Abstract
Objective
To evaluate the associations between oral corticosteroid (OCS) dose early and late in pregnancy and preterm birth (PTB) among women with RA.
Methods
Pregnant women in the MotherToBaby Pregnancy Studies (2003–2014) with RA (n = 528) were included in the primary analysis. Information was collected by phone interview and from medical records. We estimated risk ratios (RR) for OCS dose trajectories and other disease-related medications before gestational day 140 and hazard ratios (HR) for time-varying exposures after gestational day 139.
Results
PTB risk was 15.5% overall. Compared with no OCS, PTB risk was increased in high (adjusted (a)RR: 4.77 (95% CI: 2.76, 8.26)) and medium (aRR: 1.81 (95% CI: 1.10, 2.97)) cumulative OCS dose trajectories during the first 139 gestational days. The low cumulative trajectory group was associated with an increased risk of PTB that was not statistically significant (aRR: 1.38 (95% CI: 0.79, 2.38)), and DMARDs were not associated with PTB (biologic DMARDs aHR: 1.08 (95% CI: 0.70, 1.66); non-biologic DMARDs aHR: 0.87 (95% CI: 0.55, 1.38)). OCS exposure to ⩾10 mg of prednisone equivalent daily dose after gestational day 139 vs none was associated with increased PTB rate (aHR: 2.45 (95% CI: 1.32, 4.56)), whereas <10 mg was associated with a modestly increased rate of PTB that was not statistically significant (aHR: 1.18 (95% CI: 0.60, 2.30)).
Conclusion
Higher OCS doses vs no OCS use, both earlier and later in pregnancy, were associated with an increase in PTB among women with RA.
Keywords: antirheumatic agents, autoimmune diseases, oral corticosteroids, pregnancy, preterm birth, rheumatoid arthritis
Rheumatology key messages
Higher OCS doses were associated with an increase in preterm birth among women with RA.
Lower OCS doses were not clearly associated with preterm birth among women with RA.
B-DMARD and NB-DMARD use were not associated with preterm birth among women with RA.
Introduction
For pregnant women with autoimmune diseases including RA, oral corticosteroids (OCSs) may be used to manage flares or for chronic management when other treatments are discontinued [1–4]. OCSs may be used during pregnancy to treat acute asthma exacerbations or severe persistent asthma [5, 6].
Systemic corticosteroid use has been associated with serious infection in pregnant women and serious and non-serious infection in individuals with autoimmune diseases, independent of other immunosuppressive medications, especially for doses of ∼10 mg of prednisone equivalent per day and greater [7–11]. Intrauterine infection is believed to be a contributor to preterm birth (PTB) [12], and Schatz et al. proposed that immunosuppressive effects of OCSs could increase the risk of PTB through subclinical intra-amniotic infection [13]. Indeed, many studies have reported an increased risk of PTB or shorter gestational length following any OCS use during pregnancy among women with RA, systemic lupus erythematosus, IBD, asthma, and among all women regardless of autoimmune disease [13–29], yet there is limited information regarding the impact of dose and gestational timing of OCS use on PTB [23, 25]. We reported a reduction in gestational age at delivery associated with higher dose trajectories of prednisone (the most commonly used OCS during pregnancy) [30, 31] use during the first 32 gestational weeks as compared with lower dose trajectories among women with RA [32]. However, the association between OCS dose specifically during earlier and later gestational windows (i.e. during the first and second halves of pregnancy, separately) and the clinically relevant outcome of PTB has not been explored. Furthermore, there are limited data on comparisons between OCSs and other disease-related treatments and PTB risk adjusted for confounders [18, 20–22, 33, 34]. Finally, confounding by indication has been a concern for studies of OCS use and PTB as maternal autoimmune disease and control/activity are also associated with increased PTB risk [29, 35–43].
We assessed the association between OCS dose and other disease-related medication use and PTB in pregnant women with RA in the primary analysis, adjusting for disease severity at enrolment. Assuming OCS use impacts subclinical intrauterine infection, we hypothesized that higher doses of OCS later in pregnancy, as compared with no OCS use, would be associated with an increased rate of PTB, but that other disease-related medications would not be associated with PTB. We also evaluated associations between OCS use and PTB in smaller subcohorts of women with IBD and asthma in an exploratory analysis to determine whether any signals observed among women with RA would also be observed among women with other autoimmune conditions who may be treated with OCS.
Methods
The methods described below pertain to the primary analysis among women with RA. A further description for the IBD and asthma subcohorts can be found in Supplementary Methods, available at Rheumatology online.
Data sources
Data were from the MotherToBaby Pregnancy Studies [39, 44, 45], which were approved by the University of California, San Diego Institutional Review Board and the current analysis was exempt. Informed consent was obtained in the MotherToBaby Pregnancy Studies. Participants from the United States and Canada were self-referred, referred by healthcare providers, or referred by MotherToBaby, a free service of the Organization of Teratology Information Specialists providing evidence-based information regarding exposures during pregnancy and lactation.
Trained study staff conducted up to four semi-structured telephone interviews with participants: at enrolment (before gestational week 20), ∼24 and 32 weeks’ gestation, and as soon as possible after delivery. Interviewers collected data from participants regarding race, ethnicity, socio-economic status (Hollingshead categories based on maternal and paternal education and occupation; possible range from highest = 1 to lowest = 5) [46], reproductive history, pre-pregnancy weight and height, comorbidities, smoking and alcohol use, pregnancy outcomes, and name, dose and dates of medication use [44]. Furthermore, interviewers administered self-assessment questionnaires to measure disease severity. These included the HAQ (a validated measure of functional status in patients with RA; possible range from 0 = no disability to 3 = completely disabled) [47, 48], pain score (pain severity rating in the past week; possible range from 0 = no pain to 100 = severe pain), and patient’s global score (overall health rating; possible range from 0 = very well to 100 = very poor). Medical records were requested from participants to help verify maternal report.
