Abstract
Many studies have revealed the ability of the endosymbiotic bacterium Wolbachia to protect its arthropod hosts against diverse pathogens. However, as Wolbachia may also increase the susceptibility of its host to infection, predicting the outcome of a particular Wolbachia‐host–pathogen interaction remains elusive. Yet, understanding such interactions and their eco‐evolutionary consequences is crucial for disease and pest control strategies. Moreover, how natural Wolbachia infections affect artificially introduced pathogens for biocontrol has never been studied. Tetranychus urticae spider mites are herbivorous crop pests, causing severe damage on numerous economically important crops. Due to the rapid evolution of pesticide resistance, biological control strategies using entomopathogenic fungi are being developed. However, although spider mites are infected with various Wolbachia strains worldwide, whether this endosymbiont protects them from fungi is as yet unknown. Here, we compared the survival of two populations, treated with antibiotics or naturally harboring different Wolbachia strains, after exposure to the fungal biocontrol agents Metarhizium brunneum and Beauveria bassiana. To control for potential effects of the bacterial community of spider mites, we also compared the susceptibility of two populations naturally uninfected by Wolbachia, treated with antibiotics or not. In one population, Wolbachia‐infected mites had a better survival than uninfected ones in absence of fungi but not in their presence, whereas in the other population Wolbachia increased the mortality induced by B. bassiana. In one naturally Wolbachia‐uninfected population, the antibiotic treatment increased the susceptibility of spider mites to M. brunneum, but it had no effect in the other treatments. These results suggest that natural Wolbachia infections may not hamper and may even improve the success of biological control using entomopathogenic fungi. However, they also draw caution on the generalization of such effects, given the complexity of within‐host–pathogens interaction and the potential eco‐evolutionary consequences of the use of biocontrol agents for Wolbachia‐host associations.
Keywords: antibiotic treatment, bacterial community, facilitation, fungi‐induced mortality, symbiont‐mediated protection, Tetranychus urticae
This study shows that Wolbachia can increase spider‐mite susceptibility to pathogenic fungi, suggesting that this endosymbiont may not hamper, but instead improve the success of fungal‐based pest control. However, this study also highlights the complexity of such interactions and draws caution on the generalization of these findings.
1. INTRODUCTION
The maternally inherited bacterium Wolbachia is to date the best studied and probably the most common endosymbiont of arthropods. It is estimated to infect up to 52% of arthropod species (Weinert, Araujo‐Jnr, Ahmed, & Welch, 2015), a success mainly attributed to its ability to induce various types of reproductive manipulation in hosts to increase the reproductive success of infected females, thereby increasing its own transmission (Werren, Baldo, & Clark, 2008). In particular, the ability of Wolbachia to spread rapidly within and among host populations (Engelstadter & Hurst, 2009) has raised growing interests in using it in biocontrol programs (Bourtzis et al., 2014).
Possible Wolbachia‐based biocontrol strategies include the use of Wolbachia as a microbial biocontrol agent, for instance to enhance productivity of natural predators and parasites such as parasitoids (e.g., Grenier et al., 1998; Stouthamer, 1993); as a potential gene‐drive vehicle for population replacement strategies through cytoplasmic drive (which provides a mechanism for the autonomous spread of desired genes into targeted populations; e.g., Dobson, 2003; Sinkins & Godfray, 2004; Turelli & Hoffmann, 1999); or for sterile insect techniques (SIT) to suppress target pest populations by repeated sweeps with infected individuals (Calvitti, Marini, Desiderio, Puggioli, & Moretti, 2015; Zhang, Lees, Xi, Gilles, & Bourtzis, 2015; Zhong & Li, 2014). Subsequently, the discovery of the ability of Wolbachia to protect its hosts against a wide array of pathogens, including viruses, protozoan parasites, fungi, or pathogenic bacteria (reviewed by Cook & McGraw, 2010) has provided new avenues for the control of vector‐borne diseases (reviewed by Iturbe‐Ormaetxe, Walker, & Neill, 2011). For instance, deliberate introductions of Wolbachia into Aedes aegypti mosquito populations are currently being undertaken successfully in several regions worldwide to control dengue virus (e.g. Hoffmann et al., 2014; Nguyen et al., 2015). However, such ability of Wolbachia to interfere with diverse host pathogens may have undesirable effects on biocontrol strategies if, for instance, natural Wolbachia infection interferes with parasitic biocontrol agents, a possibility that has never been addressed. Alternatively, natural Wolbachia infections in several host species may also increase host susceptibility to parasite infection (e.g., Graham, Grzywacz, Mushobozi, & Wilson, 2012; Hughes, Rivero, & Rasgon, 2014), raising the possibility that Wolbachia could also facilitate the action of biocontrol agents. Moreover, potentially variable effects of Wolbachia on host susceptibility to biocontrol agents may have ecological and epidemiological consequences. For instance, artificially introduced pathogens can select Wolbachia variants that increase host resistance and counter‐selection variants that increase host susceptibility to infection, thereby potentially driving the spread of defensive Wolbachia variants (e.g., Cattel, Martinez, Jiggins, Mouton, & Gibert, 2016; Jaenike, Unckless, Cockburn, Boelio, & Perlman, 2010; Kriesner & Hoffmann, 2018). Hence, assessing the effect of natural Wolbachia infection on the efficiency of different strains and/or species of parasitic biocontrol agents is a prerequisite for the development of efficient and long‐lasting control strategies (Zindel, Gottlieb, & Aebi, 2011).
Spider mites of the genus Tetranychus (Acari: Tetranychidae) are ubiquitous major crop pests of c.a. 1,100 plant species belonging to more than 140 different plant families (Migeon & Dorkeld, 2006). Due to their short generation time and high fecundity, spider mites rapidly develop resistance to pesticides (Van Leeuwen, Vontas, Tsagkarakou, Dermauw, & Tirry, 2010), which has encouraged the development of alternative control strategies such as the use of essential oils or natural enemies (e.g., predators, entomopathogenic bacteria and fungi; Attia et al., 2013). Among them, entomopathogenic fungi have been successfully used in integrated pest management (IPM) programs, and commercial formulations are currently available to farmers in most parts of the world (Skinner, Parker, & Kim, 2014). In particular, fungi such as Beauveria bassiana, Metarhizium spp., Isaria spp. and Lecanicillium spp. have been identified as good candidates for efficient spider mite control (e.g., Bugeme, Maniania, Knapp, & Boga, 2008; Chandler, Davidson, & Jacobson, 2005; Maniania, Bugeme, Wekesa, Delalibera, & Knapp, 2008; Shin, Bae, Kim, Yun, & Woo, 2017), and their compatibility with other control methods, such as predatory mites (e.g., Dogan, Hazir, Yildiz, Butt, & Cakmak, 2017; Ullah & Lim, 2017; Wu, Xie, Li, Xu, & Lei, 2016) or pesticides (e.g., Klingen & Westrum, 2007; Shi, Jiang, & Feng, 2005) is widely studied. Curiously, however, the interaction between entomopathogenic fungi and bacterial endosymbionts of spider mites has, to our knowledge, never been investigated. This is at odds with the fact that, on the one hand, natural populations of spider mites often carry several maternally inherited endosymbiotic bacteria with variable prevalence, Wolbachia being the most prevalent (prevalence ranges from 0% to 100%; e.g. Gotoh, Sugasawa, Noda, & Kitashima, 2007; Zélé, Santos, et al., 2018; Zhang, Chen, Yang, Qiao, & Hong, 2016); and, on the other hand, Wolbachia has been shown to protect Drosophila melanogaster hosts against the mortality induced by B. bassiana (Panteleev et al., 2007), although no such effect has been found in D. simulans; (Fytrou, Schofield, Kraaijeveld, & Hubbard, 2006).
To examine the effect of the interaction between Wolbachia and fungal infection on spider mite survival, we carried out a fully factorial experiment using two naturally Wolbachia‐infected and two naturally Wolbachia‐uninfected spider mite populations belonging to two genetically differentiated forms of T. urticae (Auger, Migeon, Ueckermann, Tiedt, & Navajas Navarro, 2013) and treated or not with antibiotics. We used a strain of two generalist entomopathogenic fungi species, Beauveria bassiana and Metarhizium brunneum, as they are included in genuses that are among the most used fungi in commercial production (Vega et al., 2009), with wide geographical and host ranges (Gurlek, Sevim, Sezgin, & Sevim, 2018; Meyling & Eilenberg, 2007; Roberts & Leger, 2004). The specific aims of this work were to determine: (a) whether infection with a natural Wolbachia strain protects spider mites against fungus‐induced mortality, (b) whether this effect varies with different Wolbachia strains and/or the presence of other bacteria in spider mites, and (c) whether this effect depends on the fungus strain. We then discuss possible mechanisms leading to our results, the importance of considering the whole bacterial community of arthropods when assessing the effect of Wolbachia, as well as the potential eco‐evolutionary consequences of the presence of Wolbachia for the success of spider mite control strategies using entomopathogenic fungi.
