Abstract
Objectives
We assessed comorbidity burden in people with RA at diagnosis and early disease (3 years) and its association with early mortality and joint destruction. The association between lung disease and mortality in RA is not well studied; we also explored this relationship.
Methods
From a contemporary UK-based population (n = 1, 475 762) we identified a cohort with incident RA (n = 6591). The prevalence of comorbidities at diagnosis of RA and at 3 years was compared with age- and gender-matched controls (n = 6591). In individuals with RA we assessed the prognostic value of the Charlson Comorbidity Index and Rheumatic Disease Comorbidity Index calculated at diagnosis for all-cause mortality and joint destruction (with joint surgery as a surrogate marker). We separately evaluated the association between individual lung diseases [chronic obstructive pulmonary disease (COPD), asthma and interstitial lung disease] and mortality.
Results
Respiratory disease, cardiovascular disease, stroke, diabetes, previous fracture and depression were more common (P < 0.05) in patients with RA at diagnosis than controls. Comorbidity (assessed using RDCI) was associated with all-cause mortality in RA [adjusted hazard ratio (HR) 1.26, 95% CI 1.00–1.60]. There was no association with joint destruction. COPD, but not asthma, was associated with mortality (COPD HR 2.84, 95% CI 1.13–7.12).
Conclusion
There is an excess burden of comorbidity at diagnosis of RA including COPD, asthma and interstitial lung disease. COPD is a major predictor of early mortality in early RA. Early assessment of comorbidity including lung disease should form part of the routine management of RA patients.
Keywords: rheumatoid arthritis, mortality, joint damage, comorbidity, chronic obstructive pulmonary disease, asthma, cardiovascular diseases
Rheumatology key messages
Comorbidities, including respiratory and cardiovascular disease, are disproportionately common in patients with newly diagnosed RA.
Individuals with RA accumulate comorbidities more rapidly than controls in the 3 years after diagnosis.
Chronic obstructive pulmonary disease is a major predictor of mortality in early rheumatoid arthritis.
Introduction
The long-term prognosis of RA in terms of both function and mortality has improved with the availability of disease-modifying antirheumatic drugs (DMARDs) [1, 2], and a ‘treat to target’ approach accompanied by improved monitoring [3]. Mortality in people with RA remains higher than in the general population, with cross-sectional data from the UK suggesting that deaths from cancer, respiratory and cardiovascular causes are all increased [4, 5]. In the Early RA Study that recruited consecutive patients diagnosed with RA from nine centres in England, a modest increase in mortality was observed mainly in the first 7 years following diagnosis, although this was before the widespread introduction of biologic treatments [6]. Cause of death was similar to the general population with the exception of increases in infections, non-Hodgkin’s lymphoma, pulmonary fibrosis and cardiovascular-related deaths. While the association between RA and cardiovascular events has been studied extensively [7], there is a relative paucity of good quality epidemiological evidence quantifying the contribution of respiratory conditions on the outcomes of people with RA.
This study had two main objectives: firstly, to describe the burden of important comorbidities at diagnosis of RA compared with people without RA, and then after 3 years duration of RA, in a contemporary primary care-based population; and secondly, to evaluate the association of comorbidity using the Charlson Comorbidity Index (CCI) and Rheumatic Disease Comorbidity Index (RDCI) at diagnosis with all-cause mortality and disease progression in people with RA [8, 9], as well as the association of individual chronic lung conditions with the same outcomes.
Comorbidity indices are tools used to quantify the total burden of comorbidity based on specific conditions and have clinical applications in identifying patients with worse prognosis in their functional status, health-related quality of life, hospitalization frequency and mortality. Several generic indices have been proposed to weight specific comorbidities based on the strength of their association with outcome, in order to more accurately predict the association of overall comorbidity and adverse outcomes. One of the most widely used comorbidity indices, the CCI was derived to predict 1-year mortality in hospitalized patients [8], but has been widely applied and validated outside of this setting [10]. In RA, the CCI has been used to measure the association between comorbidity and disability [11]. Given the significant adverse effects of comorbidities in RA and their association with poor prognosis, the disease-specific RDCI has been derived in an RA population for better estimation of outcome [9].
Methods
Data sources and cohort
The Royal College of General Practitioners (RCGP) Research and Surveillance Centre (RSC) database was used for this study. It comprises the pseudonymized primary care records of all individuals registered with a large network of general practices, and provides a broadly representative sample of the English primary care population [12]. At the time of data extraction for this study, data were available from 164 general practitioner (GP) practices across England with a total population of 1 475 762 people. The RCGP RSC contains information on clinical diagnoses, anthropometric measurements (e.g. body mass index), laboratory tests [e.g. C-reactive protein (CRP)] and prescriptions coded with the Read clinical coding systems (a thesaurus of clinical terms) [13]. Coded data are inputted by clinicians and support staff in primary care, uploaded directly from laboratories, or automatically generated by the primary care system (e.g. when entering anthropometric measurements or generating patient prescriptions). Diagnoses arising in secondary care are uploaded to the primary care record following receipt of the record of the secondary care interaction, which is almost universally sent to the patient’s registered general practitioner. UK general practice lends itself to this type of study because it is a registration-based system (each patient registers with a single general practitioner) [14, 15]; it has been computerized since the 1990s, with laboratory links and pay-for-performance from 2004 that has resulted in consistent data entry about chronic disease. Studies and outcomes based on RCGP RSC data have been published across a range of chronic diseases [16–18].
