Abstract
Objective
To evaluate the effect of secukinumab on radiographic progression through 52 weeks in patients with PsA from the FUTURE 5 study.
Methods
Patients with active PsA, stratified by prior anti-TNF use (naïve or inadequate response), were randomized to s.c. secukinumab 300 mg load (300 mg), 150 mg load (150 mg), 150 mg no load regimens or placebo at baseline, at weeks 1, 2 and 3 and every 4 weeks starting at week 4. Radiographic progression was assessed by change in van der Heijde-modified total Sharp score (vdH-mTSS; mean of two readers). Statistical analysis used a linear mixed-effects model (random slope) at weeks 24 and 52, and observed data at week 52. Assessments at week 52 included additional efficacy endpoints (non-responders imputation and mixed-effects models for repeated measures) and safety.
Results
The majority (86.6%) of patients completed 52 weeks of treatment. The proportion of patients with no radiographic progression (change from baseline in vdH-mTSS ⩽0.5) was 91.8, 85.2 and 87.2% in 300, 150 and 150 mg no load groups, respectively, at week 52. The change in vdH-mTSS from baseline to week 52 using random slope [mean change (s.e.)] was –0.18 (0.17), 0.11 (0.18) and –0.20 (0.18) in 300, 150 and 150 mg no load groups, respectively; the corresponding observed data [mean change (s.d.)] was –0.09 (1.02), 0.13 (1.39) and 0.21 (1.15). Clinical efficacy endpoints were sustained, and no new or unexpected safety signals were reported through 52 weeks.
Conclusion
Secukinumab 300 and 150 mg with or without s.c. loading regimen provided sustained low rates of radiographic progression through 52 weeks of treatment.
Trial registration
ClinicalTrials.gov, http://clinicaltrials.gov, NCT02404350.
Keywords: spondylarthropathies (including psoriatic arthritis), biological therapies, immunotherapy, cytokines and inflammatory mediators, inflammation, pain assessment and management
Rheumatology key messages
Secukinumab demonstrated sustained low rates of radiographic progression through 52 weeks of treatment.
The proportion of patients with no radiographic progression ranged from 85 to 92% in this large PsA study.
Secukinumab-treated patients reported comparable radiographic data independent of the statistical method applied.
Introduction
PsA is a chronic inflammatory peripheral arthritis associated with axial disease, dactylitis, enthesitis, skin and nail psoriasis, physical disability, and reduced health-related quality of life [1, 2]. More prominently, it is associated with structural changes to the joints (erosive or proliferative bone changes, erosive joint destruction, joint space narrowing, fluffy periostitis and pencil-in-cup deformities), which are detected and characterized radiographically [3–5]. Approximately 50% of PsA patients are diagnosed with erosive joint destruction within 2 years of disease onset [6]. Assessment of radiographic disease progression in PsA is an important outcome from a clinical perspective, to assess disease severity and to quantify the effect of treatment on disease progression [7]. IL-17A plays a crucial role in the pathogenesis of PsA; structural damage and radiographic disease progression are strongly correlated with the presence of IL-17A-producing immune cells at the inflamed joints [8–10].
Secukinumab, a human monoclonal IgG1κ antibody that directly inhibits IL-17A, has demonstrated rapid and sustained efficacy in patients with PsA across Phase 3 FUTURE studies [11–14]. Secukinumab 300 and 150 mg s.c. doses have been approved for treatment of PsA. In the FUTURE 1 study, secukinumab 150 mg reported sustained clinical responses with a low rate of radiographic progression over 3 years [15]. In FUTURE 5, secukinumab 300 and 150 mg s.c. dosing regimens significantly lowered the rate of radiographic disease progression vs placebo at week 24 [12]. Herein, we present the effects of secukinumab 300 and 150 mg dosing regimens on radiographic disease progression through 52 weeks from the FUTURE 5 study.
Methods
Patients and study design
FUTURE 5 (NCT02404350) is an ongoing randomized placebo-controlled 2-year Phase 3 trial. The detailed inclusion and exclusion criteria and study design have been reported previously [12]. Patients ⩾18 years of age, fulfilling the CASPAR (ClASsification criteria for Psoriatic ARthritis) criteria [16], with symptoms of active PsA for at least 6 months: three or more tender joints (TJC) and three or more swollen joints (SJC) despite treatment with NSAIDs, conventional synthetic DMARDs (csDMARDs) and/or anti-TNF agents. Concomitant use of glucocorticoids (⩽10 mg/day), NSAIDs and MTX (⩽25 mg/week) were allowed. Patients with an inadequate response (IR) or who stopped treatment due to safety or intolerance to an anti-TNF agent (hereafter collectively referred as anti-TNF-IR) were included in this study. Key exclusion criteria included active/history of ongoing infection, prior use of a biologic other than an anti-TNF agent, use of greater than three different anti-TNF agents and active inflammatory disease other than PsA.
