Abstract
BACKGROUND
Cardioplegia is one of the main post-operative cardiac protective factors widely used in recent decades in the form of crystalloid (St. Thomas) and bloody solutions [del Nido (DN)]. The purpose of this study was to compare the effect of a crystalloid cardioplegic agent (St. Thomas) with that of a bloody cardioplegic agent (DN) in pediatric cardiac surgery among children with Tetralogy of Fallot (TOF).
METHODS
This study was performed on 60 children with TOF, who were candidates for heart repair surgery. The participants were randomly divided into two groups of crystalloid cardioplegic agent and bloody cardioplegic agent. Operative outcomes such as required time for onset of heart arrest, duration of returning to normal heart rhythm, and cardiopulmonary bypass (CPB) time, and operative complications were compared between the two groups.
RESULTS
The duration of returning to normal heart rhythm (50.43 ± 10.93 seconds vs. 43.03 ± 16.35 seconds; P = 0.044) and duration of inotropy (80.40 ± 27.14 hours vs. 63.20 ± 26.91 hours; P = 0.017) were significantly higher in the DN group compared to the St. Thomas group. However, there were no significant differences between the two groups in terms of heart arrest time, cross-clamp time, CPB time, supplementary lasix time, duration of intubation, and intensive care unit (ICU) and hospital length of stay (LOS) (P > 0.050).
CONCLUSION
The use of St. Thomas cardioplegic solution was more effective in reducing the duration of returning to normal heart rhythm and inotropy compared with DN cardioplegic agent, and a single dose of these two cardioplegic agents can keep the mean cardiac arrest duration within the range of 50-70 minutes. It seems that the use of St. Thomas cardioplegic solution can be suggested in pediatric heart surgery.
Keywords: Cardioplegic Solutions, Tetralogy of Fallot, Cardiac Surgical Procedures, Child
Introduction
Protection of the myocardium is one of the main factors in cardiac surgery. In the 1950s, myocardial protection against ischemia was not considered in cardiac surgeries, which led to an irreversible damage called Stone heart.1,2 Today, there are various methods including the use of bloody or crystalloid cardioplegia, changing of the cardioplegia temperature (cold or warm), injection of single-dose or repeated cardioplegia, and addition of specific agents to the cardioplegic solution to protect the myocardium during cardiac surgery.3
Overall, one of the most important steps in heart protection is electromechanical arrest. The protection and management of the myocardium is influenced by various factors such as surgical technique and the surgeon's experiences and skills, trying to provide a motionless and bloodless heart for the surgical procedure, lack of necrosis during surgery, institutional equipment, and costs.4
Various cardioplegic solutions are used for full cardiac arrest. The main characteristics of these solutions include inducing quick and effective myocardial arrest, protection of the heart against myocardial ischemia, being reversible when the coronary arteries left during solvent washing, and their low toxicity.5,6 Presently, different cardioplegic solutions are available with various concentrations; the St. Thomas and del Nido (DN) solutions can be considered as the two main cardioplegic solutions.7
The St. Thomas solution is an extracellular cardioplegic solution containing sodium and calcium similar to plasma, and Custodial solution is a similar intracellular solution with lower sodium and calcium that is commonly used in transplant surgery.8
In October 2014, following a change in cardioplegic solution from St. Thomas to DN, anecdotally, reduced rates of defibrillation was observed after cross-clamp. Therefore, it was hypothesized that a significant decrease would be observed in the rates of fibrillation after cross-clamp among all patients that received the DN solution based on the weight categories compared to the St. Thomas solution.9
In the DN solution, potassium chloride causes the cellular membrane to peel away and the lidocaine solution with sodium channels causes cardiac arrest in the hyperpolarized state. Its magnesium content acts as a calcium channel blocker (CCB) and prevents muscle contractility.7,9 Single-dose DN injection has been recently used in pediatric surgery. Custodial solution contains tryptophan amino acids and its single-dose injection induces cardiac protection for up to 180 minutes.10 Although there is yet no consensus among surgeons on single-dose usage or repetition of cardioplegia, DN is one of the most commonly used cardioplegic solutions in pediatric surgery. Various studies have been conducted with different volumes of blood/DN percentage. For example, in a study, a DN solution and blood ratio of 1 to 1 was used as a result of which patients did not need repeated cardiac injection for up to 2 hours.11 In some other studies, the addition of various drugs and materials, such as high-concentration glucose, was evaluated.12,13
The use of cardioplegia in cardiac surgery, both in adults and children, is associated with numerous controversies. In this regard, many studies have been performed in adults on the type of cardioplegia, and the amount and number of repetitions of cardioplegia necessary.9,14-16
However, there are a limited number of studies in pediatric cardiac surgery, no study has assessed the effect of single-dose cardioplegia, and less attention has been paid to post-operative and intraoperative outcomes. For example, use of various types of cardioplegic solutions (DN, customized solutions, St. Thomas, Plegisol, Baxter, and microplegia) by surgeons has been studied in pediatric cardiac surgery and the results have indicated that DN/custodial and St. Thomas in different crystalloids or solutions with bloody forms were the most commonly used solutions. DN was the most widely used solution; however, only the types of cardioplegia have been assessed, but their postoperative outcomes and complications have not been investigated.1
Since injection of a cardioplegic solution is generally repeated within 15-30 minutes in adults,15 a congenital heart disease called Tetralogy of Fallot (TOF) was selected for physiological investigation because it is less likely to have pulmonary hypertension, and has the least effect on the conductivity system and a duration of cardiac arrest of at least 50-55 minutes. Thus, the current study was carried out to evaluate the effect of a single-dose injection of a crystalloid cardioplegic agent (St. Thomas) compared with a bloody cardioplegic agent (DN) in pediatric cardiac surgery on patients with TOF.