Inclusion criteria specified individuals aged 18 years and older with a confirmed diagnosis of RA prior to 1 January 2017. RA was identified using a validated algorithm (sensitivity 84% and specificity 86% for RA) [19] developed using UK primary care data that has more recently been updated to incorporate recent treatment and clinical codes [20]. We reviewed these code lists and added further medication codes for newer DMARDs that had not previously been available. The study start date was the date of diagnosis for individuals with RA, indicated by first diagnostic code. Individuals with RA were age- and sex-matched at general practice level with individuals without either RA or a diagnosis of psoriatic arthritis, ankylosing spondylitis, other spondyloarthropathy or connective tissue disease. This latter cohort was used to form a control population. Matching was performed using a 1: 1 ratio. A subset of individuals with at least 3 years’ follow-up after the study start date was also defined, to examine whether incident comorbidities were more common in individuals with RA than in the control group. The follow-up period extended to the study end: either 1 January 2017 or the date of patient transfer from an included practice, or death. We report patient smoking status using the most recently recorded smoking status in the GP record prior to the index date. We report ethnicity identified from ethnicity codes recorded in the patient record using an approach previously reported [21].
Study approval was granted by the Research Committee of the RCGP RSC. The study did not meet the requirements for formal ethics board review as defined using the NHS Health Research Authority research decision tool (http://www.hra-decisiontools.org.uk/research/).
Comorbidity measures
Comorbidity variables were selected based on their inclusion in either the RDCI or CCI comorbidity index. The CCI comprises 19 conditions: myocardial infarction, congestive cardiac failure, peripheral artery disease, cerebrovascular disease, dementia, chronic pulmonary disease, rheumatic or connective tissue disease, peptic ulcer disease, mild and moderate–severe liver disease, diabetes with and without complications, hemiplegia, renal disease, solid tumours with and without metastatic disease, leukaemia, lymphoma, and AIDS [8]. In the CCI each condition is assigned a score of 1, 2, 3, or 6. The RDCI comprises lung disease, heart attack, stroke, other cardiovascular disease, hypertension, fracture, depression, diabetes, cancer, and peptic ulcer or stomach problems. The RDCI assigns a score of 1 or 2 to each condition although heart attack, stroke, other cardiovascular disease and hypertension can only score a maximum of 2 points in total; the score ranges from 0 to 9 [9]. Three chronic lung diseases were also evaluated separately: asthma, chronic obstructive pulmonary disease (COPD) and interstitial lung disease (ILD).
Mortality and joint destruction outcome measures
We analysed the association of comorbidity burden and mortality using the time to all-cause mortality as the outcome in regression models. Time to incident joint surgery was used as a surrogate measure of joint damage in separate regression models. Joint surgery was defined as any joint procedure including arthroplasty, arthrodesis, synovectomy, osteotomy and arthroscopy. Joint surgeries were classified as shoulder, elbow, hip, knee and distal joint (wrist, ankle, hand and foot) operations. Two joint surgery outcome definitions were evaluated: a composite of any joint surgery (excluding joints not listed above such as the spine) and a composite of medium and small joint surgery—shoulder, elbow and distal joints. The rationale for using incident joint surgery as a surrogate marker for joint destruction was a pragmatic decision as it was the only available measure of joint damage that would have been accurately recoded in the primary care record. Within the record there was a lack of routinely collected data on a validated disease activity measure, such as the Disease Activity Score in 28 joints (DAS28) [22] or radiographic joint damage.
Statistical analysis
We evaluated differences in baseline characteristics, including prevalent comorbidities, between those with and without RA using the χ2 test for categorical variables and the unpaired t test for continuous data. All reported P-values are two-sided. We also compared the incidence of new (first-ever) comorbidities within the first 3 years post-diagnosis across the cohorts. Those with complete data for the first 3 years after the index date were eligible for inclusion in this latter analysis, i.e. those who remained registered throughout the follow-up period and did not die during follow-up. We also report the incidence rates of the same comorbidities in the complete cohort over the first 3 years post-diagnosis including those with a shorter duration of follow-up.
We calculated event rates for all-cause mortality and first joint surgery as the number of events divided by the total person-years of follow-up and expressed as the number per 1000 person-years. The association between comorbidity burden, as reflected separately by the two comorbidity indices (CCI and RDCI), and the primary and secondary outcomes was evaluated in both unadjusted analyses and in age and gender adjusted Cox proportional hazard models. As a measure of model performance, the discrimination ability of each comorbidity index was estimated using Harrell’s C-index. The association of each individual chronic lung disease (COPD, asthma and ILD) with the same outcomes was evaluated using Cox proportional hazard models adjusted for comorbidity index (with respiratory components of the indices removed), age and gender.
Whilst conducting the analysis described above we found substantial differences in the prevalence of lung diseases in those with RA at diagnosis compared with those without. Therefore, we performed a post hoc analysis to assess by how long these conditions predated the diagnosis of RA. We report the median time from diagnosis of lung disease (asthma, COPD and ILD) to RA diagnosis with interquartile range (IQR) and the proportion diagnosed in the 2 years preceding RA diagnosis. An equivalent analysis was performed for those control patients with these lung diseases. All statistical analyses were performed in R statistical package software version 3.2.1 (R Foundation for Statistical Computing, Vienna, Austria).
Results
There is an excess of common comorbidities, including respiratory conditions, at RA diagnosis
From the RCGP RSC database, 6591 incident cases of RA were identified, and matched with 6591 controls without RA. The mean age was 59.8 years and 32.5% were male. Individuals with RA were more likely to be of white ethnicity compared with controls. Smoking was more prevalent in people with RA. Several comorbidities were more common among patients with RA when compared with controls: stroke, hemiplegia, chronic lung disease, diabetes, previous fracture, liver disease, peptic ulcer disease and depression (Table 1). Mean comorbidity scores were significantly greater among patients with RA compared with those without when measured by CCI and RDCI, and this difference persisted after excluding ‘connective tissue disease’ from the CCI calculation (Table 2). All respiratory conditions evaluated (COPD, asthma, interstitial lung disease) were more common in individuals with RA than in the control population.