2. MATERIALS AND METHODS
2.1. Spider mite populations and rearing
Four populations were used in this study, two belonging to the “red” form (AlRo and AMP), and two belonging to the “green” form (DEF and TOM) of Tetranychus urticae (Auger et al., 2013). These populations have been collected in the Iberian Peninsula from 2010 to 2017, on different plant species. Upon collection from the field, the populations AMP and TOM were found to be naturally and fully infected by two different strains of Wolbachia. The population AMP is infected by the Wolbachia strain ST481 (isolate “Turt_B_wUrtAmp,” id: 1858 in the PubMLST Wolbachia database; http:// http://www.pubmlst.org/wolbachia/), which is very similar to strain ST219 belonging to supergroup B and found in China by Zhang, Ding, Zhang, and Hong (2013); and the population TOM is infected by the Wolbachia strain ST280 (isolate “Turt_B_wUrtTom,” id: 1857), which has also been previously found in China by Zhang, Ding, et al. (2013). These strains are very closely related, having 1 SNP difference on the sequences of both the fbpA and coxA genes in the multilocus sequence typing (MLST) system developed by Baldo et al. (2006) for Wolbachia. The two other populations, AlRo and DEF, were naturally uninfected by Wolbachia and none of the populations used in this study carried other maternally inherited bacterial endosymbionts (i.e., Cardinium, Rickettsia, Spiroplasma, and Arsenophonus) at the time of the experiment, as confirmed by PCR using the methods described in Zélé, Santos, et al. (2018). All the information concerning these populations is summarized in Table 1. After collection, these populations were reared in the laboratory under standard conditions (24 ± 2°C, 60% RH, 16/8h L/D) at high numbers (c.a. 500–1000 females per population) in insect‐proof cages containing bean plants (Phaseolus vulgaris, cv. Contender seedlings obtained from Germisem, Oliveira do Hospital, Portugal).
Table 1.
Populations of spider mites used in the experiment. Mites were collected in Portugal (P) and Spain (S) and were naturally infected, or not, by Wolbachia. The absence of other maternally inherited endosymbionts (Cardinium, Rickettsia, Spiroplasma, Arsenophonus) in these populations was confirmed by PCR before the onset of the experiment (using methods described in Zélé, Santos, et al., 2018; Zélé, Weill, et al., 2018)
| Name | Date | Host plant | Location | Coordinates | Wolbachia infection | Reference |
|---|---|---|---|---|---|---|
| AlRo | 09/11/2013 | Rosa spp. | Almería (S) | 36.855725, −2.320374 | no | (Zélé, Santos, et al., 2018) |
| DEF | 26/04/2017 | Solanum lycopersicum | Alvalade, Lisbon (P) | 38.75515, −9.14685 | no | – |
| AMP | 18/11/2013 | Datura stramonium | Aldeia da Mata Pequena (P) | 38.534363, −9.191163 | yes (ST481a) | (Zélé, Santos, et al., 2018) |
| TOM | ‐‐/05/2010 | Solanum lycopersicum | Carregado (P) | 39.078962,−8.993656 | yes (ST280b) | (Clemente, Rodrigues, Ponce, Varela, & Magalhães, 2016) |
Isolate “Turt_B_wUrtTom”—id: 1857, Wolbachia strain ST280. This strain has been first identified as wTurt_2 from three different populations of T. urticae in China (Zhang, Zhang, et al., 2013).
Isolate “Turt_B_wUrtAmp”—id: 1858, Wolbachia strain ST481. This is a new strain, very similar to the strain ST219 (they differ by 1 SNP on the fbpA gene: allele 444 instead of allele 4) that was found in China by Zhang, Ding, et al. (2013).
2.2. Antibiotic treatments
Roughly 2 months (ca. 4 generations) before the onset of the experiment, a rifampicin solution (0.05%, w/v) was used to treat mites (n = 70 adult females initially) from each population for one generation (see Gotoh et al., 2005). This allowed us to obtain Wolbachia‐uninfected AMP and TOM populations as well as controls for the antibiotic treatment for the naturally uninfected populations AlRo and DEF. During the treatment, mites were maintained in Petri dishes containing bean leaf fragments placed on cotton with the antibiotic solution. After one generation, 100 adult‐mated daughters from each treated population were transferred in insect‐proof cages containing bean plants, in the same laboratory conditions as the untreated populations, and these new populations were allowed to grow for 3 successive generations in the absence of antibiotics to avoid potential side effects of the treatment (e.g., Ballard & Melvin, 2007). One generation before the onset of the experiment, pools of 100 females were taken from each treated population and checked by PCR to confirm that they were uninfected by Wolbachia (detailed procedure in Zélé, Weill, & Magalhães, 2018). This method allows detecting Wolbachia infection even at low frequencies (up to 1/100; Zélé, Weill, et al., 2018).
2.3. Entomopathogenic fungi strains and preparation of inoculum
We used the strains V275 (= Met52, F52, BIPESCO 5) of Metarhizium brunneum and UPH‐1103 of Beauveria bassiana (obtained from Swansea University; UK, and from Siedlce University; Poland, respectively), as they were previously shown to have the potential to suppress T. urticae populations (Dogan et al., 2017). The procedures used for fungal growth, inoculum preparation, and spider mite infection are similar to that described in Dogan et al. (2017). Briefly, the two fungi were grown on Sabouraud Dextrose Agar (SDA) medium at 25°C for 2 weeks. Conidia were harvested from sporulating cultures with the aid of a spatula, washed with sterile distilled water and filtered through 4 layers of gauze (pore size: 20 µm) to remove any hyphae.
2.4. Spider mite infection and survival
The experiment was conducted in a growth chamber under standard conditions (25 ± 2°C, 80% RH, 16/8 hr L/D). Roughly 2 weeks prior to the experiment, 100 females were collected from each mass culture and allowed to lay eggs during 4 days on detached bean leaves placed on water‐soaked cotton. One day prior to the onset of the experiment, 20 young adult mated females (hence with similar age) were randomly collected from these cohorts and placed on a 9 cm2 bean leaf disc on wet cotton with the abaxial surface facing upwards. On the first day of the experiment, the surface of the leaf discs was sprayed using a hand sprayer with 2.5 ml of a spore suspension of M. brunneum or B. bassiana in 0.03% (v/v) aqueous Tween 20 at 1 × 107 conidia/ml (which is the most commonly used concentration in laboratory studies; Dogan et al., 2017), or, as a control, with 0.03% aqueous Tween 20 only. Subsequently, female survival was monitored every 24 hr during 10 days by counting both dead and alive individuals. A total of twelve replicates per treatment (fungi infection and antibiotic treatment) and per population were performed within 2 experimental blocks of one day difference (6 replicates of each treatment per block).
2.5. Statistical analysis
Analyses were carried out using the R statistical package (version 3.5.3). The general procedure for building the statistical models was as follows. Spider‐mite populations (AlRo, AMP, DEF, and TOM), antibiotic treatment (treated with rifampicin or not), and infection treatment (sprayed with BB: Beauveria bassiana, with MB: Metarhizium brunneum, or with Tween 20 only as control) were fitted in as fixed explanatory variables, whereas discs nested within population and block were fitted as random explanatory variables. When a significant three‐way interaction between the three fixed variables was found, each population was analyzed separately with the same model structure, except that the variable population was removed from the model.
Survival data were analyzed using Cox proportional hazards mixed‐effect models (coxme, kinship package). Hazard ratios (HR) were obtained from these models as an estimate of the difference between the rates of dying (Crawley, 2007) between the untreated controls and the BB or MB treatments for each population. Because the timing of infection is an important parameter for the fitness of parasites, an additional early measurement of survival, the proportion of dead mites at 3 days postinfection (dpi), was obtained from Kaplan–Maier estimates of the survival distribution for each disc. This timing was chosen as it is close to the median survival upon infection in most of the populations tested, and hence corresponds to a threshold time‐point to unravel differences between treatments. The numbers of dead and alive mites at 3 dpi were computed using the function cbind and analyzed with a mixed model glmmadmb procedure (glmmADMB package) with a negative binomial error distribution to correct for overdispersed errors (family “nbinom1” with a ص variance).
Maximal models, including all higher‐order interactions, were simplified by sequentially eliminating non‐significant terms and interactions to establish a minimal model (Crawley, 2007). The significance of the explanatory variables was established using chi‐squared tests (Bolker, 2008). The significant chi‐squared values given in the text are for the minimal model, whereas nonsignificant values correspond to those obtained before deletion of the variable from the minimal model.
To explore significant interactions between infection and antibiotic treatment effects on female survival and mortality at 3 dpi, the two factors were concatenated to fit a single fixed factor containing all treatment levels in the models (i.e., 6 levels for infection by antibiotic treatment effects within each population). Multiple comparisons between levels were then performed from these models using General Linear Hypotheses (glht, package multicomp) with Bonferroni corrections, which uses classical Chisq (Wald test) for testing the global hypothesis H0.
