Abstract
This study aimed to evaluate the cell mediated immune responses against Oestrus ovis (O. ovis) in sheep through measurement of the changes in mRNA expression of the tumor necrosis factor alpha (TNF-α) and gamma interferon (IFN-γ) cytokines using quantitative Real time-PCR (qRt-PCR). Also; to detect the role of Oestrus ovis infestation in the oxidative stress markers in sheep. Fifty sheep head were examined in Cairo abattoir from the period of May to August 2019. Sera were separated and collected for measurement of nitric oxide, zinc and malondialdehyde (MDA). While TNF-α and IFN-γ mRNA were extracted from nasal mucosa. Levels of IFN-γ and TNF-α were significantly higher in infested sheep than that in non-infested one. Also, oxidative stresses were indicated by high level of nitric oxide as one of reactive oxygen species (ROS) and serum MDA as oxidative stress marker and low antioxidant capacity (zinc concentration in serum) in infested sheep. The obtained results indicated that measurements of TNF-α and IFN-γ cytokines using qRT-PCR could be used as an association and reproducible quantitative method for the diagnosis of O. ovis infestation in sheep.
Keywords: Oestrus ovis, Tumor necrosis factor alpha (TNFα), Gamma interferon (IFN-γ), Sheep nasal fly, Oxidative stress in sheep
Introduction
Oestrosis is the specific nasal myiasis that infests sheep and goat livestocks (Caracappa et al. 2000; Alcaide et al. 2003; Attia et al. 2019). It causes different clinical problems in infested animals as sneezing and nasal discharges which may lead to significant economic losses (Scala et al. 2001 and El-Tahawy 2010). It is caused by O. ovis larvae which are commonly present in Mediterranean, tropical and desert environments due to their ability to adjust their development to different climatic conditions in such areas (Alcaide et al. 2005). Molecules secreted and excreted by the larvae are the main cause of oestrosis pathogenesis through induction of a hypersensitivity immune reaction. Such stimuli are responsible for pathological damage and local systemic immune-stimulation (Tabouret et al. 2003; Angulo-Valadez et al. 2011). There is increasing interest to define the role of host immune response and the immune factors that help in control of infection and disease development (Donahoe et al. 2017). So, many researches focused on illustration how host immune system attack the larvae and how the larvae modulate their immune reactions to survive and develop inside the host. Previous studies recorded by Sandeman 1996 and Attia et al. 2019 on O. ovis larvae which are obligatory parasites that harbored and feed on the host tissues for long time, so they greatly stimulate cellular and humoral immune response. Also, Alcaide et al. 2005, Angulo-Valadez et al. 2011, Silva et al. 2012 and Attia et al. 2019 studied the humeral immune responses against O. ovis. While cellular immune response in sheep which were naturally infested with O. ovis needs further studies. Dubey and Schares 2011 reported that the efficient cell-mediated Th1 immune response that affected mostly by the cytokine gamma interferon (IFN-γ) and tumor necrosis factor alpha (TNF-α) is very important for controlling disease by restriction of parasite replication and induction of chronic latent infections with Neospora caninum (N. caninum) and Toxoplasma gondii (T. gondii) through cyst formation.
Host immune system increases the generation of reactive oxygen species (ROS) as nitric oxide, superoxide radical (O.−2), hydrogen peroxide (H2O2) and hydroxyl radical (•OH) which used as defense mechanism which is very important in the host resistance to infection with parasite as in case of liver fluke (Kolodziejczyk et al. 2005), but high reactivity of ROS causes oxidative damage to host tissues causing what is called oxidative stress. So, the host cells to neutralize ROS harmful effects produce antioxidant enzymes, nutritional antioxidants and trace elements as zinc, copper, and iron. Oxidative stress was observed in several parasitic infections as the infections of sheep with D. dendriticum (Şimşek et al. 2006), Fasciola hepatica (Saleh 2008) and Echinococcus granulosus (Heidarpour et al. 2013). So, in the present study, the cell mediated immune responses against O. ovis in sheep was evaluated through measurements of the changes in mRNA expression of the tumor necrosis factor alpha (TNF-α) and gamma interferon (IFN-γ) cytokines using Real time-PCR (qRT-PCR). Also, to measure the nitric oxide level and evaluate the relationship between malondialdehyde (MDA) as one of the oxidative stress markers due to infestation with O. ovis larvae in the nostrils, and to compare the trace elements (Zinc) in parasitized and healthy animals to evaluate the immunological response of the animals.