Eligible patients were randomized (2:2:2:3) to one of four treatment groups: self-administered s.c. secukinumab 300 mg with loading dose (300 mg), 150 mg with loading dose (150 mg), 150 mg without loading dose (150 mg no load) or placebo. All patients received s.c. secukinumab 300 mg, 150 mg or placebo at baseline, at weeks 1, 2 and 3, and every 4 weeks starting at week 4. Patients, investigators and assessors remained blinded to the treatment assignment until all patients reached the week 52 visit. At week 16, non-responders (<20% reduction in TJC and/or SJC) in the placebo group were switched to s.c. secukinumab 300 or 150 mg and all remaining patients (responders) on placebo were switched at week 24. Patients were stratified according to prior use of anti-TNF therapy status [i.e. patients who were naïve to anti-TNF therapy (anti-TNF-naïve) vs those who were anti-TNF-IR]. The study was planned to enrol no more than 30% anti-TNF-IR patients.
The study was conducted in accordance with the Declaration of Helsinki [17] and was approved by institutional review boards or independent ethics committees at each participating centre. Written informed consent was obtained from all enrolled patients. Data were collected in accordance with Good Clinical Practice guidelines by the study investigators and were analysed by the sponsor. Data presented here are from the week 52 (1 year) analysis of the study.
Efficacy outcomes
Radiographic disease progression was assessed by change from baseline in van der Heijde-modified total Sharp score [vdH-mTSS; sum of bone erosion (0–5 in the hands and 0–10 in the feet) and joint space narrowing (0–4) scores] for PsA [18]. Radiographic scores were assessed from two reading sessions: reading session 1: baseline, weeks 16 and 24; and reading session 2: baseline, weeks 16, 24 and 52. The vdH-mTSS assessment was based on hands/wrists/feet radiographs obtained from reading session 1 for 24-week analysis and from reading session 2 for 52-week analysis. Mean scores were assessed by two blinded readers independently (if there was an adjudicator involved then three readers were used) who were blinded to all patient information, treatment allocation and order of radiographs. The total radiographic score (hands and feet combined) ranged from 0 to 528, with higher scores indicating more articular damage. Data are shown for weeks 24 and 52. As recommended by van der Heijde et al., a change from baseline in vdH-mTSS ⩽0.5 was used to define no structural progression; the proportion of patients with no structural progression was determined [18] at week 52.
Other assessments at week 52 included the ACR 20, 50 and 70 responses, Psoriasis Area and Severity Index 75 and 90 responses, mean change from baseline in HAQ–Disability Index, 28-joint DAS using CRP (DAS28-CRP), and Short Form 36 Physical Component Summary Score (SF-36 PCS), and resolution of enthesitis and dactylitis [19–25].
Pre-specified subgroup analyses based on previous use of anti-TNF therapy status (naïve vs IR) on radiographic disease progression was also performed.
Overall safety and tolerability of secukinumab over the 52 weeks was assessed by monitoring adverse events (AEs), serious AEs, laboratory assessments and vital signs.
Radiography study population
The 52-week analysis
At week 52, data are presented by linear mixed-effects model (random intercept and random slopes, which will be referred to as random slope hereafter) and as observed analysis. The random slope model at week 52 included patients with baseline and at least one post-baseline radiographic assessment (evaluable radiographs) from week 16, 24 or 52. Observed analysis included patients who had radiographic assessments at baseline and week 52.
The 24-week analysis
In addition to week 52, data at week 24 are presented using linear extrapolation and random slope models. Linear extrapolation and random slope analyses at week 24 included patients with baseline and at least one post-baseline radiographic assessment from week 16 or 24. This 24-week linear extrapolation was reanalysed after an incorrect mapping of the radiographic data was identified in the interim analysis.[12] It was confirmed that 10/936 (1.1%) patients with baseline and post-baseline radiographs were impacted by the incorrect mapping and were included in this 24-week linear extrapolation reanalysis.
Statistical analyses
Data are presented for patients originally randomized to secukinumab and placebo. In the linear mixed-effects model, the random slope and random intercept was used to calculate the changes in radiographic structural progression score over 52 weeks. The week 24 random slope excluded data after escape for placebo patients who received escape therapy at week 16. The model assumed approximately linear progression over time and estimated a difference in rates (slopes) of progression over 52 weeks to compare treatment arms. The model accounted for covariates of time, treatment, treatment-by-time interaction, baseline mTSS score, body weight and prior anti-TNF status.
The linear extrapolation approach used the change rate from baseline to week 16 to impute the missing change rate for week 24; that is, for patients who met the criteria for early escape at week 16 and did not have week 24 measurement on the same treatment. Linear extrapolation was used to impute the missing values at week 24 if at least two assessments existed before week 24 (including baseline assessment).
Cumulative probability plot was generated as described previously [26] to show the vdH-mTSS for all patients according to dose group from baseline to week 52.
Clinical efficacy analyses used logistic regression for binary variables, and mixed-effects models for repeated measures (MMRM) for continuous variables. All analyses included treatment and randomization stratum (anti-TNF status) as a factor (time, treatment-by-time and baseline-by-time interaction were also used for MMRM), and body weight and the corresponding baseline value as a covariate. Missing values and placebo patients rescued at week 16 were imputed as non-responders for binary variables (rescue penalty) and the missing at random assumption of the MMRM analysis was applied for continuous variables.
Safety analysis included all patients who received one or more dose of study drug and were summarized descriptively. Patients were evaluated according to the treatment they received. All analyses were computed with SAS version 9.4.