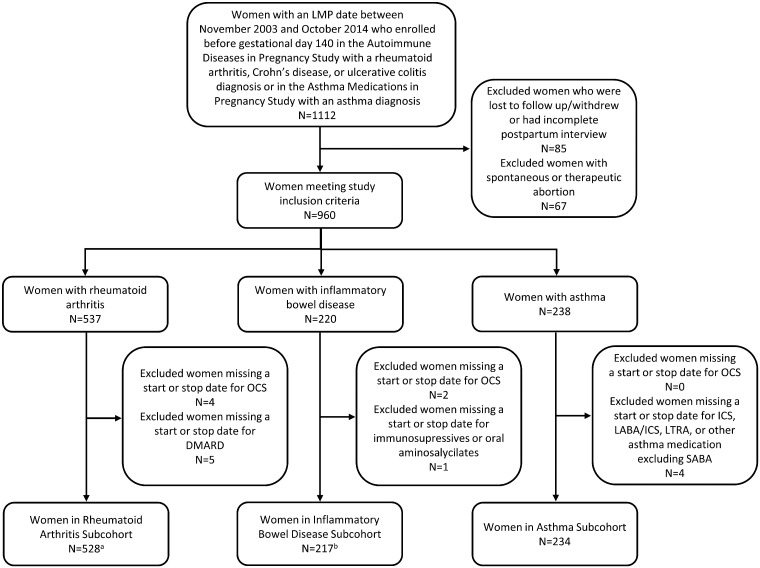
Pregnant women with a last menstrual period between 2003–2014 were eligible if they enrolled before gestational day 140 (Fig. 1) in either (i) MotherToBaby Autoimmune Diseases in Pregnancy Study and reported having RA or IBD (either Crohn’s disease or ulcerative colitis) or (ii) MotherToBaby Asthma Medications in Pregnancy Study and reported having asthma (n = 1112). Women who were lost to follow-up, withdrew, had an incomplete postpartum interview or had a spontaneous or therapeutic abortion were excluded. Women meeting the basic inclusion criteria (n = 960) were included in RA, IBD or asthma subcohorts according to diagnoses. Women were excluded if missing a start or stop date for medications of interest (Fig. 1). For analyses considering OCS dose, we excluded women missing OCS dose information during the relevant exposure windows (Fig. 1; n = 4 for RA, n = 1 for IBD).
Fig. 1.
Study cohort diagram
Fifteen women were in both the rheumatoid arthritis and IBD subcohorts, 11 women were in both the RA and asthma subcohorts, seven women were in both the IBD and asthma subcohorts, and one woman was in all three subcohorts. LMP: last menstrual period; OCS: oral corticosteroid; ICS: inhaled corticosteroid; LABA: long-acting beta-agonist; LTRA: leukotriene receptor agonist; SABA: short-acting beta-agonist. an = 524 women with oral corticosteroid dose information available before gestational day 140, n = 525 women with oral corticosteroid dose information available after gestational day 109. bn = 216 women with oral corticosteroid dose information available before gestational day 140.
Outcome
Gestational age at delivery was calculated from the last menstrual period date with adjustment for discrepant ultrasound measurements. Still birth and live birth deliveries were classified as preterm (primary outcome) based on delivery between 20 (140 gestational days, i.e. after the timeframe for spontaneous abortion) and <37 gestational weeks (<259 gestational days) and as early PTB (exploratory outcome) based on delivery between 20 and <34 weeks.
Exposure modeling
OCS use was the main exposure of interest. OCS daily dose on each gestational day was calculated based on reported dates of use, dose and frequency of use, and doses were converted to prednisone equivalent dose [49]. OCS average daily dose was the mean daily dose across days with any OCS use. OCS cumulative dose on each day was calculated by summing daily dose on each previous day. Other RA-related medications of interest were biologic DMARD (B-DMARD) and non-biologic DMARD (NB-DMARD).k-means is a statistical method used to group individuals with similar patterns of a measurement [50]. To evaluate the impact of OCS dose earlier in pregnancy on PTB risk, we used k-means to cluster women with similar trajectories of OCS dose during the first 139 days of pregnancy with the R statistical software package ‘kml’ [32, 50, 51]. We selected this method because it makes no a priori assumptions about trajectory shape or membership [50], and it has been used previously to characterize patterns of medications in pregnancy [32, 52–54]. We considered daily and cumulative dose on each gestational day allowing up to k = 3 clusters; we selected three clusters based on sample size of the smallest cluster. We plotted the mean dose for each cluster (i.e. trajectory group) on each day as well as each woman’s trajectory. Because of pronounced differences between individual trajectories and daily dose cluster means (Supplementary Fig. S1, available at Rheumatology online) due to sporadic OCS use, we focused the trajectory-PTB analysis on cumulative dose.
Because PTB can occur as early as gestational day 140, we could not apply the trajectory method when considering OCS dose later in pregnancy, as pregnancies would need the same gestational length to avoid including postpartum days for those with shorter gestations [32]. Furthermore, we avoided assessing cumulative dose or a fixed exposure window after gestational day 139 because pregnancies with shorter gestations would have less opportunity for exposure, which could cause immortal time bias [55]. Therefore, to evaluate the impact of OCS use later in pregnancy on PTB, we assessed time-dependent exposure on each gestational day between day 140 and 259, allowing for daily changes in use (any/none) and dose (high/low/none). In the primary analysis, an additional 30 days of exposure was included after OCS and DMARD end dates to allow for potential residual immunosuppressive or anti-inflammatory effects to impact intrauterine infection. Therefore, in addition to use after gestational day 139, we also considered use of these medications in the 30 days before gestational day 140 (i.e. starting on gestational day 110) to define exposure. Daily doses equivalent to ⩾10 mg of prednisone were considered to be high, as previous studies indicated increased infection risk around 10 mg of prednisone [7–11]. In sensitivity analyses, we took two other approaches to time-dependent exposure modeling. First, exposed person-time ended on the reported end dates, i.e. the 30 additional days of exposure were removed. Second, in an intention-to treat analysis, exposed person-time began with the earliest reported start date of medication use after gestational day 139 and continued until censoring (described below) [55]. In sensitivity and dose analyses, we collapsed B-DMARD and NB-DMARD exposures into DMARD exposure given similar PTB rates.
Statistical analysis
We used modified Poisson regression to estimate risk ratios (RR) and 95% CI between trajectory group before gestational day 140 and PTB [56]. With time since gestational day 140 as the time scale, we used Cox proportional hazard models to estimate hazard ratios (HR) for medication exposure after gestational day 139 and PTB. Women were censored at gestational day 259 if their delivery date was after that (i.e. not preterm). Due to the limited number of PTBs, we used propensity scores, described below, to adjust for potential confounders [57]. First, we estimated crude associations using one model for each medication exposure. Then, we adjusted each model for the propensity score. Finally, we mutually adjusted for all medications and propensity scores within one model. Due to limited size, we reported early PTB risk by OCS exposure during the first half of pregnancy only.
