretreatment was significantly better in the pregnant group. Smoking habit did not have a significant impact on IUI outcome. No significant difference was found according to COS protocols used (hMG or rFSH), types, and doses of gonadotropins (data not shown). A multivariate logistic regression model, including all the potential factors of IUI success, showed that clinical PR and LBR were significantly correlated to female age [OR 0.97 (CI 95% 0.95–0.99) and 0.95 (CI 95% 0.93–0.97), respectively] and to day 3 FSH values [OR 0.91 (CI 95% 0.87–0.94) and 0.90 (CI 95% 0.87–0.94), respectively]. Also, anovulation resulted to be a positive factor for CPR [OR 1.46 (CI 95% 1.12–1.91)] but not for LBR.
Table 1.
Univariable and multivariable results for pregnancy rate and live birth rate
| Pregnancy rate | Live birth rate | |||||||
|---|---|---|---|---|---|---|---|---|
| Univariable | Multivariable | Univariable | Multivariable | |||||
| Parameter | OR (95% CI) | p | OR (95% CI) | p | OR (95% CI) | p | OR (95% CI) | p |
| Female age (years) | 0.96 (0.95–0.98) | 0.001 | 0.97 (0.95–0.99) | 0.031 | 0.95 (0.93–0.97) | < 0.001 | 0.95 (0.93–0.98) | < 0.001 |
| Male age (years) | 0.98 (0.96–1.00) | 0.014 | 0.96 (0.94–0.98) | < 0.001 | ||||
| Female BMI (kg/m2) | 1.04 (1.02–1.07) | 0.002 | 1.05 (1.02–1.08) | 0.002 | ||||
| Male BMI (kg/m2) | 0.94 (0.85–1.05) | 0.251 | 0.94 (0.84–1.06) | 0.332 | ||||
| Smoking (yes/no) | 0.88 (0.71–1.09) | 0.231 | 0.79 (0.62–1.00) | 0.054 | ||||
| Infertility duration (months) | 1.00 (1.00–1.01) | 0.849 | 1.00 (1.00–1.01) | 0.631 | ||||
| FSH d3 (mUI/mL) | 0.91 (0.87–0.94) | < 0.001 | 0.89 (0.85–0.93) | < 0.001 | 0.90 (0.87–0.94) | < 0.001 | 0.88 (0.84–0.93) | < 0.001 |
| Etiology | ||||||||
| Anovulation | 1.21 (0.96–1.53) | 0.111 | 1.46 (1.12–1.91) | 0.005 | 1.15 (0.87–1.52) | 0.318 | ||
| Partial tubal factor | 1.02 (0.68–1.52) | 0.929 | 1.03 (0.69–1.56) | 0.870 | ||||
| Endometriosis | 0.66 (039–1.11) | 0.114 | 0.63 (0.34–1.17) | 0.145 | ||||
| Mild male factor | 1.02 (0.83–1.25) | 0.836 | 0.93 (0.75–1.17) | 0.553 | ||||
| Male and Female Factors | 1.18 (0.70–1.98) | 0.526 | 1.31 (0.77–2.24) | 0.325 | ||||
| Unexplained | 1.00 (0.85–1.18) | 0.970 | 1.07 (0.90–1.29) | 0.437 | ||||
| Multiple female factor | 0.92 (0.25–3.37) | 0.900 | 0.59 (0.10–3.40) | 0.551 | ||||
| Recurrent miscarriage | 2.28 (0.69–7.53) | 0.176 | 3.05 (0.92–10.09) | 0.067 | ||||
| Reduced ovarian reserve | 0.51 (0.30–0.87) | 0.013 | 0.45 (0.24–0.83) | 0.010 | ||||
| TPMC pre (× 106) | 1.07 (1.00–1.15) | 0.039 | 1.11 (1.03–1.21) | 0.009 | 1.09 (1.01–1.18) | 0.023 | 1.13 (1.04–1.23) | 0.006 |