Materials and Methods
This single-blind, randomized, clinical trial was conducted on all children with TOF who were candidates of complete heart repair surgery referred to Chamran Hospital, Isfahan, Iran, from January 2017 to June 2018. Considering the sample size formula in comparison of two groups, and a confidence level of 95%, power of 80%, error level of 0.1, and the results of previous studies regarding the rate of use of custodial (7%) and DN (38%) cardioplegic solutions,1 the sample size was determined to be 30 patients in each group.
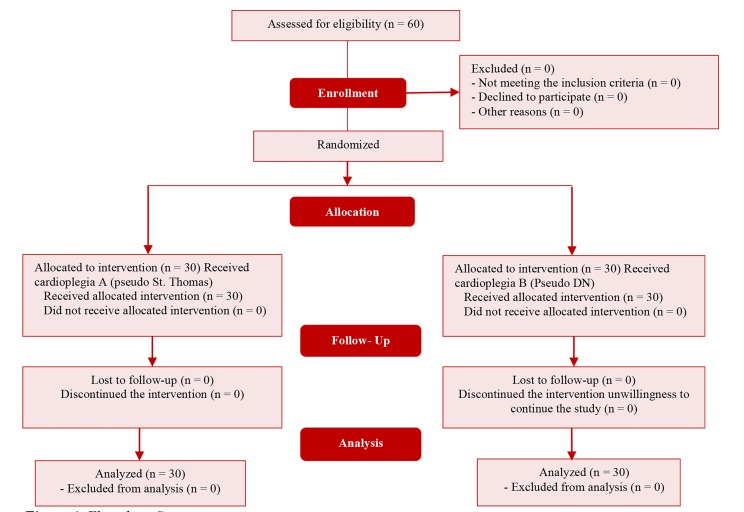
This study was approved by the ethics committee of Isfahan University of Medical Sciences, Isfahan, Iran, with the code IR.MUI.REC.1396.3.590. Written informed consent was obtained from the parents of the studied children for participation in the study. In addition, patients who had undergone previous primary cardiac surgery (including pulmonary artery banding or Blalock-Taussig shunt), the purpose of their surgery was not complete heart repair, or the surgeon decided against complete heart repair during the surgery based on their condition were excluded from the study. The excluded participants were replaced by other participants, so that there was no drop in the number of participants. Using convenience random sampling, 60 children with TOF were selected. They were divided into two groups using Random Allocation Software (RAS) (Figure 1).
Figure 1.
Flowchart Consort
Since it was not possible to use original cardioplegia in our cardiovascular center, cardioplegia with electrolyte combinations similar to DN and St. Thomas were used and the combination was prepared as follows.
To prepare cardioplegic solution A (pseudo St. Thomas), 2.5 cc magnesium sulfate electrolytes 50%, 2.5 cc lidocaine 1%, 6 cc potassium chloride (2 meq/ml), and 7.5 cc sodium bicarbonate 8.4% were dissolved in 500 cc Ringer’s lactate and used as crystalloid for cardioplegia and injected at 20 cc/kg.
To prepare cardioplegic solution B (pseudo DN) 2.5 cc magnesium electrolytes 50%, 3 cc lidocaine 2%, 8 cc mannitol 20%, 6.5 cc potassium chloride (2 meq/ml), and 6.5 cc sodium bicarbonate 8.4% were mixed in a 500 cc normal saline serum and 4 units of this solution were mixed with one blood unit and injected at 20 cc/kg (Table 1).
Table 1.
St. Thomas and del Nido cardioplegic solutions
| ST cardioplegia | Original | Pseudo* |
|---|---|---|
| Na+ | 110 mmol/l | -* |
| K+ | 16 mmol/l | 6 cc (2 meq/ml) |
| Mg2+ | 16 mmol/l | 50%, 2.5 cc |
| Ca2+ | 1.2 mmol/l | -* |
| NaHCO3- | 10 mmol/l | 8.4%, 7.5 cc |
| Lidocaine | - | 1%, 2.5 cc |
| DN cardioplegia | Original | Pseudo** |
| Mannitol | 20%, 16.3 ml, 3.26 g | 20%, 8 cc |
| Magnesium sulfate | 50%, 4 ml, 2g | 50%, 2.5 cc |
| Sodium bicarbonate | 8.4%, 13 ml, 13 mEq | 8.4%, 6.5 cc |
| Lidocaine | 1%, 13 ml, 130 mg | 2%, 3cc |
| Potassium chloride (2 mEq/ml) | 13 ml, 26 mEq | 6.5 cc (2 meq/ml) |
This cardioplegic solution was solved in a 0.5 l ringer (containing 73 Eq Na+, 2 Eq K+, 2 Eq Ca+, 77 Eq Cl-);
This cardioplegic solution was solved in a 0.5 l normal saline (containing 77 Eq Na+, 77 Eq Cl-)
Following preparation of cardioplegic solutions, they were tagged as code A and B and the person who gathered the data was not aware of the cardioplegic solution used to make the study single-blinded. Cardioplegia A was administered in the first group and the second group received cardioplegia B.