Potential confounders considered for adjustment by propensity score included: last menstrual period year, gestational age at enrolment, maternal age, race and ethnicity, socio-economic status (Hollingshead categories [46]), primiparity, multiple gestation, ⩾5 servings of alcohol in the first trimester, cigarette smoking in the first trimester, pre-pregnancy overweight or obesity, history of diabetes, history of hypertension, autoimmune disease comorbidities, NSAID use during the first half of pregnancy, and disease severity measures at enrolment as continuous variables. For analyses of exposures after gestational day 139, we also considered for adjustment cumulative OCS dose trajectory (high/low/none) and use of other medications of interest (any/none) during pregnancy and before the start of the exposure window. We estimated propensity scores for each medication exposure group during each exposure window. For time-varying exposures, the propensity score was calculated based on any exposure to a medication of interest after gestational day 139, classifying those with both high and low dose exposures as having high dose. We included in the propensity score models variables that were associated with both the outcome and the exposures, with the exception of race/ethnicity and maternal age, which we included regardless of their association with exposure and outcome. We imputed missing values with single imputation for HAQ (n = 3), pain score (n = 2), and global patient score (n = 2), using predictors of the variables. To explore the impact of adjusting for factors related to disease severity, we removed self-assessed measures of disease severity at the time of enrolment and use of medications of interest before the start of the exposure window from the propensity score models predicting exposure after gestational day 139.
Results
The median gestational age at enrolment was 75 days for RA (n = 528), 70 days for IBD (n = 217), and 90 days for asthma (n = 234) subcohorts. Maternal characteristics are described by non-mutually exclusive medication exposure groups in the second half of pregnancy in Table 1 (RA) and Supplementary Tables S1–2, available at Rheumatology online (IBD and asthma). Women were primarily non-Hispanic White with the highest levels of socio-economic status, and the average maternal age was at least 31 years across all medication groups. The highest RA severity and lowest asthma control were observed among women with OCS exposure. PTB risk was 15.5% (n = 82) for RA, 14.3% (n = 31) for IBD and 8.6% (n = 20) for asthma.
Table 1.
Characteristics among women in the RA subcohort by exposures (not mutually exclusive), n = 528
| Any exposure after gestational day 139a | ||||||
|---|---|---|---|---|---|---|
| Oral corticosteroid | B-DMARD | NB-DMARD | ||||
| Yes | No | Yes | No | Yes | No | |
| Characteristic | n=250 | n=278 | n=208 | n=320 | n=96 | n=432 |
| LMP Year 2009–2014, n (%) | 121 (48.4) | 155 (55.8) | 118 (56.7) | 158 (49.4) | 65 (67.7) | 211 (48.8) |
| Maternal age, mean (SD) | 32.3 (4.6) | 32.3 (4.7) | 32.6 (4.5) | 32.1 (4.7) | 32.3 (4.6) | 32.3 (4.6) |
| Non-Hispanic White, n (%) | 196 (78.4) | 221 (79.5) | 174 (83.7) | 243 (75.9) | 76 (79.2) | 341 (78.9) |
| Socioeconomic status, Hollingshead categories 1 or 2b, n (%) | 197 (78.8) | 213 (76.6) | 166 (79.8) | 244 (76.3) | 74 (77.1) | 336 (77.8) |
| Primiparous, n (%) | 120 (48.0) | 129 (46.4) | 99 (47.6) | 150 (46.9) | 42 (43.8) | 207 (47.9) |
| Multiple gestation, n (%) | 16 (6.4) | 7 (2.5) | 11 (5.3) | 12 (3.8) | 2 (2.1) | 21 (4.9) |
| ≥5 servings of alcohol in the first trimester, n (%) | 41 (16.4) | 56 (20.1) | 28 (13.5) | 69 (21.6) | 14 (14.6) | 83 (19.2) |
| First trimester cigarette smoking, n (%) | 14 (5.6) | 19 (6.8) | 9 (4.3) | 24 (7.5) | 4 (4.2) | 29 (6.7) |
| Pre-pregnancy hypertension, n (%) | 20 (8.0) | 17 (6.1) | 20 (9.6) | 17 (5.3) | 11 (11.5) | 26 (6.0) |
| IBD, lupus, or ankylosing spondylitis, n (%) | 16 (6.4) | 13 (4.7) | 15 (7.2) | 14 (4.4) | 3 (3.1) | 26 (6.0) |
| NSAID use before gestational day 110, n (%) | 102 (40.8) | 86 (30.9) | 76 (36.5) | 112 (35.0) | 41 (42.7) | 147 (34.0) |
| Oral corticosteroid use before gestational day 110, n (%) | 205 (82.0) | 39 (14.0) | 83 (39.9) | 161 (50.3) | 53 (55.2) | 191 (44.2) |
| B-DMARD use before gestational day 110, n (%) | 172 (68.8) | 183 (65.8) | 207 (99.5) | 148 (46.3) | 63 (65.6) | 292 (67.6) |
| NB-DMARD use before gestational day 110, n (%) | 88 (35.2) | 71 (25.5) | 58 (27.9) | 101 (31.6) | 88 (91.7) | 71 (16.4) |
| HAQ at enrollment, mean (SD)c | 0.67 (0.67) | 0.40 (0.52) | 0.49 (0.60) | 0.56 (0.62) | 0.47 (0.58) | 0.54 (0.62) |
| Pain score at enrollment, mean (SD)d | 34.4 (29.5) | 23.7 (25.3) | 27.2 (27.7) | 29.7 (28.0) | 24.8 (26.5) | 29.6 (28.2) |
| Global score at enrollment, mean (SD)d | 29.9 (27.1) | 19.9 (23.1) | 22.7 (24.5) | 26.0 (26.2) | 20.5 (22.9) | 25.6 (26.0) |
An additional 30 days of exposure was added to reported end dates of use.
Calculated using Hollingshead categories based on maternal and paternal education and occupation; Possible Range: 1, highest to 5, lowest. Missing for eight women.
Imputed for three women; possible range: 0, no disability to 3, completely disabled.
Imputed for two women; pain score possible range: 0, no pain to 100, severe pain; patient’s global score possible range: 0, very well to 100, very poor.
B-DMARD: biologic disease modifying antirheumatic drug; LMP: last menstrual period; NB-DMARD: non-biologic disease modifying antirheumatic drug.
