Abstract
Purpose
Metformin is the most commonly prescribed drug in the management of metabolic disorders such as polycystic ovarian syndrome (PCOS) and gestational diabetes in women of reproductive age. Insulin-sensitizing effect of metformin helps in improving from PCOS features such as hyperandrogenism, anovulation, and infertility. However, its ability to cross placental barrier raises concern about safety of the drug on early embryonic development. In this study, we evaluated the effect of metformin on the ovarian function and embryo development.
Methods
Adult Swiss albino female mice were administered with metformin (0, 50, 100, and 200 mg/kg body weight) for 4 weeks and assessed for reproductive function and preimplantation embryo development. Further, effect of metformin (0, 10, 25, 50, 100, 250, and 500 μg/mL) exposure to 2-cell-stage embryos was tested under in vitro conditions.
Results
Metformin did not alter the body weight, blood glucose, ovarian weight, and follicular reserve. However, the early embryo development was significantly affected in mice treated with metformin in vivo at highest dose. Moreover, embryos which were exposed to metformin in vitro showed dose-dependent decline in blastocyst rate and hatching rate. Furthermore, at highest concentration of metformin (500 μg/mL), all the embryos were arrested at compaction stage.
Conclusion
The study revealed that metformin affects the early embryonic development and raises concern about its use during conception.
Electronic supplementary material
The online version of this article (10.1007/s10815-020-01709-6) contains supplementary material, which is available to authorized users.
Keywords: Metformin, Polycystic ovarian syndrome, Blastocyst rate, In vitro maturation
Introduction
Metformin (dimethylbiguanide) is the most common and effective drug used in the treatment of type II diabetes mellitus and its associated metabolic complications. It exerts anti-hyperglycemic effect by decreasing intestinal absorption and hepatic production of glucose as well as increasing the peripheral glucose uptake [1]. Metformin is known to inhibit electron transport chain complex I (ETC I) and thereby decrease cellular adenosine tri-phosphate (ATP) level [2, 3]. The decreased ATP level in turn activates adenosine monophosphate (AMP)–activated protein kinase (AMPK), a key energy sensor, which triggers multiple signaling cascades to maintain energy homeostasis [4].
Owing to the vital role of metformin in metabolic and endocrine modulations, currently it is the best drug of choice for treatment of polycystic ovarian syndrome (PCOS), the condition which is observed in 10–15% females of reproductive age group [5]. PCOS is characterized by hyperandrogenism, insulin resistance, and obesity which results in anovulation and formation of cystic ovaries. Metformin inhibits steroidogenesis and androgen production by decreasing ATP and nicotinamide adenine dinucleotide (NAD+) levels [6, 7].
Metformin treatment regimen usually lasts for long duration in women of reproductive age group in order to minimize obstetric and pregnancy complications associated with PCOS. Studies have shown that metformin lowers pregnancy loss, reduces gestational diabetes and risk of birth defects [8–10]. On the contrary, Cochrane database showed that metformin treatment before and during assisted reproductive technology (ART) did not improve the live birth rate [11]. Further, a recent randomized double-blind controlled trial reported that metformin administration does not improve in vitro fertilization (IVF) outcomes in terms of live birth, fertilization, and implantation rate and showed similar multiple pregnancy and miscarriage rate compared with placebo [12].
It is well known that ATP is very essential for the resumption of meiotic events in oocytes [13], and therefore, reduction of ATP induced by metformin is speculated to interfere with oocyte and embryo development. It has been observed that metformin exposure causes arrest of bovine and porcine oocytes in germinal vesicle (GV) stage during in vitro maturation [14, 15]. Further, in vitro exposure of 2-cell-stage embryos to 5-aminoimidazole-4-carboxamide ribonucleotide (AICAR) has shown to affect the blastocyst formation by decreasing the expression of tight junction proteins and trophectoderm differentiation which is associated with activation of AMPK [16]. Similarly, metformin-mediated AMPK activation has led to the reduction in expression of pluripotency genes such as octamer-binding transcription factor 4 (OCT4) and reduced expression protein 1 (REX1) in the blastocyst when 2-cell-stage embryos were exposed [17] suggesting its possible adverse effects on early embryo development. However, it is not clear whether metformin affects the ovarian function and whether maternal exposure to metformin has any detrimental effects on early embryos. The present study was designed to understand the consequences of metformin administration on ovarian reserve and pre- and post-implantation embryo development using a mouse model.
Materials and methods
Animal treatment
Healthy Swiss albino mice (8 to 10 weeks) maintained in Central Animal Facility, Kasturba Medical College, Manipal, Manipal Academy of Higher Education, Manipal, were used in the study. The mice were maintained under standard conditions of temperature (25 ± 2 °C), humidity (45–55%), light (12:12 h of light and dark), and food and water ad libitum. Animal handling and experimental procedures were done according to the institutional guidelines and the study protocols were approved by the Institutional Animal Ethics Committee of Kasturba Medical College (IAEC/KMC/25/2014). Metformin (1,1-dimethylbiguanide hydrochloride, Cat. No. D150959, Sigma Aldrich, USA) was dissolved in normal saline (0.9% NaCl) and injected at a dose of 50, 100, and 200 mg/kg body weight for 4 weeks, daily by intraperitoneal (i.p.) route. The doses of metformin used in this study are clinically relevant [18] and are based on previous studies [19, 20]. Control group mice received normal saline. The animals were monitored for body weight changes every week during the course of metformin administration. After the completion of treatment, the mice were sacrificed and assessed for various parameters (Fig. 1). A minimum of 6 animals were used for each independent experiment.
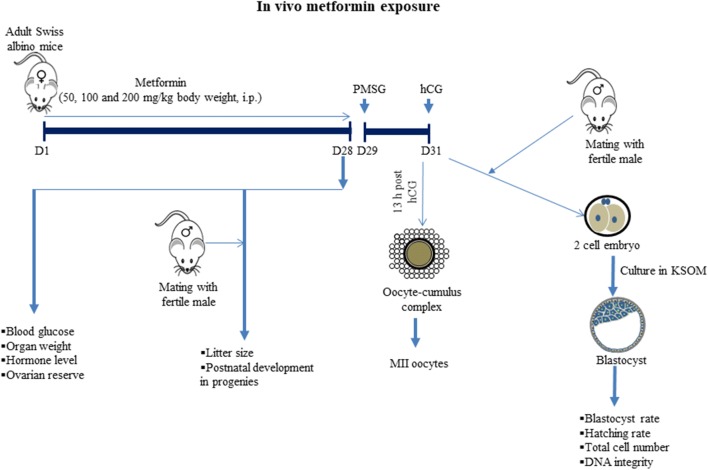
Fig. 1.
The study outline to understand the effect of in vivo exposure of metformin on female reproductive system, ovarian reserve, preimplantation embryo development, and transgenerational changes
Estimation of blood glucose
After the completion of metformin treatment, random blood glucose was analyzed in the morning (between 9 and 11 am) using a glucometer (Roche Diagnostics, Germany) by placing a drop of blood obtained by cutting the tail tip over the strips attached to the glucometer. Glucose concentration was expressed as milligrams per deciliter.
Estimation of serum testosterone and estradiol level
The blood was collected by puncturing the heart and incubated at room temperature for 2 h. The serum was separated by centrifuging the blood at 2000 rpm for 10 min at room temperature and stored at − 80 °C until further analysis. Enzyme-linked immunosorbent assay (ELISA) was performed using specific kits to assess the concentration of testosterone (Cat. No. DE1559, Demeditec Diagnostics Gmbh, Germany) and estradiol (Cat. No. DEH3355, Demeditec Diagnostics Gmbh, Germany). Briefly, standard solutions and serum (25 μL) were mixed with enzyme conjugate solution (200 μL) in 96-well plates and incubated for 1 h at room temperature. The contents were removed and washed 3 times with washing solution. The substrate solution (200 μL) was added and incubated for 15 min at room temperature. The reaction was stopped by adding stop solution (100 μL) and absorbance was read at 450 nm using an ELISA plate reader (LISA Plus, Rapid Diagnostics, India). The concentrations of hormones in serum were calculated by standard curve obtained. The ELISA kits used for the study were able to detect testosterone and estradiol in the range as low as 0.083 ng/mL and 3.94 pg/mL respectively. The antibodies coated in wells were having 100% specificity for respective hormones with negligible non-specific interaction to other steroids.