In addition to age, gender, and weight of the child, blood and metabolic tests were implemented before cardiopulmonary bypass (CPB) initiation, during CPB, 6 hours later, and 1 day after surgery and recorded. Operative outcomes, such as the time required for cardiac arrest, the time necessary to revert to normal heart rhythm, and CPB time, and operative complications were measured and recorded. Furthermore, the intensive care unit (ICU) length of stay (LOS), duration of hospitalization, and incidence of arrhythmia were recorded.
Finally, the collected data were entered into SPSS software (version 22; IBM Corporation, Armonk, NY, USA). Qualitative and quantitative data are demonstrated in the forms of frequency and frequency percentage, and mean and standard deviation, respectively. Fisher’s exact test and chi-square test were applied to compare frequency distribution of qualitative data between the two groups. The results of Kolmogorov-Smirnov (KS) test indicated normal distribution of variables. Moreover, independent sample t-test was used to compare the means of continuous variables between the two groups and repeated measures ANOVA was used to compare the two groups at different times. Significance level was considered as less than 0.05 in all analyses.
Results
The group B participants consisted of 18 (60%) boys and 12 (40%) girls with the mean age of 19.8 ± 16.39 months and group A participants consisted of 16 (53.3%) boys and 14 (46.7%) girls with the mean age of 22.26 ± 15.62 month. There was no significant difference in the distribution of gender and age between the two groups (P> 0.050) (Table 2).
Table 2.
Demographic characteristics of patients
| Characteristics | Group B (n = 30) | Group A (n = 30) | P | |
|---|---|---|---|---|
| Sex [n (%)] | Male | 18 (60) | 16 (53.3) | 0.602* |
| Female | 12 (40) | 14 (46.7) | ||
| Age (month) (mean ± SD) | 19.80 ± 16.39 | 22.26 ± 15.62 | 0.553** | |
| Weight (kg) (mean ± SD) | 9.63 ± 2.87 | 10.60 ± 5.19 | 0.374** | |
Use of chi-square for comparison between the groups
Use of independent t-test for comparison between the groups
SD: Standard deviation
As shown in table 3, repeated measures ANOVA showed that the main effect of group was not significant on any of the blood and metabolic outcomes (P > 0.050), in fact, there was no significant difference in the mean of these variables between the two groups. Significant changes were observed in ejection fraction (EF), HCO3, base excess (BE), lactate level, Cl, creatine phosphokinase (CPK), Na (P< 0.001), pH (P = 0.001), and calcium (P = 0.008) at the different measurement stages of the study. Moreover, there was only a significant difference in calcium with the passage of time between the groups (P = 0.025), so that group A had a higher mean than group B 6 hours after surgery.
Table 3.
Blood and metabolic outcomes before and after surgery
| Blood and metabolic outcomes | Time of surgery | Group B (n = 30) | Group A (n = 30) | P* time | P* group | P* time×group |
|---|---|---|---|---|---|---|
| EF | Before | 70.63 ± 5.18 | 70.53 ± 4.71 | < 0.001 | 0.645 | 0.162 |
| 1 day after | 64.57 ± 4.57 | 63.83 ± 4.04 | ||||
| 1 week after | 64.57 ± 4.56 | 63.80 ± 4.02 | ||||
| CO2 | Before | 33.92 ± 7.23 | 34.03 ± 8.97 | 0.124 | 0.354 | 0.744 |
| During the pump | 32.42 ± 5.96 | 33.23 ± 6.51 | ||||