RA: OCS dose earlier in pregnancy and PTB
For clusters of OCS cumulative dose trajectories during the first 139 gestational days, the low trajectory had a mean cumulative dose of 264.9 mg (Table 2; Fig. 2), the medium trajectory had a mean cumulative dose of 883.0 mg, and the high trajectory, with only 15 women, had a mean cumulative dose of 2, 208.6 mg prednisone equivalent, with the highest total number of days of OCS use of 137 days and the highest average daily dose of 16.1 mg prednisone equivalent. Compared with no OCS use before gestational day 140, PTB risk was increased in the high (adjusted (a) RR: 4.77 (95% CI: 2.76, 8.26); Table 3) and medium (aRR: 1.81 (95% CI: 1.10, 2.97)) cumulative dose trajectory groups. The low cumulative dose trajectory group was associated with an increased PTB risk that was not statistically significant (aRR: 1.38 (95% CI: 0.79, 2.38)). DMARD use before gestational day 140 was not associated with PTB (b-DMARD aHR: 1.08 (95% CI: 0.70, 1.66); nb-DMARD aHR: 0.87 (95% CI: 0.55, 1.38)).
Table 2.
OCS exposure between LMP and day 139 by trajectory group for RA (n = 254)
| Trajectory group | Total number of days of oral corticosteroid use | Average daily prednisone equivalent dosea (mg) | Total cumulative prednisone equivalent dose (mg) | ||||
|---|---|---|---|---|---|---|---|
| n | Min, Max | Mean (s.d.) | Min, Max | Mean (s.d.) | Min, Max | Mean (s.d.) | |
| High dose trajectory | 15 | 134, 139 | 137 (1.5) | 11.5, 25.0 | 16.1 (3.2) | 1580, 3350 | 2208.6 (432.1) |
| Medium dose trajectory | 122 | 13, 139 | 123.7 (26.7) | 4.6, 55.4 | 8.0 (5.8) | 410, 2010 | 883.0 (273.5) |
| Low dose trajectory | 117 | 1, 139 | 45.4 (36.5) | 1.0, 41.7 | 7.0 (5.2) | 10, 960 | 264.9 (206.4) |
Average dose on days with oral corticosteroid use.
max: maximum; min: minimum.
Fig. 2.
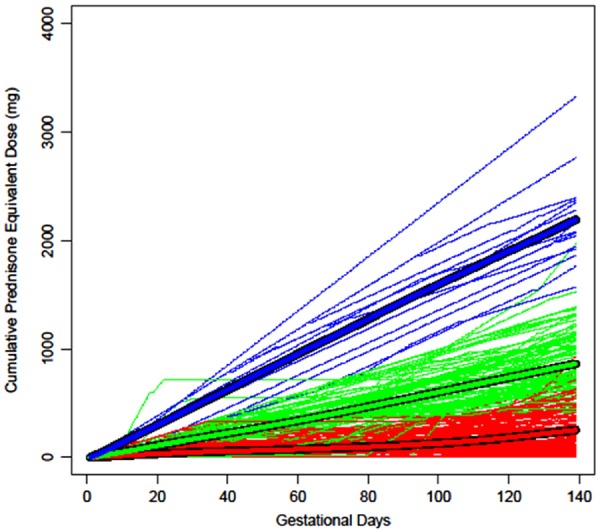
Oral corticosteroid cumulative dose trajectories between the last menstrual period and gestational day 139 (n = 254)
The thick lines represent the mean cumulative dose on each gestational day for each group and the thin lines represent each woman’s observed trajectory; blue = high, green = medium and red = low dose trajectories.
Table 3.
Association between exposures before day 140 and preterm birth for RA (n = 524)
| Medication exposure before gestational Day 140a | n | Preterm birth | % Preterm birth | Crude RR (95% CI) | Adjusted RRb (95% CI) | Mutually adjusted RRc (95% CI) |
|---|---|---|---|---|---|---|
| Oral corticosteroid | ||||||
| High dose trajectory | 15 | 10 | 66.7 | 6.92 (4.15, 11.55) | 4.77 (2.76, 8.26) | 4.74 (2.72, 8.25) |
| Medium dose trajectory | 122 | 28 | 23.0 | 2.38 (1.46, 3.89) | 1.81 (1.10, 2.97) | 1.87 (1.13, 3.10) |
| Low dose trajectory | 117 | 17 | 14.5 | 1.51 (0.85, 2.67) | 1.38 (0.79, 2.38) | 1.38 (0.80, 2.40) |
| No oral corticosteroid | 270 | 26 | 9.6 | Reference | Reference | Reference |
| B-DMARD | 354 | 55 | 15.5 | 1.02 (0.66, 1.56) | 1.08 (0.70, 1.66) | 1.07 (0.71, 1.61) |
| No B-DMARD | 170 | 26 | 15.3 | Reference | Reference | Reference |
| NB-DMARD | 161 | 23 | 14.3 | 0.89 (0.57, 1.40) | 0.87 (0.55, 1.38) | 0.85 (0.55, 1.30) |
| No NB-DMARD | 363 | 58 | 16.0 | Reference | Reference | Reference |
| No medication of interest | 56 | 4 | 7.1 | NA | NA | NA |
Mutually exclusive oral corticosteroid trajectory groups and any vs none B-DMARD and NB-DMARD exposure groups.
One model for each medication of interest. RR adjusted for quintiles of the propensity score: last menstrual period year (<2009, ≤2009), maternal age, race and ethnicity (Non-Hispanic White, Other), multiple gestation, ≥5 servings of alcohol in the first trimester, autoimmune disease comorbidity (IBD, lupus or ankylosing spondylitis), non-steroidal anti-inflammatory drug use before gestational day 140 (any, none), and HAQ Disability Index, pain score and global score at enrolment.
One model, adjusting for each medication exposure group of interest and each associated propensity score.
B-DMARD, biologic disease modifying antirheumatic drug; NB-DMARD, non-biologic disease-modifying antirheumatic drug; RR, risk ratio.
Risk of early PTB was 2.2% (n = 6) among women with no OCS use before day 140, and was 26.7% (n = 4), 3.3% (n = 4) and 3.4% (n = 4), respectively, in the high, medium and low trajectory groups.
RA: OCS exposure later in pregnancy and PTB
The aHR for OCS exposure after gestational day 139, as compared with no OCS exposure during that time was 1.64 (95% CI: 0.92, 2.92; Table 4). DMARDs were not associated with an increased PTB rate (B-DMARD aHR: 0.91 (95% CI: 0.56, 1.47); NB-DMARD aHR: 0.90 (95% CI: 0.48, 1.68)). Exposure to ⩾10 mg of prednisone equivalent dose after gestational day 139 was associated with an increased PTB rate (aHR: 2.45, 95% CI: 1.32, 4.56; Table 4), whereas <10 mg was associated with a modest increased PTB rate that was not statistically significant (aHR: 1.18 (95% CI: 0.60, 2.30)).