Follicle count
To understand the effect of metformin on ovarian reserve, the number of follicles was assessed by the method described earlier [21]. Briefly, one ovary from each mouse was subjected to enzymatic digestion by incubating in dissociation medium containing collagenase type IV (5 mg/mL, Cat. No. 17104019, Invitrogen, USA), trypsin (1 mg/mL, Cat. No. RM173, Himedia, India), and hyaluronidase (0.1 mg/mL, Cat. No. H4272, Sigma Aldrich, USA) for 1 h at 37 °C and then dispersed by gentle mechanical agitation. The follicles were counted manually under an inverted microscope (1X70, Olympus, Tokyo) and categorized into primordial, primary, and secondary follicles.
Superovulation
To collect mature oocytes and 2 cell embryos, female mice were primed with 5 IU pregnant mare serum gonadotropin (PMSG, Cat. No. G4877, Sigma Aldrich, USA) 24 h post last metformin injection. At 48 h post-PMSG, 10 IU human chorionic gonadotropin (hCG, Ovutrig VHB Life Sciences Inc. GenBiotech, Mumbai, India) was injected to each mouse to induce ovulation [22].
Oocyte collection
At 12-13 h post-hCG, the oocyte cumulus complexes (OCCs) were collected in potassium-supplemented simplex optimized medium (KSOM), and the oocytes were denuded by incubating OCCs in KSOM media containing hyaluronidase (0.1 mg/mL, Cat. No. H4272, Sigma Aldrich, USA) for 1 min at 37 °C. The oocytes were segregated into GV, MI (metaphase I), MII (metaphase II), degenerated, and fragmented oocytes. The number of MII (mature) oocytes was recorded and expressed as MII oocytes retrieved per animal.
Embryo developmental potential
After the administration of hCG, superovulated female mice were mated overnight with 10–12-week-old proven fertile Swiss albino male mice. Mating was confirmed by the presence of vaginal plug on the next morning which is considered 0.5 days post-coitus (dpc). Females were humanely sacrificed by cervical dislocation at 1.5 dpc, and 2-cell-stage embryos were retrieved from oviducts in Earle’s balanced salt solution (EBBS) medium containing 0.1% BSA. The embryos were cultured in KSOM media at 37 °C and 5% CO2 till blastocyst stage. The number of 2-cell-stage embryos cultured and number of blastocyst obtained were used to calculate blastocyst rate and hatching rate.
Total cell number and apoptotic index in blastocyst
The total cell number and DNA integrity in the blastocyst were analyzed by terminal deoxyribonucleotidyl transferase–mediated dUTP (Deoxyuridine triphosphate) nick end labeling (TUNEL) assay as described earlier [21]. The blastocysts were fixed in 4% paraformaldehyde (PFA) and permeabilized in 0.5% Triton X-100 in 0.1% sodium citrate. Later, these blastocysts were incubated with TUNEL reaction mixture (Cat. No. 1215792910, Roche GmbH, Germany). Labeled blastocysts were counter-stained with nuclear stain 4′6-diamidino-2-phenylindole (DAPI, Cat. No. D9542, Sigma Aldrich, USA) and mounted on a slide using fluorescence mounting medium (Cat No. S3023, DAKO, USA). Total cell number and apoptotic nuclei (TMR red–positive nucleus) were scored for each blastocyst by observing under a fluorescent microscope (Imager-A1, Zeiss, Gottingen, Germany), and the apoptotic index (apoptotic cell/total cell number × 100) was calculated.
Fertility assessment of metformin-treated female mice and transgenerational changes
The metformin-treated female mice were mated with proven fertile males by housing them in a ratio of 1:1. The mating was confirmed by observing vaginal plug. The mating efficiency, pregnancy rate, and litter size were noted. The post-natal development was assessed by monitoring developmental milestones such as eye opening, fur development, body weight, and any other phenotypic anomalies.
Effect of metformin exposure on early embryo development: in vitro studies
A schematic representation of in vitro metformin treatment is depicted in Fig. 2. To understand the effect of metformin on the development of oocytes and embryos, these were cultured in medium containing different concentrations of metformin (0, 10, 25, 50, 100, 250, and 500 μg/mL). The concentrations were selected based on the earlier study [23].
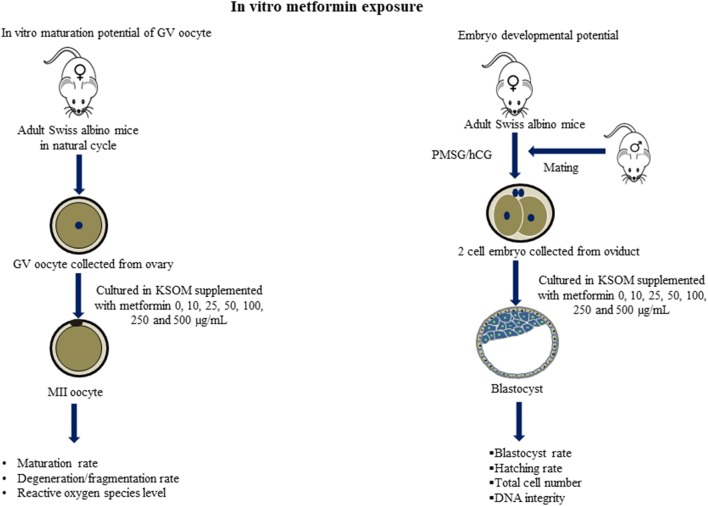
Fig. 2.
The study outline to understand the effect of in vitro exposure of metformin on oocyte maturation and early embryo development
GV oocyte collection and in vitro maturation
The ovaries were collected from mice in natural cycle (unstimulated ovary). The cortical region of the ovaries was gently teased using a fine needle to obtain germinal vesicle (GV) stage oocytes from the secondary/tertiary follicles. GV oocytes were further subjected to in vitro maturation by culturing in 20-μL droplets of Dulbecco’s modified Eagle’s medium (DMEM, Cat. No. D5648, Sigma Aldrich, USA) supplemented with 1% non-essential α-amino acids (Cat. No. M7145, Sigma Aldrich, USA), 1% insulin-transferrin-selenium (ITS, Cat. No. 51500–056, Gibco, USA), 0.05% pyruvate, and 0.3% bovine serum albumin (BSA). Oocytes were incubated at 37 °C in 5% CO2 for 24 h and then assessed for nuclear maturity. Oocytes with one polar body (PB) in perivitelline space were considered as mature (MII) oocytes.
Estimation of reactive oxygen species in MII oocytes by 2′,7′-dichlorofluorescin diacetate staining
MII oocytes were incubated with 10 μM 2′,7′-dichlorofluorescin diacetate (DCFDA) (Cat. No. D6883, Sigma Aldrich, USA) in culture medium (DMEM) droplet for 30 min at 37 °C and 5% CO2. The oocytes were washed 3 times with media and transferred to a clean microscopic slide immediately and visualized under a fluorescent microscope (Imager-A1, Zeiss, Gottingen, Germany). The images were grabbed for all the oocytes on the same day to avoid variations in fluorescence intensity using Q imaging (Micropublisher 5.0 RTV, Canada) by keeping constant exposure time. ROS intensity was measured using Q-capture software (Q Capture Pro 7, USA). The intensity corresponds to the ROS level in the oocytes and expressed as lum units.
Developmental potential of embryos following metformin exposure in vitro
Healthy female mice were superovulated by administering 5 IU PMSG and 10 IU hCG after a gap of 48 h and mated with proven fertile healthy male mice (10–12 weeks) overnight. Mating was confirmed by observing vaginal plug. At 36 h post-hCG, 2-cell-stage embryos were collected from the oviduct of female mice. The embryos were washed in KSOM medium droplets (50 μL) and cultured in media containing different concentrations of metformin (0, 10, 25, 50, 100, 250, and 500 μg/mL) till 4.5 dpc at 37 °C and 5% CO2. Embryos were scored at every 24 h interval by monitoring them under an inverted microscope (IX70, Olympus, Tokyo) fitted with a thermoregulated stage. The developmental potential of the embryos was assessed by calculating blastocyst rate and hatching rate.
