Abstract
Objectives
To gain insight into SSc patients’ perspective on quality of care and to survey their preferred quality indicators.
Methods
An online questionnaire about healthcare setting, perceived quality of care (CQ index) and quality indicators, was sent to 2093 patients from 13 Dutch hospitals.
Results
Six hundred and fifty patients (mean age 59 years, 75% women, 32% limited cutaneous SSc, 20% diffuse cutaneous SSc) completed the questionnaire. Mean time to diagnosis was 4.3 years (s.d. 6.9) and was longer in women compared with men (4.8 (s.d. 7.3) vs 2.5 (s.d. 5.0) years). Treatment took place in a SSc expert centre for 58%, regional centre for 29% or in both for 39% of patients. Thirteen percent of patients was not aware of whether their hospital was specialized in SSc. The perceived quality of care was rated with a mean score of 3.2 (s.d. 0.5) (range 1.0–4.0). There were no relevant differences between expert and regional centres. The three prioritized process indicators were: good patient-physician interaction (80%), structural multidisciplinary collaboration (46%) and receiving treatment according to SSc guidelines (44%). Absence of disease progression (66%), organ involvement (33%) and digital ulcers (27%) were the three highest rated outcome indicators.
Conclusion
The perceived quality of care evaluated in our study was fair to good. No differences between expert and regional centres were observed. Our prioritized process and outcome indicators can be added to indicators suggested by SSc experts in earlier studies and can be used to evaluate the quality of care in SSc.
Keywords: systemic sclerosis, healthcare organization, quality of care, quality indicators, patients, perspective, patient-reported outcome measurement
Rheumatology key messages
This study identified preferred process and outcome indicators in systemic sclerosis from a patient’s perspective.
No relevant differences in perceived quality of care between expert and regional centres were observed.
Patient education, definitions of and transparency about expert centres in systemic scleroses should be improved.
Introduction
Providing optimal care for patients with rare, chronic and heterogeneous conditions, like SSc, can be challenging [1, 2]. Patients may present with different signs and symptoms, and may experience high morbidity as well as increased mortality. Medical treatments are applied with varying results and the patient’s journey towards diagnosis and treatment varies greatly among individual patients.
Evaluation of the quality of the currently provided care can help to identify aspects for improvement. Furthermore, it enables comparison of care between centres. In literature, multiple definitions of quality of care exist. In general, it may be defined as the evaluation of values and goals present in the medical care system [3, 4]. When quality of care is evaluated, both outcomes of care and the process itself can be assessed. Furthermore, different stakeholders (e.g. patients, physicians, hospital staff and policy makers) can have diverse perspectives on how healthcare should be evaluated. Ideally, the selection of criteria for good healthcare should be based on agreement between these groups. So far, no clear consensus has been reached on quality indicators in SSc [5]. Two Delphi exercises with physicians resulted in a list of preferred process indicators [6, 7]. This is an important first step in making quality of SSc care tangible. However, criteria for quality of care from the perspective of patients with SSc are still missing and would be of value.
Previous small studies in SSc have identified unmet needs in patient education and organization of hospital visits [8–12]. Patient information, especially about disease progression, and non-pharmacological care was an important unmet need reported by 155 Dutch SSc patients in a previous study [10]. In a qualitative analysis of 25 interviews and a cross-sectional study using surveys (n = 77), patients reported that they preferred improvement in organization of care with regard to the diagnostic process and follow-up visits [8, 11]. Annually, the number of hospital visits is high in SSc patients [9]. This puts a huge strain on patients, especially on those with functional disabilities. Although these studies illustrate patients’ experience and preferences on an individual or regional level, none of these studies assessed quality of care nationwide in multiple hospitals or compared quality of care between expert and regional hospitals.
In 2017, as a nationwide effort to improve healthcare for patients with rare systemic autoimmune diseases, including SSc, the Arthritis Research and Collaboration Hub (ARCH) was initiated in the Netherlands (information about the Dutch healthcare system in general is provided in Table 1). As a starting point, ARCH evaluated the organization and quality of care in SSc from the perspective of patients and healthcare professionals. The present study was part of this first ARCH project. The aim of this study was to evaluate the patients’ perspective of currently provided healthcare for patients with SSc and to identify quality indicators from a patient’s perspective.