Materials and methods
Animal and sampling
Fifty sheep head (aged from 10 months to 2 years old) were examined in Cairo abattoir from the period of May to August 2019. Blood was collected from each examined sheep during slaughtering from jugular vein in two tubes; one tube containing 0.5 mg/ml EDTA (ethylene diamine tetra acetic acid) as an anticoagulant and other tube without EDTA for sera collection. The clotted blood was centrifuged at 3500 rpm for 15 min, and the sera were then collected and preserved at − 20 °C until used. Sagittal section of each examined sheep head was done for the detection of O. ovis larvae (all the collected larvae were 3rd stage larvae). All the collected larvae were identified according to Zumpt 1965 and Attia et al. 2019. Mucous from nasal passages and nasal sinuses were collected for oxidative stress markers measurement. All samples (sera, whole blood, feces, mucous from nasal passages and nasal sinuses) were transferred into Faculty of Veterinary Medicine; Cairo University for further analysis. All Institutional and National Guidelines for the care and use of animals were followed.
Hematological and parasitological examination
The anti-coagulated blood was used to prepare thin blood film from each anti-coagulated blood sample and stained with Giemsa stain to be examined for presence of any blood parasites. Fecal samples were examined for detection of any parasitic infestation using concentration techniques as described by (Soulsby 1986).
Biochemical analysis
Concentration of zinc in the collected serum samples (fifty sera samples were collected in which 30 sera were positive for O. ovis and twenty were negative for O. ovis); these sera were measured using ionized coupled plasma using mass spectrometry method (Page et al. 2018).
Measurement of oxidative stress markers
The level of malondialdehyde (MDA) in sera (the positive and negative sera); all the sera were measured according to Aktas et al. 2017. While nitric oxide level in positive and negative sera was detected according to Aytekin and Unubol Aypak (2011).
Evaluation of tumor necrosis factor alpha (TNF-α) and gamma interferon (IFN-γ) activity
Mucous samples from the infested nasal sinuses and its passages; which had the 3rd larval stages with a number of 3rd stage larvae ranged from 5 to 35 larvae; were aseptically dissected. Samples (mucous samples in the nasal sinuses and its passages) from 5 uninfected sheep were collected in a similar manner and used as negative controls. All the mucous were aseptically preserved in − 20 °C.
RNA isolation
Isolation of RNA from 100 mg of mucous and nasal sinuses were performed by total RNA kit (Ambion, Applied Biosystems), following the manufacturer’s instructions. Homogenization of the tissues were applied in Lysing Matrix D tubes (MP Biomedicals) using a FastPrep-24 homogenizer (MP Biomedicals, 2 cycles of 30 s at 6 m/s). Nanodrop (Thermo Scientific) were used to measure the RNA purity and quantity. A 500 ng of RNA were made with DNaseI amplification grade (Invitrogen) following the manufacturer’s instructions. The reverse transcription of treated RNA was performed by High-Capacity cDNA Archive Kit (Applied Biosystems) (Picard-Sánchez et al. 2019).
Quantitive real-time PCR protocol (qRT-PCR)
PCR primer sets specific for tumor necrosis factor alpha (TNFα) and gamma interferon (IFN-γ) specific for sheep were designed and based on sequences deposited in the GeneBank (Table 1). β-actin was used as a reference gene and for sample normalization. The genes expression included in this study was tested on a separate pool of cDNA, generated from five un infested sheep previously examined for presence of any parasites.