| End | 31.55 ± 5.90 | 32.07 ± 5.28 | ||||
| 6 hours after | 32.60 ± 5.50 | 34.88 ± 5.50 | ||||
| pH (n) | Before | 7.38 ± 0.08 | 7.35 ± 0.09 | 0.001 | 0.109 | 0.704 |
| During the pump | 7.47 ± 0.07 | 7.44 ± 0.09 | ||||
| End | 7.43 ± 0.08 | 7.43 ± 0.09 | ||||
| 6 hours after | 7.41 ± 0.09 | 7.38 ± 0.08 | ||||
| HCO3 | Before | 20.40 ± 3.36 | 20.01 ± 4.18 | < 0.001 | 0.182 | 0.851 |
| During the pump | 24.68 ± 2.98 | 24.19 ± 4.39 | ||||
| End | 27.20 ± 0.28 | 25.84 ± 2.82 | ||||
| 6 hours after | 27.69 ± 3.98 | 27.23 ± 3.03 | ||||
| Lactate level (n) | Before | 1.09 ± 0.40 | 1.16 ± 0.45 | < 0.001 | 0.743 | 0.305 |
| During the pump | 3.38 ± 1.13 | 3.35 ± 1.72 | ||||
| End | 3.06 ± 1.02 | 3.53 ± 1.64 | ||||
| 6 hours after | 2.73 ± 1.34 | 2.45 ± 0.91 | ||||
| BE (mmol/l) | Before | -4.00 ± 2.71 | -4.32 ± 2.57 | < 0.001 | 0.341 | 0.415 |
| During the pump | 0.97 ± 3.10 | -0.55 ± 2.86 | ||||
| End | 4.90 ± 4.25 | 4.47 ± 3.88 | ||||
| 6 hours after | 4.49 ± 3.54 | 4.67 ± 2.53 | ||||
| Calcium | Before | 4.08 ± 0.36 | 4.00 ± 0.58 | 0.008 | 0.537 | 0.025 |
| During the pump | 3.99 ± 0.21 | 4.04 ± 0.37 | ||||
| End | 3.89 ± 0.22 | 3.91 ± 0.30 | ||||
| 6 hours after | 3.79 ± 0.26 | 3.92 ± 0.43 | ||||
| Cl | Before | 103.53 ± 2.40 | 104.13 ± 1.89 | < 0.001 | 0.669 | 0.174 |
| During the pump | 102.77 ± 3.51 | 102.50 ± 2.73 | ||||
| End | 101.13 ± 2.85 | 102.20 ± 2.52 | ||||
| 6 hours after | 104.40 ± 3.76 | 103.90 ± 2.02 | ||||
| CPK | Before | 36.37 ± 23.95 | 36.17 ± 11.12 | < 0.001 | 0.593 | 0.555 |
| End | 184.57 ± 43.42 | 177.43 ± 23.13 | ||||
| 6 hours after | 144.93 ± 41.39 | 142.40 ± 20.49 | ||||
| Na | Before | 132.77 ± 3.88 | 134.72 ± 2.83 | < 0.001 | 0.871 | 0.641 |
| During the pump | 133.50 ± 2.74 | 132.45 ± 3.01 | ||||
| End | 135.92 ± 6.11 | 135.85 ± 3.93 | ||||
| 6 hours after | 142.43 ± 7.09 | 142.00 ± 6.37 | ||||
| K | Before | 3.74 ± 0.51 | 3.57 ± 0.39 | 0.797 | 0.406 | 0.266 |
| During the pump | 3.51 ± 0.64 | 3.68 ± 0.82 | ||||
| End | 3.78 ± 0.85 | 3.51 ± 0.74 | ||||
| 6 hours after | 3.57 ± 0.57 | 3.56 ± 0.59 |
Use of repeated measures ANOVA;
BE: Base excess; EF: Ejection fraction; CPK: Creatine phosphokinase
No significant difference was observed in the duration of heart arrest, clamp time, CPB time, and duration of supplementary lasix between the two groups (P > 0.050). However, the mean duration of time necessary to revert to normal heart rhythm was significantly higher in group B (50.43 ± 10.93 seconds) than that in group A (43.3 ± 16.35 seconds) (P = 0.044). Moreover, 96.7% of children in group B and all children in group A required inotropic agent, while the duration of inotropy in group B with the mean of 80.40 ± 27.14 hours was significantly higher than that in group A with the mean of 63.20 ± 26.91 hours (P = 0.017). It should be noted that no patient required shock in group B, but 40% of patients needed electrolyte during the pumping and 16.7% had arrhythmia in the ICU. In group A, 3.3% needed shock, 46.7% required electrolyte during pumping, and 6.7% had arrhythmia in the ICU. No significant difference was observed between the two groups in these variables (P > 0.050). In addition, intubation duration, ICU LOS, and duration of hospitalization were slightly higher in group B compared to group A (P > 0.050) (Table 4).
Table 4.