Table 4.
Association between exposures after day 139 and preterm birth for RA
| Time-dependent medication exposure after gestational day 139a | n | Preterm birth | Person- weeks | Rate (per 1000/ week) | Crude HR (95% CI) | Adjusted HRb (95% CI) | Mutually adjusted HRc (95% CI) |
|---|---|---|---|---|---|---|---|
| Oral corticosteroid | 253 | 51 | 4530 | 11.3 | 2.81 (1.80, 4.39) | 1.64 (0.92, 2.92) | 1.83 (1.01, 3.32) |
| No oral corticosteroid | 368 | 31 | 7415 | 4.2 | Reference | Reference | Reference |
| B-DMARD | 215 | 25 | 4146 | 6.0 | 0.90 (0.56, 1.44) | 0.91 (0.56, 1.47) | 0.85 (0.52, 1.37) |
| No B-DMARD | 389 | 57 | 7798 | 7.3 | Reference | Reference | Reference |
| NB-DMARD | 102 | 12 | 2092 | 5.7 | 0.83 (0.45, 1.53) | 0.90 (0.48, 1.68) | 0.94 (0.49, 1.78) |
| No NB-DMARD | 447 | 70 | 9852 | 7.1 | Reference | Reference | Reference |
| No medication of interest | 234 | 18 | 4044 | 4.5 | NA | NA | NA |
| Oral corticosteroid ≥10 mg/dayd | 141 | 32 | 1836 | 17.4 | 4.57 (2.79, 7.49) | 2.45 (1.32, 4.56) | 2.47 (1.32, 4.63) |
| Oral corticosteroid <10 mg/dayd | 177 | 19 | 2610 | 7.3 | 1.80 (1.02, 3.19) | 1.18 (0.60, 2.30) | 1.23 (0.63, 2.41) |
| No oral corticosteroid | 371 | 31 | 7469 | 4.2 | Reference | Reference | Reference |
| DMARD | 266 | 33 | 5263 | 6.3 | 0.90 (0.58, 1.39) | 0.89 (0.57, 1.41) | 0.88 (0.56, 1.40) |
| No DMARD | 335 | 49 | 6606 | 7.4 | Reference | Reference | Reference |
| No medication of interest | 232 | 18 | 4074 | 4.4 | NA | NA | NA |
An additional 30 days of exposure was added to reported end dates of use for oral corticosteroids and DMARDs.
One model for each medication of interest. HR adjusted for quintiles of the propensity score: last menstrual period year (<2009, ≤2009), maternal age, race and ethnicity (Non-Hispanic White, Other), multiple gestation, ≥5 servings of alcohol in the first trimester, autoimmune disease comorbidity (IBD, lupus or ankylosing spondylitis), oral corticosteroid cumulative dose trajectory before gestational day 110 (high, low, none), non-steroidal anti-inflammatory drug before gestational day 110 (any, none) and HAQ Disability Index, pain score and global score at enrolment.
One model, adjusting for each medication exposure group of interest and each associated propensity score.
Prednisone equivalent dose.
B-DMARD, biologic disease modifying antirheumatic drug; HR, hazard ratio; NA, not applicable; NB-DMARD, non-biologic disease-modifying antirheumatic drug.
Point estimates for OCS exposure tended to attenuate with adjustment for potential confounders and then strengthen slightly when including all medication exposures in the same model. Compared with including factors related to disease severity, adjusted associations for OCS exposure and PTB tended to be less attenuated when they were omitted (Supplementary Table S3, available at Rheumatology online). In sensitivity analyses, results tended to be strengthened when removing the 30 additional days of exposure following end dates or when classifying as exposed all person-time following the earliest medication use after gestational day 139 (Supplementary Table S4, available at Rheumatology online).
Exploratory analysis for IBD and asthma
For IBD, estimates of the association between cumulative dose trajectory group during the first 139 days (Supplementary Table S5 and Supplementary Fig. S2, available at Rheumatology online) and PTB were imprecise and were unadjusted (Supplementary Table S6, available at Rheumatology online; high group HR: 2.17 (95% CI: 0.63, 7.45); low group HR 1.34 (95% CI: 0.59, 3.05)). For asthma, any OCS exposure during the first half of pregnancy was associated with a suggested increased PTB risk (Supplementary Table S6, available at Rheumatology online; aRR 2.15 (95% CI: 0.83, 5.60)). Estimates were imprecise (Supplementary Table S7, available at Rheumatology online); however, exposure to OCSs overall after day 139 was not strongly associated with PTB in the IBD (Supplementary Table S7, available at Rheumatology online; aHR 1.09 (95% CI: 0.45, 2.60)) and asthma (aHR 1.22 (95% CI: 0.27, 5.50)) subcohorts. For IBD, unadjusted HRs for exposure to ⩾20 mg of prednisone equivalent dose after day 139 were 2.06 (95% CI: 0.84, 5.04) and 0.68 (95% CI: 0.09, 5.04)) for <20 mg (Supplementary Table S8, available at Rheumatology online). Other medications of interest for IBD and asthma were not strongly associated with an increased PTB risk (Supplementary Tables S6–8, available at Rheumatology online).
Comment
Among women with RA, there was a suggestion of trend for increasing cumulative OCS dose during the first half of pregnancy and increased PTB risk. High and medium dose trajectories were associated with an increased PTB risk compared with no OCS exposure. However, the lowest trajectory was associated with a more modest increased PTB risk that was not statistically significant. Doses of OCS ⩾10 mg of prednisone equivalent later in pregnancy were associated with an ∼2.5-fold increased PTB rate. However, PTB rate was not significantly increased for doses <10 mg. B-DMARD and NB-DMARD exposure earlier and later in pregnancy were not associated with PTB.
Exploratory analysis for early PTB suggested an increased risk among those with the highest OCS doses; however, the association should be confirmed in a study with adequate power for PTB subtypes. In exploratory analyses for IBD and asthma, estimates were imprecise. Nevertheless, for asthma, there was a suggested increase in PTB following OCS use, especially during the first half of pregnancy. For IBD, there was a suggested increase in PTB for high OCS dose, although estimates were imprecise and were unadjusted for confounders. Overall, IBD and asthma exploratory analyses align with the direction of the associations in the RA analysis despite limitations of precision and inability to adjust for IBD severity.