Table 1.
The sequences of the forward and reverse primer used in the quantitative real-time PCR
| Gene | Sequence [5-3] | Accession number | References |
|---|---|---|---|
| INF-γ | F-CAGAGCCAAATTGTCTCCTTC R-ATCCACCGGAATTTGAATCAG | NM_001009803 | Puech et al. (2015) |
| TNFα | F-CCAGAGGGAAGAGCAGTCC R-GGCTACAACGTGGGCTACC | NM_001024860 | Puech et al. (2015) |
| β-Actin | F-TGGGCATGGAATCCTG R-GGCGCGATGATCTTGAT | NM_001009784 | Puech et al. (2015) |
PCR cycling conditions
Amplification was carried out for 40 cycles as follows: denaturation for 30 s at 94 °C, annealing for 30 s at 60 °C and extension for 45 s at 72 °C. Real-time PCR protocol run according to Puech et al. (2015).
Statistical analysis
Results were analyzed using Predictive Analytics Software (PASW) Statistics, Version 18.0 software (SPSS Inc., Chicago, IL, USA). Blood parameters in diseases and control groups were compared by independent sample T test or Wilcoxon-Mann–Whitney test according to the normality of data. A P-value < 0.05 was considered statistically significant.
Results
Thirty examined sheep were positive for O. ovis larvae by postmortem examination of the heads with 60% prevalence rate during May to August 2019, the collected larvae were full mature 3rd stage larvae. The larvae were collected from the nasal passages; its sinuses and on the base of the two horns. The 3rd stage larvae were 15–25 (20 ± 0.5) mm in length and yellow to brown in colour. On the dorsal surface; the larvae had broad transverse brown to blackish bands which supported with several small denticles. On the ventral surface, each segment supported by 3 to 4 rows of irregularly placed strong spines.
The intensity of infestation with the total number of the 3rd instar of O. ovis larvae was recorded in 30 (60%) of the infested sheep during the period from May to August 2019. A total of 15 sheep had 5–10 larvae while 11 sheep had 11–20 larvae and 4 sheep harbored 21–35 larvae (Table 2).
Table 2.
The intensity of infestation with O. ovis in sheep
| Intensity of infestation | No. of sheep | % of sheep |
|---|---|---|
| 5–10 larvae | 15 | 50 |
| 11–20 | 11 | 36.6 |
| 21–35 | 4 | 13.3 |
Examined sheep showed significantly higher in nitric oxide [mean difference = 14.30 (± 10.44, ± 95% C.I.); t (4) = 3.802, p = 0.019] and serum MDA [18.00 (± 5.65, ± 95% C.I.); t(4) = 8.848, p = 0.001] levels than that in non-infected sheep (Table 2). Whereas we found that Zinc levels in infested sheep were -62.33 (± 8.38, ± 95% C.I.) lower than that of non-infested sheep (t (4) = − 20.651, p < 0.0001); Table 3.
Table 3.
