Abstract
This study was undertaken to understand the impact of climate change on the ecology of infection of Clinostomum complanatum, a model trematode parasite. We analysed climate change data and data from infected fish over a period of seven years (2007–2013) from the Aligarh region (India) in this retrospective study. We show that infection of the trematode parasite Clinostomum complanatum (Rudolphi, 1814) in the forage fish Trichogaster facsiatus (Bloch & Schneider, 1801) is dependent on surface air temperature amongst the (ecologically) relevant climate change variables for both the parasite and its host. This study is the first to implicate surface air temperature as an environmental variable that may contribute towards parasitism, particularly for parasites with a piscine host. The biological relevance of changing climate on the ecology of this parasite is discussed.
Keywords: Climate change, Parasite, Fish, surface air temperature, Clinostomum, Trichogaster
The progressive warming of the earth has led to shifts in patterns of infections with observable changes in geographic loci of distribution of parasites (Harvell et. al. 2002; Chen et. al. 2011; Altizer et. al. 2013). The physiological and the immunological status of the host understandably plays a major role in the success of the infection. Variability in temperature for instance has been experimentally demonstrated to affect vital rates of parasites (Raffel 2013; Paull and Johnson 2014). Modelling dynamics of infection has shown that risk of infection and transmission can be affected by changes in temperature (Brady 2014; McCreesh 2015). The non-linear and often mathematically complex relationship that exists between that parasite, its host and the climate is not understood in great details since the variables involved in the process are difficult of quantify and often hold complex relationships amongst one another, further compounding the problem. Experimental manipulation presents an interesting paradigm to understand changes in parasite ecology with reference to climate change for organisms that have a relatively uncomplicated life cycle, however for trematode parasites, experimental manipulation of the complete life cycle is practically unfeasible since their life cycle usually involves several larval stages which often infect more than one intermediate hosts. To this end we propose that understanding the environmental ecology of one larval stage at a time will yield simpler results, which would then add to the big picture and a better understanding of the situation.
Clinostomum complanatum (Rudolphi, 1814) is a model trematode parasite which has been used extensively to understand the biology of parasitism (Rizvi et. al. 2012; Larson et. al. 1990). The parasite causes the yellow grub disease in several species of commercially important fishes such as Channa punctatus and Trichogaster faciatus in tropical and subtropical regions (Dias et.al. 2003), including the Indian subcontinent and Africa. The parasite shows a typical tematode lifecycle. The natural definitive host of this parasite are ardeiid birds, which feed on infected fish. Within the definitive host, the worms undergo migration from the crop to the buccal cavity, where they remain attached to the mucosa, actively feeding on blood and mucous (Rizvi et. al. 2011). Upon attaining the ovigerous state, the eggs are released in the faeces of the bird, which subsequently hatch, into miracidia that infect the snail host. After a series of transformations within the snail, the cercaria emerge from the snail and infect fish, where the parasite resides as a progenetic metacercaria within the fish until the fish eaten by an ardieed bird, thus completing its lifecycle. The parasite has also shown clinical importance since it causes zoonotic infection in humans resulting in laryngopharyngitis (Rizvi et. al. 2012).
In this study we draw upon the monthly data collected by one of us (AR) and the previously available data (Khan 2018) on the incidence of this parasite from the district of Aligarh in India (27.75°–28° N; 78°–78.25° E.), over a period of seven years (2007 to 2013). It is pertinent to point out that this is retrospective study. The fish data used for this study came from fish that were locally procured from Aligarh region, while several other projects on the biology of C. complanatum were underway. No fish were specifically captured or sacrificed for this study.
We chose precipitation, specific humidity, surface air temperature, and anomaly of surface air temperature (anomaly of SAT) as predictor variables which could affect the incidence of parasitism because of their obvious role in the natural history of both C. complanatum and its host Trichogaster fasciatus. The monthly climate data for our area of interest was procured from the NASA website for data on climate (https://giovanni.gsfc.nasa.gov/giovanni/). The precipitation data is in 0.25° × 0.25° resolution. The specific humidity data was taken in 0.25° × 0.25° resolution. The surface air temperature and the anomaly of SAT data was taken in 0.1° × 0.1° resolution and the values were averaged for the selected region.