Table 1.
Presence of comorbidities among individuals with and without RA, at diagnosis and after 3 years
| RA at diagnosis (n = 6591) | Controlsa (n = 6591) | P-value | RA at 3 years (n = 4646) | Controlsa at 3 years (n = 4667) | P-value | |
|---|---|---|---|---|---|---|
| Age, years | 59.8 (15.8) | 59.8 (15.8) | 1.000 | 64.1 (15.5) | 64.4 (15.7) | 0.445 |
| Male sex | 2142 (32.5) | 2142 (32.5) | 1.000 | 1475 (31.7) | 1468 (31.5) | 0.778 |
| Ethnicity | 0.026 | 0.004 | ||||
| White | 4883 (91.6) | 4428 (92.8) | 3451 (91.8) | 3136 (93.3) | ||
| Asian | 255 (4.8) | 174 (3.6) | 181 (4.8) | 118 (3.5) | ||
| Black | 128 (2.4) | 125 (2.6) | 79 (2.1) | 84 (2.5) | ||
| Mixed | 26 (0.5) | 24 (0.5) | 22 (0.6) | 11 (0.3) | ||
| Other | 36 (0.7) | 21 (0.4) | 25 (0.7) | 11 (0.3) | ||
| Smoking status | <0.001 | <0.001 | ||||
| Active smoker | 1499 (23.0) | 1231 (18.9) | 812 (17.5) | 725 (15.7) | ||
| Ex-smoker | 3090 (47.4) | 2968 (45.6) | 2560 (55.2) | 2410 (52.1) | ||
| Never smoked | 1926 (29.6) | 2307 (35.5) | 1265 (27.3) | 1492 (32.2) | ||
| Myocardial infarction | 202 (3.1) | 182 (2.8) | 0.325 | 37 (0.8) | 29 (0.6) | 0.377 |
| Heart failure | 108 (1.6) | 89 (1.4) | 0.196 | 45 (1.0) | 27 (0.6) | 0.042 |
| Hypertension | 2063 (31.3) | 1964 (29.8) | 0.064 | 278 (6.0) | 229 (4.9) | 0.025 |
| Peripheral artery disease | 103 (1.6) | 78 (1.2) | 0.072 | 23 (0.5) | 15 (0.3) | 0.249 |
| Stroke or TIA | 254 (3.9) | 209 (3.2) | 0.037 | 60 (1.3) | 45 (1.0) | 0.162 |
| Hemiplegia | 26 (0.4) | 11 (0.2) | 0.021 | 5 (0.1) | 3 (0.1) | 0.719 |
| Other cardiovascular disease | 600 (9.1) | 509 (7.7) | 0.005 | 125 (2.7) | 86 (1.8) | 0.007 |
| Chronic lung disease | 1390 (21.1) | 1033 (15.7) | <0.001 | 158 (3.4) | 86 (1.8) | <0.001 |
| COPD | 466 (7.1) | 255 (3.9) | <0.001 | 123 (2.6) | 75 (1.6) | 0.001 |
| Asthma | 1118 (17.0) | 891 (13.5) | <0.001 | 49 (1.1) | 40 (0.9) | 0.382 |
| Interstitial lung disease | 52 (0.8) | 3 (0.0) | <0.001 | 40 (0.9) | 4 (0.1) | <0.001 |
| Diabetes | 660 (10.0) | 542 (8.2) | <0.001 | 81 (1.7) | 61 (1.3) | 0.102 |
| Diabetes with no end-organ damage | 217 (3.3) | 136 (2.1) | <0.001 | 28 (0.6) | 30 (0.6) | 0.909 |
| Diabetes with end-organ damage | 443 (6.7) | 406 (6.2) | 0.201 | 133 (2.9) | 82 (1.8) | <0.001 |
| Fracture | 1609 (24.4) | 1465 (22.2) | 0.003 | 146 (3.1) | 129 (2.8) | 0.309 |
| Malignancy without metastases | 366 (5.6) | 342 (5.2) | 0.374 | 34 (0.7) | 32 (0.7) | 0.806 |
| Malignancy with metastases | 6 (0.1) | 7 (0.1) | 1.000 | 7 (0.2) | 2 (0.0) | 0.180 |
| Mild liver disease | 138 (2.1) | 92 (1.4) | 0.003 | 38 (0.8) | 22 (0.5) | 0.050 |
| Moderate-severe liver disease | 13 (0.2) | 2 (0.0) | 0.010 | 2 (0.0) | 0 (0.0) | 0.477 |
| Peptic ulcer disease | 250 (3.8) | 199 (3.0) | 0.021 | 41 (0.9) | 12 (0.3) | <0.001 |
| Rheumatic or connective tissue diseasea | 6591 (100.0) | 0 (0.0) | NA | NA | NA | NA |
| Dementia | 33 (0.5) | 33 (0.5) | 1.000 | 44 (0.9) | 22 (0.5) | 0.009 |
| Depression | 1855 (28.1) | 1519 (23.0) | <0.001 | 235 (5.1) | 124 (2.7) | <0.001 |
Data are mean (s.d.) or n (%). P-values from χ2 test or t test are provided. The following variables had missing values: ethnicity in RA at diagnosis, n = 1263; no RA, n = 1921; RA at 3 years, n = 888; no RA at 3 years, n = 1307; smoking status in RA at diagnosis, n = 76; no RA, n = 85; RA at 3 years, n = 9; no RA at 3 years, n = 40. aPeople with RA, psoriatic arthritis, ankylosing spondylitis, other spondyloarthropathies or connective tissue disease, e.g. systemic lupus erythematosus, are excluded from the controls by definition. Non-RA connective tissue disease were also excluded from the cases by definition. NA: not applicable; TIA: transient ischaemic attack.