Table 1.
The Dutch National Health System
| The Netherlands is a small but densely populated country. The number of physicians per head is relatively low in comparison to other European countries: 329 per 100 000 people [13]. Total expenditure on health as % of Gross Domestic Product was 10.1% in 2018 in the Netherlands. |
| The Dutch system is universal. Primary care plays a major role and is characterized by the gatekeeping principle: hospital care requires referral from a general practitioner (GP) (only 7% of the contacts result in a referral). After receiving a referral, patients can choose in which centre they want to be treated. Basic health insurance is mandatory and covers medical care, medicines and hospital stays, including all medical care for SSc. GPs are affiliated with primary health care centres and most medical specialists are working in hospitals. Tertiary hospitals are most often associated with a university. All patients diagnosed with SSc receive medical care in secondary or tertiary centres. |
| The Dutch National Health System has three managed markets for a universal health insurance package, healthcare purchasing and provision. Health insurers and providers negotiate on price and quality of care. Hospitals are paid through an adapted type of diagnosis-related group system. In most centres, healthcare providers do not financially benefit or lose from an increase in referrals or diagnostic tests. |
| The government aims to enable patients to make choices between insurers and providers and stresses the importance of transparency with regard to quality of care and the development of reliable quality indicators. Choosing these indicators is also a task of the Dutch scientific organizations. |
Methods
Study design
In this cross-sectional study, an online survey was used to gather information from patients with SSc across the Netherlands. Ethical approval was obtained from participating centres and all participants provided written informed consent. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) guidelines were followed (supplementary material, STROBE Checklist section, available at Rheumatology online) [14].
Patients and setting
Setting and participants
Fifteen physicians in 13 different hospitals (seven regional hospitals and six expert centres, median number of 150 SSc patients per hospital (range 40–800)) were asked to invite their SSc patients to participate in the study. An information letter about the study was enclosed to the invitation, explaining the aim and methods of the study. By returning a reply card or sending an email, patients received a link to enter the online survey. A total of 2093 invitations to patients were sent. If patients were treated in two participating hospitals, they could receive this invitation twice. Patients could only take the survey once. The online survey was accessible from 15 December 2017 until 21 February 2018.
Content
The survey was composed by three rheumatologists, one senior researcher and one patient. The questions were discussed in a pilot group, which included three independent researchers, two representatives from the Dutch organization of health professionals (NHPR), two representatives from the Dutch patient organization for systemic autoimmune diseases (NVLE) and one representative from the Dutch Society of Rheumatology. Next, the survey was tested on five patients with SSc.
Questions per theme
Sociodemographic questions included sex, age, status of living (living alone, with partner, with parents, with partner and children, with children, with (a) friend(s)), educational level (primary education and vocational education/university), paid employment (no paid job, <12 h a week, 12 h–30 h a week, >30 h a week).
Disease related questions included the disease subset (limited cutaneous, diffuse cutaneous SSc (lcSSc or dcSSc respectively), other or unknown, disease duration since onset of first symptoms (years) and time since diagnosis (years)).
Multiple-choice questions about healthcare setting included hospital type (Are you treated in an SSc expert centre, regional centre? Are you treated in one, two or more than two hospitals for SSc related symptoms?), travelling time to hospital (<15 min, 15–30 min, 30–60 min, longer than 60 min), number of hospitals visited for SSc treatment last year (1, 2 or >2). Expert centres were defined as hospitals where experts in the field of SSc are working; the five expert centres in the Netherlands were mentioned in the survey.
The Consumer Quality Index for rheumatoid arthritis was used and adapted to measure the quality of care as perceived by patients. This is an instrument validated in patients with rheumatoid arthritis and has also been used in SSc [15, 16]. Subscales on cooperation, data exchange and interaction with the healthcare provider are assessed on a five-point Likert scale (never, sometimes, most of the time, always, I don’t know); the index has a range from 1.0–4.0. Higher Consumer Quality Index scores indicate higher satisfaction with the received healthcare.
Additional questions were based on the results from three focus group interviews with 23 SSc patients and interviews with 12 rheumatologists and five specialized nurses, that took place in September 2017 (for further details about the methods and results of the focus group interviews, see Supplementary Material, Survey Preparation: A Qualitative Study section, and supplementary Tables S1–S5, available at Rheumatology online).