Statistical analysis
All the data were presented as mean and standard error (mean ± SE) except for embryo and oocyte developmental parameters which were represented in percentage. The statistical analysis was carried out using one-way ANOVA followed by Tukey’s multiple comparison for the data which obey Gaussian distributions and Kruskal-Wallis test for the data which did not obey Gaussian distributions using GraphPad InStat 3.0 statistical package (GraphPad Inc., version 3.06, USA). The percentage values were analyzed between the groups by contingency table followed by Fisher’s exact test.
Results
Effect of metformin on body weight, organ weight, blood glucose level, and hormone level
Daily administration of metformin for 28 days did not alter the body weight in female mice at any of the doses injected. Mice from all the groups gained ~ 2 g at the end of injection period (4 weeks) at which stage animals were 12–14 weeks old. Blood glucose level, ovarian weight, and liver weight were not altered in any of the metformin-treated groups. However, a significant reduction was observed in weight of spleen (P < 0.05) in highest dose of metformin (200 mg/kg) compared with control. Interestingly, liver to spleen (L/S) ratio was increased in a dose-dependent manner where the highest dose group showed 1.7-fold increase compared with control. However, these changes were statistically non-significant. The level of reproductive hormones such as estradiol and testosterone were not altered by metformin treatment when compared with control (Table 1).
Table 1.
The effect of various doses of metformin on body weight, organ weight, blood glucose, and hormone level in Swiss albino mice
| Metformin dose (mg/kg) | Body weight gain (g) (n = 36) | Blood glucose (mg/dL) (n = 8) | Ovary weight (mg) (n = 32) | Spleen weight (mg) (n = 6) | Liver weight (g) (n = 6) | Liver/spleen ratio | Estradiol (pg/mL) (n = 5) | Testosterone (ng/mL) (n = 5) |
|---|---|---|---|---|---|---|---|---|
| 0 | 2.59 ± 0.24 | 122.63 ± 7.81 | 9.54 ± 0.54 | 188.53 ± 18.70 | 1.31 ± 0.72 | 6.96 | 19.51 ± 3.52 | 0.34 ± 0.05 |
| 50 | 2.63 ± 0.37 | 120.25 ± 4.31 | 10.16 ± 0.59 | 154.15 ± 15.30 | 1.11 ± 0.59 | 7.18 | 18.85 ± 2.56 | 0.25 ± 0.03 |
| 100 | 2.39 ± 0.27 | 119.63 ± 6.58 | 10.10 ± 0.63 | 140.52 ± 19.25 | 1.12 ± 0.45 | 8.02 | 16.06 ± 3.11 | 0.22 ± 0.04 |
| 200 | 2.83 ± 0.37 | 131.88 ± 5.49 | 9.36 ± 0.30 | 105.98 ± 8.76a | 1.26 ± 0.47 | 11.98 | 17.34 ± 3.65 | 0.20 ± 0.02 |
Data were expressed as mean ± SE. aP < 0.05 compared with the control group
Effect of metformin on ovarian reserve
Metformin treatment did not affect follicular reserve as indicated by similar number of primordial, primary, and secondary follicles in the control and metformin group (Table 2). Similarly, the number of ovulated MII oocytes retrieved from superovulated mice was not altered by metformin administration at all three doses compared with control (Fig. 3).
Table 2.
The effect of various doses of metformin on follicular reserve in Swiss albino mice (n = 9)
| Metformin dose (mg/kg) | Total number of follicles | Number of primordial follicles | Number of primary follicles | Number of secondary follicles |
|---|---|---|---|---|
| 0 | 197.56 ± 15.31 | 83.78 ± 8.03 | 50.78 ± 6.66 | 37.00 ± 5.50 |
| 50 | 197.22 ± 24.80 | 93.89 ± 8.98 | 49.89 ± 9.27 | 35.00 ± 6.30 |
| 100 | 206.33 ± 22.64 | 92.89 ± 14.09 | 53.78 ± 6.34 | 41.44 ± 7.17 |
| 200 | 189.67 ± 21.04 | 84.33 ± 10.48 | 47.67 ± 5.84 | 34.89 ± 5.52 |
Data were expressed as mean ± SE
Fig. 3.
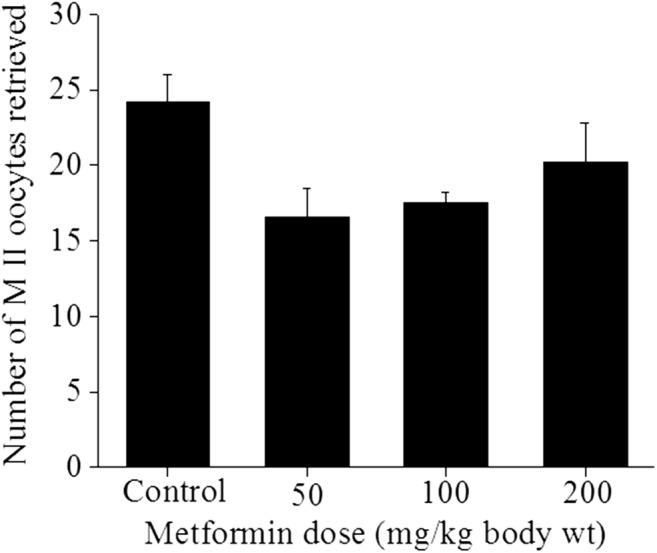
Effect of in vivo treatment of metformin on ovulation pattern. Swiss albino mice were treated with 50, 100, and 200 mg/kg body weight of metformin daily for 4 weeks. After the completion of the treatment, mice were superovulated with PMSG (5 IU) and hCG (10 IU). The OCCs were collected from the oviduct in KSOM media and denuded by incubating OCCs in KSOM media supplemented with hyaluronidase (0.1 mg/mL). MII oocytes were collected and the data was expressed as mean ± SE of MII oocytes retrieved per animal (n = 8)
Effect of in vitro exposure of metformin on oocyte maturation
The effect of metformin on the nuclear maturation of GV oocytes was assessed by subjecting them to in vitro maturation. Maturation rate in control oocytes was 55.7% which was not affected by metformin up to 500-μg/mL concentration. Significantly high percentage of oocyte degeneration was observed in 500 μg/mL concentration (P < 0.01) indicating that metformin at high dose has toxic effects on the oocytes (Fig. 4). At lower concentrations, metformin induced significant increase in the intracellular reactive oxygen species (ROS) level (10 to 50 μg/mL, P < 0.001, and 100 μg/mL, P < 0.01). At higher doses (250 and 500 μg/mL), there was no significant difference in the intracellular ROS level (Fig. 5).
Fig. 4.
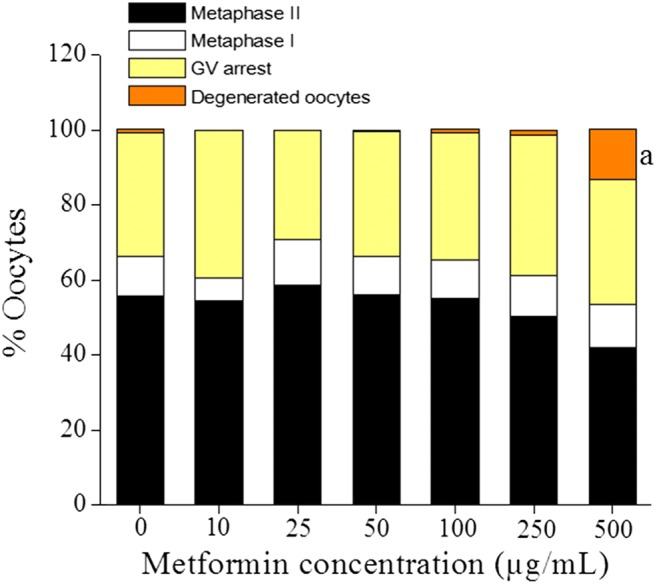
Effect of in vitro treatment of metformin on oocyte maturation. GV oocytes were retrieved from the ovary by gently teasing the cortical region of the ovary and cultured in DMEM media supplemented with 1% non-essential α-amino acids, 1% ITS, 0.05% pyruvate, 0.3% BSA, and different concentrations of metformin (0, 10, 25, 50, 100, 250, and 500 μg/mL). Oocytes were incubated at 37 °C and 5% CO2 for 24 h and the number of MII, MI, GV, and degenerated oocytes was recorded. The data was expressed as percentage (n = 269, 99, 99, 269, 269, 269, and 234 GV oocytes for 0, 10, 25, 50, 100, 250, and 500 μg/mL group respectively). aP < 0.01 compared with the control group
Fig. 5.