Intraoperative and postoperative outcomes
| Outcomes | Group B (n = 30) | Group A (n = 30) | P |
|---|---|---|---|
| Arrest duration (second) (mean ± SD) | 46.30 ± 13.58 | 42.47 ± 13.11 | 0.271* |
| Cardiopulmonary bypass time (minute) (mean ± SD) | 98.33 ± 13.76 | 92.87 ± 11.84 | 0.105* |
| Aortic cross-clamp time (minute) (mean ± SD) | 71.33 ± 13.12 | 67.47 ± 9.07 | 0.190* |
| return to normal heart rhythm (second) (mean ± SD) | 50.43 ± 10.93 | 43.03 ± 16.35 | 0.044* |
| Supplementary lasix duration (hour) (mean ± SD) | 57.10 ± 25.18 | 49.30 ± 26.20 | 0.249* |
| Inotropic time (hour) (mean ± SD) | 80.40 ± 27.14 | 63.20 ± 26.91 | 0.017* |
| Duration of intubation (hour) (mean ± SD) | 63.20 ± 27.65 | 51.33 ± 29.69 | 0.115* |
| ICU LOS (day) (mean ± SD) | 5.23 ± 1.07 | 4.77 ± 1.13 | 0.107* |
| Total hospital LOS (day) (mean ± SD) | 10.53 ± 1.59 | 10.03 ± 1.50 | 0.215* |
| Arrhythmias [n (%)] | 5 (16.7) | 2 (6.7) | 0.212*** |
| Need for supplementary electrolyte during CPB [n (%)] | 12 (40.0) | 14 (46.7) | 0.397** |
| Inotropic requirement [n (%)] | 29 (96.7) | 30 (100) | 0.999** |
| Delayed sternal closure [n (%)] | 0 (0) | 0 (0) | - |
| Need for intraoperative DC shocks [n (%)] | 0 (0) | 1 (3.3) | 0.999*** |
SD: Standard deviation; ICU: Intensive care unit; LOS: Length of stay; CPB: Cardiopulmonary bypass; DC: Direct current
Use of independent sample t-test for comparison of mean variables between the two groups
Use of chi-square test for comparison of variables’ frequency distribution between the groups
Use of Fisher’s exact test for comparison of variables’ frequency distribution between the groups
Discussion
Clinical research has been focused on myocardial protection in open-heart surgery for many years, but debate is still ongoing about the ultimate cardioprotective strategy and optimal cardioplegic solution. In the early 1990s, at the University of Pittsburgh, Dr. Pedro del Nido and his colleagues introduced a cardioplegic solution to show the specific requirements of immature myocardium during neonatal and pediatric cardiac surgery. It is commonly known as DN cardioplegic solution, induces a depolarizing arrest during cardiac surgery, and has been increasingly used recently.17
It is an improvised form of cardioplegia, which includes the beneficial effect of crystalloid cardioplegia, as well as blood components.
The current study aimed to compare the effect of single-dose crystalloid cardioplegic agent (pseudo St. Thomas) with blood cardioplegic solution (pseudo Del Nido) in pediatrics cardiac surgery. It was performed on 60 children with TOF that were divided into two groups (n = 30) of stimulated crystalloid cardioplegia and bloody cardioplegia. A statistically significant difference was not observed between the two groups in terms of age and gender. The results of metabolic and blood changes before, during, and after surgery showed EF and acidity reduction, and increased sodium ion and BE in both groups.
In the present study, the crystalloid and bloody cardiopelgic solutions used were similar to the original solutions, but it should be noted that we solved crystalloid St. Thomas in a 0.5 l ringer (including 73 Eq Na+, 2 Eq K+, 2 Eq Ca+, and 77 Eq Cl-) and bloody DN in 0.5 l normal saline (including 77 Eq Na+, and 77 Eq Cl-).
Different components in cardioplegia play different roles, for instance potassium plays the role of arresting agent, glucose acts as substrate, bicarbonate as buffer, mannitol as oncotic agent and free radical scavenger, and magnesium and lidocaine as membrane stabilizers, and only traces of calcium are seen. Addition of magnesium and lidocaine increases the potassium content and magnesium helps to counteract the effects of calcium and lidocaine by blocking the sodium channels. It causes a decrease in the adverse effects of prolonged membrane depolarization and prevents cellular edema. Use of lidocaine in the DN cardioplegia prolongs the heart arrest duration through its effect on the cell membrane.3,18
O’Brien et al. conducted the first clinical study on DN cardioplegia in Halifax in 2009.19 Their results approved DN cardioplegia effects on lower troponin T and improvement of calcium management in pediatric patients.19 A clinical investigation was carried out by Charette et al. on 34 children with a cross-clamp time of longer than 90 minutes and single-dose of DN cardioplegia or modified multi-dose of cardioplegia solution.11 They observed no significant differences in the risk of congenital heart surgery, CPB times, aortic cross-clamp time, weight, or number of intraoperative exogenous blood units. However, they reported significant differences in cardioplegic solution doses and perioperative glucose levels.11
A survey among pediatric cardiothoracic surgeons in North America showed that regardless of cross-clamp time a single shot of DN cardioplegic solution is the most commonly used cardioprotective method (38%).1 In a randomized trial on 100 patients of younger than 12 years who had undergone elective repair of ventricular septal defects (VSD) and TOF, a single-dose DN solution or repeated doses of St. Thomas solution at 30-minute intervals were used.20 Electron microscopic ultrastructural alterations were assessed through myocardial biopsy. The results showed that the cardiac index was higher in the DN group than the St. Thomas group at 2, 6, and 24 hours. Mechanical ventilation, and ICU and hospital LOS were significantly lower in the DN group and there was lower troponin I release at the 24-hour interval. Electron microscopic studies illustrated more myofibrillar disarray in the St. Thomas solution group.20
Cardioplegic solutions have an important role in protecting the heart from myocardial injury during open-heart surgery and in pediatric cardiac surgery; DN solution has been successfully used to his end.21
Among the suggested benefits of this solution, the decreased need for repetition of several doses of standard cardioplegia that results in shorter cross-clamp time, and lower postoperative complications and mortality rate can be mentioned, but the reported differences were not statistically significant.22
Accelerated accumulation of intracellular Ca2+ can mediate the occurrence of reperfusion injury during cardiac surgery in myocardial ischemia. The myocardial cell prevents the accumulation of these high intracellular ions through energy consuming active transport mechanisms, and ultimately, introducing accelerated accumulation of intracellular Ca2+ as myocardial dysfunction upon reperfusion.23 The DN solution contains lidocaine, a membrane-stabilizing agent that increases Na+ channel blockade and lowers the potential of Na+. In addition, Mg2+ acts as a Ca2+ antagonist that is a suggested mechanism of myocardium protection against high rate of intracellular Ca2+.24
It can be stated that reduced cellular acidity caused the activation of the hydrogen-sodium pump (Na+-H+) and sodium-calcium pump (Na+-Ca2+) for removing the hydrogen ion, which led to increased intercellular calcium and sodium ions. In addition, it should be noted that in the case of acidity reduction, patients under electrolyte during pumping (group B: 40%, group Z: 46.7%) are controlled. Thus, the changes therapy in pH were not considered as significant.