3. RESULTS
Overall, depending on whether they were naturally infected or uninfected by Wolbachia, the survival of females from different populations was not evenly affected by fungal infection and by rifampicin treatment (fungal infection x rifampicin treatment x population interaction: X26 = 36.16, p < .0001 and X26 = 18.33, p = .005 on the overall survival of spider mites and on their mortality at 3 days postinfection, respectively). Thus, to understand this three‐way interaction, we looked at the effect of fungal infection and of the antibiotic treatment in each population separately.
3.1. Effect of fungal infection and of antibiotic treatment in the naturally Wolbachia‐uninfected population AlRo
In the population AlRo, the two fungal strains affected differently the survival of spider mites depending on whether they were treated with antibiotics or not (fungal infection x rifampicin treatment interaction: X22 = 9.53, p = .009; Figure 1a). Indeed, M. brunneum (MB) induced a stronger mortality in rifampicin‐treated mites than in untreated mites (z = 2.80, p = .05), while B. bassiana (BB) induced the same mortality in both rifampicin‐treated and untreated mites (z = −1.20, p = 1.00 Figure 1b; Table 2a). In both cases, however, M. brunneum induced a stronger mortality than B. bassiana (MB vs. BB: z = 6.72, p < .0001 and z = 2.81, p = .05 in rifampicin‐treated and untreated mites, respectively). At 3 days postinfection (dpi), however, no significant interaction between fungal infection and rifampicin treatment (X22 = 1.78, p = .41; Figure 1c) and no effect of the antibiotics treatment alone (X21 = 0.90, p = .34) on female mortality were found. Both fungi strains severely increased the mortality of both rifampicin‐treated and rifampicin‐untreated mites at this early age of infection (X22 = 135.68, p < .00014; see Table 3a for all multiple comparisons).
Figure 1.
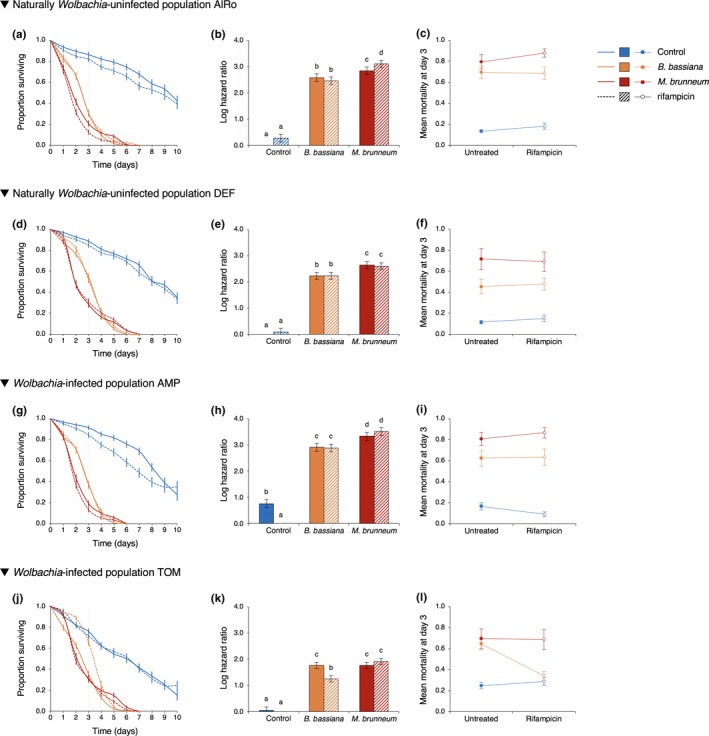
Survival curves (proportion surviving ± s.e.) (a,d,g,j), relative mortality (estimated log hazard ratio ± s.e.) (b,e,h,k), and average survival (± s.e.) at 3 dpi (c,f,i,l) of spider mites from the naturally Wolbachia‐uninfected populations AlRo (a,b,c) and DEF (d,e,f), and the naturally Wolbachia‐infected populations AMP (g,h,i) and TOM (j,k,l). Adult females were treated (dashed lines, dashed bars, and empty circles) or not (solid lines, filled bars, and circles) with rifampicin, and sprayed with B. bassiana (orange), M. brunneum (red), or Tween 20 only as control (blue)
Table 2.
Results of multiple comparisons (with Bonferroni correction) between hazard ratios obtained for the naturally Wolbachia‐uninfected populations (a) AlRo, and (b) DEF, and for the naturally Wolbachia‐infected populations (c) AMP, and (d) TOM sprayed or not with fungi (BB: Beauveria bassiana; MB: Metarhizium brunneum; Control: Tween 20 only) and treated or not with antibiotics (rif: rifampicin‐treated; nt: untreated)
| (a) Naturally Wolbachia‐uninfected population AlRo | ||||||
|---|---|---|---|---|---|---|
| Treatments compared | Estimate | Std. Error | z value | p‐value | ||
| Control_rif | versus | Control_nt | 0.272 | 0.146 | 1.869 | .554 |
| BB_rif | versus | BB_nt | −0.110 | 0.092 | −1.200 | 1.000 |
| MB_ rif | versus | MB_nt | 0.259 | 0.093 | 2.802 | .046a |
| BB_ nt | versus | Control_nt | 2.578 | 0.144 | 17.931 | <2e‐16a |
| MB_nt | versus | Control_nt | 2.840 | 0.144 | 19.718 | <2e‐16a |
| MB_ nt | versus | BB_nt | 0.262 | 0.093 | 2.810 | .045a |
| BB_ rif | versus | Control_rif | 2.195 | 0.134 | 16.345 | <2e‐16a |
| MB_rif | versus | Control_rif | 2.827 | 0.139 | 20.410 | <2e‐16a |
| MB_ rif | versus | BB_rif | 0.632 | 0.094 | 6.718 | 1.66E‐10a |
| (b) Naturally Wolbachia‐uninfected population DEF | ||||||
|---|---|---|---|---|---|---|
| Treatments compared | Estimate | Std. Error | z value | p‐value | ||
| Control_rif | versus | Control_nt | 0.091 | 0.141 | 0.648 | 1.000 |
| BB_rif | versus | BB_nt | −0.001 | 0.092 | −0.015 | 1.000 |
| MB_ rif | versus | MB_nt | −0.044 | 0.091 | −0.487 | 1.000 |
| BB_ nt | versus | Control_nt | 2.238 | 0.136 | 16.435 | <2e‐16a |
| MB_nt | versus | Control_nt | 2.647 | 0.138 | 19.240 | <2e‐16a |
| MB_ nt | versus | BB_nt | 0.410 | 0.096 | 4.246 | 1.96E‐04a |
| BB_ rif | versus | Control_rif | 2.145 | 0.132 | 16.220 | <2e‐16a |
| MB_rif | versus | Control_rif | 2.512 | 0.134 | 18.685 | <2e‐16a |
| MB_ rif | versus | BB_rif | 0.367 | 0.095 | 3.845 | .001a |
| (c) Naturally Wolbachia‐infected population AMP | ||||||
|---|---|---|---|---|---|---|
| Treatments compared | Estimate | Std. Error | z value | p‐value | ||
| Control_rif | versus | Control_nt | −0.756 | 0.154 | −4.916 | 7.93E‐06a |
| BB_rif | versus | BB_nt | −0.024 | 0.091 | −0.258 | 1.000 |
| MB_ rif | versus | MB_nt | 0.172 | 0.092 | 1.875 | .547 |
| BB_ nt | versus | Control_nt | 2.151 | 0.136 | 15.819 | <2e‐16a |
| MB_nt | versus | Control_nt | 2.581 | 0.136 | 18.921 | <2e‐16a |
| MB_ nt | versus | BB_nt | 0.430 | 0.093 | 4.599 | 3.81E‐05a |
| BB_ rif | versus | Control_rif | 2.883 | 0.150 | 19.195 | <2e‐16a |
| MB_rif | versus | Control_rif | 3.508 | 0.154 | 22.828 | <2e‐16a |
| MB_ rif | versus | BB_rif | 0.625 | 0.093 | 6.702 | 1.85E‐10a |
| (d) Naturally Wolbachia‐infected population TOM | ||||||
|---|---|---|---|---|---|---|
| Treatments compared | Estimate | Std. Error | z value | p‐value | ||
| Control_rif | versus | Control_nt | −0.050 | 0.130 | −0.385 | 1.000 |
| BB_rif | versus | BB_nt | −0.510 | 0.092 | −5.539 | 2.74E‐07a |
| MB_ rif | versus | MB_nt | 0.145 | 0.092 | 1.579 | 1.000 |
| BB_ nt | versus | Control_nt | 1.711 | 0.120 | 14.237 | <2e‐16a |
| MB_nt | versus | Control_nt | 1.715 | 0.120 | 14.302 | <2e‐16a |
| MB_ nt | versus | BB_nt | 0.004 | 0.093 | 0.045 | 1.000 |
| BB_ rif | versus | Control_rif | 1.250 | 0.118 | 10.603 | <2e‐16a |
| MB_rif | versus | Control_rif | 1.909 | 0.121 | 15.839 | <2e‐1a |
| MB_ rif | versus | BB_rif | 0.659 | 0.096 | 6.878 | 5.48E‐11a |
* p‐value < .05,
** p‐value < .01,
*** p‐value < .001.
Table 3.