Table 2.
Mean scores for comorbidity indices among patients with RA at diagnosis and age- and sex-matched controls
| RA (n = 6591) | Controla (n = 6591) | P-value | |
|---|---|---|---|
| RDCI, mean (s.d.) | 1.63 (1.48) | 1.38 (1.37) | <0.001 |
| CCI, mean (s.d.) | 1.68 (1.06) | 0.55 (0.97) | <0.001 |
| CCI excluding connective tissue diseasea, mean (s.d.) | 0.68 (1.06) | 0.55 (0.97) | <0.001 |
People with RA, psoriatic arthritis, ankylosing spondylitis, other spondyloarthropathies or connective tissue disease, e.g. systemic lupus erythematosus, are excluded from the controls by definition. Non-RA connective tissue diseases were also excluded from the cases by definition. CCI: Charlson Comorbidity Index; RDCI: Rheumatic Disease Comorbidity Index.
There is an excess of incident comorbidities after the diagnosis of RA
The majority of people in both cohorts had complete follow-up for 3 years after the index date: 4646 (70.5%) cases and 4667 (70.8%) controls (P = 0.688). There was a greater incidence of several comorbidities within 3 years of diagnosis in RA patients compared with controls (Table 1 and Supplementary Table S1, available at Rheumatology online), including heart failure, hypertension, peptic ulcer disease and depression. Incident diagnoses of COPD and ILD were also substantially more common within the first 3 years in RA patients. There was no difference in incident asthma diagnoses.
Greater comorbidity is associated with mortality but not joint surgery
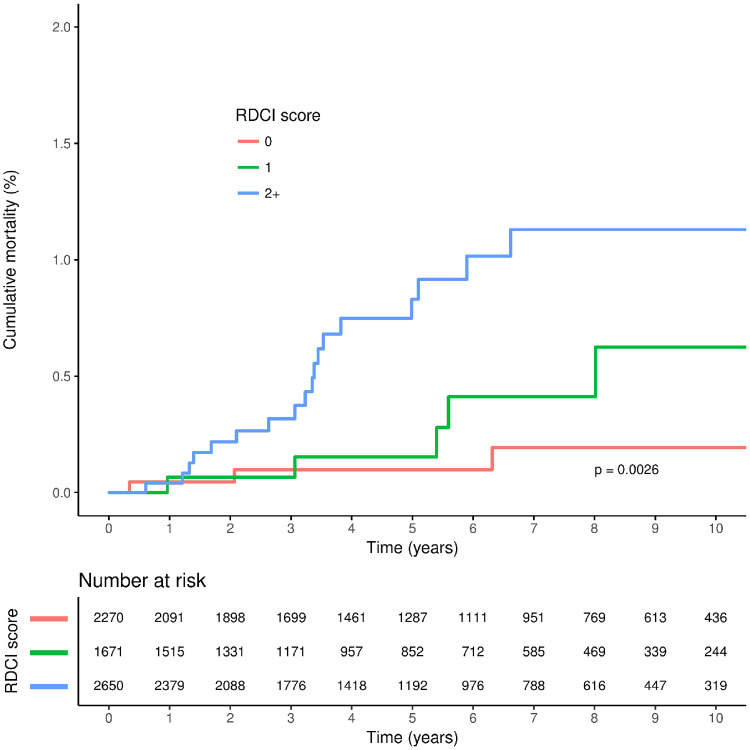
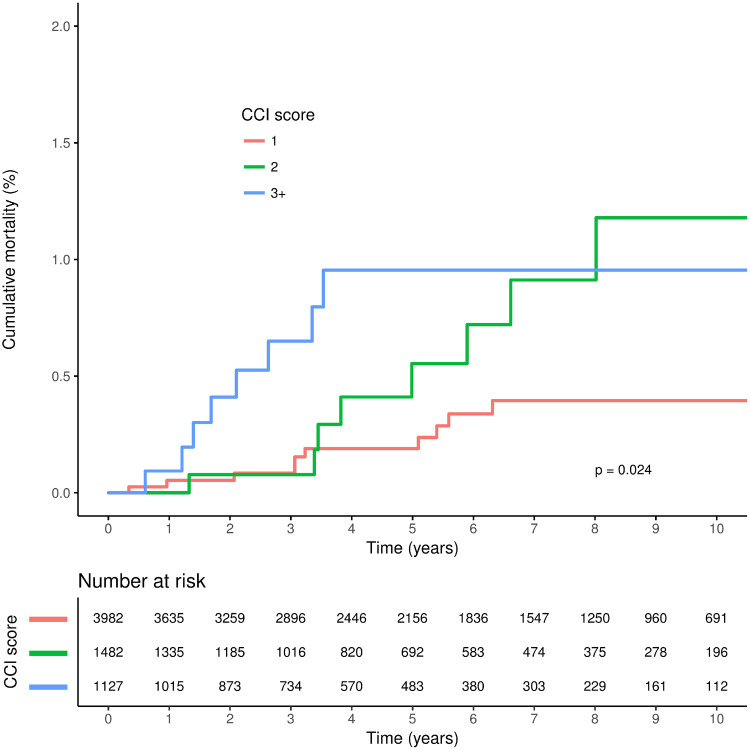
A total follow-up duration of 36 745 person-years was available for people with RA: a mean (s.d.) follow-up of 5.58 (3.59) years per patient. The proportion of patients who had some form of joint surgery in the 3 years following RA diagnosis was 10.4% (Table 3). Cumulative rates of all-cause mortality were greater with higher RDCI and CCI scores, although separation of the cumulative incidence curves was less marked for the CCI score (Figs 1 and 2). In unadjusted analyses, higher CCI and RDCI scores were both significantly associated with an increased risk of all-cause mortality, and risk was similar for both indices in adjusted analyses (Table 4). Higher RDCI but not CCI scores were associated with an increased risk of any joint surgery in unadjusted but not adjusted analysis. Model performance was similar for both the RDCI and CCI score models (Table 4). There was no evidence of an association between either index and risk of medium and small joint surgery.