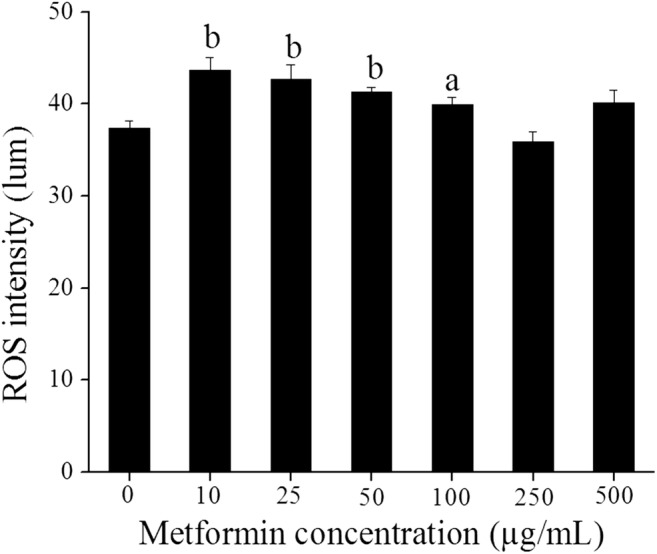
The effect of in vitro treatment of metformin on the ROS level in MII oocytes. MII oocytes obtained from post-IVM of GV oocytes exposed to different concentrations of metformin (0, 10, 25, 50, 100, 250, and 500 μg/mL) were incubated with 10 μM DCFDA in DMEM for 30 min at 37 °C and 5% CO2. The oocytes were washed and visualized under a fluorescent microscope. The images were captured and ROS intensity was measured using Q-capture software. The intensity corresponds to the ROS level in the oocytes and expressed as lum units. The data was represented as mean ± SE (n = 150, 54, 58, 151, 148, 135, and 98 MII oocytes for 0, 10, 25, 50, 100, 250, and 500 μg/mL group respectively). aP < 0.01, bP < 0.001 compared with the control group
Effect of metformin on preimplantation embryo development
The number of 2-cell-stage embryos retrieved from female mice administered with different doses of metformin was similar to that of the control group suggesting that metformin did not have any effect on ovulation and fertilization (Table 3). The incidence of fragmented oocytes was increased by 2-fold in the 200 mg/kg metformin group compared with control. On 3.5 dpc, the number of embryos that reached blastocyst stage was similar in the control and 50 mg/kg group. In the 100 mg/kg group, the blastocyst rate was non-significantly lower compared with control. However, a significant reduction (P < 0.01) in blastocyst rate was observed in the 200 mg/kg metformin group compared with control. On 4.5 dpc, the blastocyst rate was similar in the 50 and 100 mg/kg metformin group compared with control, while in the 200 mg/kg group, the embryos reaching blastocyst stage was significantly lower (P < 0.0001) compared with control with higher percentage of fragmentation or degeneration (P < 0.0001) rate. Similarly, the number of blastocysts hatched on 3.5 dpc was significantly lower in the 100 and 200 mg/kg group (P < 0.01). On 4.5 dpc, hatching rate was significantly lower only in 200 mg/kg (P < 0.01) which suggests slow development of embryos induced by metformin. In addition, the blastocysts from the 200 mg/kg group showed significantly lower total cell number (P < 0.01) than control. However, metformin treatment did not have any significant effect on the DNA integrity of the blastocysts.
Table 3.
The effect of various doses of metformin on developmental potential of 2-cell-stage embryos cultured in KSOM
| Metformin dose (mg/kg body weight) | 0 | 50 | 100 | 200 | |
| No. of females | 07 | 05 | 08 | 08 | |
| No. of 2-cell-stage embryos retrieved | 25.29 ± 4.05 | 24.00 ± 3.21 | 22.13 ± 3.96 | 30.63 ± 5.34 | |
| No. of unfertilized oocytes | 1.14 ± 0.86 | 1.00 ± 0.63 | 0.38 ± 0.26 | 0.63 ± 0.38 | |
| No. of fragmented oocytes | 1.43 ± 0.81 | 1.00 ± 1.00 | 1.63 ± 0.60 | 3.00 ± 1.20 | |
| No. of 2-cell-stage embryos cultured | 177 | 120 | 177 | 247 | |
| Morula rate (%) | 99.44 (176) | 100 (120) | 98.87 (175) | 93.12 (230) | |
| Blastocyst rate (%) | 3.5 dpc | 66.67 (118) | 71.67 (86) | 59.32 (105)e | 50.61 (125)b,g |
| 4.5 dpc | 98.31 (174) | 100 (120) | 97.18 (172) | 85.43 (211)c,h,k | |
| Hatching rate (%) | 3.5 dpc | 5.08 (9) | 4.17 (5) | 0.57 (1)a | 1.21 (3)a |
| 4.5 dpc | 48.59 (86) | 55.83 (67) | 44.07 (78) | 33.20 (82)b,h,i | |
| Fragmented embryos at 4.5 dpc (%) | 1.69 (3) | 0 | 2.26 (4) | 12.55 (31)c,h,k | |
| Total cell number | 108.12 ± 4.58 | 95.92 ± 2.42 | 95.28 ± 3.62 | 89.53 ± 2.45b | |
| Apoptotic index (%) | 2.51 ± 0.45 | 1.98 ± 0.37 | 3.18 ± 0.44 | 1.87 ± 0.27 | |
Data were expressed as mean ± SE and percentage. Data in parentheses represents the actual number of embryos at respective stages of development
aP < 0.05, bP < 0.01, cP < 0.0001 vs control
eP < 0.05, fP < 0.01, gP < 0.001, hP < 0.0001 vs the 50 mg/kg group
iP < 0.05, jP < 0.01, kP < 0.001 vs the 100 mg/kg group
Effect of in vitro exposure of metformin on the development potential of 2-cell-stage embryo
To confirm this observation made on effect of metformin on embryo development, we carried out an in vitro experiment where 2-cell-stage embryos (1.5 dpc) were exposed to various concentrations of metformin (10–500 μg/mL) and assessed for their developmental potential (Table 4). At 24 h later, there was no significant difference observed in metformin-treated embryos except in the highest concentration (500 μg/mL) in which there was a significant decrease in the embryos which progressed to 6–8 cell stage (P < 0.01). Similarly, at 48 h later (3.5 dpc), compaction rate was significantly lower in this concentration (P < 0.0001). In the rest of the concentration of metformin, even though the morula rate was not affected, reduction in blastocyst rate was observed. At and above 25 μg/mL concentration, the blastocyst rate decreased in a dose-dependent manner. However, a significant reduction (P < 0.001) was observed only in 250 and 500 μg/mL concentration. On 4.5 dpc, blastocyst rate was similar in lower concentrations of metformin (10–100 μg/mL) compared with control. In 250 μg/mL, only 7.69% (P < 0.0001) of the 2-cell embryos reached blastocyst stage, and in the 500 μg/mL group, none of the embryos developed to blastocyst (P < 0.0001) compared with control. Hatching rate was significantly decreased in 100 μg/mL (P < 0.01), 250 μg/mL (P < 0.001), and 500 μg/mL (0%, P < 0.001) concentration compared with control. The analysis of total cell number in the blastocyst revealed that the total cell number was decreased in 50 μg/mL and 100 μg/mL metformin concentration compared with control. However, values are statistically significant only in the 100 μg/mL (P < 0.01) group. TUNEL assay in blastocyst showed that metformin exposure did not affect the DNA integrity of the blastocysts in any of the concentrations tested.
Table 4.