| TPMC post (× 106) | 1.02 (0.99–1.05) | 0.214 | 1.03 (0.99–1.05) | 0.111 | ||||
| Δ TMC % | 0.99 (0.94–1.04) | 0.728 | 1.01 (0.96–1.06) | 0.631 | ||||
| Normal morphology pre % | 1.02 (1.01–1.04) | 0.008 | 1.03 (1.01–1.05) | < 0.001 | ||||
| Normal morphology post % | 1.01 (0.99–1.02) | 0.363 | 1.01 (1.00–1.03) | 0.186 | ||||
| Δ normal morphology % (× 100) | 0.94 (0.86–1.03) | 0.175 | 0.92 (0.82–1.03) | 0.153 | ||||
| E&N test % | 1.00 (0.99–1.02) | 0.712 | 1.01 (0.99–1.02) | 0.547 | ||||
TPMC total progressive motile count, E&N eosin and nigrosin staining technique
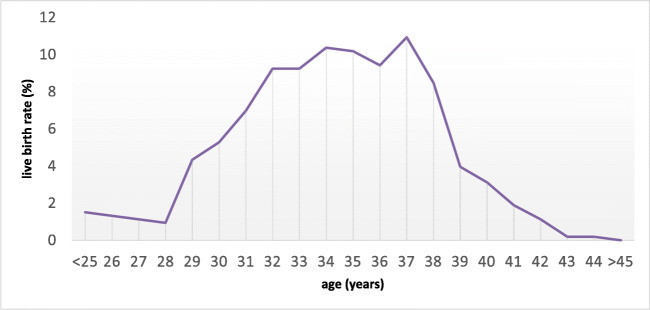
Figure 2 shows a significantly higher LBR for women up to 37 years compared with women 38 years or older. Moreover, a lower LBR was reported in patient under 30 years old. In our study, the AUC for the fitted logistic model was found to be 0.52. Table 2 shows a sub-analysis of clinical PR and LBR according to different group of ages. In the 21-year period considered, the treated population showed some changes: the female age gradually decreased (from a mean age of 36.2 in 1997 to 33.4 in 2017); meanwhile the mean TPMC post treatment improved (from 19 × 106/ml in 1997 to 28 × 106/ml in 2017). A progressively significant reduction in the daily dosing of medications for COS protocols was not associated with significant changes in PR and LBR.
Fig. 2.
Live birth rate according to female age
Table 2.
Pregnancy rate and live birth rate sub-analysis according to different group of ages
| Pregnancy rate | Live birth rate | |
|---|---|---|
| Age ≤ 35 | 11.25 | 9.11 |
| Age 36–38 | 11.45 | 8.61 |
| Age 39–40 | 9.03 | 5.49 |
| Age > 40 | 7.17 | 4.03 |
All estimated rates are corrected for TPMC pretreatment and day 3 FSH and clustered for female ID number
Discussion
As a first step in ART, IUI treatments keep a central place in the management of infertile couples for its simplicity and acceptability [10]. However, the overall success rates per cycle are rather low if compared with IVF/ICSI, with 10–20% clinical PR per cycle across various etiology of infertility [18]. Nevertheless clinical practice provides evidence that it is a worthy first step for acceptance for new couples who interface for their first time to ART. Results from our study on gonadotropin COS/IUI showed an overall clinical PR per cycle of 9.27% and a multiple pregnancy rate of 14.33%, which are respectively in agreement with and higher than the lately results reported by the Italian Registry for Assisted Procreation [19].