Table 3.
Event rates for joint surgery outcomes among patients with RA
| RA (n = 6591) | ||
|---|---|---|
| Outcome | No events | Events per 1000 person-years (95% CI) |
| Shoulder operations | 49 (0.7) | 1.34 (0.99–1.77) |
| Elbow operations | 14 (0.2) | 0.38 (0.21–0.64) |
| Hip operations | 236 (3.6) | 6.54 (5.73–7.44) |
| Knee operations | 371 (5.6) | 10.40 (9.36–11.52) |
| Distal jointa operations | 94 (1.4) | 2.59 (2.09–3.17) |
| Medium or small jointb operations | 155 (2.4) | 4.29 (3.65–5.03) |
| Any jointc operation | 686 (10.4) | 20.11 (18.63–21.69) |
Distal joints comprise wrist, ankle, hand and foot joints.
Medium and small joints comprise shoulder, elbow and distal joints.
Any joint includes all joints listed in the table; spinal and other joints not listed are excluded.
Fig. 1.
Cumulative all-cause mortality according to RDCI score among patients at diagnosis of RA
RDCI: Rheumatic Disease Comorbidity Index.
Table 4.
Hazard ratio for the association between comorbidity index as assessed at diagnosis and outcome in RA
| All-cause mortality | Model C-statistic | Any joint surgery | Model C-statistic | Medium or small joint surgery | Model C-statistic | |
|---|---|---|---|---|---|---|
| CCI unadjusted HR (95% CI; P-value) | 1.53 (1.21–1.93; P < 0.001) | 0.650 | 1.01 (0.94–1.09; P = 0.764) | 0.502 | 0.84 (0.69–1.02; P = 0.073) | 0.541 |
| CCI adjusteda HR (95% CI; P-value) | 1.30 (0.99–1.70; P = 0.057) | 0.814 | 0.94 (0.87–1.01; P = 0.101) | 0.600 | 0.83 (0.68–1.01; P = 0.068) | 0.582 |
| RDCI unadjusted HR (95% CI) | 1.50 (1.20–1.87; P < 0.001) | 0.682 | 1.08 (1.03–1.14; P = 0.002) | 0.542 | 0.96 (0.86–1.08; P = 0.500) | 0.505 |
| RDCI adjusteda HR (95% CI) | 1.26 (1.00–1.59; P = 0.049) | 0.815 | 1.03 (0.97–1.08; P = 0.334) | 0.600 | 0.96 (0.85–1.08; P = 0.461) | 0.568 |
Adjusted for age and gender. CCI: Charlson Comorbidity Index; HR: hazard ratio; RDCI: Rheumatic Disease Comorbidity Index.
Fig. 2.
Cumulative all-cause mortality according to CCI score among patients at diagnosis of RA
CCI: Charlson Comorbidity Index.
COPD is an important predictor of all-cause mortality in RA
COPD was associated with a near 3-fold increase in the risk of mortality [hazard ratio (HR) 2.84, 95% CI 1.13–7.12; P = 0.027], whereas there was no evidence of an association for asthma (HR 1.79, 95% CI 0.75–4.30; P = 0.192). Numbers of individuals with ILD were insufficient to assess the association between ILD and risk of mortality.
Where interstitial lung disease predates RA diagnosis it usually occurs in the preceding few years
Almost half of ILD cases (23 of 52; 44%) were diagnosed within the 2 years preceding the diagnosis of RA, and a first diagnostic code for ILD predated RA by a median of 2.2 (IQR 0.6–6.3) years. The three cases of ILD in controls predated the matched index date by a median of 10.2 years (P = 0.032 for comparison vs cases). Where COPD preceded a diagnosis of RA, only 25.8% (120 of 466) occurred in the preceding 2 years, with COPD preceding RA by a median of 4.5 (IQR 1.9–8.2) years. COPD preceded the index date in controls by a median of 5.1 (IQR 1.9–8.6) years (P = 0.407 for comparison vs cases). Just 7.0% (78 of 1118) of asthma cases that preceded RA occurred in the 2 years before diagnosis, with asthma preceding RA by a median of 12.5 (IQR 6.9–20.7) years. Asthma preceded the index date in controls by a median of 13.2 (IQR 7.4–20.4) years (P = 0.471 for comparison vs cases).
Discussion
We found that people with RA have a substantially higher burden of comorbidity at diagnosis compared with matched individuals without RA. The incidences of several comorbidities, including heart failure, peptic ulcer disease, depression, COPD and ILD, were also greater in the first 3 years after diagnosis than in controls. Our study shows that even in early RA, both the RDCI and CCI comorbidity indices were associated with an increased risk of all-cause mortality. Age and gender adjusted models using both these scores had good discriminative ability to predict mortality. However, neither index was associated with risk of disease progression, as measured by the need for joint surgery as a surrogate marker of joint destruction. Respiratory diseases (COPD, asthma and ILD) were all more common at diagnosis than in controls. COPD at diagnosis, but not asthma, was associated with a near 3-fold increased risk of early mortality. The sample size for ILD was insufficient to assess for any association.