The effect of in vitro exposure of various concentrations of metformin on developmental competence of 2-cell-stage embryos cultured in KSOM medium (24 embryos per group)
| Metformin concentration (μg/mL) | 2.5 dpc | 3.5 dpc | 4.5 dpc | ||||
|---|---|---|---|---|---|---|---|
| 6–8 cell rate (%) | Morula rate (%) | Blastocyst rate (%) | Blastocyst rate (%) | Hatching rate (%) | Total cell number | TUNEL index | |
| 0 | 95.83 (23) | 95.83 (23) | 70.83 (17) | 91.67 (22) | 29.17 (7) | 83.04 ± 3.78 | 5.72 ± 0.77 |
| 10 | 100 (24) | 100 (24) | 79.17 (19) | 95.83 (23) | 33.33 (8) | 90.09 ± 3.65 | 4.38 ± 0.76 |
| 25 | 95.83 (23) | 95.83 (23) | 50.00 (12) | 83.33 (20) | 12.50 (3) | 88.30 ± 4.18 | 7.37 ± 1.27 |
| 50 | 100 (24) | 100 (24) | 54.17 (13) | 95.83 (23) | 25.00 (6) | 71.82 ± 4.33 | 5.36 ± 1.63 |
| 100 | 100 (24) | 100 (24) | 41.67 (10) | 95.83 (23) | 8.33 (2)a | 53.61 ± 2.90a | 2.05 ± 0.89 |
| 250 | 100 (24) | 100 (24) | 7.69 (2)c | 7.69 (2)c | 0b | - | - |
| 500 | 37.50 (9)a | 25 (6)c | 0c | 0c | 0b | - | - |
The developmental potential data were expressed as percentage (%). The total cell number and TUNEL index were expressed as mean ± SE
aP < 0.01, bP < 0.001, cP < 0.0001 compared with the control group
Transgenerational changes induced by maternal exposure to metformin
The fertility potential of female mice treated with metformin was assessed by mating with healthy fertile males and mating was confirmed by the presence of vaginal plug. Compared with control (86%), mating efficiency was not altered significantly in the 50 mg/kg (71%), 100 mg/kg (86%), and 200 mg/kg (100%) metformin-treated group. The litter size in metformin-treated mice was also similar to that of control. There was no significant difference in the post-natal development of litters born to metformin-treated mothers with respect to survival and other developmental milestones such as fur development and eye opening. The litters born to mothers treated with 200 mg/kg of metformin showed decrease in body weight at 4 weeks compared with other groups. However, when they reached 8 weeks, the weight was similar to that of control (Table 5).
Table 5.
Effect of various doses of metformin on the reproductive potential of female mice and the post-natal development in progenies
| Metformin (mg/kg) | Mating efficiency (%) | Total number of litters | Average litter size | Body weight of progenies (g) | |||
|---|---|---|---|---|---|---|---|
| Parturition | 2 weeks | 4 weeks | 8 weeks | ||||
| 0 | 86 | 93 | 8.50 ± 0.40 | 1.49 ± 0.01 | 5.92 ± 0.11 | 13.21 ± 0.30 | 23.60 ± 0.40 |
| 50 | 71 | 85 | 8.50 ± 0.45 | 1.43 ± 0.02 | 6.24 ± 0.09 | 14.67 ± 0.31a | 24.15 ± 0.43 |
| 100 | 86 | 99 | 9.00 ± 0.41 | 1.43 ± 0.01a | 6.23 ± 0.11 | 13.63 ± 0.31 | 24.81 ± 0.40 |
| 200 | 100 | 93 | 8.38 ± 0.43 | 1.46 ± 0.02 | 6.38 ± 0.11b | 11.75 ± 0.41a,c,d | 25.08 ± 0.47 |
The data were expressed as mean ± SE [n = 12 females and 12 males (1:1 ratio)] for all parameter except for mating efficiency which is expressed in percentage
aP < 0.05, bP < 0.01 compared with control; cP < 0.001 compared with 50 mg/kg; dP < 0.001 compared with 100 mg/kg
Discussion
In the present study, we observed that the daily administration of metformin at 50, 100, and 200 mg/kg for 4 weeks did not alter the body weight and the blood glucose level. Our finding agrees with a previous report [24] which also demonstrated that metformin administration in healthy subjects did not alter glucose but decreased the post-prandial insulin secretion. Earlier studies have reported that the plasma level of metformin is highly variable in humans ranging from 54 to 4133 ng/mL which varies with the individual and dose of metformin administered [25]. Since the half-life of the compound is approximately 5 h [26], frequent administration of metformin is essential for maintaining the threshold level in blood to achieve reduction in blood glucose. In our study, daily administration of metformin did not cause any change in the liver weight but spleen weight was found to be decreased. Metformin administration to normal mice at high dose can induce metabolic stress which could be the reason for decreased spleen weight. Liver/spleen ratio is known to change in several pathological conditions such as liver cirrhosis [27] and biliary cirrhosis [28]. We observed a dose-dependent increase in liver/spleen (L/S) ratio post-metformin treatment. Similar finding was observed in an earlier study in which metformin increased liver to spleen (L/S) ratio in patients with non-alcoholic fatty liver disease [29]. This may be due to the anti-inflammatory function of metformin which is already reported. Even though there are several mechanisms by which metformin modulates immune response in auto-immune disorder [30, 31] and cancer condition [32, 33], altering the gut microbiome is considered to be more predominant [34].
The administration of metformin did not affect ovulation, follicle reserve, and endocrine profile. Metformin has shown to decrease the androgen production by thecal [6] and granulosa cells [35] due to decreased ATP and activation of AMPK which in turn inhibit anabolic process like steroidogenesis. Furthermore, a systemic review and meta-analysis revealed that metformin increased SHBG level leading to the further reduction in free testosterone [36]. Earlier studies have shown that metformin did not alter ovulation rate in women with PCOS [37] whereas other studies have reported adverse effects on nuclear maturation in porcine and bovine oocytes [14, 15, 38]. In our study, in vivo exposure of metformin did not alter the number of ovulated oocytes following priming with PMSG and hCG. It has been shown that metformin causes arrest of bovine oocytes in germinal vesicle stage during in vitro maturation through activation of AMPK [14]. AMPK is a master regulatory protein which is generally activated when there is any stress in the form of elevated oxidative stress [39, 40] and increased AMP/ATP ratio [41]. However, other study has demonstrated that the inhibitory effect of metformin on nuclear maturation during IVM of porcine oocytes is not mediated through activation of AMPK [15]. In this study, Bilodeau-Goeseels et al. observed that compound C was not able to reverse the negative effect of metformin on oocyte maturation. In vitro exposure data on oocyte maturation in our study indicates that at higher doses of metformin, the nuclear maturation is inhibited with higher incidence of oocyte degeneration and higher ROS level which might have resulted in activation of AMPK as reported in literature [40, 42].
Effect of metformin on oocyte and embryo development is contradictory. The major effect of in vivo administration of metformin was observed on the early embryo development where metformin significantly hindered blastocyst formation and decreased the hatching potential. An earlier study has demonstrated the presence of metformin transporters such as organic cation transporters (OCT) and multidrug and toxin extrusion proteins (MATE) in the endometrial epithelium of humans and rats suggesting the exposure of metformin to the embryos [43]. Our in vitro metformin exposure data agrees with an earlier study, where blastocyst rate was decreased drastically when frozen thawed 2-cell-stage embryos were exposed to metformin in vitro at a concentration of 100 μg/mL [23]. On the contrary, an in vitro study has used low concentrations of metformin ranging from 10−6 to 10−12 M [44] which are lower than what we used in the study, and these concentrations of metformin can inhibit the secretion of estradiol, progesterone, and androgen by human granulosa and thecal cells, whereas another study has claimed that metformin did not affect the development of embryos due to the lack of metformin transporter and unaltered AMPK level [45]. In vitro exposure to metformin revealed that 2-cell embryos were highly susceptible to metformin which is in agreement with a previous study where they compared 2-cell versus blastocyst exposure [46]. However, it was not clear whether similar degree of sensitivity is expressed at all stages of pre-compaction development. To understand this aspect, we assessed the effect of metformin on different stages of development (supplementary Table 1). The blastocyst forming potential was lowest in embryos exposed to metformin at 2-cell stage, and the sensitivity gradually decreased with their progression to 4, 6–8-cell, and morula stages. Even at the lowest concentration, metformin significantly decreased the blastocyst rate in embryos exposed at 2-cell stage suggesting its extreme sensitivity to metformin. These observations provide clues on possible shift in their sensitivity to metformin post-embryonic genome activation. The adverse effect of metformin on embryo development could also be due to the direct inhibitory action of metformin on ETC which in turn affects the production of ATP [2, 3]. In addition, activation of AMPK is known to alter the expression of gap junction proteins which regulate the key events during compaction [16] and subsequent formation of blastocyst. This could be the reason for the arresting embryos at compaction stage post-exposure to metformin which is an AMPK activator. In addition, an earlier study has reported the reduction in stemness, proliferation, and metabolic pattern in mouse embryos exposed to metformin [17] which could be another cause for the developmental arrest of embryo.