Results
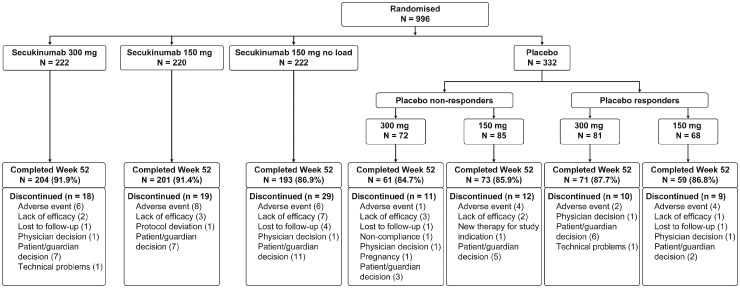
Of the 996 patients randomized at baseline, 86.6% (862/996) completed 52 weeks of treatment [300 mg: 91.9% (204/222), 150 mg: 91.4% (201/220), 150 mg no load: 86.9% (193/222) and placebo-secukinumab: 79.5% (264/332)]. A total of 108 (10.8%) patients discontinued before or at week 52. The detailed disposition up to week 52 including reasons for discontinuation is presented in Fig. 1. Demographics and baseline disease characteristics were balanced between treatment arms and have been reported previously [12]. At baseline, ∼70% of patients were anti-TNF-naïve and 50% were receiving concomitant MTX. The number of patients with evaluable radiographs at both baseline and week 52 were as follows: 207 (93.2%) in 300 mg, 203 (92.3%) in 150 mg and 195 (87.8%) in 150 mg no load. The number of patients included in the linear extrapolation and random slope analyses at week 24 were: 217 (97.7%) in the 300 mg, 213 (96.8%) in 150 mg, 210 (94.6%) in 150 mg no load and 296 (89.2%) in the placebo group.
Fig. 1.
Patient disposition through week 52
Patients received s.c. secukinumab 300 or 150 mg, or placebo at baseline, weeks 1, 2 and 3, and every 4 weeks starting at week 4. Patients in the 150 mg no load arm were administered placebo at weeks 1, 2 and 3. At week 16, non-responders (<20% reduction in TJC and/or SJC) in the placebo group were switched to s.c. secukinumab 300 or 150 mg in a double-blind manner, and similarly, responders were switched at week 24. N: number of randomized patients; SJC: swollen joint count; TJC: tender joint count.
Efficacy
Radiographic progression
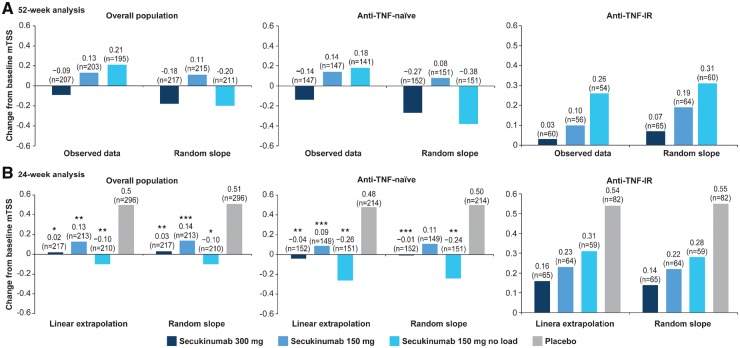
In the overall population, the radiographic progression rate was low at week 52 across all treatment groups. Mean changes from baseline in vdH-mTSS using observed data and random slope analysis at week 52 are presented in Fig. 2A. Random slope data (s.e.) showed numerically lower progression in the 300 mg [–0.18 (0.17)] and 150 mg no load [–0.20 (0.18)] groups compared with the 150 mg [0.11 (0.18)] group. Observed data (s.d.) showed lower [–0.09 (1.02)] progression in the 300 mg group compared with the 150 [0.13 (1.39)] and 150 mg no load [0.21 (1.15)] groups.
Fig. 2.
Mean changes from baseline in vdH-mTSS
*P < 0.001; **P < 0.01; ***P < 0.05 un-adjusted P-values versus placebo (P-values are from non-parametric ANCOVA). Overall population: N = 222, 220, 222 and 332; anti-TNF-naïve: N = 154, 155, 158 and 234; anti-TNF-IR: N = 68, 65, 64 and 98 patients in secukinumab 300 mg, 150 mg, 150 mg no load and placebo groups, respectively. IR: inadequate response; N: total number of patients randomized; n: number of patients included in the linear extrapolation and random slope at week 24 and evaluable patients at week 52; vdH-mTSS: van der Heijde-modified total Sharp score.
Mean changes (s.d.) from baseline in vdH-mTSS by linear extrapolation were 0.02 (1.34; P < 0.001), 0.13 (1.22; P < 0.01) and –0.10 (2.87; P < 0.01) in the 300, 150 and 150 mg no load groups, respectively, vs 0.50 (1.71) in the placebo group at week 24 (Fig. 2B). Corresponding mean changes (s.e.) from baseline by random slope analysis were very similar, with 0.03 (0.13; P < 0.01), 0.14 (0.13; P < 0.05) and –0.10 (0.13; P < 0.001), respectively, vs 0.51 (0.11) at week 24 (Fig. 2B).