Building upon our previous report that patterns of higher vs lower prednisone dose during the first 32 gestational weeks were associated with shorter gestation among women with RA [32], we found that higher OCS doses in two time windows (i.e. earlier and later in pregnancy) vs no OCS use were positively associated with the clinically-relevant outcome of PTB. Although unadjusted for confounders, other studies reported that prednisone dose >10 mg/day anytime during pregnancy was associated with PTB in women with systemic lupus erythematosus [25], whereas gestational age at delivery did not differ significantly between prednisone dose >7.5 mg/day vs ⩽7.5 mg/day anytime during pregnancy in women with RA [23].
OCS dose during pregnancy is tightly linked with disease activity [3], and maternal autoimmune disease and disease control/activity are also associated with increased PTB risk [29, 33–43]. Therefore, confounding by disease severity would be expected to bias the OCS dose–PTB association upward. We adjusted for self-assessed RA severity at enrolment, usually during the first trimester, and for disease-related medication exposures earlier in pregnancy when considering exposures later in pregnancy. Adjusting for factors related to disease severity tended to attenuate point estimates. Ideally, we would have measures of disease severity at the time of every medication start, stop or dose change to account for time-varying confounding later in pregnancy [58].
In accordance with our hypothesis, high OCS doses later in pregnancy were associated with an increase in PTB. This positive association could be due to a true effect of OCS on PTB, for example by OCS contributing to subclinical intrauterine infection and triggering PTB as previously proposed [13], in combination with residual confounding by time-varying severity. However, the link between OCS dose and PTB was also observed for OCS exposures earlier in pregnancy. This could be due to a direct effect of OCS dose early in pregnancy on PTB development, an indirect effect of OCS dose early in pregnancy on PTB through OCS dose later in pregnancy, and/or time-varying confounding by disease severity. Lending support to the hypothesis that OCS-related infection contributes to PTB, Desai et al. reported that increasing steroid dose was associated with an increased serious infection risk among pregnant women with autoimmune conditions [7]. This association was independent of NB-DMARDs and tumor necrosis factor inhibitors, which were not associated with infection, just as they were not associated with PTB in our study.
Associations were strongest when removing the additional 30 days of exposure in sensitivity analyses indicating that future studies of OCS and PTB should not take this approach. Our study had limitations in addition to being unable to account for time-varying confounding of OCS dose and PTB by disease severity changes. First, we did not systematically collect clinical information to characterize type of RA, such as presence of erosions and rheumatoid factor and anti-citrullinated protein antibody positivity. Second, the limited study size led to imprecise results, especially in our exploratory analyses for IBD and asthma. It also prevented us from thorough examination of PTB subtypes (early/late, spontaneous/indicated) [59], which have been differentially associated with subclinical chorioamnionitis [60], and the interaction between OCS and B-DMARDs, as infection risk may be greatest among individuals using both treatments [61]. Third, women may have under-reported doses or medication use duration, possibly resulting in upward bias for the associations between low OCS dose and PTB. Nevertheless, maternal self-report of OCS use among women with RA and asthma appears to be more complete than information from medical records alone [44]. Fourth, results may reflect selection bias if loss to follow-up and pregnancy loss are associated with the exposures and PTB. Fifth, women with RA tended to have relatively low disease severity, and the observed associations may not generalize to women with greater disease activity. Women were primarily non-Hispanic white with older maternal age and higher socio-economic status, although these factors are not expected to modify the associations. Finally, we were unable to evaluate the hypothesized mechanism between OCS and PTB through subclinical intrauterine infection because placental pathology was unavailable. Combining all OCS doses together into one exposure may result in a positive or null association between OCS and PTB depending on the distribution of OCS doses used in a study population, and evaluating OCS dose instead of any OCS use provides nuanced information about associations with perinatal outcomes. The positive association between high dose OCS and PTB observed among women with RA in this study may reflect residual confounding by underlying disease severity or an effect of OCS at high doses or a combination of confounding and medication effect. However, the lack of a clear positive association between low OCS dose, b-DMARDs, and nb-DMARDs and PTB is reassuring for women who are able to manage RA with low OCS doses and DMARDs during pregnancy.
Supplementary Material
Acknowledgements
G.B. was supported by the National Institutes of Health (TL1TR001443).
Funding: This study was supported by a career development award from the Eunice Kennedy Shriver National Institute of Child Health & Human Development, National Institutes of Health (R00HD082412, K. Palmsten).
Disclosure statement : MotherToBaby Pregnancy Studies are or have been funded by research grants from AbbVie, Amgen, Apotex, Barr, Bristol-Myers Squibb (BMS), Par, Kali, Sandoz, Teva, Roche/Genetech, GSK, UCB, Pfizer, Janssen, Celgene, Regeneron, Takeda, Sanofi-Aventis/Genzyme and by a grant from the Agency for Healthcare Research and Quality (R18HS018474, CD Chambers). The funders had no role in the study design, in the data collection, in the analysis and interpretation of data, in the writing of the report, or in the decision to submit the article for publication.
Supplementary data
Supplementary data are available at Rheumatology online.