Some previous studies have shown superior cardiac index values in the DN group indicating better myocardial protection that in part can be associated with the lidocaine content. It abolishes all electrical activity, reduces the incidence of arrhythmias, and prevents intracellular calcium accumulation. This action results in the prevention of cell injury and probably safeguards the cells from the harmful effects of intracellular calcium accumulation thus providing better myocardial protection.25 It has also been hypothesized that single-dose cardioplegia offers better myocardial protection than multiple-dose cardioplegia.26 The DN solution leads to prolonged heart arrest that allows the surgeon to complete most of the routine procedures with a single dose. Although some studies have suggested the provision of a better ultrastructural preservation by multiple doses assigned to metabolic end products,27 the results of the present study and some previous studies have shown better functional recovery with long-acting single-dose DN cardioplegia.28,29
In a randomized control trial, the DN cardioplegia and modified St. Thomas cardioplegia were compared in terms of the inflammatory cytokine response and cardiac troponin I changes in patients undergoing TOF surgery and there were no significant differences in Tumor necrosis factor-alpha (TNF-α), Interleukin 6 (IL-6), or Interleukin-8 (IL-8)cytokine levels.17 A moderately significant increase was seen in IL-10 level in the St. Thomas group, postoperative lactate level was significantly higher in the DN group, and no differences were detected in troponin levels. It was finally concluded that anti-inflammatory cytokine response in the St. Thomas solution group was significantly better than the DN group, which may be due to the shorter intervals of St. Thomas solution administration.17
In the current study, the level of lactate increased from the beginning until the end of surgery, but it again decreased after 6 hours. The level of CPK increased immediately and 6 hours after surgery compared with preoperative CPK.
Generally, it should be noted that none of these metabolic and blood changes were significant in the two groups. Evaluating intraoperative and postoperative outcomes showed that although cardiac arrest duration, CBP, and aortic cross-clamp were higher in group B than group A, this difference was not significant. It has also been showed that returning to normal heart rhythm was significantly higher in group B than group A. In addition, the need for inotropy in groups A and B was 100 and 96.7%, respectively, but the duration of inotropy was higher in group B than group A. It should be noted that these two factors were also dependent upon the child’s weight, as the duration of returning to normal heart rhythm and need for inotropy increased with weight.
The findings of some previous studies are in agreement with that of the present study, there is evidence regarding the advantages of single-dose DN compared with multi-dose cardioplegia in terms of mechanical ventilation time, cross-clamp time, and pumping time.18,19,29-31
Charette et al. reported no significant differences in cross-clamp and CPB times between DN and multi-dose cardioplegia groups and revealed that multiple doses of cardioplegia could not significantly increase the duration of cross-clamp;11 this finding was in accordance with that of Kim et al. in adults undergoing CPB.25
Moreover, significant differences were not observed in these variables between the DN and St. Thomas groups in the present study. Considering the variable complexity of repairing TOF, the slightly longer, but not significant cross-clamp time in the DN group may be due to the complexity of the defect and the related reparative operation.
Mishra et al. reported lower rates of immediate postoperative complications and mortality with DN usage, but the differences were not significant.15
In the current study, no death or arrhythmia in the ICU occurred in the groups. There were also no significant differences in the need and duration of intubation and shock, length of hospitalization, and ICU LOS between the two groups.
One of the limitations of this study was that only one type of disease (TOF) was evaluated, but some studies have evaluated various types of heart disorders. The evaluation of single-dose cardioplegia in children can be considered as the advantage of this study. To the researchers’ knowledge, there are very few studies in this field, so it is a very important topic in countries such as Iran due to some deprivations and their economic conditions, and further studies are needed in this area. It should be noted that we had no hospital mortality cases and this can be considered as the most important advantage of this study.
Conclusion
The present study results indicate that a single dose of a routine cardioplegic agent can be used in children and a cardiac arrest time of 50-70 minutes be achieved. Considering that the duration of returning to normal heart rhythm and need for inotropy were lower in the St. Thomas group compared to the DN group, the St. Thomas cardioplegic solution can be suggested for children within the age group under the age of 5 years. However, further studies with larger sample sizes and different types of cardiac diseases are needed.
Acknowledgments
This article is part of a Cardiac Surgery Specialist dissertation funded by Isfahan University of Medical Sciences. This research was approved by the Ethics Committee of Isfahan University of Medical Sciences with the code IR.MUI.MED.REC.1396.3.590.