In patients with prior anti-TNF therapy status, the mean changes from baseline in vdH-mTSS by random slope, observed data and linear extrapolation analyses followed a similar trend of low radiographic progression to that in the overall population (Fig. 2A and B). The anti-TNF-naïve group showed lower progression than the anti-TNF-IR group.
Additionally, the mean changes from baseline at week 24 in vdH-mTSS by linear extrapolation from patients with baseline and at least one post-baseline radiographic assessment from week 16, 24 or 52 are presented in supplementary Fig. S1, available at Rheumatology online (from reading session 2).
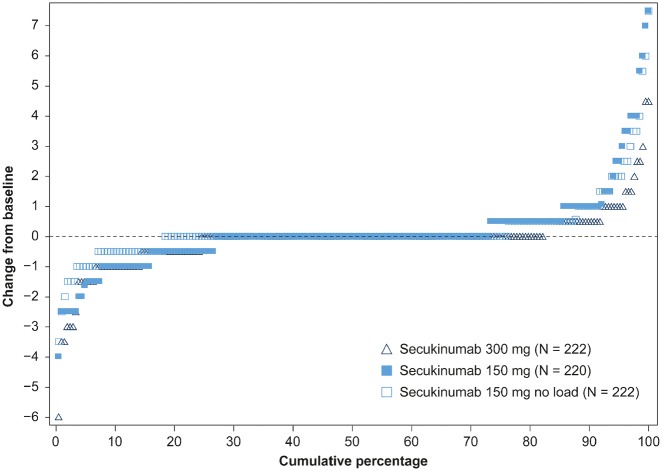
The proportion of patients with no radiographic progression (change from baseline in vdH-mTSS ⩽0.5) with secukinumab at week 52 were 91.8% (300 mg), 85.2% (150 mg) and 87.2% (150 mg no load). Cumulative probability plots in vdH-mTSS change from baseline to week 52 showed the scores of the individual patients in the different secukinumab doses over 52 weeks of therapy (Fig. 3). The findings in the pre-specified subgroup of patients by anti-TNF therapy status through 52 weeks were similar. The proportion of anti-TNF-naïve patients with no radiographic progression at week 52 were: 94.6% (300 mg), 85.7% (150 mg) and 88.7% (150 mg no load), and in anti-TNF-IR patients were: 85.0% (300 mg), 83.9% (150 mg) and 83.3% (150 mg no load).
Fig. 3.
Cumulative probability plot in vdH-mTSS at week 52
The proportion of patients with no radiographic progression (change from baseline in vdH-mTSS ≤0.5) with secukinumab at week 52 were 91.8% (n = 207; 300 mg), 85.2% (n = 203; 150 mg) and 87.2% (n = 195; 150 mg no load). Cumulative probability in each dose group in the range of 0 to 100%. The lower score indicates more inhibition achieved. N: total number of patients randomized; n: number of evaluable patients; vdH-mTSS: van der Heijde-modified total Sharp score.
Clinical efficacy
The ACR20 response rates reported at week 16 [12] were sustained through 52 weeks of secukinumab treatment. At week 52, the ACR20 response was 68.9, 64.1 and 65.8% in secukinumab 300, 150 and 150 mg no load groups, respectively (Table 1). At week 52, the ACR20 response rate in anti-TNF-naïve patients was 72.7, 74.2 and 69.6% in the secukinumab 300 mg, 150 mg and 150 mg no load groups, respectively; in comparison, a numerically lower response (60.3, 40.0 and 56.3%) was observed in anti-TNF-IR patients (Table 1). In the overall population, the least squares mean change (s.e.) from baseline in DAS28-CRP score was –1.92 (0.07), –1.72 (0.07) and –1.83 (0.07) in the 300, 150 and 150 mg no load groups, respectively, at week 52. Corresponding mean change in SF-36 PCS score was 7.16 (0.46), 6.31 (0.46) and 7.03 (0.47), respectively, at week 52. Improvements reported across all other efficacy endpoints at week 16 were also sustained at week 52 (Table 1).
Table 1.