The assessment of unmet needs and preferences included: questions about the burden of disease with regard to traveling time (Do you experience problems with traveling time: yes/no, if yes because of financial, social, physical or logistical reasons?), the choice of location of preferred treatment and out-patient visits. (Do you prefer treatment nearby, in a regional centre only, shared care (combined treatment with regional physician and someone from an expert centre) or by an expert in an expert centre only?).
Questions about patient education and information provision about SSc were evaluated by multi-response questions about their main information source and used devices for patient education (leaflet, book, magazine, internet (computer, smartphone, other), internet (website patient organization, website Dutch Arthritis Society, other), physician, specialized nurse, patients with SSc, I never ask/look for information about SSc, other). Patients were asked if they received information about SSc (yes/no), if this information was understandable (yes/no), if there was any information about SSc on the website of the hospital (yes/no) and whether patients were introduced with patient organizations (yes/no).
Process and outcome indicators previously mentioned in the focus groups were prioritized by using multi-response questions; participants could choose a maximum of three options, including an option to add a new item. The choice of process indicators were: good accessibility of healthcare, satisfaction with interaction between patient and physician, getting the appropriate treatment (defined by receiving treatment according to the Dutch guideline for SSc), number of patients correctly diagnosed, structured multidisciplinary collaboration, collaboration between healthcare providers, annual evaluation of skin score, annual evaluation of pulmonary function, access to a specialized nurse, percentage of patients informed about non-pharmacological care, or other suggestions (free text option). Listed outcome indicators were: improved quality of life, improved daily functioning, decreased fatigue score, decreased pain, improved hand function, absence of disease progression, absence of organ involvement, absence of digital ulcers, absence of recurrent digital ulcers, or other suggestions (free text option). The option ‘I do not know’ was also available in the multi-response questions about quality indicators.
Data collection and analyses
Descriptive statistics were used to summarize patient characteristics. Missing data were not imputed. Means and standard deviations were calculated and compared using t test. Associations between the healthcare satisfaction (Consumer Quality Index subscales) and treatment in an expert centre were investigated. All analyses were performed using Statistical Package for Social Sciences (SPSS) version 25.
Results
Participants
Six hundred and fifty patients (31%) (mean age 59 years, 164 (25%) men and 486 (75%) women) completed the survey. All characteristics are shown in Table 2. LcSSc was reported as disease subset in 207 (32%) patients and 132 (20%) had dcSSc. Remarkably, 254 (39%) patients did not know the subset of their disease. Disease duration was significantly longer in women than in men (mean difference 1.8 years, P = 0.01 [95% CI 0.4, 3.2]). The mean time between onset of first symptoms, including Raynaud’s phenomenon, and diagnosis was 4.3 years (s.d. 6.9). Women reported a significantly longer period between onset of symptoms and diagnosis than men (4.8 vs 2.5 years respectively, mean difference 2.3 years, P < 0.001 [95% CI 1.1, 3.5]). There were no correlations observed between the time to diagnosis and educational level, disease subtype or treatment received in expert centres or regional centres.
Table 2.
Patient characteristics
| n = 650 | |
|---|---|
| Age, mean (s.d.), years | 59 (11) |
| Male, n (%) | 164 (25) |
| Living with partner, n (%) | 359 (55) |
| Educational level, n (%) | |
| Low | 19 (3) |
| Medium | 425 (65) |
| High | 207 (32) |
| Paid employement n (%) | 245 (38) |
| SSc subset, n (%) | |
| LcSSc | 207 (32) |
| DcSSc | 132 (20) |
| Other | 65 (10) |
| Unknown | 250 (38) |
| Time between onset symptoms and diagnosis, mean (s.d.), years | 4.3 (7) |
| Disease duration after diagnosis, (s.d.), years | 8.0 (8) |
| Patients treated in, n (%) | |
| SSc expert centres | 360 (58) |
| Regional hospitals | 182 (29) |
| Unknown | 83 (13) |
Quality of healthcare
Healthcare setting
A total of 252 (39%) patients visited two or more centres for the treatment of SSc. More than half (58%) of the patients was treated in an expert centre. Interestingly, 13% of patients did not know if treatment took place in an expert centre. Traveling time to the hospital was <30 min in 36% of the patients and more than one h in 30%. Traveling time was experienced as a problem by 15% of the patients, mainly due to physical limitations (64%). Shared care was the preferred model of care for 49% (n = 159) of patients; 332 patients (51%) wished to be treated by an SSc expert only.