Results of multiple comparisons (with Bonferroni correction) between mortality at 3 dpi of the naturally Wolbachia‐uninfected populations (a) AlRo, and (b) DEF, and for the naturally Wolbachia‐infected populations (c) AMP, and (d) TOM sprayed or not with fungi (BB: Beauveria bassiana; MB: Metarhizium brunneum; Control: Tween 20 only) and treated or not with antibiotics (rif: rifampicin‐treated; nt: untreated)
| (a) Naturally Wolbachia‐uninfected population AlRo | ||||||
|---|---|---|---|---|---|---|
| Treatments compared | Estimate | Std. Error | z value | p‐value | ||
| Control_rif | versus | Control_nt | 0.327 | 0.236 | 1.387 | 1.000 |
| BB_rif | versus | BB_nt | −0.012 | 0.247 | −0.049 | 1.000 |
| MB_ rif | versus | MB_nt | 0.085 | 0.175 | 0.489 | 1.000 |
| BB_ nt | versus | Control_nt | 1.684 | 0.196 | 8.607 | <2e−16a |
| MB_nt | versus | Control_nt | 1.818 | 0.194 | 9.386 | <2e−16a |
| MB_ nt | versus | BB_nt | 0.134 | 0.232 | 0.579 | 1.000 |
| BB_ rif | versus | Control_rif | 1.345 | 0.291 | 4.614 | 3.55E−05a |
| MB_rif | versus | Control_rif | 1.576 | 0.276 | 5.702 | 1.06E−07a |
| MB_ rif | versus | BB_rif | 0.232 | 0.202 | 1.145 | 1.000 |
| (b) Naturally Wolbachia‐uninfected population DEF | ||||||
|---|---|---|---|---|---|---|
| Treatments compared | Estimate | Std. Error | z value | p‐value | ||
| Control_rif | versus | Control_nt | 0.177 | 0.328 | 0.539 | 1.000 |
| BB_rif | versus | BB_nt | 0.089 | 0.368 | 0.241 | 1.000 |
| MB_ rif | versus | MB_nt | −0.020 | 0.258 | −0.077 | 1.000 |
| BB_ nt | versus | Control_nt | 1.255 | 0.277 | 4.525 | 5.44E‐05a |
| MB_nt | versus | Control_nt | 1.738 | 0.265 | 6.550 | 5.17E‐10a |
| MB_ nt | versus | BB_nt | 0.482 | 0.339 | 1.423 | 1.000 |
| BB_ rif | versus | Control_rif | 1.167 | 0.416 | 2.806 | .045a |
| MB_rif | versus | Control_rif | 1.541 | 0.393 | 3.925 | .001a |
| MB_ rif | versus | BB_rif | 0.374 | 0.304 | 1.231 | 1.000 |
| (c) Naturally Wolbachia‐infected population AMP | ||||||
|---|---|---|---|---|---|---|
| Treatments compared | Estimate | Std. Error | z value | p‐value | ||
| Control_rif | versus | Control_nt | −0.644 | 0.270 | −2.390 | .152 |
| BB_rif | versus | BB_nt | 0.013 | 0.235 | 0.056 | 1.000 |
| MB_ rif | versus | MB_nt | 0.070 | 0.170 | 0.411 | 1.000 |
| BB_ nt | versus | Control_nt | 1.322 | 0.178 | 7.424 | 1.03E‐12a |
| MB_nt | versus | Control_nt | 1.579 | 0.174 | 9.088 | < 2e‐16a |
| MB_ nt | versus | BB_nt | 0.257 | 0.219 | 1.175 | 1.000 |
| BB_ rif | versus | Control_rif | 1.979 | 0.316 | 6.258 | 3.50E‐09a |
| MB_rif | versus | Control_rif | 2.293 | 0.305 | 7.529 | 4.58E‐13a |
| MB_ rif | versus | BB_rif | 0.314 | 0.195 | 1.608 | .970 |
| (d) Naturally Wolbachia‐infected population TOM | ||||||
|---|---|---|---|---|---|---|
| Treatments compared | Estimate | Std. Error | z value | p‐value | ||
| Control_rif | versus | Control_nt | 0.125 | 0.177 | 0.706 | 1.000 |
| BB_rif | versus | BB_nt | −0.637 | 0.234 | −2.717 | .059. |
| MB_ rif | versus | MB_nt | −0.012 | 0.175 | −0.069 | 1.000 |
| BB_ nt | versus | Control_nt | 0.949 | 0.152 | 6.239 | 3.96E‐09a |
| MB_nt | versus | Control_nt | 1.024 | 0.151 | 6.798 | 9.56E‐11a |
| MB_ nt | versus | BB_nt | 0.075 | 0.200 | 0.374 | 1.000 |
| BB_ rif | versus | Control_rif | 0.187 | 0.250 | 0.751 | 1.000 |
| MB_rif | versus | Control_rif | 0.887 | 0.220 | 4.033 | 4.95E‐04a |
| MB_ rif | versus | BB_rif | 0.699 | 0.205 | 3.414 | .006a |
* p‐value < .05,
** p‐value < .01,
*** p‐value < .001.
3.2. Effect of fungal infection and of antibiotic treatment in the naturally Wolbachia‐uninfected population DEF
In the population DEF, we did not find a significant interaction between fungal infection and rifampicin treatment (X22 = 0.65, p = .72; Figure 1d), neither a significant effect of rifampicin treatment (X21 = 0.003, p = .96), but only a significant effect of fungal infection (X22 = 879.17, p < .0001). Indeed, both fungi induced the same mortality in rifampicin‐treated and in rifampicin‐untreated mites, with an overall stronger effect of M. brunneum than of B. bassiana (Figure 1e; Table 2b for all multiple comparisons). Similarly, at 3 dpi, no significant interaction between fungal infection and rifampicin treatment (X22 = 0.40, p = .82; Figure 1f), neither a significant effect of rifampicin treatment (X21 = 0.14, p = .71) was found. As for the population AlRo, only fungal infection affected the spider‐mite survival (X22 = 64.89, p < .0001; Table 3b for all multiple comparisons).
3.3. Effect of fungal infection and of antibiotic treatment in the naturally Wolbachia‐infected population AMP
In the population AMP, we found a significant interaction between infection and rifampicin treatment (X22 = 26.61, p < .0001; Figure 1g). This interaction was due to a lower survival of Wolbachia‐infected controls compared with rifampicin‐treated controls (z = −4.92, p < .0001) only, as Wolbachia‐infected and rifampicin‐treated mites had the same overall survival upon infection with each fungal strain (for B. bassiana: z = −0.26, p = 1.00; for M. brunneum: z = 1.88, p = .55; Figure 1h and Table 2c). However, accounting for this difference between Wolbachia‐infected and rifampicin‐treated controls reveals that, relative to their respective control, both fungi induced higher mortality in rifampicin‐treated mites (HR = 17.87 and HR = 33.39, for BB and MB, respectively) than in Wolbachia‐infected ones (HR = 8.60 and HR = 13.21, respectively). A significant interaction between infection and rifampicin treatment was also found at 3 dpi (X22 = 6.5, p = .04; Figure 1i). However, this interaction was relatively weak at this time‐point and could not be explained by multiple comparisons between factor levels (i.e., no differences were found between Wolbachia‐infected and rifampicin‐treated mites when sprayed with Tween 20 only, B. bassiana, or M. brunneum; Table 3c for all multiple comparisons).
3.4. Effect of fungal infection and of antibiotic treatment in the naturally Wolbachia‐infected population TOM
In the population TOM, we also found a significant interaction between infection and rifampicin treatment (X22 = 26.00, p < .0001; Figure 1j). In this population, the effect of B. bassiana was weaker in rifampicin‐treated (HR = 3.49) than Wolbachia‐infected mites (HR = 5.53; z = −5.54, p < .0001; Figure 1k and Table 2d), while M. brunneum had the same effect in both rifampicin‐treated and nontreated mites (HR = 6.75 and HR = 5.56, respectively; z = 1.58, p = 1.00). Moreover, whereas both fungi had the same effect on nontreated mites (MB vs. BB: z = 0.05, p = 1.00), B. bassiana did not decrease the survival of rifampicin‐treated mites as much as M. brunneum (MB vs. BB: z = −6.88, p < .0001). This effect was even stronger at 3 dpi (fungal infection x rifampicin interaction: X22 = 15.44, p < .001). At this time‐point, B. bassiana induced the same mortality as M. brunneum in Wolbachia‐infected mites (BB vs. Control: z = 6.24, p < .0001), but did not affect significantly the survival of rifampicin‐treated mites (BB vs. Control: z = 0.75, p = 1.00; Figure 1l and Table 3d).