Summary of clinical efficacy at week 52
| Overall population | Anti-TNF-naïve | Anti-TNF-IR | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Secukinumab | |||||||||
| Efficacy endpoints | 300 mg | 150 mg | 150 mg no load | 300 mg | 150 mg | 150 mg no load | 300 mg | 150 mg | 150 mg no load |
| (N = 222) | (N = 220) | (N = 222) | (N = 154) | (N = 155) | (N = 158) | (N = 68) | (N = 65) | (N = 64) | |
| ACR20 response, % | 68.9 | 64.1 | 65.8 | 72.7 | 74.2 | 69.6 | 60.3 | 40.0 | 56.3 |
| ACR50 response, % | 46.8 | 41.4 | 43.2 | 53.2 | 49.7 | 48.1 | 32.4 | 21.5 | 31.3 |
| ACR70 response, % | 28.8 | 25.9 | 24.8 | 33.1 | 30.3 | 26.6 | 19.1 | 15.4 | 20.3 |
| PASI 75 response (BL psoriasis ≥3% body surface area), % | (n = 110) 74.5 | (n = 125) 63.2 | (n = 117) 59.8 | (n = 75) 77.3 | (n = 95) 69.5 | (n = 92) 63.0 | (n = 35) 68.6 | (n = 30) 43.3 | (n = 25) 48.0 |
| PASI 90 response (BL psoriasis ≥3% body surface area), % | (n = 110) 57.3 | (n = 125) 47.2 | (n = 117) 41.0 | (n = 75) 56.0 | (n = 95) 54.7 | (n = 92) 43.5 | (n = 35) 60.0 | (n = 30) 23.3 | (n = 25) 32.0 |
| HAQ-DI score, LS mean change from BL (s.e.) | −0.56 (0.04) | −0.50 (0.04) | −0.52 (0.04) | −0.59 (0.04) | −0.57 (0.04) | −0.57 (0.04) | −0.57 (0.08) | −0.40 (0.08) | −0.46 (0.08) |
| Enthesitis resolution (enthesitis subset), % | (n = 140) 70.7 | (n = 141) 63.1 | (n = 129) 61.2 | (n = 97) 76.3 | (n = 93) 69.9 | (n = 87) 64.4 | (n = 43) 58.1 | (n = 48) 50.0 | (n = 42) 54.8 |
| Dactylitis resolution (dactylitis subset), % | (n = 82) 69.5 | (n = 80) 72.5 | (n = 103) 68.9 | (n = 55) 80.0 | (n = 52) 80.8 | (n = 79) 72.2 | (n = 27) 48.1 | (n = 28) 57.1 | (n = 24) 58.3 |
Missing values were imputed as non-response for binary variables and MMRM analysis for continuous variables. BL: baseline; DAS28-CRP: 28-joint DAS using CRP; HAQ-DI: HAQ-Disability Index; LS: least squares; PASI: Psoriasis Area and Severity Index; MMRM: mixed-effect model repeated measure; N: total number of patients; n: number of patients with that symptom at baseline.
Safety
The safety data were consistent with that reported previously [12]. Over the entire treatment period, mean secukinumab exposure was 309.0 days (patient-years: 815.5). The most commonly reported treatment-emergent AEs were nasopharyngitis and upper respiratory tract infection (Table 2). Most serious AEs represented single events with no discernible pattern. Serious AE rates were low and comparable in both any secukinumab 300 mg (8.1%) and 150 mg (8.3%) dose groups. The rate of discontinuation due to any AE was also low and comparable between any secukinumab 300 mg (2.4%) and 150 mg (3.4%) dose groups. The rate of selected AEs of interest are shown in Table 2. No new or unexpected safety signals, no tuberculosis infections and no deaths were reported.
Table 2.
Clinical safety with secukinumab during the entire treatment period through week 52a
| Variable | Any secukinumab 300 mg | Any secukinumab 150 mg |
|---|---|---|
| (N = 371)b | (N = 593)b | |
| Total exposure | ||
| Mean (s.d.) | 298.5 (83.22) | 315.5 (83.21) |
| Patient-years | 303.2 | 512.2 |
| Any AE, n (%) | 259 (69.8) | 436 (73.5) |
| Any SAE, n (%) | 30 (8.1) | 49 (8.3) |
| Discontinuation due to AE, n (%) | 9 (2.4) | 20 (3.4) |
| Death, n (%) | 0 | 0 |
| Most frequent AEs, n (EAIR per 100 patient-years)c | ||
| Nasopharyngitis | 41 (14.4) | 67 (14.1) |
| Upper respiratory tract infection | 24 (8.2) | 55 (11.4) |
| Hypertension | 14 (4.7) | 28 (5.6) |
| Diarrhoea | 17 (5.8) | 24 (4.8) |
| Influenza | 16 (5.4) | 15 (3.0) |
| Headache | 10 (3.4) | 25 (5.0) |
| Selected AEs of interest, n (EAIR per 100 patient-years) | ||
| Serious infections | 5 (1.7) | 8 (1.6) |
| Fungal infection | 0 (0.0) | 2 (0.4) |
| Candida infection (HLT) | 9 (3.0) | 9 (1.8) |
| Oral candidiasis | 6 (2.0) | 3 (0.6) |
| Ulcerative colitis | 0 (0.0) | 1 (0.2) |
| Crohn’s disease | 0 (0.0) | 2 (0.4) |
| MACE | 1 (0.3) | 1 (0.2) |
| Malignant/unspecified tumours | 2 (0.7) | 3 (0.6) |
| Neutropenia | 3 (1.0) | 8 (1.6) |
The entire treatment period for safety data was from baseline through to the week 52 visit of each patient enrolled in this study.
Any secukinumab group represents each originally randomized secukinumab patient plus placebo patients who switched to active treatment at week 16 or 24.
AEs that occurred with an EAIR of at least 5.0 cases per 100 patient-years in either any secukinumab 300 or 150 mg group over the entire treatment period. Events listed according to preferred term in the Medical Dictionary for Regulatory Activities (MedDRA version 20.1), sorted in descending order of EAIR. AE: adverse event; EAIR: exposure-adjusted incidence rate; HLT: high-level term; MACE: major adverse cardiovascular event; N: total number of patients; SAE: serious adverse event.