The prevalence of comorbidity, including cardiovascular and respiratory disease, has previously been reported to be higher in individuals with RA at diagnosis than in the general population [23] and has been increasing over time [24]. In contrast, Swedish case–control studies have observed that although cardiovascular risk increases rapidly following RA diagnosis [25], there is no evidence of an increase in ischaemic heart disease prior to the onset of RA [26]. However, an important limitation of this previous comparative work is that methods of assessment of comorbidity were different in cases and controls; cases were recruited to the study, with assessment of comorbidity via patient self-reporting, clinical assessment and medical records review, whereas population controls and their comorbidities were identified using electronic health records. We were able to corroborate these findings in our study, which has the strength of applying the same approach to assess comorbidity in both individuals with and those without RA. Our matched analysis also benefits from identifying controls from the same GP practice as cases. Our data also support analyses using the Finnish nationwide disease register, which suggested elevated cardiovascular risk precedes the clinical onset of RA [27]. Several studies have also demonstrated that RA-associated lung disease can precede the onset of clinically significant RA [28], but to our knowledge the duration between lung disease onset and RA diagnosis has not previously been explored in a community-based cohort. The differences in temporal association between COPD, asthma and RA most likely reflect the differences in age of onset of these conditions. However, it is interesting to observe that where ILD does predate RA it usually occurs within the few years immediately before RA diagnosis.
COPD is associated with a 3-fold increase in mortality in the general population and asthma with a very small increased mortality risk in adults [29]. These results are consistent with our findings in those with RA; we cannot exclude the possibility of a small association between asthma and mortality given our sample size. Given the high overall mortality in people with RA the additional 3-fold risk conferred by the presence of COPD is of particular importance in this group. This contrasts, for example, with the situation in patients with heart failure, where the presence of COPD confers only a small additional mortality risk (HR 1.19; 95% CI 1.02–1.39) [30].
The excess burden of COPD and perhaps cardiovascular disease in people with RA at diagnosis may be, at least in part, related to the greater incidence of smoking we observed in people with RA. Multiple previous studies have found that smoking is a risk factor for the development of RA, which is consistent with our results [31]. This underscores the importance of smoking cessation advice and support as part of the routine management of people with and at risk of RA. A holistic and patient-centric approach to the care of people with RA is vital.
Our study corroborates the findings of England and colleagues who compared the RDCI with other comorbidity indices for predicting disease outcomes; in their study, the RCDI performed well as a predictor of mortality [32]. We find this holds true in early RA. To our knowledge, no other studies have assessed whether the RDCI is associated with the need for joint surgery.
This study benefits from the large sample size and the ability to use data routinely collected prior to a diagnosis of RA to assess baseline comorbidities. UK primary care provides a high level of data quality in the recording and monitoring of chronic disease and the quality of data within the RCGP RSC network is high in this regard [12, 33, 34].
There are several limitations to the present study. Although the cohort is derived from a nationally representative sample of England [12], the results may not be generalizable to other populations. The possibility of residual confounding cannot be excluded in any observational study of this type. In common with other studies using routinely collected data, our study relies on accurate recording of disease by clinicians at participating practices. This limitation was mitigated against by using a recommended approach for variable definition, defining comprehensive code lists to maximize correct disease identification [13, 35]. No secondary care data linkage was used for this study and therefore we rely on primary care recording of events; this may have led to some underestimation of comorbidities. Our analyses were also limited by the lack of data on cause-specific mortality. Joint surgery type is often recorded using high-level Read codes in primary care, i.e. the specific procedure is not recorded just the joint operated upon. We were therefore unable to restrict our analysis to joint replacement surgery and relied on joint surgery as a surrogate marker for joint damage. In our analysis of the prevalence of comorbidity between cases and controls 3 years after diagnosis, we cannot exclude the possibility of under-identification of conditions in the control group due to reduced surveillance. There is also a possible survival bias in this assessment of incident comorbidities at 3 years as continuous registration was an a priori requirement for this subset; we therefore may have slightly underestimated the incident comorbidity burden in both cases and controls.
Implications of our findings
With increasing life-expectancy and improved control of joint disease in RA the relative importance of comorbidity in RA is increasing [36]. However, globally the quality of evaluation, monitoring and treatment of comorbidities in RA is variable with considerable scope for improvement [37]. Our findings highlight the importance of assessing comorbidity in individuals with newly diagnosed RA, in line with existing literature [24, 38, 39]. In particular, we found individuals with RA were more likely to have existing cardiovascular disease, respiratory disease and depression. We also found, incident comorbidities were much more common in the years subsequent to a diagnosis of RA compared with matched individuals without RA, suggesting a need for clinicians to reassess comorbidity frequently post-diagnosis in the RA population, especially as medications used for RA treatment are associated with several comorbidities. Early identification of these comorbidities should enable early intervention to minimize future morbidity and mortality and to help avoid RA treatments that may worsen some comorbidities. Future research should explore the impact of this early intervention. Further research is also needed to explore whether the excess burden of comorbidities at diagnosis of RA varies between those who are seropositive and those who are seronegative. Our findings also provide good ground for the future development of a tool to calculate individual mortality risk in RA using comorbidity burden, for use in routine clinical practice.
Conclusions
There is an excess burden of comorbidity around the time of diagnosis of RA, including increased respiratory disease (COPD, asthma and ILD). Both the CCI and RDCI comorbidity indices predict risk of all-cause mortality in early RA but not joint surgery. We also found that the presence of COPD was a major predictor of early mortality in RA. Individuals with RA also appear to accumulate many comorbidities more rapidly than controls in the first few years after diagnosis. Early and repeated assessment of comorbidities, including respiratory disease, should form part of the routine care of RA patients. Management of comorbidities, especially preventable and modifiable ones and associated risk factors, early on in the disease course, may improve outcomes and quality of life.