Patient education
The main source and provider of information about SSc was the physician in 70% (n = 450) of patients. Two-thirds of patients (67%, n = 427) used the internet. The website of the Dutch Arthritis Foundation, the Dutch patient society and other sources were used equally (29%, 28% and 27%, respectively). In 48% of patients, support and education by a specialized nurse was provided. Only 26% received information from a specialized nurse. There were no significant differences in used information resources and age or level of education, although a higher percentage of patients with a lower education level used a specialized nurse as their main information source compared with higher educated patients (42 vs 30%). There was no difference between expert centres and regional hospitals with regard to patient education.
Consumer quality index
The rating of the perceived quality of care and the differences between patients treated in expert centres and regional hospitals are shown in Table 3. The perceived quality of care provided by the physician was rated with a mean score of 3.2 (s.d. 0.5) (scale 1.0–4.0). The majority of patients thought that their physician took them seriously, listened carefully and provided enough opportunity to ask questions, provided clear explanation and had enough time during hospital visits for them. With regard to follow-up outpatient visits, the quality of care perceived in expert centres was significantly better compared with regional hospitals (mean difference −0.35, 95% CI (−0.49, −0.22), P < 0.01). There were no differences between expert and regional centres on the other subscales.
Table 3.
Evaluation of quality of health care by patients with SSc
| Mean CQI (s.d.), range 1.0–4.0 | |||||
|---|---|---|---|---|---|
| Total n = 640 | Expert centre n = 357 | Regional hospital n = 176 | Mean difference (CI) | P-value | |
| Care provided by physician | 3.2 (0.5) | 3.2 (0.8) | 3.2 (0.7) | −0.03 (−0.4, 0.1) | 0.66 |
| n = 620 | n = 353 | n = 167 | |||
| Outpatients follow up visits | 3.3 (0.7) | 3.4 (0.6) | 3.0 (0.7) | −0.35 (−0.49, −0.22) | <0.01 |
| n = 570 | n = 323 | n = 156 | |||
| Collaboration | 3.0 (0.7) | 3.0 (0.7) | 2.9 (0.8) | −0.15 (−0.29, −0.01) | 0.03 |
| n = 255 | n = 167 | n = 50 | |||
| Care provided by nurse | 3.3 (0.9) | 3.4 (0.8) | 3.2 (1.1) | −0.23 (−0.50, 0.05) | 0.10 |
| n = 323 | n = 193 | n = 76 | |||
| Care provided by health professional | 3.3 (0.6) | 3.2 (0.6) | 3.1 (0.7) | −0.05 (−0.22, 0.11) | 0.53 |
CQI, Consumer Quality Index.
Quality indicators
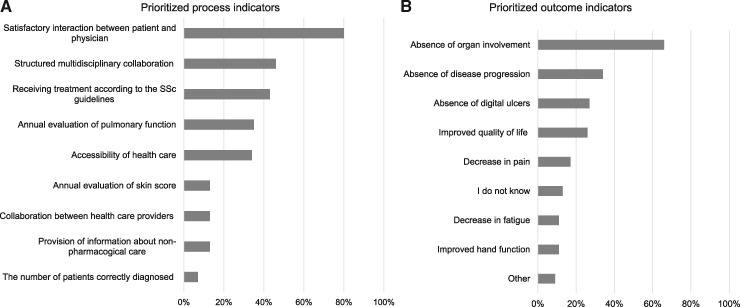
A good patient–physician relationship (80%, n = 513), structural multidisciplinary collaboration (46%, n = 298) and receiving adequate treatment (44%, n = 283) were the three highest rated process indicators. No disease progression (66%, n = 425), no organ involvement (33%, n = 215) and the absence of digital ulcers (27%, n = 171) were the three highest rated outcome indicators. All results are shown in Fig. 1.
Fig. 1.