Discussion
This randomized placebo-controlled large Phase 3 study of s.c. secukinumab 300 mg and 150 mg, with or without loading regimen, demonstrated sustained low rates of radiographic disease progression, with consistent clinical efficacy and a favourable safety profile, in patients with active PsA over 52 weeks of treatment. The majority (87%) of patients enrolled at baseline remained in the study for 52 weeks, reflecting a high retention rate. The results reported in this paper further substantiate previously published data from the FUTURE 1 and 5 studies that demonstrated significant and sustained inhibition of radiographic progression in patients with PsA [12, 15]. Furthermore, this study validates the treatment effect of secukinumab on radiographic disease progression by utilizing different statistical methodologies to analyse the radiographic data, and obtaining very similar results from each of these methods. The radiographic assessment in this study used random slope, evaluable radiograph and linear extrapolation. The random slope analysis accounted for more baseline covariates and handled statistical uncertainty more robustly than single imputation, which may be a preferred way of handing missing data. The linear extrapolation approach handled inter-current events (i.e. placebo escape patients’ data) and used linearity to model vdH-mTSS over time. This model assumes a linear rate of progression for all patients and uses single imputation. However, the uncertainty surrounding the missing data may not be sufficiently accounted for due to slightly larger CIs.
The majority (85–92%) of secukinumab-treated patients in the overall population showed no radiographic progression at week 52. The vdH-mTSS were slightly different between random slope and observed analyses presented at week 52, with random slope analysis showing lower radiographic progression scores across all secukinumab doses compared with observed data. At week 24, the vdH-mTSS showed significant reduction of radiographic progression in secukinumab-treated patients compared with placebo; the scores were almost identical across all dose groups as measured by linear extrapolation and random slope.
Similar to the overall population, low rates of radiographic progression were also observed in secukinumab-treated patients regardless of prior anti-TNF therapy status. In the subgroup of TNF-naïve patients, there was a significant reduction of radiographic progression in the secukinumab-treated patients at week 24 compared with placebo, which was lower through 52 weeks of secukinumab treatment. In the (smaller) group of anti-TNF-IR patients, higher progression was observed with secukinumab compared with those observed in TNF-naïve patients. The lower response in the anti-TNF-IR group may be attributed to a relatively small number patients and the heterogeneity of the anti-TNF subgroup, which included patients with treatment failure due to lack of efficacy, IR, intolerance or safety issues [27] with anti-TNF therapies as specified in the inclusion criteria of the current study. An additional reason may be that these patients are harder to treat. Previous studies confirm the inhibition of radiographic disease progression in PsA patients treated with anti-TNF agents [28–32]. The radiographic findings from the current study are aligned with those reported in the anti-TNF trials and further establish the therapeutic effect of secukinumab in patients with PsA.
Sustained improvement was observed in secukinumab-treated patients across all efficacy endpoints including disease activity and physical function through week 52 in the overall population. Clinical improvements observed at week 16 were sustained through 52 weeks of secukinumab treatment, with numerically greater efficacy in anti-TNF-naïve vs -IR patients. The safety profile up to 52 weeks was consistent with that reported in prior secukinumab trials [11–14], with no new or unexpected safety signals reported.
Limitations included that (i) this study was not designed to identify a difference between treatment regimens (load vs no load) or to assess treatment differences in response to previous anti-TNF therapy status and (ii) the data presented in the anti-TNF-IR group should be interpreted with caution due the small patient population.
In conclusion, this study demonstrated that secukinumab 300 and 150 mg provided significant inhibition of radiographic disease progression at week 24, with sustained low rates of progression at week 52. This study confirms the robustness of the radiographic data as there is very low or minimal impact on the outcome irrespective of the statistical methodology used. These results extend previous findings confirming sustained low rates of radiographic progression, clinical efficacy and safety of secukinumab for the treatment of patients with active PsA [11–14].
Supplementary Material
Acknowledgements
The authors thank the patients who participated in this study, the study investigators and John Gallagher, medical consultant, Novartis Pharma AG, Switzerland. Medical writing support, under the guidance of the authors, was provided by Niladri Maity, senior scientific writer for Novartis, India, and Neeta Pillai, scientific editor for Novartis, India. The first draft of this manuscript was written by Niladri Maity based on input from all the authors. The datasets generated during and/or analysed during the current study are not publicly available. Novartis is committed to sharing with qualified external researchers access to patient-level data and supporting clinical documents from eligible studies. These requests are reviewed and approved the basis of scientific merit. All data provided are anonymized to respect the privacy of patients who have participated in the trial, in line with applicable laws and regulations. The data may be requested from the corresponding author.
Funding: The study was sponsored by Novartis Pharma AG, Basel, Switzerland, and designed by the scientific steering committee and Novartis personnel. Manuscript processing charges were funded by Novartis Pharma AG, Basel, Switzerland.