Supplementary Material
Acknowledgements
This paper presents independent research conducted by K.R. and C.B., who are supported by the NIHR Birmingham Biomedical Research Centre at the University Hospitals Birmingham NHS Foundation Trust and the University of Birmingham. C.M. is funded by the National Institute for Health Research (NIHR) Collaborations for Leadership in Applied Health Research and Care West Midlands, the NIHR School for Primary Care Research and an NIHR Research 95 Professorship in General Practice (NIHR-RP-2014-04-026). The views expressed in this article are those of the authors and not necessarily those of the NHS, the NIHR, our funding bodies or the Department of Health and Social Care. Medical writing and statistical support was provided by John Dennis at Momentum Data and was funded by Pfizer. We acknowledge additional medical writing and statistical support from Andrew McGovern and Jack Brownrigg (Momentum Data) and project management support from Filipa Ferreira (University of Surrey).
Funding: This work was supported by Pfizer UK.
Disclosure statement: E.N. has received speaker honoraria and has participated in advisory boards for Pfizer, Sanofi, 110 Gilead, Celltrion, AbbVie and Lilly. K.R. has received research funding from AbbVie and Pfizer and honoraria/consultancy fees from AbbVie, Sanofi, Lilly, Bristol-Myers Squibb, UCB, Pfizer, Janssen and Roche Chugai. J.G. has received honoraria and/or sponsorships for conferences from AbbVie, Celgene, Janssen, Pfizer and UCB. K.K., G.B. and J.R. are employees of Pfizer. The other authors have declared no conflicts of interest.
Supplementary data
Supplementary data are available at Rheumatology online.
References
- 1. Shourt CA, Crowson CS, Gabriel SE. et al. Orthopedic surgery among patients with rheumatoid arthritis 1980–2007: a population-based study focused on surgery rates, sex, and mortality. J Rheumatol 2012;39:481–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Salliot C, van der Heijde D.. Long-term safety of methotrexate monotherapy in patients with rheumatoid arthritis: a systematic literature research. Ann Rheum Dis 2009;68:1100–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Abhishek A, Nakafero G, Kuo C-F. et al. Rheumatoid arthritis and excess mortality: down but not out. A primary care cohort study using data from Clinical Practice Research Datalink. Rheumatology 2018;57:977–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Hall F, Dalbeth N.. Disease modification and cardiovascular risk reduction: two sides of the same coin? Rheumatology 2005;44:1473–82. [DOI] [PubMed] [Google Scholar]
- 5. Symmons D, Jones M, Scott D. et al. Longterm mortality outcome in patients with rheumatoid arthritis: early presenters continue to do well. J Rheumatol 1998;25:1072–7. [PubMed] [Google Scholar]
- 6. Young A, Koduri G, Batley M. et al. Mortality in rheumatoid arthritis. Increased in the early course of disease, in ischaemic heart disease and in pulmonary fibrosis. Rheumatology 2007;46:350–7. [DOI] [PubMed] [Google Scholar]
- 7. Kitas GD, Gabriel SE.. Cardiovascular disease in rheumatoid arthritis: state of the art and future perspectives. Ann Rheum Dis 2011;70:8–14. [DOI] [PubMed] [Google Scholar]
- 8. Charlson ME, Pompei P, Ales KL. et al. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis 1987;40:373–83. [DOI] [PubMed] [Google Scholar]
- 9. Michaud K, Wolfe F.. Comorbidities in rheumatoid arthritis. Best Pract Res Clin Rheumatol 2007;21:885–906. [DOI] [PubMed] [Google Scholar]
- 10. Yurkovich M, Avina-Zubieta JA, Thomas J. et al. A systematic review identifies valid comorbidity indices derived from administrative health data. J Clin Epidemiol 2015;68:3–14. [DOI] [PubMed] [Google Scholar]
- 11. Radner H, Smolen JS, Aletaha D.. Comorbidity affects all domains of physical function and quality of life in patients with rheumatoid arthritis. Rheumatology (Oxford) 2011;50:381–8. [DOI] [PubMed] [Google Scholar]
- 12. Correa A, Hinton W, McGovern A. et al. Royal College of General Practitioners Research and Surveillance Centre (RCGP RSC) sentinel network: a cohort profile. BMJ Open 2016;6:e011092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. de Lusignan S, Liaw S-T, Michalakidis G. et al. Defining datasets and creating data dictionaries for quality improvement and research in chronic disease using routinely collected data: an ontology-driven approach. J Innov Health Inform 2011;19:127–34. [DOI] [PubMed] [Google Scholar]
- 14. de Lusignan S, Metsemakers J, Houwink P. et al. Routinely collected general practice data: goldmines for research? A report of the European Federation for Medical Informatics Primary Care Informatics Working Group (EFMI PCIWG) from MIE2006, Maastricht, the Netherlands. J Innov Health Inform 2006;14:203–9. [DOI] [PubMed] [Google Scholar]
- 15. de Lusignan S, van Weel C.. The use of routinely collected computer data for research in primary care: opportunities and challenges. Fam Pract 2006;23:253–63. [DOI] [PubMed] [Google Scholar]
- 16. Kumar S, de Lusignan S, McGovern A. et al. Ischaemic stroke, haemorrhage, and mortality in older patients with chronic kidney disease newly started on anticoagulation for atrial fibrillation: a population based study from UK primary care. BMJ 2018;360:k342. [DOI] [PubMed] [Google Scholar]