Oxidative stress markers, trace elements levels in healthy and O. ovis infested sheep (Mean ± SE)
| Healthy | Infested | Change (%)a | P-value | |
|---|---|---|---|---|
| Nitric oxide (nmol/mg protein) | 48.37 ± 0.19 | 62.67 ± 3.76 | 29.6 | 0.019* |
| MDA (nmol/ml) | 12.33 ± 0.17 | 30.33 ± 2.03 | 146.0 | 0.001* |
| Zinc (µg/dl) | 118.00 ± 0.58 | 55.67 ± 2.96 | − 52.8 | <0.0001* |
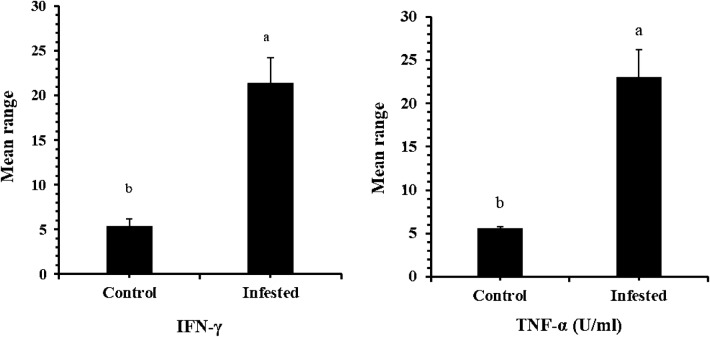
| IFN-γ | 5.33 ± 0.88 | 21.33 ± 2.91 | 300.2 | 0.006* |
| TNF-α (U/ml) | 5.50 ± 0.29 | 23.00 ± 3.21 | 318.2 | 0.006* |
aPercentage of change between the mean values of the infested group (O. ovis infested sheep) and the healthy control group (group of non- infested sheep; with no O. ovis infestation and no any parasites); *Indicate significant difference at p-value < 0.05; SE Standard error
Infested sheep showed significantly higher in INF-γ [mean difference = 16.00 (± 8.43, ± 95% C.I.); t(4) = 5.269, p = 0.006] and TNFα [17.50 (± 8.96, ± 95% C.I.); t(4) = 5.422, p = 0.006] levels than that in non-infested sheep (Table 3; Figs. 1;2).
Fig. 1.
Gamma interferon (IFN-γ) and tumor necrosis factor-alpha (TNF-α) activities in healthy and infested sheep with O. ovis. (a,b) The difference between healthy and infested group is significant (P < 0.05)
Fig. 2.
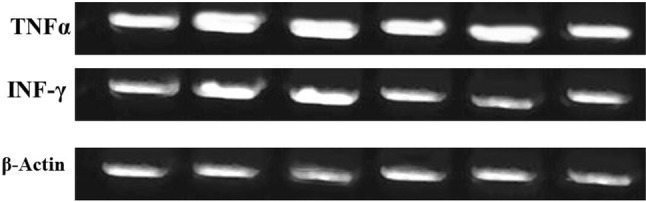
Electrophoretic mobility of q RT-PCR products of TNFα and INF-γ versus β- actin as control (internal control) on 2% agarose gel
Discussion
In Egypt, Sheep breeding is very important as most peoples in village and bed wins in the desert breed variable flocks of sheep due to its high fertility rate and low raising cost. Ovine parasitic infestations cause an important problem of economic losses (Ramadan et al. 2013). O. ovis larva is one of these parasites that affect the hosts performance, growth and animal production. In this study infestation rate of 3rd stage larvae of O. ovis during summer was found to be 60%. This result was in agreement with Ramadan et al. 2013 who recorded that infestation rate was at peak during summer in Egypt. Also; Ahaduzzaman 2019 reported that O. ovis was a parasite of high prevalence rate in Africa and Europe and, Hidalgo et al., 2015 found that the prevalence of O. ovis in sheep slaughtered in Chile abattoirs was 60.9%. The high percentages of L3point out to the year-round development and that the flies present with at least three generations in year (Ramadan et al. 2013).
Many researches were done on prevalence, morphology and serodiagnosis of oestrosis in Egypt. As, that the study recorded by Gaaboub 1978, Hassanin et al. 1989, Amin et al. 1997, Ramadan et al. 2013 and Attia et al. 2019. But no researches were done on the cell mediated immune response against O. ovis in sheep. Cytokines play a key role in the immune system response to infectious parasites. So, lately cytokine level measurement was used in many researches to detect immune response against parasitic infestation in ruminants (Puech et al. 2015). As, they control the activation, differentiation of different types of cells and antibodies secretion (Mosmann and Coffman, 1989). So, differential cytokine production and expression is commonly used method to characterize disease pathogenesis in cattle (Coussens et al. 2004 and Boddu-Jasmine et al. 2008), goats (Singh et al. 2013) and sheep (Smeed et al. 2007 and Channappanavar et al. 2012). The cytokine balance is analyzed by detection of the secreted cytokine proteins quantity or by the analysis of cytokine mRNA expression (Giulietti et al. 2001). So, our study was performed to evaluate the cell mediated immune responses against O. ovis in sheep through measurement of the changes in mRNA expression of the tumor necrosis factor alpha (TNF-α) and gamma interferon (IFN-γ) cytokines using Real time-PCR (qRT-PCR).