Disclosure statement: D.v.d.H. has received consulting fees from AbbVie, Amgen, Astellas, AstraZeneca, BMS, Boehringer Ingelheim, Celgene, Daiichi, Eli-Lilly, Galapagos, Gilead, Glaxo-Smith-Kline, Janssen, Merck, Novartis, Pfizer, Regeneron, Roche, Sanofi, Takeda and UCB. D.v.d.H. is Director of Imaging Rheumatology. P.J.M. has received research grants from AbbVie, Amgen, BMS, Celgene, Janssen, Lilly, Novartis, Pfizer, SUN and UCB; consulting fees from AbbVie, Amgen, BMS, Celgene, Covagen, Crescendo, Janssen, LEO, Lilly, Merck, Novartis, Pfizer, SUN and UCB; and speakers’ bureau for AbbVie, Amgen, BMS, Celgene, Genentech, Janssen, Lilly, Pfizer and UCB. R.B.M.L. has provided consultation or participation in advisory boards: Abbott/AbbVie, Ablynx, Amgen, Astra-Zeneca, Bristol-Myers Squibb, Centocor, GlaxoSmithKline, Novartis, Merck, Pfizer, Roche, Schering-Plough, UCB and Wyeth; received research grants from Abbott, Amgen, Centocor, Novartis, Pfizer, Roche, Schering-Plough, UCB and Wyeth; received speakers fees from Abbott, Amgen, Bristol-Myers Squibb, Centocor, Merck, Pfizer, Roche, Schering-Plough, UCB and Wyeth. R.B.M.L. is the director of Rheumatology Consultancy BV, which is a registered company under Dutch law. P.R. has received consulting fees for Abbott, AbbVie, Amgen, BMS, Celgene, Janssen, Novartis, Pfizer and Roche; and received research grant from Janssen. H.T. has provided consultation or participation in advisory boards: AbbVie, Novartis, Pfizer, UCB, Eli-Lilly and Janssen; and received Education Grants from Novartis and Pfizer. A.S. has received research/clinical trial grants from AbbVie, Gilead, Sanofi, Regeneron, Amgen, Roche, BMS, Janssen, Lilly, Novartis, Pfizer, UCB, Astra Zeneca, MedImmune, FujiFilm, Nichi-Iko and Mallinckrodt; and speakers’ bureau for AbbVie. E.B. has received consulting and speaking fees from Amgen, Roche, Eli Lilly, Pfizer, MSD and Novartis. S.N. has received consulting and speaker fees from Pfizer, Novartis, Astra-Zeneca, Janssen, Astellas and Roche. X.Z. is an employee of Novartis, with Novartis stock. G.L. is an employee of Novartis, with Novartis stock. A.R. is an employee of Novartis, with Novartis stock. S.M. is an employee of Novartis, with Novartis stock. L.P. is an Employee of Novartis, with Novartis stock.
Supplementary data
Supplementary data are available at Rheumatology online.
References
- 1. Gladman DD, Antoni C, Mease P, Clegg DO, Nash P.. Psoriatic arthritis: epidemiology, clinical features, course, and outcome. Ann Rheum Dis 2005;64(Suppl 2):ii14–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Rosen CF, Mussani F, Chandran V. et al. Patients with psoriatic arthritis have worse quality of life than those with psoriasis alone. Rheumatology (Oxford) 2012;51:571–6. [DOI] [PubMed] [Google Scholar]
- 3. Bond SJ, Farewell VT, Schentag CT, Gladman DD.. Predictors for radiological damage in psoriatic arthritis: results from a single centre. Ann Rheum Dis 2007;66:370–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Cresswell L, Chandran V, Farewell VT, Gladman DD.. Inflammation in an individual joint predicts damage to that joint in psoriatic arthritis. Ann Rheum Dis 2011;70:305–8. [DOI] [PubMed] [Google Scholar]
- 5. Day MS, Nam D, Goodman S, Su EP, Figgie M.. Psoriatic arthritis. J Am Acad Orthop Surg 2012;20:28–37. [DOI] [PubMed] [Google Scholar]
- 6. Kane D, Stafford L, Bresnihan B, FitzGerald O.. A prospective, clinical and radiological study of early psoriatic arthritis: an early synovitis clinic experience. Rheumatology (Oxford) 2003;42:1460–8. [DOI] [PubMed] [Google Scholar]
- 7. Gottlieb A, Korman NJ, Gordon KB. et al. Guidelines of care for the management of psoriasis and psoriatic arthritis: section 2. Psoriatic arthritis: overview and guidelines of care for treatment with an emphasis on the biologics. J Am Acad Dermatol 2008;58:851–64. [DOI] [PubMed] [Google Scholar]
- 8. Mease P, McInnes IB.. Secukinumab: a new treatment option for psoriatic arthritis. Rheumatol Ther 2016;3:5–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Menon B, Gullick NJ, Walter GJ. et al. Interleukin-17+CD8+ T cells are enriched in the joints of patients with psoriatic arthritis and correlate with disease activity and joint damage progression. Arthritis Rheumatol 2014;66:1272–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Rossini M, Viapiana O, Adami S. et al. Focal bone involvement in inflammatory arthritis: the role of IL17. Rheumatol Int 2016;36:469–82. [DOI] [PubMed] [Google Scholar]
- 11. McInnes IB, Mease PJ, Kirkham B. et al. Secukinumab, a human anti-interleukin-17A monoclonal antibody, in patients with psoriatic arthritis (FUTURE 2): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet 2015;386:1137–46. [DOI] [PubMed] [Google Scholar]