References
- 1. Ferguson CB, Mahsud-Dornan S, Patterson RN.. Inflammatory bowel disease in pregnancy. BMJ 2008;337:a427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Østensen M, Förger F.. Management of RA medications in pregnant patients. Nat Rev Rheumatol 2009;5:382–90. [DOI] [PubMed] [Google Scholar]
- 3. Mitchell K, Kaul M, Clowse ME.. The management of rheumatic diseases in pregnancy. Scand J Rheumatol 2010;39:99–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Ateka-Barrutia O, Khamashta MA.. The challenge of pregnancy for patients with SLE. Lupus 2013;22:1295–308. [DOI] [PubMed] [Google Scholar]
- 5. Gregersen TL, Ulrik CS.. Safety of bronchodilators and corticosteroids for asthma during pregnancy: what we know and what we need to do better. J Asthma Allergy 2013;6:117–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Namazy JA, Schatz M.. The safety of asthma medications during pregnancy: an update for clinicians. Ther Adv Respir Dis 2014;8:103–10. [DOI] [PubMed] [Google Scholar]
- 7. Desai RJ, Bateman BT, Huybrechts KF. et al. Risk of serious infections associated with use of immunosuppressive agents in pregnant women with autoimmune inflammatory conditions: cohort study. BMJ 2017;356:j895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Schneeweiss S, Setoguchi S, Weinblatt ME. et al. Anti-tumor necrosis factor alpha therapy and the risk of serious bacterial infections in elderly patients with rheumatoid arthritis. Arthritis Rheum 2007;56:1754–64. [DOI] [PubMed] [Google Scholar]
- 9. Dixon WG, Kezouh A, Bernatsky S, Suissa S.. The influence of systemic glucocorticoid therapy upon the risk of non-serious infection in older patients with rheumatoid arthritis: a nested case-control study. Ann Rheum Dis 2011;70:956–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Grijalva CG, Chen L, Delzell E. et al. Initiation of tumor necrosis factor-alpha antagonists and the risk of hospitalization for infection in patients with autoimmune diseases. JAMA 2011;306:2331–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Winthrop KL. Infections and biologic therapy in rheumatoid arthritis: our changing understanding of risk and prevention. Rheum Dis Clin North Am 2012;38:727–45. [DOI] [PubMed] [Google Scholar]
- 12. Goldenberg RL, Culhane JF, Iams JD, Romero R.. Epidemiology and causes of preterm birth. Lancet 2008;371:75–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Schatz M, Dombrowski MP, Wise R. et al. The relationship of asthma medication use to perinatal outcomes. J Allergy Clin Immunol 2004;113:1040–5. [DOI] [PubMed] [Google Scholar]
- 14. Schatz M, Patterson R, Zeitz S, O'Rourke J, Melam H.. Corticosteroid therapy for the pregnant asthmatic patient. JAMA 1975;233:804–7. [PubMed] [Google Scholar]
- 15. Perlow JH, Montgomery D, Morgan MA, Towers CV, Pronto M.. Severity of asthma and perinatal outcome. Am J Obstet Gynecol 1992;167:963–7. [DOI] [PubMed] [Google Scholar]
- 16. Schatz M, Zeiger RS, Hoffman CP. et al. Perinatal outcomes in the pregnancies of asthmatic women: a prospective controlled analysis. Am J Respir Criti Care Med 1995;151:1170–4. [DOI] [PubMed] [Google Scholar]
- 17. Olesen C, Thrane N, Nielsen GL, Sørensen HT, Olsen J.. A population-based prescription study of asthma drugs during pregnancy: changing the intensity of asthma therapy and perinatal outcomes. Respiration 2001;68:256–61. [DOI] [PubMed] [Google Scholar]
- 18. Bracken MB, Triche EW, Belanger K. et al. Asthma symptoms, severity, and drug therapy: a prospective study of effects on 2205 pregnancies. Obstet Gynecol 2003;102:739–52. [DOI] [PubMed] [Google Scholar]
- 19. Bakhireva LN, Jones KL, Schatz M, Johnson D, Chambers CD.. Asthma medication use in pregnancy and fetal growth. J Allergy Clin Immunol 2005;116:503–9. [DOI] [PubMed] [Google Scholar]
- 20. Norgard B, Pedersen L, Christensen LA, Sorensen HT.. Therapeutic drug use in women with Crohn's disease and birth outcomes: a Danish nationwide cohort study. Am J Gastroenterol 2007;102:1406–13. [DOI] [PubMed] [Google Scholar]
- 21. Boyd HA, Basit S, Harpsoe MC, Wohlfahrt J, Jess T.. Inflammatory bowel disease and risk of adverse pregnancy outcomes. PLoS One 2015;10:e0129567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Broms G, Granath F, Stephansson O, Kieler H.. Preterm birth in women with inflammatory bowel disease - the association with disease activity and drug treatment. Scand J Gastroenterol 2016;51:1462–9. [DOI] [PubMed] [Google Scholar]
- 23. de Man YA, Hazes JM, van der Heide H. et al. Association of higher rheumatoid arthritis disease activity during pregnancy with lower birth weight: results of a national prospective study. Arthritis Rheum 2009;60:3196–206. [DOI] [PubMed] [Google Scholar]
- 24. Chambers CD, Johnson DL, Robinson LK. et al. Birth outcomes in women who have taken leflunomide during pregnancy. Arthritis Rheum 2010;62:1494–503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Clark CA, Spitzer KA, Nadler JN, Laskin CA.. Preterm deliveries in women with systemic lupus erythematosus. J Rheumatol 2003;30:2127–32. [PubMed] [Google Scholar]
- 26. Chakravarty EF, Colon I, Langen ES. et al. Factors that predict prematurity and preeclampsia in pregnancies that are complicated by systemic lupus erythematosus. Am J Obstet Gynecol 2005;192:1897–904. [DOI] [PubMed] [Google Scholar]
- 27. Al Arfaj AS, Khalil N.. Pregnancy outcome in 396 pregnancies in patients with SLE in Saudi Arabia. Lupus 2010;19:1665–73. [DOI] [PubMed] [Google Scholar]
- 28. Gur C, Diav-Citrin O, Shechtman S, Arnon J, Ornoy A.. Pregnancy outcome after first trimester exposure to corticosteroids: a prospective controlled study. Reprod Toxicol 2004;18:93–101. [DOI] [PubMed] [Google Scholar]
- 29. Bandoli G, Palmsten K, Forbess Smith CJ, Chambers CD.. A review of systemic corticosteroid use in pregnancy and the risk of select pregnancy and birth outcomes. Rheum Dis Clin North Am 2017;43:489–502. PMC5604866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Palmsten K, Hernandez-Diaz S, Chambers CD. et al. The most commonly dispensed prescription medications among pregnant women enrolled in the U.S. Medicaid Program. Obstet Gynecol 2015;126:465–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Andrade SE, Gurwitz JH, Davis RL. et al. Prescription drug use in pregnancy. Am J Obstet Gynecol 2004;191:398–407. [DOI] [PubMed] [Google Scholar]