The total cell number in blastocyst determines the quality of the blastocyst and is decreased at high-dose metformin indicating that high concentration of metformin affects the embryonic cell division. Further, delay in development of embryos exposed to metformin indicates its possible effect on cell cycle progression. Several studies done on tumor cells [47–49] and on keratinocytes [50] have shown that metformin induces G0/G1 cell cycle arrest and apoptosis and affects the proliferation. Since there are no reports regarding the effect of metformin on cell cycle during embryo development, the further studies need to be carried out in this direction.
Metformin freely crosses the placental barrier exposing a developing fetus to therapeutic concentration of the drug [51]. An earlier study revealed that metformin decreases the circulating insulin in women with PCOS [52] and insulin is known to inhibit hepatic SHBG synthesis [53]. Therefore, metformin-mediated increase in SHBG can cause reduction in testosterone and AMH affecting sexual differentiation and early gonadal development. In our study, we did not observe any change in pregnancy rate, litter size, and developmental anomalies in the progenies born. Earlier follow-up studies have shown that the children born to mothers with PCOS treated with metformin exhibited increased BMI, obesity, and altered metabolism at 4 and 9 years of age [54, 55]. However, body weight was decreased in litters at 4 weeks of post-natal life but it was comparable at 8 weeks when compared with control.
In conclusion, from the present study, it appears that metformin is a safe drug as far as ovarian function is concerned. However, the early embryo development seems to be severely affected especially at higher dose/concentration of metformin used in the present study (Fig. 6). The major limitation of the study is that we have not assessed the level of metformin in the blood or in the oviduct of metformin-injected mice which could have given us a hint about the appropriate concentration of metformin to be used for embryo toxicity assessment. The doses used for this study was based on an earlier report where the human embryos were exposed to metformin in the range of 10 to 100 μg/mL [23]. A systematic review has shown that the therapeutic plasma concentration can be as high as 90 μg/mL [56]. On the contrary, majority of the studies report much lower concentration of metformin in serum [25, 57, 58]. Even though our study demonstrates embryotoxic effect of metformin at higher doses, one may argue that the lowest dose used in our in vitro study (10 μg/mL) is much higher than the highest serum concentration reported in the literature. Therefore, further studies are essential to draw conclusive evidence to confirm that metformin at the therapeutic dose has embryo toxic effects. Future studies on assessing the possible effects of metformin on the function of key metabolic enzymes involved in early embryo metabolism, expression of gap junction proteins during early embryo development, and whether disruption of AMPK-mediated signaling cascades caused by metformin can be reversed by using AMPK inhibitor such as compound C can be important research avenues to explore. Therefore, even though metformin is considered to be safe and used extensively in treating various human disorders, the results obtained from the present study draw attention about using it in fertility treatments routinely. This warrants further extensive research to establish the safety of the drug, especially during the early embryo development and fetal gonadogenesis.
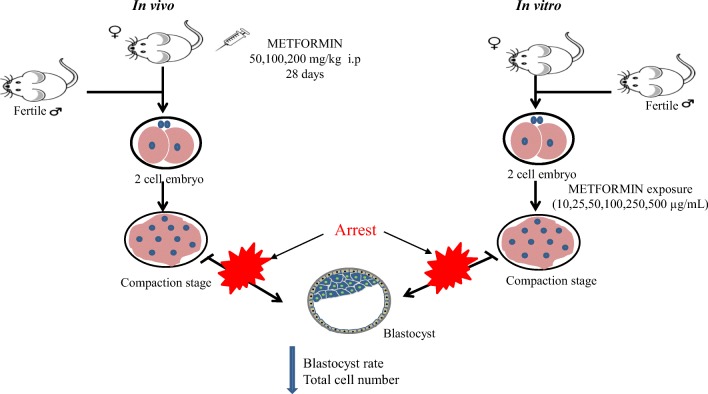
Fig. 6.
Schematic representation indicating conclusion of the study. The metformin exposure at higher concentration both in vivo and in vitro in mice revealed significant adverse effects on the early embryo development by decreasing blastocyst rate and also arresting embryo at compaction stage
Electronic supplementary material
(DOCX 14.4 kb)
Author’s contribution
GN and SRS contributed equally to the manuscript. GN, SRS, PA, PSP, AR, and SK performed experiments; SGK performed histological studies and edited the manuscript; ABS and SM helped in in vitro studies and statistical evaluation of data; SKA edited the manuscript; GK conceptualized and designed the study.
Funding information
This study was funded by Science and Engineering Research Board (SERB), Department of Science and Technology, Government of India, Grant. No. EMR/2016/002077.
Compliance with ethical standards
Animal handling and experimental procedures were done according to the institutional guidelines, and the study protocols were approved by the Institutional Animal Ethics Committee of Kasturba Medical College (IAEC/KMC/25/2014).
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Grzybowska M, Bober J, Olszewska M. Metformin - mechanisms of action and use for the treatment of type 2 diabetes mellitus. Postepy Hig Med Dosw. 2011;65:277–285. doi: 10.5604/17322693.941655. [DOI] [PubMed] [Google Scholar]
- 2.El-Mir MY, Nogueira V, Fontaine E, Averet N, Rigoulet M, Leverve X. Dimethylbiguanide inhibits cell respiration via an indirect effect targeted on the respiratory chain complex I. J Biol Chem. 2000;275:223–228. doi: 10.1074/jbc.275.1.223. [DOI] [PubMed] [Google Scholar]
- 3.Stephenne X, Foretz M, Taleux N, van der Zon GC, Sokal E, Hue L, et al. Metformin activates AMP-activated protein kinase in primary human hepatocytes by decreasing cellular energy status. Diabetologia. 2011;54:3101–3110. doi: 10.1007/s00125-011-2311-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Foretz M, Guigas B, Bertrand L, Pollak M, Viollet B. Metformin: from mechanisms of action to therapies. Cell Metab. 2014;20:953–966. doi: 10.1016/j.cmet.2014.09.018. [DOI] [PubMed] [Google Scholar]
- 5.Sirmans SM, Pate KA. Epidemiology, diagnosis, and management of polycystic ovary syndrome. Clin Epidemiol. 2013;6:1–13. doi: 10.2147/CLEP.S37559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Attia GR, Rainey WE, Carr BR. Metformin directly inhibits androgen production in human thecal cells. Fertil Steril. 2001;76:517–524. doi: 10.1016/s0015-0282(01)01975-6. [DOI] [PubMed] [Google Scholar]
- 7.Hirsch A, Hahn D, Kempna P, Hofer G, Nuoffer JM, Mullis PE, et al. Metformin inhibits human androgen production by regulating steroidogenic enzymes HSD3B2 and CYP17A1 and complex I activity of the respiratory chain. Endocrinology. 2012;153:4354–4366. doi: 10.1210/en.2012-1145. [DOI] [PubMed] [Google Scholar]
- 8.De Leo V, Musacchio MC, Piomboni P, Di Sabatino A, Morgante G. The administration of metformin during pregnancy reduces polycystic ovary syndrome related gestational complications. Eur J Obstet Gynecol Reprod Biol. 2011;157:63–66. doi: 10.1016/j.ejogrb.2011.03.024. [DOI] [PubMed] [Google Scholar]
- 9.Glueck CJ, Goldenberg N, Wang P, Loftspring M, Sherman A. Metformin during pregnancy reduces insulin, insulin resistance, insulin secretion, weight, testosterone and development of gestational diabetes: prospective longitudinal assessment of women with polycystic ovary syndrome from preconception throughout pregnancy. Hum Reprod. 2004;19:510–521. doi: 10.1093/humrep/deh109. [DOI] [PubMed] [Google Scholar]