Prioritized quality indicators (n = 640)
Results from a multi-response question: Which three outcomes are most appropriate to evaluate the quality of care? 1A. process indicators, 1B. outcome indicators.
Discussion
This multicentre study evaluated the currently provided healthcare and quality of care in a large group of SSc patients in the Netherlands and identified quality indicators from a patient’s perspective.
The overall quality of care evaluated in our study using the CQ index was fair to good. The ratings on care provided by the physician and collaboration were comparable to the rating that was given in a smaller Dutch single-centre study among 198 SSc patients published five years ago [15]. In our study, aside from a small difference in outpatient follow-up visits, the quality of care was not perceived lower in regional hospitals compared with SSc expert centres.
This equality in quality of care of centres could have several reasons. Firstly, the standard of care in general is high in the Netherlands, and all participating centres in our study were large hospitals that offer multidisciplinary care. Patients could therefore experience a similar quality of care, regardless of the expert status or performance with regard to SSc care of their hospital. Secondly, 13% of patients stated that they did not know the expert status of their hospital, despite the definitions provided in our survey. At present, there are several definitions on expert centres in the Netherlands, i.e. the definitions according to university centres, ‘top-clinical’ hospitals and SSc expert centres defined in the Dutch SSc guideline. This complicates answering this question for patients and limits comparison of results between expert and regional hospitals. The lack of clarity on the definition of expert centres was a point identified in a national and international debate on how to improve care for SSc patients [17, 18]. For both patients and physicians, it is important that there is one single and clear definition on SSc expert centres. Patients prefer to know where expertise is present and are increasingly involved in their own healthcare nowadays. Moreover, in the Netherlands, patients are able to freely choose in which hospital they want to be treated, so insight into where expert centres for SSc are situated is important to make that choice. Also, this insight helps physicians finding and consulting expert centres. Future initiatives should therefore focus on reaching agreement on a single definition of SSc expert centres and developing strategies to make the expert status of hospitals across the Netherlands accessible and transparent.
The second objective of our study was to identify indicators relevant from a patient’s perspective to evaluate the quality of care in SSc. The three prioritized process indicators were; (i) good patient-physician relationship, (ii) structural multidisciplinary collaboration and (iii) receiving treatment according to the SSc guidelines. Some of our identified indicators are compatible with the indicators resulted from a Delphi round with SSc experts that took place in 2011 [7]. Yearly pulmonary function testing and skin score assessment and adequate treatment were preferred process indicators in both the expert consensus-meeting and the present study.
Additionally, we introduce two new process indicators that meet the values reported in earlier investigations on care from a patient point of view in SSc. Structural multidisciplinary collaboration was reported as an important point for improvement by 77 SSc patients [11]. Also, the importance of the patient–practitioner relationship was emphasized in a qualitative study in SSc patients [11]. The latter indicator was selected as an important process indicator by the vast majority of patients (80%) in our study. In several studies in primary and secondary care patients, patient–practitioner interaction was also identified as a very relevant dimension of service quality [19–21].
There are no established disease-specific outcome indicators to assess quality of care in SSc. Yet, establishing a few ‘hard’ SSc specific outcomes could be very useful. In our study, outcome indicators prioritized by patients were the absence of (i) disease progression, (ii) organ involvement and (iii) digital ulcers. The results from our study are a valuable addition to the existing list of process indicators selected by physicians and provide suggestions for outcome indicators as well. Furthermore, numerous disease-specific PROs have been developed for SSc in the last few years, on several domains [22–24]. Our results can help in deciding which outcome domains are most relevant for patients in the evaluation of the quality of medical practice. We emphasize the importance of a core set of indicators to evaluate quality of care, which are supported by both patients and physicians.
Several other findings related to quality of care came up in our study. The lack of knowledge among patients about their disease subtype (unknown in respectively 39%) was one remarkable observation and could be the result of limited information provision or patient education. Several previous studies have reported on the unmet needs with regard to information in patients with SSc [10, 18, 25].
Besides general information on the disease, the need for additional support or counselling on physical and psychological consequences of SSc have also been reported [1, 11, 26]. These unmet needs fall within the scope of health professionals including specialized nurses. In some Dutch centres, nurses are already involved in SSc care, and satisfaction of this provided care was good in our study. To improve overall care for patients with SSc, access to specialized nurses in all centres seems warranted.