4. DISCUSSION
In this study, we found variable effects of infection by B. bassiana and M. brunneum following antibiotic treatment, depending on the spider mite population and on whether spider mites were naturally infected by Wolbachia or not. Indeed, the mortality induced by both fungi did not differ between Wolbachia‐infected and rifampicin‐treated mites in the population AMP, despite Wolbachia infection being costly in absence of fungal infection. Similarly, the mortality induced by M. brunneum was not affected by Wolbachia infection in the population TOM, but the mortality induced by B. bassiana increased in presence of Wolbachia. These results suggest that Wolbachia may buffer, or conversely increase, the effect of fungal infection depending on the fungi strains, the Wolbachia strain and/or the host genetic background. Moreover, in absence of natural Wolbachia infection, we found a relatively small effect of the antibiotic treatment on mite susceptibility to infection: The antibiotic treatment had no effect on the outcome of infection by fungi, with the exception of a higher mortality in rifampicin‐treated mites from the population AlRo when infected with M. brunneum. This effect, although significant, is of relatively low amplitude and in the opposite direction than that observed in the Wolbachia‐infected population TOM following B. bassiana infection. This suggests that the effect of Wolbachia in the population TOM may not be explained by an alteration of the whole bacterial community in mites following antibiotic treatment. However, because the effect of fungal infection and antibiotic treatment vary between populations independently of the presence of Wolbachia, we draw caution on the generalization of such results.
In different arthropod host species, Wolbachia may either protect (e.g., Braquart‐Varnier et al., 2015; Hughes, Koga, Xue, Fukatsu, & Rasgon, 2011; Kambris, Cook, Phuc, & Sinkins, 2009; Moreira et al., 2009; Panteleev et al., 2007; Teixeira, Ferreira, & Ashburner, 2008), have no effect (e.g., Tortosa, Courtiol, Moutailler, Failloux, & Weill, 2008; Wong, Hedges, Brownlie, & Johnson, 2011; Zouache, Michelland, Failloux, Grundmann, & Mavingui, 2012), or even increase the susceptibility (e.g., Graham et al., 2012; Hughes et al., 2014) of its arthropod hosts to infection depending on the pathogens tested, the Wolbachia strain (e.g., Chrostek et al., 2013; Martinez et al., 2017; Osborne, Leong, O'Neill, & Johnson, 2009), but also the host genetic background (although to a lesser extent; e.g. Martinez et al., 2017). In several of these studies the effect of Wolbachia on host susceptibility to pathogens has been assessed following artificial Wolbachia infection (e.g., Joubert et al., 2016; Moreira et al., 2009; Walker et al., 2011), which prevents a direct alteration of the host bacterial community but may not accurately reflect the effect of natural Wolbachia infections. Indeed, novel Wolbachia‐host associations are often costly for hosts (e.g., McGraw, Merritt, Droller, & O'Neill, 2002), mainly due to the activation of the host immune system, which in turn prevents subsequent infections by other pathogens (reviewed by Zug & Hammerstein, 2015). Conversely, the effect of natural Wolbachia infections on host susceptibility to pathogens is usually assessed by using antibiotic treatments. However, antibiotics do not affect Wolbachia only, but also the entire bacterial community in hosts (e.g., Lehman, Lundgren, & Petzke, 2009; Zouache, Voronin, Tran‐Van, & Mavingui, 2009), which raises the necessity to assess the effect of the antibiotic treatment per se.
In T. truncatus spider mites, Zhu et al. (2018) showed that antibiotic treatment affects the composition of the bacterial community even after more than 20 generations without antibiotics. In particular, bacteria from different families increased in proportion in tetracycline‐treated mites in absence of the Anaplasmataceae (which includes Wolbachia). Hence, in our study, the lower mortality observed for antibiotic‐treated mites following infection by B. bassiana in the naturally Wolbachia‐infected population TOM cannot be unambiguously attributed to Wolbachia only. This result could be explained, for instance, by Wolbachia outcompeting bacteria that contribute to the host homeostasis and immunity (reviewed by Shapira, 2016; Vavre & Kremer, 2014; Weiss & Aksoy, 2011), thereby increasing the success of B. bassiana infection (i.e., indirect facilitation; Zélé, Magalhães, Kéfi, & Duncan, 2018). In contrast, in the Wolbachia‐uninfected population AlRo, antibiotic‐treated mites have a higher mortality than untreated mites when infected with M. brunneum. One possible explanation is that, in the absence of natural Wolbachia infection, the antibiotic treatment affected differently the bacterial community, potentially eliminating bacteria that interfere with M. brunneum.
The apparent facilitation of B. bassiana by Wolbachia in the TOM population may also be due to Wolbachia interacting directly with the host immune system. Indeed, Wolbachia can downregulate autophagy‐associated genes in naturally infected hosts, possibly as an immune evasion strategy (Chevalier et al., 2012; Kremer et al., 2009). Under such scenario, the elimination of Wolbachia with antibiotics may result in overall higher autophagic processes in the host, to which B. bassiana could be susceptible. Moreover, in diverse native hosts, including T. urticae, Wolbachia also plays a role in redox homeostasis (e.g., Zhang, Ding, Rong, & Hong, 2015; Zug & Hammerstein, 2015). The elimination of Wolbachia with antibiotics in coevolved T. urticae hosts may thus potentially lead to a disruption of redox homeostasis and higher production of reactive oxygen species (ROS), which are involved in host immunity (e.g., encapsulation, melanisation; reviewed by Zug & Hammerstein, 2015), thereby increasing host resistance to infection. However, all these different scenarios would only explain our results if such mechanisms affect differently the two fungal strains and are specific to the Wolbachia strain and/or the host population.
As stated above, the host genetic background also plays a major role in determining host susceptibility to infection. First, not all populations (independently of their status of infection by Wolbachia) are equally affected by the infection by the two fungi (e.g., the mortality induced by both fungi is stronger in the population DEF than in the population TOM). Indeed, we have previously shown both inter‐ and intraspecific variability in spider mite susceptibility to infection by the same two fungi (Zélé et al. in press). Second, host susceptibility to infection may also result from Genotype x Genotype interactions with their endosymbionts (e.g., Martinez et al., 2017). Here, the different effects of Wolbachia observed in the populations TOM and AMP cannot be unambiguously attributed to the Wolbachia strain only, but likely result from their interaction with the host genetic background. Hence, although further investigations on the respective role of the Wolbachia strain and of the host genome in the susceptibility to different fungal strains (e.g., by using several spider mite populations infected by the same and different Wolbachia strains and by different fungal strains belonging to different species), as well as on the composition of the bacterial communities in each of the population tested would be necessary to shed light on the mechanisms involved, these results show that the outcome of infection strongly depends on complex interactions between multiple microorganisms and their host.
Irrespective of the underlying mechanisms, the variable effects of Wolbachia on spider mite susceptibility observed here raise important questions about the potential consequences of the use of biocontrol agents for both the ecology and epidemiology of naturally occurring Wolbachia infection in arthropod pests. Indeed, the artificial introduction of pathogens for biocontrol may counter‐select susceptible Wolbachia‐host combinations and potentially select for defensive Wolbachia variants, leading to their spread across host populations (e.g., Cattel et al., 2016; Jaenike et al., 2010; Kriesner & Hoffmann, 2018). A better understanding of the variability in the outcome of Wolbachia‐host–pathogens interactions, as well as its consequences for the ecology and evolution of all players of such interaction, is thus a challenge with both fundamental and applied interests, and future work should go in that direction.
In conclusion, our results show variable effects of Wolbachia on spider mite susceptibility to fungi‐induced mortality using two generalist fungi, B. bassiana and M. brunneum. To our knowledge, this is the first study investigating the interaction between natural Wolbachia infections and widely used biocontrol agents. As Wolbachia was found to have either no effect or to increase spider mite susceptibility to fungal infection, these results suggest that it may improve the success of biological control using entomopathogenic fungi. However, these results also highlight the complexity of within‐host–pathogens interaction and caution against the generalization of such effects as (a) the outcome of these interactions may vary depending on the fungal strain, the Wolbachia strain, and the host genetic background, and (b) these interactions may evolve at a rapid pace with potentially important consequences for the ecology and epidemiology of Wolbachia infection in arthropod pests. Finally, our findings also point to the importance of considering the whole bacterial community of arthropods when assessing the effect of Wolbachia in a particular system.
CONFLICT OF INTEREST
None declared.
AUTHORS’ CONTRIBUTIONS
FZ and SM conceived and designed the experiment; IS was involved in the maintenance of spider mite populations and plants; MA acquired the data; FZ performed statistical analyses; FZ and SM wrote the manuscript, with input from all authors; IC and SM funded the study. All authors have read and approved the final version of the manuscript.