- 12. Mease P, van der Heijde D, Landewé R. et al. Secukinumab improves active psoriatic arthritis symptoms and inhibits radiographic progression: primary results from the randomised, double-blind, phase III FUTURE 5 study. Ann Rheum Dis 2018;77:890–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Mease PJ, McInnes IB, Kirkham B. et al. Secukinumab inhibition of interleukin-17A in patients with psoriatic arthritis. N Engl J Med 2015;373:1329–39. [DOI] [PubMed] [Google Scholar]
- 14. Nash P, Mease PJ, McInnes IB. et al. Efficacy and safety of secukinumab administration by autoinjector in patients with psoriatic arthritis: results from a randomized, placebo-controlled trial (FUTURE 3). Arthritis Res Ther 2018;20:47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Mease PJ, Kavanaugh A, Reimold A. et al. Secukinumab in the treatment of psoriatic arthritis: efficacy and safety results through 3 years from the year 1 extension of the randomised phase III FUTURE 1 trial. RMD Open 2018;4:e000723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Taylor W, Gladman D, Helliwell P. et al. Classification criteria for psoriatic arthritis: development of new criteria from a large international study. Arthritis Rheum 2006;54:2665–73. [DOI] [PubMed] [Google Scholar]
- 17.World Medical Association. World Medical Association Declaration of Helsinki: ethical principles for medical research involving human subjects. JAMA 2013;310:2191–4. [DOI] [PubMed] [Google Scholar]
- 18. van der Heijde D, Landewé R, Klareskog L. et al. Presentation and analysis of data on radiographic outcome in clinical trials: experience from the TEMPO study. Arthritis Rheum 2005;52:49–60. [DOI] [PubMed] [Google Scholar]
- 19. Felson DT, Anderson JJ, Boers M. et al. American College of Rheumatology. Preliminary definition of improvement in rheumatoid arthritis. Arthritis Rheum 1995;38:727–35. [DOI] [PubMed] [Google Scholar]
- 20. Fries JF, Spitz P, Kraines RG, Holman HR.. Measurement of patient outcome in arthritis. Arthritis Rheum 1980;23:137–45. [DOI] [PubMed] [Google Scholar]
- 21. Healy PJ, Helliwell PS.. Measuring clinical enthesitis in psoriatic arthritis: assessment of existing measures and development of an instrument specific to psoriatic arthritis. Arthritis Rheum 2008;59:686–91. [DOI] [PubMed] [Google Scholar]
- 22. Helliwell PS, Firth J, Ibrahim GH. et al. Development of an assessment tool for dactylitis in patients with psoriatic arthritis. J Rheumatol 2005;32:1745–50. [PubMed] [Google Scholar]
- 23. Ware JE Jr, Sherbourne CD.. The MOS 36-item short-form health survey (SF-36). I. Conceptual framework and item selection. Med Care 1992;30:473–83. [PubMed] [Google Scholar]
- 24. Weisman S, Pollack CR, Gottschalk RW.. Psoriasis disease severity measures: comparing efficacy of treatments for severe psoriasis. J Dermatolog Treat 2003;14:158–65. [DOI] [PubMed] [Google Scholar]
- 25. Wells G, Becker JC, Teng J. et al. Validation of the 28-joint Disease Activity Score (DAS28) and European League Against Rheumatism response criteria based on C-reactive protein against disease progression in patients with rheumatoid arthritis, and comparison with the DAS28 based on erythrocyte sedimentation rate. Ann Rheum Dis 2009;68:954–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Landewé R, van der Heijde D.. Radiographic progression depicted by probability plots: presenting data with optimal use of individual values. Arthritis Rheum 2004;50:699–706. [DOI] [PubMed] [Google Scholar]
- 27. Mease PJ, Armstrong AW.. Managing patients with psoriatic disease: the diagnosis and pharmacologic treatment of psoriatic arthritis in patients with psoriasis. Drugs 2014;74:423–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Gladman DD, Mease PJ, Ritchlin CT. et al. Adalimumab for long-term treatment of psoriatic arthritis: forty-eight week data from the adalimumab effectiveness in psoriatic arthritis trial. Arthritis Rheum 2007;56:476–88. [DOI] [PubMed] [Google Scholar]
- 29. Kavanaugh A, Antoni CE, Gladman D. et al. The Infliximab Multinational Psoriatic Arthritis Controlled Trial (IMPACT): results of radiographic analyses after 1 year. Ann Rheum Dis 2006;65:1038–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Kavanaugh A, McInnes IB, Mease P. et al. Clinical efficacy, radiographic and safety findings through 5 years of subcutaneous golimumab treatment in patients with active psoriatic arthritis: results from a long-term extension of a randomised, placebo-controlled trial (the GO-REVEAL study). Ann Rheum Dis 2014;73:1689–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Mease PJ, Kivitz AJ, Burch FX. et al. Continued inhibition of radiographic progression in patients with psoriatic arthritis following 2 years of treatment with etanercept. J Rheumatol 2006;33:712–21. [PubMed] [Google Scholar]
- 32. van der Heijde D, Fleischmann R, Wollenhaupt J. et al. Effect of different imputation approaches on the evaluation of radiographic progression in patients with psoriatic arthritis: results of the RAPID-PsA 24-week phase III double-blind randomised placebo-controlled study of certolizumab pegol. Ann Rheum Dis 2014;73:233–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.