An unexpected finding in our study was the time to diagnosis, which was found to be longer compared with another large study performed in Canada (mean patient-reported time to diagnosis was 2.4 years, n = 813) [27]. Moreover, in our survey the time to establish the diagnosis was twice as long in women compared with men. Further investigation of the diagnostic process in SSc to confirm our findings is needed. In both male and female participants, better recognition of the disease by physicians was indicated as the most important point that should be improved in a cross-sectional survey study we performed in the same group of patients [18]. ‘Time to diagnosis’ could therefore also be suggested as a relevant process indicator reflecting the quality of care in SSc.
Our study has some limitations. Firstly, there could be selection bias, for both the qualitative part of the study and the survey. Only patients who were willing to discuss their condition with other patients and were able to travel attended the focus groups. In addition, a relatively large percentage of respondents to the survey received treatment at a specialty centre (58%). It is possible that these patients have different preferences compared with patients in small, local hospitals who did not participate. Secondly, in order to recruit a large group of patients, we could only send patients one invitation without a reminder, which might explain the estimated response rate of 31%. The response rate will, however, be somewhat higher, because patients who are treated in shared care (39% of patients) could have received the invitation twice if both centres participated in the study. Thirdly, because we could not ask patients in which specific hospital they were treated, due to privacy protection regulations, we do not have data from the non-responders to the invitation, in order to estimate generalizability of our findings. Finally, inherent to a survey study, is that we collected patient-reported information. We were not able to check the provided information in medical records, because it was an anonymous questionnaire. Researchers who intend to perform similar studies should consider offering patients different ways to participate in the study, i.e. online, on paper or at the hospital together with a nurse or patient partner, in order to decrease the risk of selection bias. Also, we suggest including data from medical records on the provided treatment and diagnostic workup. In this way, a broader assessment of quality of care could be accomplished.
Conclusion
This study provides insight into the care currently provided for SSc patients in the Netherlands and the preferred quality indicators, from a patient point of view. We did not observe relevant differences in the perceived quality of care between patients treated in SSc expert centres and regional hospitals. Several points for improvement, particularly with regard to patient education and definition of expertise, were identified. The reported quality indicators added and prioritized by patients to evaluate the quality of healthcare complement the indicators composed by SSc experts in earlier studies and can be considered in the evaluation of care in SSc.
Supplementary Material
Acknowledgements
The authors would like to thank the Dutch Arthritis Foundation (ReumaNederland) for funding the ARCH initiative.
Funding: This work was supported by the Arthritis Research and Collaboration Hub (ARCH) foundation.
Disclosure statement: The authors have declared no conflicts of interest.
Supplementary data
Supplementary data are available at Rheumatology online.
References
- 1. Nakayama A, Tunnicliffe DJ, Thakkar V. et al. Patients’ perspectives and experiences living with systemic sclerosis: a systematic review and thematic synthesis of qualitative studies. J Rheumatol 2016;43:1363–75. [DOI] [PubMed] [Google Scholar]
- 2. Wagner EH, Austin BT, Von Korff M.. Organizing care for patients with chronic illness. Milbank Q 1996;74:511–44. [PubMed] [Google Scholar]
- 3. Donabedian A. Evaluating the quality of medical care. 1966. Milbank Q 2005;83:691–729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Donabedian A. The quality of care. How can it be assessed? JAMA 1988;260:1743–8. [DOI] [PubMed] [Google Scholar]
- 5. Gazi H, Pope JE, Clements P. et al. Outcome measurements in scleroderma: results from a delphi exercise. J Rheumatol 2007;34:501–9. [PubMed] [Google Scholar]