- 32. Palmsten K, Rolland M, Hebert MF. et al. Patterns of prednisone use during pregnancy in women with rheumatoid arthritis: daily and cumulative dose. Pharmacoepidemiol Drug Saf 2018;27:430–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Norgard B, Hundborg HH, Jacobsen BA, Nielsen GL, Fonager K.. Disease activity in pregnant women with Crohn's disease and birth outcomes: a regional Danish cohort study. Am J Gastroenterol 2007;102:1947–54. [DOI] [PubMed] [Google Scholar]
- 34. Kammerlander H, Nielsen J, Kjeldsen J. et al. The effect of disease activity on birth outcomes in a nationwide cohort of women with moderate to severe inflammatory bowel disease. Inflamm Bowel Dis 2017;23:1011–8. [DOI] [PubMed] [Google Scholar]
- 35. Reed SD, Vollan TA, Svec MA.. Pregnancy outcomes in women with rheumatoid arthritis in Washington State. Matern Child Health J 2006;10:361–6. [DOI] [PubMed] [Google Scholar]
- 36. Stephansson O, Larsson H, Pedersen L. et al. Crohn's disease is a risk factor for preterm birth. Clin Gastroenterol Hepatol 2010;8:509–15. [DOI] [PubMed] [Google Scholar]
- 37. Firoozi F, Lemiere C, Beauchesne MF. et al. Impact of maternal asthma on perinatal outcomes: a two-stage sampling cohort study. Eur J Epidemiol 2012;27:205–14. [DOI] [PubMed] [Google Scholar]
- 38. Norgaard M, Larsson H, Pedersen L. et al. Rheumatoid arthritis and birth outcomes: a Danish and Swedish nationwide prevalence study. J Int Med 2010;268:329–37. [DOI] [PubMed] [Google Scholar]
- 39. Bharti B, Lee SJ, Lindsay SP. et al. Disease severity and pregnancy outcomes in women with rheumatoid arthritis: results from the organization of teratology information specialists autoimmune diseases in pregnancy project. J Rheumatol 2015;42:1376–82. [DOI] [PubMed] [Google Scholar]
- 40. Chen C-Y, Chen Y-H, Lin H-C, Chen S-F, Lin H-C.. Increased risk of adverse pregnancy outcomes for hospitalisation of women with lupus during pregnancy: a nationwide population-based study. Clin Exp Rheumatol 2010;28:49–55. [PubMed] [Google Scholar]
- 41. Wei S, Lai K, Yang Z, Zeng K.. Systemic lupus erythematosus and risk of preterm birth: a systematic review and meta-analysis of observational studies. Lupus 2017;26:563–71. [DOI] [PubMed] [Google Scholar]
- 42. Broms G, Granath F, Linder M. et al. Birth outcomes in women with inflammatory bowel disease: effects of disease activity and drug exposure. Inflamm Bowel Dis 2014;20:1091–8. [DOI] [PubMed] [Google Scholar]
- 43. Bakhireva LN, Schatz M, Jones KL, Chambers CD. et al. Asthma control during pregnancy and the risk of preterm delivery or impaired fetal growth. Ann Allergy Asthma Immunol 2008;101:137–43. [DOI] [PubMed] [Google Scholar]
- 44. Palmsten K, Hulugalle A, Bandoli G. et al. Agreement between maternal report and medical records during pregnancy: medications for rheumatoid arthritis and asthma. Paediatr Perinat Epidemiol 2018;32:68–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Chambers C, Johnson DL, Kiernan E.. Approach to evaluating pregnancy safety of anti-rheumatic medications in the OTIS MotherToBaby pregnancy studies: what have we learned? Rheumatology 2018;57:v34–v39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Hollingshead AB. Four factor index of social status, unpublished working paper, 1975. New Haven, CT: Yale University. https://artlesstanzim.files.wordpress.com/2014/05/hollinghead-four-factors-2.pdf (2 September 2019, date last accessed).
- 47. Fries JF, Spitz P, Kraines RG, Holman HR.. Measurement of patient outcome in arthritis. Arthritis Rheum 1980;23:137–45. [DOI] [PubMed] [Google Scholar]
- 48. Bruce B, Fries JF.. The Stanford Health Assessment Questionnaire: dimensions and practical applications. Health Qual Life Outcomes 2003;1:20–165587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Schimmer BP, Funder JW.. ACTH, adrenal steroids, and pharmacology of the adrenal cortex In: Brunton LL, Chabner BA, Knollmann BC, eds. Goodman & Gilman's: the pharmacological basis of therapeutics. 12th edn. New York: McGraw-Hill Medical, 2011: 1209–36. [Google Scholar]
- 50. Genolini C, Falissard B.. KmL: a package to cluster longitudinal data. Comput Methods Programs Biomed 2011;104:e112–121. [DOI] [PubMed] [Google Scholar]
- 51. Genolini C, Falissard B. (2016) Package “kml.” https://cran.r-project.org/web/packages/kml/kml.pdf (24 May 2018, date last accessed).
- 52. Bandoli G, Kuo GM, Sugathan R. et al. Longitudinal trajectories of antidepressant use in pregnancy and the postnatal period. Arch Womens Ment Health 2018;21:411–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Hurault-Delarue C, Chouquet C, Savy N. et al. How to take into account exposure to drugs over time in pharmacoepidemiology studies of pregnant women? Pharmacoepidemiol Drug Saf 2016;25:770–7. [DOI] [PubMed] [Google Scholar]
- 54. Hurault-Delarue C, Chouquet C, Savy N. et al. Interest of the trajectory method for the evaluation of outcomes after in utero drug exposure: example of anxiolytics and hypnotics. Pharmacoepidemiol Drug Saf 2017;26:561–9. [DOI] [PubMed] [Google Scholar]
- 55. Matok I, Azoulay L, Yin H, Suissa S.. Immortal time bias in observational studies of drug effects in pregnancy. Birth Defects Res A Clin Mol Teratol 2014;100:658–62. [DOI] [PubMed] [Google Scholar]
- 56. Zou G. A modified poisson regression approach to prospective studies with binary data. Am J Epidemiol 2004;159:702–6. [DOI] [PubMed] [Google Scholar]
- 57. Glynn RJ, Schneeweiss S, Sturmer T.. Indications for propensity scores and review of their use in pharmacoepidemiology. Basic Clin Pharmacol Toxicol 2006;98:253–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Bodnar LM, Davidian M, Siega-Riz AM, Tsiatis AA.. Marginal structural models for analyzing causal effects of time-dependent treatments: an application in perinatal epidemiology. Am J Epidemiol 2004;159:926–34. [DOI] [PubMed] [Google Scholar]
- 59. Kramer MS, Papageorghiou A, Culhane J. et al. Challenges in defining and classifying the preterm birth syndrome. Am J Obstet Gynecol 2012;206:108–12. [DOI] [PubMed] [Google Scholar]
- 60. Palmsten K, Nelson KK, Laurent LC. et al. Subclinical and clinical chorioamnionitis, fetal vasculitis, and risk for preterm birth: a cohort study. Placenta 2018;67:54–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Strangfeld A, Eveslage M, Schneider M. et al. Treatment benefit or survival of the fittest: what drives the time-dependent decrease in serious infection rates under TNF inhibition and what does this imply for the individual patient?. Ann Rheum Dis 2011;70:1914–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.