In this study, infested sheep showed significantly higher IFN-γ and TNF-α levels than that in non-infested sheep. These results agree with that recorded in different parasitic infections as (Toxoplasma gondii; Neospora caninum; Demodex mites and malaria infection) by Donahoe et al. 2017; Lacey et al. 2018 and Nasr et al. 2014 which recorded significant high IFN-γ and TNF-α levels. This data was considered as a beginning of further researches on the functional and genetic analysis of the TNF-α and IFN-γ genomic region in the oestrosis infestation of sheep.
The fecal and blood samples reveal no other associated parasites which prove that the risen in the gene expression was due to O. ovis infection.
Moreover, oxidative stress and changes in antioxidants capacity was studied. Infested sheep showed significantly higher nitric oxide level as one of ROS than that of non-infested sheep. High level of ROS causes the production of MDA by initiating the peroxidation of membrane un-saturated fatty acids (Uner et al. 2001). This explain the higher level of serum MDA in infested sheep than that of non-infested sheep. While antioxidant capacity (Zinc level) in infested sheep was lower than that of non-infested sheep. As the host body needs an adequate amount of trace elements for the structure and function of some antioxidant enzymes that is important in the cells protection against toxic effect of ROS (e.g., zinc and copper for superoxide dismutase and iron for catalase (Munoz et al. 2007). Also, Samadieh et al. 2017 reported decreased zinc and iron concentrations in the parasitized animals with dicrocoeliasis explaining that stress could result in losses of trace elements during parasitic infections. This result showed that oestrosis in sheep cause a case of oxidative stress indicated by high level of serum MDA and low antioxidant capacity (zinc concentration in serum). Other studies reported oxidative stress in sheep suffering from dicrocoeliasis (Şimşek et al. 2006 and Samadieh et al. 2017).
Conclusion
In this study, the cell mediated immune response against infestation with O. ovis larvae in sheep was studied. The higher of TNF-α and IFN-γ cytokines levels measured by qRT-PCR could be associated with positive oestrosis diagnosis even in low levels of cytokine mRNA. This study pointed out to the role of O. ovis larvae in the production of a case of oxidative stress indicated by high level of nitric oxide and MDA with low antioxidant capacity by zinc concentration in sera of infested sheep.
Authors Contribution
All authors sharing in the aim of works; Marwa M. Attia: collect the samples and identify the clinical sign on the sheep; Marwa M. Attia and Sohila M. El-Gameel Identify the parasites; applying the clinical works; Elshaimaa Ismael applying of statistical analysis of the results. All authors sharing in writing and revised this manuscript.
Funding
No funding supporting this work.