We used two different approaches in order to assess the impact of the predictor variables on the response variable i.e. number of infected fish. Using Akaike Information Criteria (AIC) (Burnham et al. 2011) we selected the variables which best explain the predictions for response variable. We used a forward addition approach, utilizing Leaps in R where different models of predictor variables are run, selecting variables which contribute the most to the model. This eventually left us with the variable which exerts the maximum effect on our response variable and has the lowest AIC value.
To further validate our results, we used multiple regression (Ajemian et. al. 2018; de Araujo Lima Constantino 2016; Lewin et. al. 2017; Nath et. al. 2019) in order to assess the significance of the predictor variables on the response variable. Standardization of data was carried out and multicollinearity was assessed among the predictor variables using the pearson product moment correlation coefficient. To assess the model fit we used Adjusted R square values while to evaluate the multiple regression model, we used residual plot and QQ plot. All analysis was carried out using Programme R (R v3.2.2.).
Among the predictor variables we found a strong positive correlation only among the precipitation and surface humidity (Table 1A). But we retained both the predictor variables due to their ecological relationship with the response variable. Both precipitation and surface humidity contribute to abundance of tematode parasites, since the larval stages which use a snail host are heavily dependent on precipitation and moisture to perpetuate their lifecycle (Lockyer et al. 2004).
Table 1.
(A) Correlation between the predictor variables (precipitation, specific humidity, surface area temperature and anomaly of surface area temperature). (B) Selection of sets of predictor variables using model selection with the least Akiek Information Criterion (AIC) values. Lower AIC values imply the maximum effect of the predictor variable (precipitation, specific humidity, surface area temperature and anomaly of surface area temperature) which may influence the response variable (number of infected fish) (C) Effect of predictor variables (precipitation, specific humidity, surface area temperature and anomaly of surface area temperature) on response variable (number of infected fish) using multiple regression analysis
| S.No | Variables | R value | p value |
|---|---|---|---|
| (A) | |||
| 1 | Surface humidity and precipitation | 0.88 | < 0.01 |
| 2 | Surface air temperature and precipitation | 0.35 | < 0.01 |
| 3 | Anomaly of SAT and precipitation | − 0.32 | < 0.01 |
| 4 | Anomaly of SAT and specific humidity | − 0.26 | < 0.01 |
| 5 | Surface air temperature and specific humidity | 0.41 | < 0.01 |
| 6 | Anomaly of SAT and Surface air temperature | 0.28 | < 0.01 |
| S.No | Model | AIC value |
|---|---|---|
| (B) | ||
| 1 | Surface area temperature + Anomaly of SAT + Specific humidity + Precipitation | 0.99 |
| 2 | Surface area temperature | − 9.9 |
| S.No | Predictor variable | Regression coefficients | Standard error | t value | p value |