In the multivariate logistic analysis, clinical PR and LBR were found to be significantly correlated with female age and FSH levels. Several studies have illustrated the decline in pregnancy with an increasing age in IUI treatment [15, 20, 21], and moderate quality evidence-based data show a sharp decline in success rate for women older than 40 years, likely related to a decrease in oocyte quality [22]. In our study, we observed a significant decrease in PR already in women older than 38 years, as shown in Table 2. Our data showed that ovarian reserve (day 3 FSH values) seems to influence the outcome of IUI’s independent of age. In the literature, few studies have assessed the impact of ovarian reserve on IUI outcome. Merviel et al. [23] found no significant difference in PR according to the basal FSH values (threshold value of 9.4 IU/L). The same conclusion was reported by Mullin et al. [24]. However, Soria et al. [25] observed that women with basal FSH levels < 9 IU/L had 3.17 times higher chances to become pregnant after IUIs than women with basal FSH levels > 9 IU/L. Another factor impacting IUI success rate has been related to the duration of infertility, noting that the longer the infertility duration, the lower the likelihood of pregnancy [26, 27]. Nuojua-Huttunen et al. [28] reported significant differences in pregnancy rates according to whether the length of infertility was below or above 6 years (14.2% vs. 6.1%). However, in agreement with the findings of Goverde et al. [29] and Merviel et al. [30], our study did not reveal differences in PR according to the duration of infertility. There was also no significant difference across infertility types for both PR and LBR, as confirmed by several other reports [15, 31]. However, we could not demonstrate higher live birth rates when the indications for IUI were cervical factor [23] and anovulation [25, 32].
Despite considering our inclusion criteria of post-processing TPMC > 1 million, semen parameters also showed no impact on clinical outcomes. Our results confirm the data of a meta-analysis of 16 studies [33] in which receiver operating characteristics (ROC) curves indicated a reasonable predictive performance of IUI outcome, at cutoff levels between 0.8 and 5 total million motile spermatozoa in the post-wash sample. At these cutoff levels, the authors reported a specificity of the post-wash TMC, defined as the ability to predict failure to become pregnant, as high as 100%, but a very limited sensitivity of the test, defined as the ability to predict pregnancy. The negative predictive value of a pre-wash TMC less than 2 million has been recently confirmed by a retrospective study on 655 IUI cycles [34]. As commented by Pereira et al., despite its retrospective nature and heterogeneous patient population, the study provided pertinent clinical data about the poor prognostic value of pre-washed TMC in predicting LBR in IUI cycles [35].
Because there is no agreement on the minimal values of sperm concentration and motility, it is still difficult to predict which couples would unequivocally benefit from IUI treatments. A Cochrane systematic review by Bensdorp et al. analyzed IUI (with or without COS) in patients with male infertility [36]. However, studies with different definitions of male infertility were included, and the authors concluded that there was insufficient evidence to whether recommend or not IUI in male infertility, mainly because large high-quality randomized trials are lacking. A recent large RCT showed that IUI with COS is non-inferior to IVF in couples with mild male infertility, defined as a TMC between 3 and 10 million before sperm processing [12].
The strength of the present work is the huge number of cycles analyzed, the length of the study period, and the population heterogeneity that may bring the applicability of reading data to general conclusions. However, it presents some limitations, such as the retrospective study design, the partial incompleteness of data due to the length of the study period, the selection of only gonadotropin COS, and the limited population over 40 years old.
In summary, from the analysis of our large database, female age and day 3 FSH values were the only variables significantly associated with IUI success in gonadotropin-stimulated cycles. Semen parameters showed no impact on clinical outcomes, if they were considered suitable for IUI (> 1 million posttreatment TPMCs).
Due to its affordability and when accompanied by appropriate patient selection, IUI remains an effective method among the available options for infertile couples. Clinicians, however, must be aware of the limits of this method particularly when female age is over 38 years. We would suggest, from our own experience, to limit to no more than 3 IUIs during a 6-month span, before considering further assisted reproductive options.
Electronic supplementary material
(DOCX 17 kb)
Data availability
The datasets generated for this study are available on request to the corresponding author.
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
The study was approved by the Independent Ethical Committee of our Institution. Consent was obtained from each patient after full explanation of the purpose and nature of all procedures used.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Templeton A. Infertility-epidemiology, aetiology and effective management. Health Bull (Edinb) 1995;53:294–298. [PubMed] [Google Scholar]
- 2.World Health Organization (WHO). Infertility is a Global Public Health Issue. In: Sexual and Reproductive Health; 2014.