Compliance with ethical standards
Conflict of interest
All authors declare that they have no conflict of interest.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Ahaduzzaman M. The global and regional prevalence of oestrosis in sheep and goats: a systematic review of articles and meta-analysis. Parasit Vectors. 2019;12(1):346. doi: 10.1186/s13071-019-3597-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aktas MS, Kandemir FM, Kirbas A, Hanedan B, Aydin MA. Evaluation of oxidative stress in sheep infected with Psoroptes Ovis using total antioxidant capacity, total oxidant status, and malondialdehyde level. J. Vet. Res. 2017;61(2):197–201. doi: 10.1515/jvetres-2017-0025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alcaide M, Reina D, Sánchez J, Frontera E, Navarrete I. Seasonal variations in the larval burden distribution of Oestrus ovis in sheep in the southwest of Spain Vet. Parasitol. 2003;30(8):235–241. doi: 10.1016/j.vetpar.2003.09.021. [DOI] [PubMed] [Google Scholar]
- Alcaide M, Reina D, Sánchez-López J, Frontera E, Navarrete I. Seroprevalence of Oestrus ovis (Diptera, Oestridae) infestation and associated risk factors in ovine livestock from southwestern Spain. J Med Entomol. 2005;42(3):327–331. doi: 10.1093/jmedent/42.3.327. [DOI] [PubMed] [Google Scholar]
- Amin AR, Morsy TA, Shoukry A, Mazyad SA. Oestrid head maggots in slaughtered sheep in Cairo abattoir. J Egypt Soc Parasitol. 1997;27:855–861. [PubMed] [Google Scholar]
- Angulo-Valadez CE, Ascencio F, Jacquiet P, Dorchies P, Cepeda-Palacios R. Sheep and goat immune responses to nose bot infestation: a review. Med Vet Entomol. 2011;25(2):117–125. doi: 10.1111/j.1365-2915.2010.00911.x. [DOI] [PubMed] [Google Scholar]
- Attia MM, Soliman SM, Salaeh NMK. Advanced and rapid serodiagnosis of oestrosis (Oestrus ovis; Diptera: Oestridae) in sheep using indirect and Dot-ELISA. Jordan J Biol Sci. 2019;12(3):275–281. [Google Scholar]
- Aytekin I, Unubol Aypak S. Levels of selected minerals, nitric oxide, and vitamins in aborted Sakis sheep raised under semitropical conditions. Trop Anim Health Prod. 2011;43:511–514. doi: 10.1007/s11250-010-9724-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boddu-Jasmine HC, Witchell J, Vordermeier M, Wangoo A, Goyal M. Cytokine mRNA expression in cattle infected with different dosages of Mycobacterium bovis. Tuberculosis (Edinb). 2008;88(6):610–615. doi: 10.1016/j.tube.2008.06.003. [DOI] [PubMed] [Google Scholar]
- Caracappa S, Rilli S, Zanghi P, Di Marco V, Dorchies Ph. Epidemiology of ovine oestrosis (Oestrus ovis Linné 1761, Diptera: Oestridae) in Sicily. Vet Parasitol. 2000;92:233–237. doi: 10.1016/s0304-4017(00)00317-4. [DOI] [PubMed] [Google Scholar]
- Channappanavar R, Singh KP, Singh R, Umeshappa CS, Ingale SL, Pandey AB. Enhanced proinflammatory cytokine activity during experimental bluetongue virus-1 infection in Indian native sheep. Vet Immunol Immunopathol. 2012;145(1–2):485–492. doi: 10.1016/j.vetimm.2011.10.013. [DOI] [PubMed] [Google Scholar]