- 10.Nawaz FH, Khalid R, Naru T, Rizvi J. Does continuous use of metformin throughout pregnancy improve pregnancy outcomes in women with polycystic ovarian syndrome? J Obstet Gynaecol Res. 2008;34:832–837. doi: 10.1111/j.1447-0756.2008.00856.x. [DOI] [PubMed] [Google Scholar]
- 11.Tso LO, Costello MF, Albuquerque LE, Andriolo RB, Macedo CR. Metformin treatment before and during IVF or ICSI in women with polycystic ovary syndrome. Cochrane Database Syst Rev. 2014:CD006105. [DOI] [PMC free article] [PubMed]
- 12.Abdalmageed OS, Farghaly TA, Abdelaleem AA, Abdelmagied AE, Ali MK, Abbas AM. Impact of metformin on IVF outcomes in overweight and obese women with polycystic ovary syndrome: a randomized double-blind controlled trial. Reprod Sci. 2018;1933719118765985. [DOI] [PubMed]
- 13.Dumollard R, Carroll J, Duchen MR, Campbell K, Swann K. Mitochondrial function and redox state in mammalian embryos. Semin Cell Dev Biol. 2009;20:346–353. doi: 10.1016/j.semcdb.2008.12.013. [DOI] [PubMed] [Google Scholar]
- 14.Tosca L, Uzbekova S, Chabrolle C, Dupont J. Possible role of 5′AMP-activated protein kinase in the metformin-mediated arrest of bovine oocytes at the germinal vesicle stage during in vitro maturation. Biol Reprod. 2007;77:452–465. doi: 10.1095/biolreprod.107.060848. [DOI] [PubMed] [Google Scholar]
- 15.Bilodeau-Goeseels S, Magyara N, Collignon C. Characterization of the effects of metformin on porcine oocyte meiosis and on AMP-activated protein kinase activation in oocytes and cumulus cells. Zygote. 2014;22:275–285. doi: 10.1017/S0967199413000075. [DOI] [PubMed] [Google Scholar]
- 16.Calder MD, Edwards NA, Betts DH, Watson AJ. Treatment with AICAR inhibits blastocyst development, trophectoderm differentiation and tight junction formation and function in mice. Mol Hum Reprod. 2017;23:771–785. doi: 10.1093/molehr/gax050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bolnick A, Abdulhasan M, Kilburn B, Xie Y, Howard M, Andresen P, et al. Commonly used fertility drugs, a diet supplement, and stress force AMPK-dependent block of stemness and development in cultured mammalian embryos. J Assist Reprod Genet. 2016;33:1027–1039. doi: 10.1007/s10815-016-0735-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nair AB, Jacob S. A simple practice guide for dose conversion between animals and human. J Basic Clin Pharm. 2016;7:27–31. doi: 10.4103/0976-0105.177703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Han X, Tao YL, Deng YP, Yu JW, Cai J, Ren GF, et al. Metformin ameliorates insulitis in STZ-induced diabetic mice. PeerJ. 2017;5:e3155. doi: 10.7717/peerj.3155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Anisimov VN, Piskunova TS, Popovich IG, Zabezhinski MA, Tyndyk ML, Egormin PA, Yurova MV, Rosenfeld SV, Semenchenko AV, Kovalenko IG, Poroshina TE, Berstein LM. Gender differences in metformin effect on aging, life span and spontaneous tumorigenesis in 129/Sv mice. Aging (Albany NY) 2010;2:945–958. doi: 10.18632/aging.100245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nair R, Singh VJ, Salian SR, Kalthur SG, D’Souza AS, Shetty PK, Mutalik S, Kalthur G, Adiga SK. Methyl parathion inhibits the nuclear maturation, decreases the cytoplasmic quality in oocytes and alters the developmental potential of embryos of Swiss albino mice. Toxicol Appl Pharmacol. 2014;279:338–350. doi: 10.1016/j.taap.2014.07.004. [DOI] [PubMed] [Google Scholar]
- 22.Kalthur G, Salian SR, Nair R, Mathew J, Adiga SK, Kalthur SG, et al. Distribution pattern of cytoplasmic organelles, spindle integrity, oxidative stress, octamer-binding transcription factor 4 (Oct4) expression and developmental potential of oocytes following multiple superovulation. Reprod Fertil Dev. 2015. [DOI] [PubMed]
- 23.Bedaiwy MA, Miller KF, Goldberg JM, Nelson D, Falcone T. Effect of metformin on mouse embryo development. Fertil Steril. 2001;76:1078–1079. doi: 10.1016/s0015-0282(01)02825-4. [DOI] [PubMed] [Google Scholar]
- 24.Sambol NC, Chiang J, O’Conner M, Liu CY, Lin ET, Goodman AM, et al. Pharmacokinetics and pharmacodynamics of metformin in healthy subjects and patients with noninsulin-dependent diabetes mellitus. J Clin Pharmacol. 1996;36:1012–1021. doi: 10.1177/009127009603601105. [DOI] [PubMed] [Google Scholar]
- 25.Christensen MM, Brasch-Andersen C, Green H, Nielsen F, Damkier P, Beck-Nielsen H, et al. The pharmacogenetics of metformin and its impact on plasma metformin steady-state levels and glycosylated hemoglobin A1c. Pharmacogenet Genomics. 2011;21:837–850. doi: 10.1097/FPC.0b013e32834c0010. [DOI] [PubMed] [Google Scholar]
- 26.Graham GG, Punt J, Arora M, Day RO, Doogue MP, Duong JK, Furlong TJ, Greenfield JR, Greenup LC, Kirkpatrick CM, Ray JE, Timmins P, Williams KM. Clinical pharmacokinetics of metformin. Clin Pharmacokinet. 2011;50:81–98. doi: 10.2165/11534750-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 27.Chen XL, Chen TW, Zhang XM, Li ZL, Zeng NL, Li T, et al. Quantitative assessment of the presence and severity of cirrhosis in patients with hepatitis B using right liver lobe volume and spleen size measured at magnetic resonance imaging. PLoS One. 2014;9:e89973. doi: 10.1371/journal.pone.0089973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Murata Y, Abe M, Hiasa Y, Azemoto N, Kumagi T, Furukawa S, Matsuura B, Michitaka K, Horiike N, Onji M. Liver/spleen volume ratio as a predictor of prognosis in primary biliary cirrhosis. J Gastroenterol. 2008;43:632–636. doi: 10.1007/s00535-008-2202-9. [DOI] [PubMed] [Google Scholar]
- 29.Yabiku K, Mutoh A, Miyagi K, Takasu N. Effects of oral antidiabetic drugs on changes in the liver-to-spleen ratio on computed tomography and inflammatory biomarkers in patients with type 2 diabetes and nonalcoholic fatty liver disease. Clin Ther. 2017;39:558–566. doi: 10.1016/j.clinthera.2017.01.015. [DOI] [PubMed] [Google Scholar]
- 30.Lee SY, Moon SJ, Kim EK, Seo HB, Yang EJ, Son HJ, et al. Metformin suppresses systemic autoimmunity in Roquin(san/san) mice through inhibiting B cell differentiation into plasma cells via regulation of AMPK/mTOR/STAT3. J Immunol. 2017;198:2661–2670. doi: 10.4049/jimmunol.1403088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kim EK, Lee SH, Lee SY, Kim JK, Jhun JY, Na HS, et al. Metformin ameliorates experimental-obesity-associated autoimmune arthritis by inducing FGF21 expression and brown adipocyte differentiation. Exp Mol Med. 2018;50:e432. doi: 10.1038/emm.2017.245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schuiveling M, Vazirpanah N, Radstake T, Zimmermann M, Broen JCA. Metformin, a new era for an old drug in the treatment of immune mediated disease? Curr Drug Targets. 2018;19:945–959. doi: 10.2174/1389450118666170613081730. [DOI] [PubMed] [Google Scholar]