Footnotes
Conflicts of Interests
Authors have no conflict of interests.
REFERENCES
- 1.Kotani Y, Tweddell J, Gruber P, Pizarro C, Austin EH, Woods RK, et al. Current cardioplegia practice in pediatric cardiac surgery: A North American multiinstitutional survey. Ann Thorac Surg. 2013;96(3):923–9. doi: 10.1016/j.athoracsur.2013.05.052. [DOI] [PubMed] [Google Scholar]
- 2.Cooley DA, Reul GJ, Wukasch DC. Ischemic contracture of the heart: "stone heart". Am J Cardiol. 1972;29(4):575–7. doi: 10.1016/0002-9149(72)90454-7. [DOI] [PubMed] [Google Scholar]
- 3.Yamamoto H, Yamamoto F. Myocardial protection in cardiac surgery: A historical review from the beginning to the current topics. Gen Thorac Cardiovasc Surg. 2013;61(9):485–96. doi: 10.1007/s11748-013-0279-4. [DOI] [PubMed] [Google Scholar]
- 4.Kouchoukos NT, Blackstone EH, Hanley FL, Kirklin JK. Kirklin/Barratt-Boyes Cardiac Surgery E-Book. Philadelphia, PA: Elsevier Health Sciences; 2012. [Google Scholar]
- 5.Feindel CM, Tait GA, Wilson GJ, Klement P, MacGregor DC. Multidose blood versus crystalloid cardioplegia. Comparison by quantitative assessment of irreversible myocardial injury. J Thorac Cardiovasc Surg. 1984;87(4):585–95. [PubMed] [Google Scholar]
- 6.Fallouh HB, Kentish JC, Chambers DJ. Targeting for cardioplegia: Arresting agents and their safety. Curr Opin Pharmacol. 2009;9(2):220–6. doi: 10.1016/j.coph.2008.11.012. [DOI] [PubMed] [Google Scholar]
- 7.Buckberg GD. Antegrade/retrograde blood cardioplegia to ensure cardioplegic distribution: Operative techniques and objectives. J Card Surg. 1989;4(3):216–38. doi: 10.1111/j.1540-8191.1989.tb00284.x. [DOI] [PubMed] [Google Scholar]
- 8.Jonas RA. Myocardial protection. In: Jonas R, editor. Comprehensive Surgical Management of Congenital Heart Disease. Boca Raton, FL: CRC Press; 2004. pp. 175–84. [Google Scholar]
- 9.Buel ST, Striker CW, O'Brien JE. del Nido versus St. Thomas cardioplegia solutions: A single-center retrospective analysis of post cross-clamp defibrillation rates. J Extra Corpor Technol. 2016;48(2):67–70. [PMC free article] [PubMed] [Google Scholar]
- 10.Liu J, Feng Z, Zhao J, Li B, Long C. The myocardial protection of HTK cardioplegic solution on the long-term ischemic period in pediatric heart surgery. ASAIO J. 2008;54(5):470–3. doi: 10.1097/MAT.0b013e318188b86c. [DOI] [PubMed] [Google Scholar]
- 11.Charette K, Gerrah R, Quaegebeur J, Chen J, Riley D, Mongero L, et al. Single dose myocardial protection technique utilizing del Nido cardioplegia solution during congenital heart surgery procedures. Perfusion. 2012;27(2):98–103. doi: 10.1177/0267659111424788. [DOI] [PubMed] [Google Scholar]
- 12.Mimic B, Ilic S, Vulicevic I, Milovanovic V, Tomic D, Mimic A, et al. Comparison of high glucose concentration blood and crystalloid cardioplegia in paediatric cardiac surgery: A randomized clinical trial. Interact Cardiovasc Thorac Surg. 2016;22(5):553–60. doi: 10.1093/icvts/ivv391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fazelifar S, Bigdelian H. Effect of esmolol on myocardial protection in pediatrics congenital heart defects. Adv Biomed Res. 2015;4:246. doi: 10.4103/2277-9175.170241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kim K, Ball C, Grady P, Mick S. Use of del nido cardioplegia for adult cardiac surgery at the cleveland clinic: Perfusion implications. J Extra Corpor Technol. 2014;46(4):317–23. [PMC free article] [PubMed] [Google Scholar]
- 15.Mishra P, Jadhav RB, Mohapatra CK, Khandekar J, Raut C, Ammannaya GK, et al. Comparison of del Nido cardioplegia and St. Thomas Hospital solution-two types of cardioplegia in adult cardiac surgery. Kardiochir Torakochirurgia Pol. 2016;13(4):295–9. doi: 10.5114/kitp.2016.64867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gorjipour F, Dehaki MG, Totonchi Z, Hajimiresmaiel SJ, Azarfarin R, Pazoki-Toroudi H, et al. Inflammatory cytokine response and cardiac troponin I changes in cardiopulmonary bypass using two cardioplegia solutions; del Nido and modified St. Thomas':A randomized controlled trial. Perfusion. 2017;32(5):394–402. doi: 10.1177/0267659117691119. [DOI] [PubMed] [Google Scholar]