- 3.Ombelet W. The revival of intrauterine insemination: evidence-based data have changed the picture. Facts Views Vis Obgyn. 2017;9:131–132. [PMC free article] [PubMed] [Google Scholar]
- 4.Cohlen B, Bijkerk A, Van der Poel S, Ombelet W. IUI: review and systematic assessment of the evidence that supports global recommendations. Hum Reprod Update. 2018;24:300–319. doi: 10.1093/humupd/dmx041. [DOI] [PubMed] [Google Scholar]
- 5.Group ECW Intrauterine insemination. Hum Reprod Update. 2009;15:265–277. doi: 10.1093/humupd/dmp003. [DOI] [PubMed] [Google Scholar]
- 6.O'Flynn N. Assessment and treatment for people with fertility problems: NICE guideline. Br J Gen Pract. 2014;64:50–51. doi: 10.3399/bjgp14X676609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bhattacharya S, Harrild K, Mollison J, Wordsworth S, Tay C, Harrold A, et al. Clomifene citrate or unstimulated intrauterine insemination compared with expectant management for unexplained infertility: pragmatic randomised controlled trial. BMJ. 2008;337:a716. doi: 10.1136/bmj.a716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Reindollar RH, Regan MM, Neumann PJ, Levine BS, Thornton KL, Alper MM, Goldman MB. A randomized clinical trial to evaluate optimal treatment for unexplained infertility: the fast track and standard treatment (FASTT) trial. Fertil Steril. 2010;94:888–899. doi: 10.1016/j.fertnstert.2009.04.022. [DOI] [PubMed] [Google Scholar]
- 9.Wordsworth S, Buchanan J, Mollison J, Harrild K, Robertson L, Tay C, Harrold A, McQueen D, Lyall H, Johnston L, Burrage J, Grossett S, Walton H, Lynch J, Johnstone A, Kini S, Raja A, Templeton A, Bhattacharya S. Clomifene citrate and intrauterine insemination as first-line treatments for unexplained infertility: are they cost-effective? Hum Reprod. 2011;26:369–375. doi: 10.1093/humrep/deq315. [DOI] [PubMed] [Google Scholar]
- 10.Farquhar CM, Liu E, Armstrong S, Arroll N, Lensen S, Brown J. Intrauterine insemination with ovarian stimulation versus expectant management for unexplained infertility (TUI): a pragmatic, open-label, randomised, controlled, two-Centre trial. Lancet. 2018;391:441–450. doi: 10.1016/S0140-6736(17)32406-6. [DOI] [PubMed] [Google Scholar]
- 11.Nandi A, El-Toukhy T. Stimulated intrauterine insemination for unexplained subfertility. Lancet. 2018;391:404–405. doi: 10.1016/S0140-6736(17)33038-6. [DOI] [PubMed] [Google Scholar]
- 12.Bensdorp AJ, Tjon-Kon-Fat RI, Bossuyt PM, Koks CA, Oosterhuis GJ, Hoek A, et al. Prevention of multiple pregnancies in couples with unexplained or mild male subfertility: randomised controlled trial of in vitro fertilisation with single embryo transfer or in vitro fertilisation in modified natural cycle compared with intrauterine insemination with controlled ovarian hyperstimulation. BMJ. 2015;350:g7771. doi: 10.1136/bmj.g7771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tjon-Kon-Fat RI, Bensdorp AJ, Bossuyt PM, Koks C, Oosterhuis GJ, Hoek A, Hompes P, Broekmans FJ, Verhoeve HR, de Bruin JP, van Golde R, Repping S, Cohlen BJ, Lambers MD, van Bommel P, Slappendel E, Perquin D, Smeenk J, Pelinck MJ, Gianotten J, Hoozemans DA, Maas JW, Groen H, Eijkemans MJ, van der Veen F, Mol BW, van Wely M. Is IVF-served two different ways-more cost-effective than IUI with controlled ovarian hyperstimulation? Hum Reprod. 2015;30:2331–2339. doi: 10.1093/humrep/dev193. [DOI] [PubMed] [Google Scholar]