- Coussens PM, Verman N, Coussens MA, Elftman MD, McNulty AM. Cytokine gene expression in peripheral blood mononuclear cells and tissues of cattle infected with mycobacterium avium subsp. paratuberculosis: evidence for an inherent proinflammatory gene expression pattern. Infect Immun. 2004;72(3):1409–1422. doi: 10.1128/IAI.72.3.1409-1422.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donahoe SL, Phalen DN, McAllan BM, O’Meally D, McAllister MM, Ellis J, Šlapeta J. Differential gamma interferon- and tumor necrosis factor alpha-driven cytokine response distinguishes acute infection of a metatherian host with Toxoplasma gondii and Neospora caninum. Infect Immun. 2017;85(6):e00173. doi: 10.1128/IAI.00173-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubey JP, Schares G. Neosporosis in animals—the last five years. Vet Parasitol. 2011;180:90–108. doi: 10.1016/j.vetpar.2011.05.031. [DOI] [PubMed] [Google Scholar]
- El-Tahawy AS. The prevalence of selected diseases and syndromes affecting Barki sheep with special emphasis on their economic impact. Small Rumin Res. 2010;90:83–87. [Google Scholar]
- Gaaboub LA. The distribution and seasonal dynamics of Oestrus ovis Linne infesting nasal cavities and sinuses of sheep in Egypt. Vet. Parasiotl. 1978;4(1):79–82. [Google Scholar]
- Giulietti A, Overbergh L, Valckx D, Decallonne B, Bouillon R, Mathieu C. An overview of real-time quantitative PCR: applications to quantify cytokine gene expression. Methods. 2001;25(4):386–401. doi: 10.1006/meth.2001.1261. [DOI] [PubMed] [Google Scholar]
- Hassanin MA, Abdel-Gawad AM, Ramadan EI. Incidence, monthly prevalence and bionomics of Oestrus ovis infesting sheep in Egypt. J Egypt Vet Med Assoc. 1989;49:431–443. [Google Scholar]
- Heidarpour M, Mohri M, Borji H, Moghdass E. Oxidant/antioxidant status in cattle with liver cystic echinococcosis. Vet Parasitol. 2013;195(1–2):131–135. doi: 10.1016/j.vetpar.2013.01.018. [DOI] [PubMed] [Google Scholar]
- Hidalgo A, Palma H, Oberg C, Fonseca-Salamanca F. Oestrus ovis infection of grazing sheep during summer in southern Chile. Pesq. Vet. Bras. 2015;35(6):497–500. [Google Scholar]
- Kolodziejczyk L, Siemieniuk E, Skrzydlewska E. Antioxidant potential of rat liver in experi-mental infection with Fasciola hepatica. Parasitol Res. 2005;96(6):367–372. doi: 10.1007/s00436-005-1377-8. [DOI] [PubMed] [Google Scholar]
- Lacey N, Russell-Hallinan A, Zouboulis CC, Powell FC. Demodex mites modulate sebocyte immune reaction: possible role in the pathogenesis of rosacea. BJD. 2018;179:252–253. doi: 10.1111/bjd.16540. [DOI] [PubMed] [Google Scholar]
- Mosmann TR, Coffman RL. TH1 and TH2 cells: different patterns of lymphokine secretion lead to different functional properties. Annu Rev Immunol. 1989;7:145–173. doi: 10.1146/annurev.iy.07.040189.001045. [DOI] [PubMed] [Google Scholar]
- Munoz C, Rios E, Olivos J, Brunser O, Oli-vares M. Iron, copper and immunocompetence. Br J Nutr. 2007;98(Suppl 1):S24–S28. doi: 10.1017/S0007114507833046. [DOI] [PubMed] [Google Scholar]
- Nasr A, Allam G, Hamid O, Al-Ghamdi A. IFN-gamma and TNF associated with severe falciparum malaria infection in Saudi pregnant women. Malar J. 2014;13:314. doi: 10.1186/1475-2875-13-314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Page CM, Murphy TW, Van Emon ML, Bowman JGP, Wyffels SA, Stewart WC. Blood serum mineral element concentrations of weaned Montana ram lambs and their relationship with water quality characteristics. Prof Anim Sci. 2018;34(5):410–420. [Google Scholar]
- Picard-Sánchez A, Estensoro I, Del Pozo R, Piazzon MC, Palenzuela O, Sitjà-Bobadilla A. Acquired protective immune response in a fish-myxozoan model encompasses specific antibodies and inflammation resolution. Fish Shellfish Immunol. 2019;90:349–362. doi: 10.1016/j.fsi.2019.04.300. [DOI] [PubMed] [Google Scholar]