ACKNOWLEDGMENTS
We thank Catarina Pinto and João Alpedrinha for their help in collecting some data, as well as Marta Palma for technical support. We also thank all members of the SM lab for useful discussions and suggestions. This work was funded by an FCT‐Tubitak agreement (FCT‐TUBITAK/0001/2014 and TUBITAK TOVAG 115O610) to IC and SM, by the cE3c FCT Unit UID/BIA/00329/2020 to FZ and SM, and by Adnan Menderes University Research Foundation (ZRF‐17055) to IC. FZ was funded through an FCT Post‐Doc fellowship (SFRH/BPD/125020/2016). Funding agencies did not participate in the design or analysis of experiments.
Zélé F, Altıntaş M, Santos I, Cakmak I, Magalhães S. Population‐specific effect of Wolbachia on the cost of fungal infection in spider mites. Ecol Evol. 2020;10:3868–3880. 10.1002/ece3.6015
DATA AVAILABILITY STATEMENT
Full dataset has been deposited in the Dryad data repository (doi.org/10.5061/dryad.9p8cz8wc4).
REFERENCES
- Attia, S. , Grissa, K. L. , Lognay, G. , Bitume, E. , Hance, T. , & Mailleux, A. C. (2013). A review of the major biological approaches to control the worldwide pest Tetranychus urticae (Acari: Tetranychidae) with special reference to natural pesticides: Biological approaches to control Tetranychus urticae . Journal of Pest Science, 86, 361–386. 10.1007/s10340-013-0503-0 [DOI] [Google Scholar]
- Auger, P. , Migeon, A. , Ueckermann, E. A. , Tiedt, L. , & Navajas Navarro, M. (2013). Evidence for synonymy between Tetranychus urticae and Tetranychus cinnabarinus (Acari, Prostigmata, Tetranychidae): Review and new data. Acarologia, 53, 383–415. 10.1051/acarologia/20132102 [DOI] [Google Scholar]
- Baldo, L. , Hotopp, J. C. D. , Jolley, K. A. , Bordenstein, S. R. , Biber, S. A. , Choudhury, R. R. , … Werren, J. H. (2006). Multilocus sequence typing system for the endosymbiont Wolbachia pipientis . Applied and Environmental Microbiology, 72, 7098–7110. 10.1128/AEM.00731-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ballard, J. W. O. , & Melvin, R. G. (2007). Tetracycline treatment influences mitochondrial metabolism and mtDNA density two generations after treatment in Drosophila . Insect Molecular Biology, 16, 799–802. 10.1111/j.1365-2583.2007.00760.x [DOI] [PubMed] [Google Scholar]
- Bolker, B. M. (2008). Ecological models and data in R. New Jersey: Princeton University Press; [Google Scholar]
- Bourtzis, K. , Dobson, S. L. , Xi, Z. Y. , Rasgon, J. L. , Calvitti, M. , Moreira, L. A. , … Gilles, J. R. L. (2014). Harnessing mosquito‐Wolbachia symbiosis for vector and disease control. Acta Tropica, 132, S150–S163. 10.1016/j.actatropica.2013.11.004 [DOI] [PubMed] [Google Scholar]
- Braquart‐Varnier, C. , Altinli, M. , Pigeault, R. , Chevalier, F. D. , Greve, P. , Bouchon, D. , & Sicard, M. (2015). The mutualistic side of Wolbachia‐isopod interactions: Wolbachia mediated protection against pathogenic intracellular bacteria. Frontiers in Microbiology, 6, 1388 10.3389/fmicb.2015.01388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bugeme, D. M. , Maniania, N. K. , Knapp, M. , & Boga, H. I. (2008). Effect of temperature on virulence of Beauveria bassiana and Metarhizium anisopliae isolates to Tetranychus evansi . Experimental and Applied Acarology, 46, 275–285. 10.1007/s10493-008-9179-1 [DOI] [PubMed] [Google Scholar]
- Calvitti, M. , Marini, F. , Desiderio, A. , Puggioli, A. , & Moretti, R. (2015). Wolbachia density and cytoplasmic incompatibility in Aedes albopictus: Concerns with using artificial Wolbachia infection as a vector suppression tool. PLoS ONE, 10, e0121813 10.1371/journal.pone.0121813 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cattel, J. , Martinez, J. , Jiggins, F. , Mouton, L. , & Gibert, P. (2016). Wolbachia‐mediated protection against viruses in the invasive pest Drosophila suzukii . Insect Molecular Biology, 25, 595–603. [DOI] [PubMed] [Google Scholar]
- Chandler, D. , Davidson, G. , & Jacobson, R. J. (2005). Laboratory and glasshouse evaluation of entomopathogenic fungi against the two‐spotted spider mite, Tetranychus urticae (Acari : Tetranychidae), on tomato, Lycopersicon esculentum . Biocontrol Science and Technology, 15, 37–54. [Google Scholar]
- Chevalier, F. , Herbiniere‐Gaboreau, J. , Charif, D. , Mitta, G. , Gavory, F. , Wincker, P. , … Bouchon, D. (2012). Feminizing Wolbachia: A transcriptomics approach with insights on the immune response genes in Armadillidium vulgare . BMC Microbiology, 12, S1 10.1186/1471-2180-12-S1-S1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chrostek, E. , Marialva, M. S. , Esteves, S. S. , Weinert, L. A. , Martinez, J. , Jiggins, F. M. , & Teixeira, L. (2013). Wolbachia variants induce differential protection to viruses in Drosophila melanogaster: A phenotypic and phylogenomic analysis. PLoS Genetics, 9, e1003896 10.1371/journal.pgen.1003896 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clemente, S. H. , Rodrigues, L. R. , Ponce, R. , Varela, S. A. M. , & Magalhães, S. (2016). Incomplete species recognition entails few costs in spider mites, despite first‐male precedence. Behavioral Ecology and Sociobiology, 70, 1161–1170. 10.1007/s00265-016-2124-0 [DOI] [Google Scholar]
- Cook, P. E. , & McGraw, E. A. (2010). Wolbachia pipientis: An expanding bag of tricks to explore for disease control. Trends in Parasitology, 26, 373–375. 10.1016/j.pt.2010.05.006 [DOI] [PubMed] [Google Scholar]
- Crawley, M. J. (2007). The R Book. Chichester, England: John Wiley & Sons Ltd. [Google Scholar]
- Dobson, S. L. (2003). Reversing Wolbachia‐based population replacement. Trends in Parasitology, 19, 128–133. 10.1016/S1471-4922(03)00002-3 [DOI] [PubMed] [Google Scholar]
- Dogan, Y. O. , Hazir, S. , Yildiz, A. , Butt, T. M. , & Cakmak, I. (2017). Evaluation of entomopathogenic fungi for the control of Tetranychus urticae (Acari: Tetranychidae) and the effect of Metarhizium brunneum on the predatory mites (Acari: Phytoseiidae). Biological Control, 111, 6–12. 10.1016/j.biocontrol.2017.05.001 [DOI] [Google Scholar]
- Engelstadter, J. , & Hurst, G. D. D. (2009). The ecology and evolution of microbes that manipulate host reproduction. Annual Review of Ecology Evolution and Systematics, 40, 127–149. 10.1146/annurev.ecolsys.110308.120206 [DOI] [Google Scholar]
- Fytrou, A. , Schofield, P. G. , Kraaijeveld, A. R. , & Hubbard, S. F. (2006). Wolbachia infection suppresses both host defence and parasitoid counter‐defence. Proceedings of the Royal Society B‐Biological Sciences, 273, 791–796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gotoh, T. , Noda, H. , Fujita, T. , Iwadate, K. , Higo, Y. , Saito, S. , & Ohtsuka, S. (2005). Wolbachia and nuclear‐nuclear interactions contribute to reproductive incompatibility in the spider mite Panonychus mori (Acari : Tetranychidae). Heredity, 94, 237–246. 10.1038/sj.hdy.6800605 [DOI] [PubMed] [Google Scholar]
- Gotoh, T. , Sugasawa, J. , Noda, H. , & Kitashima, Y. (2007). Wolbachia‐induced cytoplasmic incompatibility in Japanese populations of Tetranychus urticae (Acari : Tetranychidae). Experimental and Applied Acarology, 42, 1–16. 10.1007/s10493-007-9072-3 [DOI] [PubMed] [Google Scholar]
- Graham, R. I. , Grzywacz, D. , Mushobozi, W. L. , & Wilson, K. (2012). Wolbachia in a major African crop pest increases susceptibility to viral disease rather than protects. Ecology Letters, 15, 993–1000. [DOI] [PubMed] [Google Scholar]
- Grenier, S. , Pintureau, B. , Heddi, A. , Lassabliere, F. , Jager, C. , Louis, C. , & Khatchadourian, C. (1998). Successful horizontal transfer of Wolbachia symbionts between Trichogramma wasps. Proceedings of the Royal Society B‐Biological Sciences, 265, 1441–1445. 10.1098/rspb.1998.0455 [DOI] [Google Scholar]