- 14.Luco SM, Agbo C, Behr B, Dahan MH. The evaluation of pre and post processing semen analysis parameters at the time of intrauterine insemination in couples diagnosed with male factor infertility and pregnancy rates based on stimulation agent. A retrospective cohort study. Eur J Obstet Gynecol Reprod Biol. 2014;179:159–162. doi: 10.1016/j.ejogrb.2014.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dinelli L, Courbière B, Achard V, Jouve E, Deveze C, Gnisci A, Grillo JM, Paulmyer-Lacroix O. Prognosis factors of pregnancy after intrauterine insemination with the husband's sperm: conclusions of an analysis of 2,019 cycles. Fertil Steril. 2014;101:994–1000. doi: 10.1016/j.fertnstert.2014.01.009. [DOI] [PubMed] [Google Scholar]
- 16.Zegers-Hochschild F, Adamson GD, de Mouzon J, Ishihara O, Mansour R, Nygren K, Sullivan E, Vanderpoel S, International Committee for Monitoring Assisted Reproductive Technology. World Health Organization International Committee for Monitoring Assisted Reproductive Technology (ICMART) and the World Health Organization (WHO) revised glossary of ART terminology, 2009. Fertil Steril. 2009;92:1520–1524. doi: 10.1016/j.fertnstert.2009.09.009. [DOI] [PubMed] [Google Scholar]
- 17.Zegers-Hochschild F, Adamson GD, Dyer S, Racowsky C, de Mouzon J, Sokol R, Rienzi L, Sunde A, Schmidt L, Cooke ID, Simpson JL, van der Poel S. The international glossary on infertility and fertility care, 2017. Fertil Steril. 2017;108:393–406. doi: 10.1016/j.fertnstert.2017.06.005. [DOI] [PubMed] [Google Scholar]
- 18.Duran HE, Morshedi M, Kruger T, Oehninger S. Intrauterine insemination: a systematic review on determinants of success. Hum Reprod Update. 2002;8:373–384. doi: 10.1093/humupd/8.4.373. [DOI] [PubMed] [Google Scholar]
- 19.Istituto Superiore di Sanità (ISS). Italian assisted reproductive technology national summer report. In: Monitoring the activity and outcomes of Italian ART centers in 2016: Italian national registry of Assisted Reproductive Technologies (ART); 2016.
- 20.Campana A, Sakkas D, Stalberg A, Bianchi PG, Comte I, Pache T, Walker D. Intrauterine insemination: evaluation of the results according to the woman's age, sperm quality, total sperm count per insemination and life table analysis. Hum Reprod. 1996;11:732–736. doi: 10.1093/oxfordjournals.humrep.a019244. [DOI] [PubMed] [Google Scholar]
- 21.Thijssen A, Creemers A, Van der Elst W, Creemers E, Vandormael E, Dhont N, et al. Predictive value of different covariates influencing pregnancy rate following intrauterine insemination with homologous semen: a prospective cohort study. Reprod BioMed Online. 2017;34:463–472. doi: 10.1016/j.rbmo.2017.01.016. [DOI] [PubMed] [Google Scholar]
- 22.Wiser A, Shalom-Paz E, Reinblatt SL, Son WY, Das M, Tulandi T, Holzer H. Ovarian stimulation and intrauterine insemination in women aged 40 years or more. Reprod BioMed Online. 2012;24:170–173. doi: 10.1016/j.rbmo.2011.11.007. [DOI] [PubMed] [Google Scholar]
- 23.Merviel P, Heraud MH, Grenier N, Lourdel E, Sanguinet P, Copin H. Predictive factors for pregnancy after intrauterine insemination (IUI): an analysis of 1038 cycles and a review of the literature. Fertil Steril. 2010;93:79–88. doi: 10.1016/j.fertnstert.2008.09.058. [DOI] [PubMed] [Google Scholar]
- 24.C M, N V, M S, G SR. Day 2 follicles stimulating hormone (FSH) and estradiol (E2): could these values be used as markers to predict pregnancy outcomes in women undergoing ovulation induction (OI) therapy with intrauterine insemination (IUI) cycles? Fertil Steril. 2005;84(Suppl):S162.