- 17.Matte GS, del Nido PJ. History and use of del Nido cardioplegia solution at Boston Children's Hospital. J Extra Corpor Technol. 2012;44(3):98–103. [PMC free article] [PubMed] [Google Scholar]
- 18.Sorabella RA, Akashi H, Yerebakan H, Najjar M, Mannan A, Williams MR, et al. Myocardial protection using del nido cardioplegia solution in adult reoperative aortic valve surgery. J Card Surg. 2014;29(4):445–9. doi: 10.1111/jocs.12360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.O'Brien JD, Howlett SE, Burton HJ, O'Blenes SB, Litz DS, Friesen CL. Pediatric cardioplegia strategy results in enhanced calcium metabolism and lower serum troponin T. Ann Thorac Surg. 2009;87(5):1517–23. doi: 10.1016/j.athoracsur.2009.02.067. [DOI] [PubMed] [Google Scholar]
- 20.Talwar S, Bhoje A, Sreenivas V, Makhija N, Aarav S, Choudhary SK, et al. Comparison of del Nido and St Thomas Cardioplegia Solutions in Pediatric Patients: A Prospective Randomized Clinical Trial. Semin Thorac Cardiovasc Surg. 2017;29(3):366–74. doi: 10.1053/j.semtcvs.2017.08.017. [DOI] [PubMed] [Google Scholar]
- 21.Suleiman MS, Hancock M, Shukla R, Rajakaruna C, Angelini GD. Cardioplegic strategies to protect the hypertrophic heart during cardiac surgery. Perfusion. 2011;26(Suppl 1):48–56. doi: 10.1177/0267659111420607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Valooran GJ, Nair SK, Chandrasekharan K, Simon R, Dominic C. del Nido cardioplegia in adult cardiac surgery - scopes and concerns. Perfusion. 2016;31(1):6–14. doi: 10.1177/0267659115608936. [DOI] [PubMed] [Google Scholar]
- 23.Tsukube T, McCully JD, Federman M, Krukenkamp IB, Levitsky S. Developmental differences in cytosolic calcium accumulation associated with surgically induced global ischemia: Optimization of cardioplegic protection and mechanism of action. J Thorac Cardiovasc Surg. 1996;112(1):175–84. doi: 10.1016/s0022-5223(96)70194-0. [DOI] [PubMed] [Google Scholar]
- 24.O'Blenes SB, Friesen CH, Ali A, Howlett S. Protecting the aged heart during cardiac surgery: The potential benefits of del Nido cardioplegia. J Thorac Cardiovasc Surg. 2011;141(3):762–70. doi: 10.1016/j.jtcvs.2010.06.004. [DOI] [PubMed] [Google Scholar]
- 25.Kim JS, Jeong JH, Moon SJ, Ahn H, Hwang HY. Sufficient myocardial protection of del Nido cardioplegia regardless of ventricular mass and myocardial ischemic time in adult cardiac surgical patients. J Thorac Dis. 2016;8(8):2004–10. doi: 10.21037/jtd.2016.06.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pourmoghadam KK, Ruzmetov M, O'Brien MC, Piggott KD, Plancher G, Narasimhulu SS, et al. Comparing del Nido and Conventional Cardioplegia in Infants and Neonates in Congenital Heart Surgery. Ann Thorac Surg. 2017;103(5):1550–6. doi: 10.1016/j.athoracsur.2016.10.070. [DOI] [PubMed] [Google Scholar]
- 27.Ramanathan R, Parrish DW, Armour TK, Brinster DR. Use of del Nido Cardioplegia in Adult Cardiac Surgery. Thorac Cardiovasc Surg. 2015;63(7):624–7. doi: 10.1055/s-0035-1545260. [DOI] [PubMed] [Google Scholar]
- 28.Smigla G, Jaquiss R, Walczak R, Bonadonna D, Kaemmer D, Schwimer C, et al. Assessing the safety of del Nido cardioplegia solution in adult congenital cases. Perfusion. 2014;29(6):554–8. doi: 10.1177/0267659114543346. [DOI] [PubMed] [Google Scholar]
- 29.Mick SL, Robich MP, Houghtaling PL, Gillinov AM, Soltesz EG, Johnston DR, et al. del Nido versus Buckberg cardioplegia in adult isolated valve surgery. J Thorac Cardiovasc Surg. 2015;149(2):626–34. doi: 10.1016/j.jtcvs.2014.10.085. [DOI] [PubMed] [Google Scholar]
- 30.Ota T, Yerebakan H, Neely RC, Mongero L, George I, Takayama H, et al. Short-term outcomes in adult cardiac surgery in the use of del Nido cardioplegia solution. Perfusion. 2016;31(1):27–33. doi: 10.1177/0267659115599453. [DOI] [PubMed] [Google Scholar]
- 31.Yerebakan H, Sorabella RA, Najjar M, Castillero E, Mongero L, Beck J, et al. Del Nido Cardioplegia can be safely administered in high-risk coronary artery bypass grafting surgery after acute myocardial infarction: A propensity matched comparison. J Cardiothorac Surg. 2014;9:141. doi: 10.1186/s13019-014-0141-5. [DOI] [PMC free article] [PubMed] [Google Scholar]