|---|---|---|---|---|---|
| (C) | |||||
| 1 | Intercept | 4.641e−15 | 1.027e−01 | 0.000 | 1.00000 |
| 2 | Precipitation | − 1.748e−01 | 2.275e−01 | − 0.768 | 0.44456 |
| 3 | Specific humidity | 9.981e−02 | 2.284e−01 | 0.437 | 0.66326 |
| 4 | Surface air temperature | 3.861e−01 | 1.263e−01 | 3.057 | 0.00305** |
| 5 | Anomaly of SAT | 4.055e−02 | 1.219e−01 | 0.333 | 0.74031 |
** Shows high statistical significance (p < 0.001), SAT is an acronym for surface air temperature.
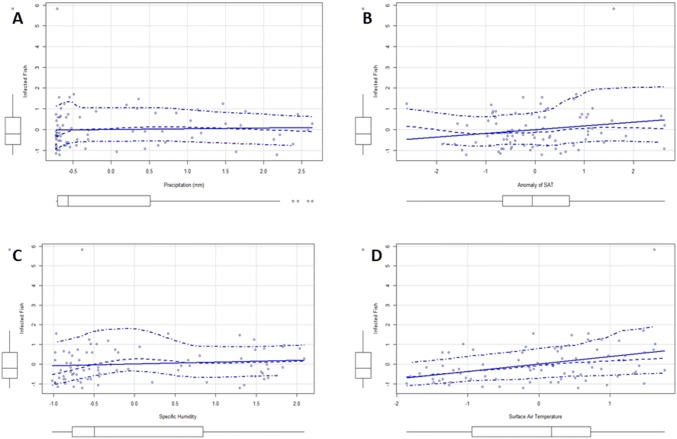
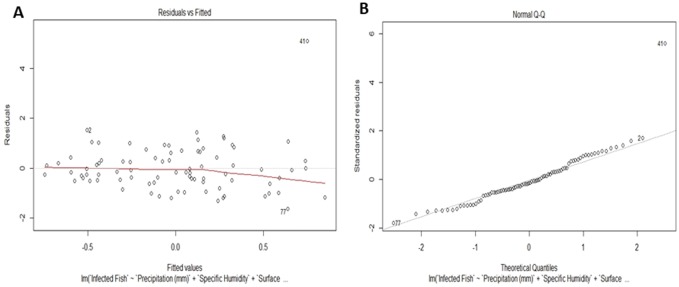
Using AIC model approach, we found two models with surface area temperature as the only variable in the models with the least AIC values (Table 1B). Multiple regression analysis also revealed the same results with surface area temperature being the only highly significant factor (p < 0.01) affecting the response variable i.e. infected fish positively (Table 1C and Fig. 1). The model fit was also significant (p < 0.05) with adjusted R2 value 0.11. Residuals plots in case of multiple regression analysis did not show any pattern in distribution of residuals value (Fig. 2A) indicating that the model retains the underlying assumptions of multiple regression analysis. The Normal QQ polt did not show significant deviation of standardised residuals from the normal values (Fig. 2B) indicating that our model is normally distributed. Taken together, these results indicate that surface air temperature significantly impacts the abundance of C. complanatum in its intermediate host T. fasciatus. Surface air temperature is one of the major factors which influences the temperature of water bodies (Gudasz et.al. 2010), such as those which are inhabited by T. facsiatus and the snail hosts of C. complanatum.
Fig. 1.
Regression plots showing relationship between predictor variable (infected fish) and selected response variables. a Preciptation, b anomaly of SAT c specific humidity d SAT
Fig. 2.
Plots to evaluate model performance a residual plot b normal QQ plot
Rise in surface air temperature can prospectively have two contributions to the life cycle of C. complanatum. Firstly, the cooling caused as a consequence of evaporation will contribute towards creation of cool mud puddles on the banks of water bodies which will provide an ideal habitat for perpetuation of the life cycle of snails that are an intermediate host of this parasite. It is worth mentioning that summer temperatures in our region of study often shoot upto 40º C where such evaporation induced cooling becomes a fundamental requirement for the survival of snails. Secondly, the evaporation induced shrinkage of natural water reservoirs such as lakes and ponds also contributes to perpetuation of infection. It is reasonable to presume that smaller volumes of water will contribute towards higher abundance of C. complanatum cercaria which will have a greater chance to infect the available fish host. The role of the host immune system and its dependence on climate has been shown to be fundamental in establishment of parasite infection (Mignatti 2016), we do not rule climate dependent immunomodulation in our system.
It has also been shown that shrinkage of water bodies leads to congregation of wild ardieed birds on the available water sources (Mishra 2012). On one hand the shrinkage of the water body will lead to an increased density of infected fish and on the other hand it will provide ample opportunities of infection of the avian hosts of this parasite.
We continue efforts to model these conditions in the laboratory to provide conclusive and concrete evidence of the impact of climate change on parasitism. Perhaps other experimental trematode model systems which can be manipulated in the laboratory, such as the Schistosoma mansoni/Biomphlaria glabrata system will help us validate the phenomenon.
This is the first report indicating that changes in the surface air temperature will contribute increased infection of C. complanatum in its fish host. We have reasons to believe that this phenomenon is not specific to this parasite and this observation has a broader implications in terms of parasitism of trematodes, including for human infections.
Compliance with ethical standards
Conflict of interest
The authors declare no conflict of interests.