- 25.Soria M, Pradillo G, García J, Ramón P, Castillo A, Jordana C, Paricio P. Pregnancy predictors after intrauterine insemination: analysis of 3012 cycles in 1201 couples. J Reprod Infertil. 2012;13:158–166. [PMC free article] [PubMed] [Google Scholar]
- 26.Collins JA, Burrows EA, Wilan AR. The prognosis for live birth among untreated infertile couples. Fertil Steril. 1995;64:22–28. doi: 10.1016/S0015-0282(16)57650-X. [DOI] [PubMed] [Google Scholar]
- 27.Snick HK, Snick TS, Evers JL, Collins JA. The spontaneous pregnancy prognosis in untreated subfertile couples: the Walcheren primary care study. Hum Reprod. 1997;12:1582–1588. doi: 10.1093/humrep/12.7.1582. [DOI] [PubMed] [Google Scholar]
- 28.Nuojua-Huttunen S, Tomas C, Bloigu R, Tuomivaara L, Martikainen H. Intrauterine insemination treatment in subfertility: an analysis of factors affecting outcome. Hum Reprod. 1999;14:698–703. doi: 10.1093/humrep/14.3.698. [DOI] [PubMed] [Google Scholar]
- 29.Goverde AJ, McDonnell J, Vermeiden JP, Schats R, Rutten FF, Schoemaker J. Intrauterine insemination or in-vitro fertilisation in idiopathic subfertility and male subfertility: a randomised trial and cost-effectiveness analysis. Lancet. 2000;355:13–18. doi: 10.1016/S0140-6736(99)04002-7. [DOI] [PubMed] [Google Scholar]
- 30.Merviel P, Cabry R, Lourdel E, Barbier F, Scheffler F, Mansouri N, Devaux A, Benkhalifa M, Copin H. Intrauterine insemination. Rev Prat. 2014;64:87–91. [PubMed] [Google Scholar]
- 31.Cabry-Goubet R, Scheffler F, Belhadri-Mansouri N, Belloc S, Lourdel E, Devaux A, et al. Effect of gonadotropin types and indications on homologous intrauterine insemination success: a study from 1251 cycles and a review of the literature. Biomed Res Int. 2017;2017:3512784. doi: 10.1155/2017/3512784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Vlahos NF, Coker L, Lawler C, Zhao Y, Bankowski B, Wallach EE. Women with ovulatory dysfunction undergoing ovarian stimulation with clomiphene citrate for intrauterine insemination may benefit from administration of human chorionic gonadotropin. Fertil Steril. 2005;83:1510–1516. doi: 10.1016/j.fertnstert.2004.11.049. [DOI] [PubMed] [Google Scholar]
- 33.van Weert JM, Repping S, Van Voorhis BJ, van der Veen F, Bossuyt PM, Mol BW. Performance of the postwash total motile sperm count as a predictor of pregnancy at the time of intrauterine insemination: a meta-analysis. Fertil Steril. 2004;82:612–620. doi: 10.1016/j.fertnstert.2004.01.042. [DOI] [PubMed] [Google Scholar]
- 34.Mankus EB, Holden AE, Seeker PM, Kampschmidt JC, McLaughlin JE, Schenken RS, Knudtson JF. Prewash total motile count is a poor predictor of live birth in intrauterine insemination cycles. Fertil Steril. 2019;111:708–713. doi: 10.1016/j.fertnstert.2018.12.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pereira N. Total motile count as predictor of live birth in intrauterine insemination cycles. Fertil Steril. 2019;111:674. doi: 10.1016/j.fertnstert.2019.02.026. [DOI] [PubMed] [Google Scholar]
- 36.Bensdorp AJ, Cohlen BJ, Heineman MJ, Vandekerckhove P. Intra-uterine insemination for male subfertility. Cochrane Database Syst Rev. 2007;(4):CD000360. 10.1002/14651858.CD000360.pub4. [DOI] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX 17 kb)
Data Availability Statement
The datasets generated for this study are available on request to the corresponding author.