- Puech C, Dedieu L, Chantal I, Rodrigues V. Design and evaluation of a unique SYBR Green real-time RT-PCR assay for quantification of five major cytokines in cattle, sheep and goats. BMC. Vet. Res. 2015;11:65. doi: 10.1186/s12917-015-0382-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramadan MY, Khater HF, Omer SF, Abdel Rahman A (2013) Epidemiology of Oestrus ovis infesting Egyptian sheep. In: International congress of mediterranean federation of health and production of ruminants, pp 19–22
- Saleh MA. Circulating oxidative stress status in desert sheep naturally infected with Fasciola hepatica. Vet Parasitol. 2008;154(3–4):262–269. doi: 10.1016/j.vetpar.2008.03.012. [DOI] [PubMed] [Google Scholar]
- Samadieh H, Mohammadi GR, Maleki M, Borji H, Azizzadeh M, Heidarpour M. Relationships between oxidative stress, liver, and erythrocyte injury, trace elements and parasite burden in sheep naturally infected with Dicrocoelium dendriticum. Iran J. Parasitol. 2017;12(1):46–55. [PMC free article] [PubMed] [Google Scholar]
- Sandeman RM. Immune responses to mosquitoes and flies. In: Wikel SK, editor. The immunology of host-ectoparasitic arthropod relationship. Wallingford: CAB International; 1996. pp. 175–203. [Google Scholar]
- Scala A, Solinas G, Citterio CV, Kramer LH, Genchi C. Sheep oestrosis (Oestrus ovis Linné 1761, Diptera: Oestridae) in Sardinia, Italy. Vet Parasitol. 2001;102(1–2):133–141. doi: 10.1016/s0304-4017(01)00515-5. [DOI] [PubMed] [Google Scholar]
- Silva BF, Bassetto CC, Amarante AF. Immune responses in sheep naturally infected with Oestrus ovis (Diptera: Oestridae) and gastrointestinal nematodes. Vet Parasitol. 2012;190(1–2):120–126. doi: 10.1016/j.vetpar.2012.06.004. [DOI] [PubMed] [Google Scholar]
- Şimşek S, Yüce A, Ütük AE. Determination of serum malondialdehyde levels in sheep naturally infected with Dicrocoelium dendriticum. F. U. Saglık Bil. Dergisi. 2006;20(3):217–220. [Google Scholar]
- Singh PK, Singh SV, Saxena VK, Singh MK, Singh AV, Sohal JS. Expression profiles of different cytokine genes in peripheral blood mononuclear cells of goats infected experimentally with native strain of Mycobacterium avium subsp. paratuberculosis. Biotechnol. 2013;24(3):187–197. doi: 10.1080/10495398.2012.762008. [DOI] [PubMed] [Google Scholar]
- Smeed JA, Watkins CA, Rhind SM, Hopkins J. Differential cytokine gene expression profiles in the three pathological forms of sheep paratuberculosis. BMC Vet Res. 2007;3:18. doi: 10.1186/1746-6148-3-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soulsby EJL. Helminths, Arthropods & Protozoa of Domesticated Animals. 7. London: Bailliere Tindall; 1986. [Google Scholar]
- Tabouret G, Bret-Bennis L, Dorchies P, Jacquiet P. Serine protease activity in excretory-secretory products of Oestrus ovis (Diptera: Oestridae) larvae. Vet Parasitol. 2003;114:305–314. doi: 10.1016/s0304-4017(03)00157-2. [DOI] [PubMed] [Google Scholar]
- Uner N, Oruç EO, Canli M, Sevgile Y. Effects of cypermethrin on antioxidant enzyme activities and lipid peroxidation in liver and kidney of the freshwater fish. Oreochromis niloticus and Cyprinus carpio (L.). . Bull Environ Contam Toxicol. 2001;67(5):657–664. doi: 10.1007/s001280174. [DOI] [PubMed] [Google Scholar]
- Zumpt F. Myiasis in man and animals in the old world. London: Butterworth; 1965. [Google Scholar]