- Gurlek, S. , Sevim, A. , Sezgin, F. M. , & Sevim, E. (2018). Isolation and characterization of Beauveria and Metarhizium spp. from walnut fields and their pathogenicity against the codling moth, Cydia pomonella (NL.) (Lepidoptera: Tortricidae). Egyptian Journal of Biological. Pest Control, 28, 50. [Google Scholar]
- Hoffmann, A. A. , Iturbe‐Ormaetxe, I. , Callahan, A. G. , Phillips, B. L. , Billington, K. , Axford, J. K. , … O'Neill, S. L. (2014). Stability of the wMel Wolbachia infection following invasion into Aedes aegypti populations. PloS Neglected Tropical Diseases, 8, e3115 10.1371/journal.pntd.0003115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes, G. L. , Koga, R. , Xue, P. , Fukatsu, T. , & Rasgon, J. L. (2011). Wolbachia infections are virulent and inhibit the human malaria parasite Plasmodium falciparum in Anopheles gambiae . PLoS Path, 7, e1002043 10.1371/journal.ppat.1002043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes, G. L. , Rivero, A. , & Rasgon, J. L. (2014). Wolbachia can enhance Plasmodium infection in mosquitoes: Implications for malaria control? PLoS Path, 10, e1004182 10.1371/journal.ppat.1004182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iturbe‐Ormaetxe, I. , Walker, T. , & Neill, S. L. O. (2011). Wolbachia and the biological control of mosquito‐borne disease. EMBO Reports, 12, 508–518. 10.1038/embor.2011.84 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaenike, J. , Unckless, R. , Cockburn, S. N. , Boelio, L. M. , & Perlman, S. J. (2010). Adaptation via symbiosis: Recent spread of a Drosophila defensive symbiont. Science, 329, 212–215. 10.1126/science.1188235 [DOI] [PubMed] [Google Scholar]
- Joubert, D. A. , Walker, T. , Carrington, L. B. , De Bruyne, J. T. , Kien, D. H. T. , Hoang, N. L. T. , … O'Neill, S. L. (2016). Establishment of a Wolbachia superinfection in Aedes aegypti mosquitoes as a potential approach for future resistance management. PLoS Path, 12, e1005434 10.1371/journal.ppat.1005434 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kambris, Z. , Cook, P. E. , Phuc, H. K. , & Sinkins, S. P. (2009). Immune activation by life‐shortening Wolbachia and reduced filarial competence in mosquitoes. Science, 326, 134–136. 10.1126/science.1177531 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klingen, I. , & Westrum, K. (2007). The effect of pesticides used in strawberries on the phytophagous mite Tetranychus urticae (Acari: Tetranychidae) and its fungal natural enemy Neozygites floridana (Zygomycetes: Entomophthorales). Biological Control, 43, 222–230. 10.1016/j.biocontrol.2007.07.013 [DOI] [Google Scholar]
- Kremer, N. , Voronin, D. , Charif, D. , Mavingui, P. , Mollereau, B. , & Vavre, F. (2009). Wolbachia interferes with ferritin expression and iron metabolism in insects. PLoS Pathogens, 5, e1000630 10.1371/journal.ppat.1000630 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kriesner, P. , & Hoffmann, A. A. (2018). Rapid spread of a Wolbachia infection that does not affect host reproduction in Drosophila simulans cage populations. Evolution, 72, 1475–1487. [DOI] [PubMed] [Google Scholar]
- Lehman, R. M. , Lundgren, J. G. , & Petzke, L. M. (2009). Bacterial communities associated with the digestive tract of the predatory ground beetle, Poecilus chalcites, and their modification by laboratory rearing and antibiotic treatment. Microbial Ecology, 57, 349–358. 10.1007/s00248-008-9415-6 [DOI] [PubMed] [Google Scholar]
- Maniania, N. K. , Bugeme, D. M. , Wekesa, V. W. , Delalibera, I. , & Knapp, M. (2008). Role of entomopathogenic fungi in the control of Tetranychus evansi and Tetranychus urticae (Acari: Tetranychidae), pests of horticultural crops. Experimental and Applied Acarology, 46, 259–274. 10.1007/s10493-008-9180-8 [DOI] [PubMed] [Google Scholar]
- Martinez, J. , Tolosana, I. , Ok, S. , Smith, S. , Snoeck, K. , Day, J. P. , & Jiggins, F. M. (2017). Symbiont strain is the main determinant of variation in Wolbachia‐mediated protection against viruses across Drosophila species . Molecular Ecology, 26, 4072–4084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGraw, E. A. , Merritt, D. J. , Droller, J. N. , & O'Neill, S. L. (2002). Wolbachia density and virulence attenuation after transfer into a novel host. Proceedings of the National Academy of Sciences of the United States of America, 99, 2918–2923. 10.1073/pnas.052466499 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyling, N. V. , & Eilenberg, J. (2007). Ecology of the entomopathogenic fungi Beauveria bassiana and Metarhizium anisopliae in temperate agroecosystems: Potential for conservation biological control. Biological Control, 43, 145–155. 10.1016/j.biocontrol.2007.07.007 [DOI] [Google Scholar]
- Migeon, A. , & Dorkeld, F. (2006). Spider Mites Web: a comprehensive database for the Tetranychidae. Retrieved from http://www.montpellier.inra.fr/CBGP/spmweb/.
- Moreira, L. A. , Iturbe‐Ormaetxe, I. , Jeffery, J. A. , Lu, G. J. , Pyke, A. T. , Hedges, L. M. , … O'Neill, S. L. (2009). A Wolbachia symbiont in Aedes aegypti limits infection with dengue, Chikungunya, and Plasmodium . Cell, 139, 1268–1278. 10.1016/j.cell.2009.11.042 [DOI] [PubMed] [Google Scholar]
- Nguyen, T. H. , Nguyen, H. L. , Nguyen, T. Y. , Vu, S. N. , Tran, N. D. , Le, T. N. , … Hoffmann, A. A. (2015). Field evaluation of the establishment potential of wMelPop Wolbachia in Australia and Vietnam for dengue control. Parasites & Vectors, 8, 563 10.1186/s13071-015-1174-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osborne, S. E. , Leong, Y. S. , O'Neill, S. L. , & Johnson, K. N. (2009). Variation in antiviral protection mediated by different Wolbachia strains in Drosophila simulans . PLoS Pathogens, 5, e1000656 10.1371/journal.ppat.1000656 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panteleev, D. Y. , Goryacheva, I. I. , Andrianov, B. V. , Reznik, N. L. , Lazebny, O. E. , & Kulikov, A. M. (2007). The endosymbiotic bacterium Wolbachia enhances the nonspecific resistance to insect pathogens and alters behavior of Drosophila melanogaster . Russian Journal of Genetics, 43, 1066–1069. 10.1134/S1022795407090153 [DOI] [PubMed] [Google Scholar]
- Roberts, D. W. , & Leger, R. J. S. (2004). Metarhizium spp., cosmopolitan insect‐pathogenic fungi: Mycological aspects In Laskin A. I., Bennet J. W., & Gadd G. M. (Eds.), Advances in Applied Microbiology (pp. 1–70). USA: Academic Press. [DOI] [PubMed] [Google Scholar]
- Shapira, M. (2016). Gut microbiotas and host evolution: Scaling up symbiosis. Trends in Ecology & Evolution, 31, 539–549. 10.1016/j.tree.2016.03.006 [DOI] [PubMed] [Google Scholar]
- Shi, W.‐B. , Jiang, Y. , & Feng, M.‐G. (2005). Compatibility of ten acaricides with Beauveria bassiana and enhancement of fungal infection to Tetranychus cinnabarinus (Acari: Tetranychidae) eggs by sublethal application rates of pyridaben. Applied Entomology and Zoology, 40, 659–666. 10.1303/aez.2005.659 [DOI] [Google Scholar]
- Shin, T. Y. , Bae, S. M. , Kim, D. J. , Yun, H. G. , & Woo, S. D. (2017). Evaluation of virulence, tolerance to environmental factors and antimicrobial activities of entomopathogenic fungi against two‐spotted spider mite, Tetranychus urticae . Mycoscience, 58, 204–212. 10.1016/j.myc.2017.02.002 [DOI] [Google Scholar]
- Sinkins, S. P. , & Godfray, H. C. J. (2004). Use of Wolbachia to drive nuclear transgenes through insect populations. Proceedings of the Royal Society B‐Biological Sciences, 271, 1421–1426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skinner, M. , Parker, B. L. , & Kim, J. S. (2014). Role of entomopathogenic fungi in integrated pest management In Abrol D. P. (Ed.), Integrated pest management: Current concepts and ecological perspective (pp. 169–191). San Diego, California: Elsevier. [Google Scholar]
- Stouthamer, R. (1993). The use of sexual versus asexual wasps in biological‐control. Entomophaga, 38, 3–6. 10.1007/BF02373133 [DOI] [Google Scholar]