Ethical statement
This study was “exempt” from the Ethical Committee of the Department of Biochemistry, Aligarh Muslim University, since it was a retrospective study and no animals were used or procured for this study.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Ajemian MJ, et al. Moving forward in a reverse estuary: habitat use and movement patterns of black drum (Pogonias cromis) under distinct hydrological regimes. Estuaries Coasts. 2018;41:1410–1421. doi: 10.1007/s12237-017-0363-6. [DOI] [Google Scholar]
- Altizer S. Climate change and infectious diseases: From evidence to a predictive framework. Science. 2013;341:514–519. doi: 10.1126/science.1239401. [DOI] [PubMed] [Google Scholar]
- Brady OJ, et al. Global temperature constraints on Aedes aegypti and Ae. albopictus persistence and competence for dengue virus transmission. Parasit Vectors. 2014;7:338. doi: 10.1186/1756-3305-7-338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burnham KP, et al. AIC Model selection and multimodel inference in behavioural ecology: some background, obervations and comparasions. Bihav Ecol Sociobiol. 2011;65:23–35. doi: 10.1007/s00265-010-1029-6. [DOI] [Google Scholar]
- Chen IC, et al. Rapid range shifts of species associated with high levels of climate warming. Science. 2011;333:1024–1026. doi: 10.1126/science.1206432. [DOI] [PubMed] [Google Scholar]
- de Araujo Lima Constantino P (2016) Deforestation and hunting effects on wildlife across amazonian indigenous lands. EcolSoc 21(2)
- Dias MLGG, et al. The life cycle of Clinostomum complanatum Rudolphi, 1814 (Digenea, Clinostomidae) on the flood plain of the high Parana’ river. Brazil Parasitol Res. 2003;89:506–508. doi: 10.1007/s00436-002-0796-z. [DOI] [PubMed] [Google Scholar]
- Gudasz C, et al. Temperature-controlled organic carbon mineralization in lake sediments. Nature. 2010;466:478–481. doi: 10.1038/nature09186. [DOI] [PubMed] [Google Scholar]
- Harvell CD, et al. Climate warming and disease risks for terrestrial and marine biota. Science. 2002;296:2158–2162. doi: 10.1126/science.1063699. [DOI] [PubMed] [Google Scholar]
- Khan S (2018) Epidemiology biochemistry and pathogenicity of Clinostomum complanatum metacercariae infecting Trichogaster fasciatus, submitted to the Aligarh Muslim University for the award of Ph.D. in Zoology. Accessed from https://shodhganga.inflibnet.ac.in/handle/10603/12849
- Larson OR, Uglem GL. Cultivation of Clinostomum marginatum (Digenea: Clinostomatidae) metacercariae in vitro, in chick embryo and in mouse coelom. J Parasitol. 1990;76:505–508. doi: 10.2307/3282829. [DOI] [PubMed] [Google Scholar]
- Lewin N, et al. Juvenile concentrations of IGF-1 predict life-history trade-offs in a wild mammal. Funct Ecol. 2017;31:894–902. doi: 10.1111/1365-2435.12808. [DOI] [Google Scholar]
- Lockyer AE, et al. Trematodes and snails: an intimate association. Can J Zool. 2004;82:251–269. doi: 10.1139/z03-215. [DOI] [Google Scholar]
- McCreesh N, et al. Predicting the effects of climate change on Schistosoma mansoni transmission in eastern Africa. Parasit Vectors. 2015;8:4. doi: 10.1186/s13071-014-0617-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mignatti A, et al. Climate change and host immunity to infections. PNAS. 2016;113:2970–2975. doi: 10.1073/pnas.1501193113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mishra N, et al. Serological evidence of West Nile virus infection in wild migratory and resident water birds in Eastern and Northern India. Comp Immunol Microbiol Infec Dis. 2012;35:591–598. doi: 10.1016/j.cimid.2012.08.002. [DOI] [PubMed] [Google Scholar]
- Nath A, et al. Sparrows in urban complexity: macro and micro-scale habitat use of sympatric sparrows in Guwahati City, India. Urban Ecosyst. 2019;22:1047–1060. doi: 10.1007/s11252-019-00876-4. [DOI] [Google Scholar]
- Paull SH, Johnson PTJ. Experimental warming drives a seasonal shift in the timing of host-parasite dynamics with consequences for disease risk. Ecol Lett. 2014;17:445–453. doi: 10.1111/ele.12244. [DOI] [PubMed] [Google Scholar]
- Raffel TR, et al. Disease and thermal acclimation in a more variable and unpredictable climate. Nat Clim Change. 2013;3:146–151. doi: 10.1038/nclimate1659. [DOI] [Google Scholar]
- Rizvi A, et al. Abandoning the ship: spontaneous mass exodus of Clinostomum complanatum (Rudolphi, 1814) progenetic metecercariae from the dying intermediate host Trichogaster fasciatus (Bloch & Schneider, 1801) J Parasit Dis. 2011;36:139–140. doi: 10.1007/s12639-011-0077-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rizvi A, et al. A rabbit eye model for in vivo transformation of progenetic metacercariae of Clinostomum complanatum into ovigerous adult worms. J Helminthol. 2012;11:1–5. doi: 10.1017/S0022149X12000752. [DOI] [PubMed] [Google Scholar]