- 17. Williams R, Alexander G, Armstrong I. et al. Disease burden and costs from excess alcohol consumption, obesity, and viral hepatitis: fourth report of the Lancet Standing Commission on Liver Disease in the UK. Lancet 2018;391:1097–1107. [DOI] [PubMed] [Google Scholar]
- 18. Woodmansey C, McGovern AP, McCullough KA. et al. Incidence, demographics, and clinical characteristics of diabetes of the exocrine pancreas (type 3c): a retrospective cohort study. Diabetes Care 2017;40:1486–93. [DOI] [PubMed] [Google Scholar]
- 19. Thomas SL, Edwards CJ, Smeeth L. et al. How accurate are diagnoses for rheumatoid arthritis and juvenile idiopathic arthritis in the general practice research database? Arthritis Care Res 2008;59:1314–21. [DOI] [PubMed] [Google Scholar]
- 20. Muller S, Hider SL, Raza K. et al. An algorithm to identify rheumatoid arthritis in primary care: a Clinical Practice Research Datalink study. BMJ Open 2015;5:e009309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Tippu Z, Correa A, Liyanage H. et al. Ethnicity recording in primary care computerised medical record systems: an ontological approach. J Innov Health Inform 2017;23:799. [DOI] [PubMed] [Google Scholar]
- 22. Prevoo M, Van'T Hof MA, Kuper H. et al. Modified disease activity scores that include twenty-eight-joint counts development and validation in a prospective longitudinal study of patients with rheumatoid arthritis. Arthritis Rheum 1995;38:44–8. [DOI] [PubMed] [Google Scholar]
- 23. Norton S, Koduri G, Nikiphorou E. et al. A study of baseline prevalence and cumulative incidence of comorbidity and extra-articular manifestations in RA and their impact on outcome. Rheumatology (Oxford) 2013;52:99–110. [DOI] [PubMed] [Google Scholar]
- 24. Nikiphorou E, Norton S, Carpenter L. et al. Secular changes in clinical features at presentation of rheumatoid arthritis: increase in comorbidity but improved inflammatory states. Arthritis Care Res 2017;69:21–7. [DOI] [PubMed] [Google Scholar]
- 25. Holmqvist ME, Wedren S, Jacobsson LT. et al. Rapid increase in myocardial infarction risk following diagnosis of rheumatoid arthritis amongst patients diagnosed between 1995 and 2006. J Intern Med 2010;268:578–85. [DOI] [PubMed] [Google Scholar]
- 26. Holmqvist ME, Wedren S, Jacobsson LT. et al. No increased occurrence of ischemic heart disease prior to the onset of rheumatoid arthritis: results from two Swedish population-based rheumatoid arthritis cohorts. Arthritis Rheum 2009;60:2861–9. [DOI] [PubMed] [Google Scholar]
- 27. Kerola AM, Kerola T, Kauppi MJ. et al. Cardiovascular comorbidities antedating the diagnosis of rheumatoid arthritis. Ann Rheum Dis 2013;72:1826–9. [DOI] [PubMed] [Google Scholar]
- 28. Shaw M, Collins BF, Ho LA. et al. Rheumatoid arthritis-associated lung disease. Eur Respir Rev 2015;24:1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Ringbaek T, Seersholm N, Viskum K.. Standardised mortality rates in females and males with COPD and asthma. Eur Respir J 2005;25:891–5. [DOI] [PubMed] [Google Scholar]
- 30. De Blois J, Simard S, Atar D. et al. COPD predicts mortality in HF: the Norwegian Heart Failure Registry. J Card Fail 2010;16:225–9. [DOI] [PubMed] [Google Scholar]
- 31. Chang K, Yang SM, Kim SH. et al. Smoking and rheumatoid arthritis. Int J Mol Sci 2014;15:22279–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. England BR, Sayles H, Mikuls TR. et al. Validation of the rheumatic disease comorbidity index. Arthritis Care Res 2015;67:865–72. [DOI] [PubMed] [Google Scholar]
- 33. Hinton W, McGovern A, Coyle R. et al. Incidence and prevalence of cardiovascular disease in English primary care: a cross-sectional and follow-up study of the Royal College of General Practitioners (RCGP) Research and Surveillance Centre (RSC). BMJ Open 2018;8:e020282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. McGovern A, Hinton W, Correa A. et al. Real-world evidence studies into treatment adherence, thresholds for intervention and disparities in treatment in people with type 2 diabetes in the UK. BMJ Open 2016;6:e012801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Davé S, Petersen I.. Creating medical and drug code lists to identify cases in primary care databases. Pharmacoepidemiol Drug Saf 2009;18:704–7. [DOI] [PubMed] [Google Scholar]
- 36. Nikiphorou E, Nurmohamed MT, Szekanecz Z.. Editorial: Comorbidity burden in rheumatic diseases. Front Med 2018;5:197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Dougados M, Soubrier M, Antunez A. et al. Prevalence of comorbidities in rheumatoid arthritis and evaluation of their monitoring: results of an international, cross-sectional study (COMORA). Ann Rheum Dis 2014;73:62–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Nikiphorou E, Norton S, Young A. et al. The association of obesity with disease activity, functional ability and quality of life in early rheumatoid arthritis: data from the Early Rheumatoid Arthritis Study/Early Rheumatoid Arthritis Network UK prospective cohorts. Rheumatology (Oxford) 2018;57:1194. [DOI] [PubMed] [Google Scholar]
- 39. Radner H, Chatzidionysiou K, Nikiphorou E. et al. 2017 EULAR recommendations for a core data set to support observational research and clinical care in rheumatoid arthritis. Ann Rheum Dis 2018;77:476–9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.