- 33.Pereira FV, Melo ACL, Low JS, de Castro IA, Braga TT, Almeida DC, Batista de Lima AGU, Hiyane MI, Correa-Costa M, Andrade-Oliveira V, Origassa CST, Pereira RM, Kaech SM, Rodrigues EG, Câmara NOS. Metformin exerts antitumor activity via induction of multiple death pathways in tumor cells and activation of a protective immune response. Oncotarget. 2018;9:25808–25825. doi: 10.18632/oncotarget.25380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ma W, Chen J, Meng Y, Yang J, Cui Q, Zhou Y. Metformin alters gut microbiota of healthy mice: implication for its potential role in gut microbiota homeostasis. Front Microbiol. 2018;9:1336. doi: 10.3389/fmicb.2018.01336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tosca L, Chabrolle C, Uzbekova S, Dupont J. Effects of metformin on bovine granulosa cells steroidogenesis: possible involvement of adenosine 5′ monophosphate-activated protein kinase (AMPK) Biol Reprod. 2007;76:368–378. doi: 10.1095/biolreprod.106.055749. [DOI] [PubMed] [Google Scholar]
- 36.Barba M, Schunemann HJ, Sperati F, Akl EA, Musicco F, Guyatt G, et al. The effects of metformin on endogenous androgens and SHBG in women: a systematic review and meta-analysis. Clin Endocrinol. 2009;70:661–670. doi: 10.1111/j.1365-2265.2008.03459.x. [DOI] [PubMed] [Google Scholar]
- 37.Ng EH, Wat NM, Ho PC. Effects of metformin on ovulation rate, hormonal and metabolic profiles in women with clomiphene-resistant polycystic ovaries: a randomized, double-blinded placebo-controlled trial. Hum Reprod. 2001;16:1625–1631. doi: 10.1093/humrep/16.8.1625. [DOI] [PubMed] [Google Scholar]
- 38.Mayes MA, Laforest MF, Guillemette C, Gilchrist RB, Richard FJ. Adenosine 5′-monophosphate kinase-activated protein kinase (PRKA) activators delay meiotic resumption in porcine oocytes. Biol Reprod. 2007;76:589–597. doi: 10.1095/biolreprod.106.057828. [DOI] [PubMed] [Google Scholar]
- 39.Han X, Tai H, Wang X, Wang Z, Zhou J, Wei X, Ding Y, Gong H, Mo C, Zhang J, Qin J, Ma Y, Huang N, Xiang R, Xiao H. AMPK activation protects cells from oxidative stress-induced senescence via autophagic flux restoration and intracellular NAD(+) elevation. Aging Cell. 2016;15:416–427. doi: 10.1111/acel.12446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hinchy EC, Gruszczyk AV, Willows R, Navaratnam N, Hall AR, Bates G, et al. Mitochondria-derived ROS activate AMP-activated protein kinase (AMPK) indirectly. J Biol Chem. 2018;293:17208–17217. doi: 10.1074/jbc.RA118.002579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Auciello FR, Ross FA, Ikematsu N, Hardie DG. Oxidative stress activates AMPK in cultured cells primarily by increasing cellular AMP and/or ADP. FEBS Lett. 2014;588:3361–3366. doi: 10.1016/j.febslet.2014.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rabinovitch RC, Samborska B, Faubert B, Ma EH, Gravel SP, Andrzejewski S, Raissi TC, Pause A, St-Pierre J, Jones RG. AMPK maintains cellular metabolic homeostasis through regulation of mitochondrial reactive oxygen species. Cell Rep. 2017;21:1–9. doi: 10.1016/j.celrep.2017.09.026. [DOI] [PubMed] [Google Scholar]
- 43.Shao R, Li X, Feng Y, Lin JF, Billig H. Direct effects of metformin in the endometrium: a hypothetical mechanism for the treatment of women with PCOS and endometrial carcinoma. J Exp Clin Cancer Res. 2014;33:41. doi: 10.1186/1756-9966-33-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mansfield R, Galea R, Brincat M, Hole D, Mason H. Metformin has direct effects on human ovarian steroidogenesis. Fertil Steril. 2003;79:956–962. doi: 10.1016/s0015-0282(02)04925-7. [DOI] [PubMed] [Google Scholar]
- 45.Lee HY, Wei D, Loeken MR. Lack of metformin effect on mouse embryo AMPK activity: implications for metformin treatment during pregnancy. Diabetes Metab Res Rev. 2014;30:23–30. doi: 10.1002/dmrr.2451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bolnick A, Abdulhasan M, Kilburn B, Xie Y, Howard M, Andresen P, Shamir AM, Dai J, Puscheck EE, Secor E, Rappolee DA. Two-cell embryos are more sensitive than blastocysts to AMPK-dependent suppression of anabolism and stemness by commonly used fertility drugs, a diet supplement, and stress. J Assist Reprod Genet. 2017;34:1609–1617. doi: 10.1007/s10815-017-1028-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wang Y, Xu W, Yan Z, Zhao W, Mi J, Li J, et al. Metformin induces autophagy and G0/G1 phase cell cycle arrest in myeloma by targeting the AMPK/mTORC1 and mTORC2 pathways. J Exp Clin Cancer Res. 2018;37:63. doi: 10.1186/s13046-018-0731-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Cai X, Hu X, Tan X, Cheng W, Wang Q, Chen X, et al. Metformin induced AMPK activation, G0/G1 phase cell cycle arrest and the inhibition of growth of esophageal squamous cell carcinomas in vitro and in vivo. PLoS One. 2015;10:e0133349. doi: 10.1371/journal.pone.0133349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Jin DH, Kim Y, Lee BB, Han J, Kim HK, Shim YM, Kim DH. Metformin induces cell cycle arrest at the G1 phase through E2F8 suppression in lung cancer cells. Oncotarget. 2017;8:101509–101519. doi: 10.18632/oncotarget.21552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ochoa-Gonzalez F, Cervantes-Villagrana AR, Fernandez-Ruiz JC, Nava-Ramirez HS, Hernandez-Correa AC, Enciso-Moreno JA, et al. Correction: metformin induces cell cycle arrest, reduced proliferation, wound healing impairment in vivo and is associated to clinical outcomes in diabetic foot ulcer patients. PLoS One. 2016;11:e0159468. doi: 10.1371/journal.pone.0150900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Vanky E, Zahlsen K, Spigset O, Carlsen SM. Placental passage of metformin in women with polycystic ovary syndrome. Fertil Steril. 2005;83:1575–1578. doi: 10.1016/j.fertnstert.2004.11.051. [DOI] [PubMed] [Google Scholar]
- 52.Mathur R, Alexander CJ, Yano J, Trivax B, Azziz R. Use of metformin in polycystic ovary syndrome. Am J Obstet Gynecol. 2008;199:596–609. doi: 10.1016/j.ajog.2008.09.010. [DOI] [PubMed] [Google Scholar]
- 53.Katz E, Ricciarelli E, Adashi EY. The potential relevance of growth hormone to female reproductive physiology and pathophysiology. Fertil Steril. 1993;59:8–34. doi: 10.1016/s0015-0282(16)55610-6. [DOI] [PubMed] [Google Scholar]
- 54.Rowan JA, Rush EC, Plank LD, Lu J, Obolonkin V, Coat S, et al. Metformin in gestational diabetes: the offspring follow-up (MiG TOFU): body composition and metabolic outcomes at 7-9 years of age. BMJ Open Diabetes Res Care. 2018;6:e000456. doi: 10.1136/bmjdrc-2017-000456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hanem LGE, Stridsklev S, Juliusson PB, Salvesen O, Roelants M, Carlsen SM, et al. Metformin use in PCOS pregnancies increases the risk of offspring overweight at 4 years of age: follow-up of two RCTs. J Clin Endocrinol Metab. 2018;103:1612–1621. doi: 10.1210/jc.2017-02419. [DOI] [PubMed] [Google Scholar]
- 56.Kajbaf F, De Broe ME, Lalau J-D. Therapeutic concentrations of metformin: a systematic review. Clin Pharmacokinet. 2016;55:439–459. doi: 10.1007/s40262-015-0323-x. [DOI] [PubMed] [Google Scholar]
- 57.Lalau J-D, Lemaire-Hurtel A-S, Lacroix C. Establishment of a database of metformin plasma concentrations and erythrocyte levels in normal and emergency situations. Clin Drug Investig. 2011;31:435–438. doi: 10.2165/11588310-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 58.Hess C, Unger M, Madea B, Stratmann B, Tschoepe D. Range of therapeutic metformin concentrations in clinical blood samples and comparison to a forensic case with death due to lactic acidosis. Forensic Sci Int. 2018;286:106–112. doi: 10.1016/j.forsciint.2018.03.003. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX 14.4 kb)