- Teixeira, L. , Ferreira, A. , & Ashburner, M. (2008). The bacterial symbiont Wolbachia induces resistance to RNA viral infections in Drosophila melanogaster . PLoS Biology, 6, 2753–2763. 10.1371/journal.pbio.1000002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tortosa, P. , Courtiol, A. , Moutailler, S. , Failloux, A. B. , & Weill, M. (2008). Chikungunya‐Wolbachia interplay in Aedes albopictus . Insect Molecular Biology, 17, 677–684. [DOI] [PubMed] [Google Scholar]
- Turelli, M. , & Hoffmann, A. A. (1999). Microbe‐induced cytoplasmic incompatibility as a mechanism for introducing transgenes into arthropod populations. Insect Molecular Biology, 8, 243–255. 10.1046/j.1365-2583.1999.820243.x [DOI] [PubMed] [Google Scholar]
- Ullah, M. S. , & Lim, U. T. (2017). Synergism of Beauveria bassiana and Phytoseiulus persimilis in control of Tetranychus urticae on bean plants. Systematic and Applied Acarology, 22, 1924–1935. 10.11158/saa.22.11.11 [DOI] [Google Scholar]
- Van Leeuwen, T. , Vontas, J. , Tsagkarakou, A. , Dermauw, W. , & Tirry, L. (2010). Acaricide resistance mechanisms in the two‐spotted spider mite Tetranychus urticae and other important Acari: A review. Insect Biochemistry and Molecular Biology, 40, 563–572. 10.1016/j.ibmb.2010.05.008 [DOI] [PubMed] [Google Scholar]
- Vavre, F. , & Kremer, N. (2014). Microbial impacts on insect evolutionary diversification: From patterns to mechanisms. Current Opinion in Insect Science, 4, 29–34. 10.1016/j.cois.2014.08.003 [DOI] [PubMed] [Google Scholar]
- Vega, F. E. , Goettel, M. S. , Blackwell, M. , Chandler, D. , Jackson, M. A. , Keller, S. , … Roy, H. E. (2009). Fungal entomopathogens: New insights on their ecology. Fungal Ecology, 2, 149–159. 10.1016/j.funeco.2009.05.001 [DOI] [Google Scholar]
- Walker, T. , Johnson, P. H. , Moreira, L. A. , Iturbe‐Ormaetxe, I. , Frentiu, F. D. , McMeniman, C. J. , … Hoffmann, A. A. (2011). The wMel Wolbachia strain blocks dengue and invades caged Aedes aegypti populations. Nature, 476, 450–453. 10.1038/nature10355 [DOI] [PubMed] [Google Scholar]
- Weinert, L. A. , Araujo‐Jnr, E. V. , Ahmed, M. Z. , & Welch, J. J. (2015). The incidence of bacterial endosymbionts in terrestrial arthropods. Proceedings of the Royal Society B‐Biological Sciences, 282, 20150249 10.1098/rspb.2015.0249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss, B. , & Aksoy, S. (2011). Microbiome influences on insect host vector competence. Trends in Parasitology, 27, 514–522. 10.1016/j.pt.2011.05.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werren, J. H. , Baldo, L. , & Clark, M. E. (2008). Wolbachia: Master manipulators of invertebrate biology. Nature Reviews Microbiology, 6, 741–751. 10.1038/nrmicro1969 [DOI] [PubMed] [Google Scholar]
- Wong, Z. S. , Hedges, L. M. , Brownlie, J. C. , & Johnson, K. N. (2011). Wolbachia‐mediated antibacterial protection and immune gene regulation in Drosophila . PLoS ONE, 6, e25430 10.1371/journal.pone.0025430 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu, S. , Xie, H. , Li, M. , Xu, X. , & Lei, Z. (2016). Highly virulent Beauveria bassiana strains against the two‐spotted spider mite, Tetranychus urticae, show no pathogenicity against five phytoseiid mite species. Experimental and Applied Acarology, 70, 421–435. 10.1007/s10493-016-0090-x [DOI] [PubMed] [Google Scholar]
- Zélé, F. , Altıntaş, M. , Santos, I. , Cakmak, I. , & Magalhães, S. (2020). Inter‐ and intra‐specific variation of spider mite susceptibility to fungal infections: implications for the long‐term success of biological control. Ecology and Evolution, in press. 10.1002/ece3.5958 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zélé, F. , Magalhães, S. , Kéfi, S. , & Duncan, A. B. (2018). Ecology and evolution of facilitation among symbionts. Nature Communications, 9, 4869 10.1038/s41467-018-06779-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zélé, F. , Santos, I. , Olivieri, I. , Weill, M. , Duron, O. , & Magalhães, S. (2018). Endosymbiont diversity and prevalence in herbivorous spider mite populations in South‐Western Europe. FEMS Microbiology Ecology, 94, fiy015 10.1093/femsec/fiy015 [DOI] [PubMed] [Google Scholar]
- Zélé, F. , Weill, M. , & Magalhães, S. (2018). Identification of spider‐mite species and their endosymbionts using multiplex PCR. Experimental and Applied Acarology, 74, 123–138. 10.1007/s10493-018-0224-4 [DOI] [PubMed] [Google Scholar]
- Zhang, D. J. , Lees, R. S. , Xi, Z. Y. , Gilles, J. R. L. , & Bourtzis, K. (2015). Combining the sterile insect technique with Wolbachia‐based approaches: II‐ A safer approach to Aedes albopictus population suppression programmes, designed to minimize the consequences of inadvertent female release. PLoS ONE, 10, e0135194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, Y. K. , Chen, Y. T. , Yang, K. , Qiao, G. X. , & Hong, X. Y. (2016). Screening of spider mites (Acari: Tetranychidae) for reproductive endosymbionts reveals links between co‐infection and evolutionary history. Scientific Reports, 6, 27900 10.1038/srep27900 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, Y. K. , Ding, X. L. , Rong, X. , & Hong, X. Y. (2015). How do hosts react to endosymbionts? A new insight into the molecular mechanisms underlying the Wolbachia‐host association. Insect Molecular Biology, 24, 1–12. [DOI] [PubMed] [Google Scholar]
- Zhang, Y. K. , Ding, X. L. , Zhang, K. J. , & Hong, X. Y. (2013). Wolbachia play an important role in affecting mtDNA variation of Tetranychus truncatus (Trombidiformes: Tetranychidae). Environmental Entomology, 42, 1240–1245. [DOI] [PubMed] [Google Scholar]
- Zhang, Y. K. , Zhang, K. J. , Sun, J. T. , Yang, X. M. , Ge, C. , & Hong, X. Y. (2013). Diversity of Wolbachia in natural populations of spider mites (genus Tetranychus): Evidence for complex infection history and disequilibrium distribution. Microbial Ecology, 65, 731–739. 10.1007/s00248-013-0198-z [DOI] [PubMed] [Google Scholar]
- Zhong, Y. , & Li, Z. X. (2014). Bidirectional cytoplasmic incompatibility induced by cross‐order transfection of Wolbachia: Implications for control of the host population. Microbial Ecology, 68, 463–471. 10.1007/s00248-014-0425-2 [DOI] [PubMed] [Google Scholar]
- Zhu, Y. X. , Song, Y. L. , Hoffmann, A. A. , Jin, P. Y. , Huo, S. M. , & Hong, X. Y. (2018). A change in the bacterial community of spider mites decreases fecundity on multiple host plants. Microbiology Open, 8(6):e743 10.1002/mbo3.743 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zindel, R. , Gottlieb, Y. , & Aebi, A. (2011). Arthropod symbioses: A neglected parameter in pest‐ and disease‐control programmes. Journal of Applied Ecology, 48, 864–872. 10.1111/j.1365-2664.2011.01984.x [DOI] [Google Scholar]
- Zouache, K. , Michelland, R. J. , Failloux, A.‐B. , Grundmann, G. L. , & Mavingui, P. (2012). Chikungunya virus impacts the diversity of symbiotic bacteria in mosquito vector. Molecular Ecology, 21, 2297–2309. 10.1111/j.1365-294X.2012.05526.x [DOI] [PubMed] [Google Scholar]
- Zouache, K. , Voronin, D. , Tran‐Van, V. , & Mavingui, P. (2009). Composition of bacterial communities associated with natural and laboratory populations of Asobara tabida infected with Wolbachia . Applied and Environmental Microbiology, 75, 3755–3764. 10.1128/AEM.02964-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zug, R. , & Hammerstein, P. (2015). Wolbachia and the insect immune system: What reactive oxygen species can tell us about the mechanisms of Wolbachia‐host interactions. Frontiers in Microbiology, 6, 1201 10.3389/fmicb.2015.01201 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Full dataset has been deposited in the Dryad data repository (doi.org/10.5061/dryad.9p8cz8wc4).