- 6. Khanna D. A standardized core set for systemic sclerosis clinical trials. First step in development of combined response index. Rheumatology 2008;47(Suppl 5):v31–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Khanna D, Kowal-Bielecka O, Khanna PP. et al. Quality indicator set for systemic sclerosis. Clin Exp Rheumatol 2011;29:S33–9. [PMC free article] [PubMed] [Google Scholar]
- 8. Schouffoer AA, Zirkzee EJ, Henquet SM. et al. Needs and preferences regarding health care delivery as perceived by patients with systemic sclerosis. Clin Rheumatol 2011;30:815–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Meijs J, Zirkzee EJ, Schouffoer AA. et al. Health-care utilization in Dutch systemic sclerosis patients. Clin Rheumatol 2014;33:825–32. [DOI] [PubMed] [Google Scholar]
- 10. Schouffoer A, Ndosi ME, Vliet Vlieland TP, Meesters JJ.. The educational needs of people with systemic sclerosis: a cross-sectional study using the Dutch version of the Educational Needs Assessment Tool (D-ENAT). Rheumatol Int 2016;36:289–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Mouthon L, Alami S, Boisard AS. et al. Patients' views and needs about systemic sclerosis and its management: a qualitative interview study. BMC Musculoskeletal Disord 2017;18:230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Rubenzik TT, Derk CT.. Unmet patient needs in systemic sclerosis. J Clin Rheumatol 2009;15:106–10. [DOI] [PubMed] [Google Scholar]
- 13. Kroneman M, Boerma W, van den Berg M. et al. Netherlands: health system review. Health Syst Transit 2016;18:1–240. [PubMed] [Google Scholar]
- 14. von Elm E, Altman DG, Egger M. et al. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. Int J Surg 2014;12:1495–9. [DOI] [PubMed] [Google Scholar]
- 15. Willems LM, Kwakkenbos L, Bode C, van den Hoogen FH, van den Ende CH.. Health care use and patients' perceptions on quality of care in systemic sclerosis. Clin Exp Rheumatol 2013;31:64–70. [PubMed] [Google Scholar]
- 16. Zuidgeest M, Sixma H, Rademakers J.. Measuring patients’ experiences with rheumatic care: the consumer quality index rheumatoid arthritis. Rheumatol Int 2009;30:159–67. [DOI] [PubMed] [Google Scholar]
- 17. Distler O, Allanore Y, Denton CP. et al. Factors influencing early referral, early diagnosis and management in patients with diffuse cutaneous systemic sclerosis. Rheumatology 2018;57:813–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Spierings J, van den Ende C, Schriemer R. et al. Optimal care for systemic sclerosis patients: recommendations from a patient-centered and multidisciplinary mixed-method study and working conference. Clin Rheumatol 2019;38:1007–15. [DOI] [PubMed] [Google Scholar]
- 19. Hudson Smith M, Smith D.. Directing improvements in primary care patient experience through analysis of service quality. Health Serv Res 2018;53:4647–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Papp R, Borbas I, Dobos E. et al. Perceptions of quality in primary health care: perspectives of patients and professionals based on focus group discussions. BMC Fam Pract 2014;15:128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Schulz LN, Zlatina K, Kent N, Peter J.. The doctor–patient relationship and patient resilience in chronic pain: a qualitative approach to patients’ perspectives. Chronic Illn 2018;14:256–70. [DOI] [PubMed] [Google Scholar]
- 22. Almeida C, Almeida I, Vasconcelos C.. Quality of life in systemic sclerosis. Autoimmun Rev 2015;14:1087–96. [DOI] [PubMed] [Google Scholar]
- 23. Ingegnoli F, Carmona L, Castrejon I.. Systematic review of systemic sclerosis-specific instruments for the EULAR Outcome Measures Library: an evolutional database model of validated patient-reported outcomes. Semin Arthritis Rheum 2017;46:609–14. [DOI] [PubMed] [Google Scholar]
- 24. Valentini G, Matucci Cerinic M.. Disease-specific quality indicators, guidelines and outcome measures in scleroderma. Clin Exp Rheumatol 2007;25:159–62. [PubMed] [Google Scholar]
- 25. van der Vaart R, Repping-Wuts H, Drossaert CH. et al. Need for online information and support of patients with systemic sclerosis. Arthritis Care Res 2013;65:594–600. [DOI] [PubMed] [Google Scholar]
- 26. Bassel M, Hudson M, Taillefer SS. et al. Frequency and impact of symptoms experienced by patients with systemic sclerosis: results from a Canadian National Survey. Rheumatology 2011;50:762–7. [DOI] [PubMed] [Google Scholar]
- 27. Johnson SR, Carette S, Dunne JV.. Scleroderma: health services utilization from patients’ perspective. J Rheumatol 2006;33:1123–7. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.