Dear Editor-in-Chief
Visceral Leishmaniasis (VL), also known as Kala-azar, is a life-threatening disease responsible for 300.000 new cases per year, more than 90% of them occurring in Bangladesh, Brazil, Ethiopia, India, Nepal, South Sudan and Sudan (1). The L. donovani complex is responsible for the worldwide burden of VL represented by L. donovani in East Africa and Indian sub-continent followed by L. infantum in Europe, North Africa and Latin America (2). Since both conventional techniques (parasitological and serological) have several limitations for VL diagnosis, scientists have explored the field of molecular biology for nucleic acid amplification tests (3). LAMP (Loop-Mediated Isothermal Amplification) is a novel nucleic acid amplification method emerged as a promising diagnostic tool for VL diagnosis amplifying a target DNA sequence with high sensitivity, specificity and efficiency under isothermal conditions (4).
We evaluated the application of a LAMP system developed in the Old World using a L. infantum reference strain from Brazil, expecting this protocol may contribute to reducing the VL incidence rate in South America (5).
We performed a BLASTn (Basic Local Alignment Search nucleotide-https://blast.ncbi.nlm.nih.gov/Blast.cgi) using the same L. infantum kDNA sequence applied to primers design (GenBank accession number Z35271). The alignment result demonstrated sequences with significant homology to L. infantum (over 97.86% of identity) such as L. donovani and L. chagasi. Hence, we used standard genomic DNA from Leishmania (L.) infantum chagasi IOC-L 3328 (MHOM/BR/2011/COS) to prepare a dilution series standard DNA (10 ng – 0.01 fg) to evaluate the limit of detection for Polymerase Chain Reaction (PCR) and LAMP system. PCR assay was performed applying the protocol developed by Gualda et al (6) using primers FLC2 (5′-GTCAGTGTCGGAAACTAATCCGC-3′) and RLC2 (5′-GGGAAATTGGCCTCCCTGAG-3′), which amplification product was 230bp.
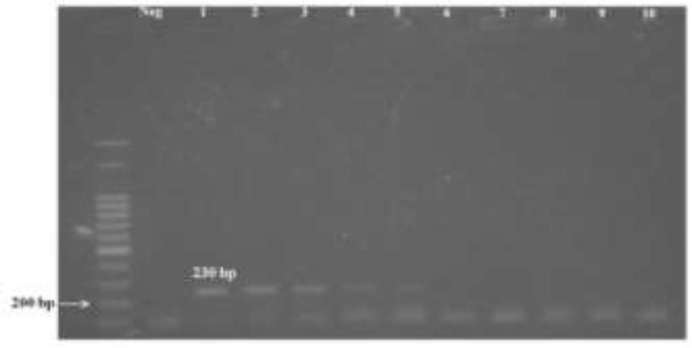
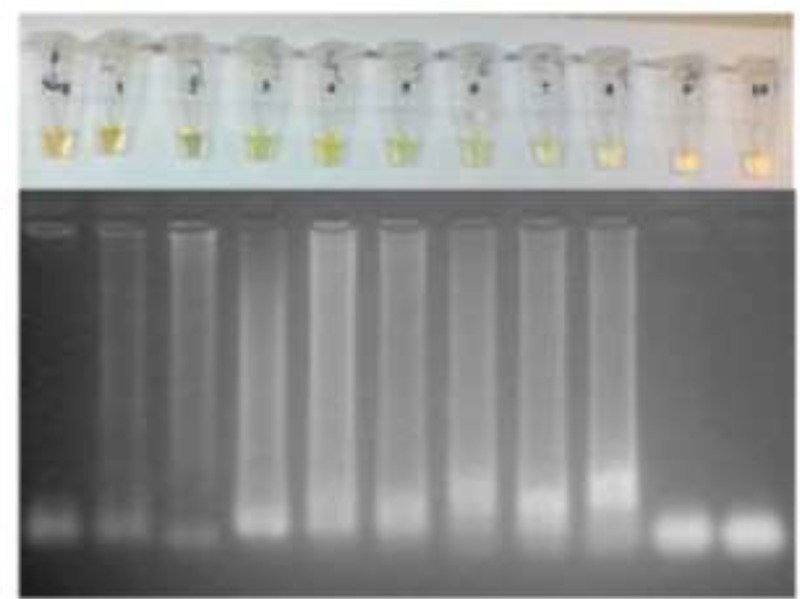
Figure 1 shows FLC2 and RLC2 limit of detection, achieving a maximum analytical sensitivity of 1 pg. In addition, it was applied the isothermal amplification protocol in our dilution series standard DNA. In Fig. 2, LAMP results could be interpreted as suggestive of nucleic acid amplification-based on changing of color and electrophoresis profile with analytical sensitivity of 1 fg, but after trying to optimize the system several times, changing magnesium concentration (2, 4, 6 and 8mM) and temperature (52, 55, 58, 61 and 64 °C), the results were not reproducible.
Fig. 1:
PCR results are displayed in 1.5% agarose gel staining with ethidium bromide visualized under UV light. The 10-fold dilution curve is constituted by: Molecular marker of 100 bp, Neg – Control negative, (1) 10ng, (2) 1ng, (3) 100pg, (4) 10pg, (5) 1pg, (6) 100fg, (7) 10fg, (8) 1fg, (9) 0.1fg and (10) 0.01fg
Fig. 2:
LAMP results in 2% agarose gel staining with ethidium bromide visualized under UV light. The 10-fold dilution curve is constituted by: Neg – Control negative, (1) 10ng, (2) 1ng, (3) 100pg, (4) 10pg, (5) 1pg, (6) 100fg, (7) 10fg, (8) 1fg, (9) 0.1fg and (10) 0.01fg. On top is seen LAMP reaction tubes after addition of SYBR Green I
Thus, there is a notorious effort from the scientific community to identify accurate and sensitive methods for diagnosing VL, which means an investment of knowledge, money, time and technical support. In Bangladesh, after develop the first LAMP system for VL diagnosis (7), a validation study was performed from this first LAMP assay with minor modifications, achieving a better clinical sensitivity performance (8). In India, also a validation study was performed on the first isothermal amplification system for VL diagnosis (9). Despite they achieved great sensitivity and specificity results, the LAMP assay was found less sensitive for strains of L. (L.) donovani originating from distinct geographical regions other than India.
Therefore, there is a meaningful point taken into consideration before trying to apply any LAMP protocol for VL diagnosis in different endemic settings, which would be the inter- and intragenic diversities of Leishmania species. Finally, we do not recommend the application of the LAMP protocol that we evaluated for VL studies in Brazil and South America based on reproducibility limitations.
Footnotes
Funding
This work was supported by Fundação de Amparo à Ciência e Tecnologia do Estado de Pernambuco (FACEPE) [grant number IBPG-0397-4.01/17] and Coordenação de Aperfeiçoamento de Pessoal de Nível Superior– Brasil (CAPES) [grant number 001].
Conflict of interest
The authors declare that there is no conflict of interests.
References
- 1.World Health Organization (2015). Investing to overcome the global impact of neglected tropical diseases: third WHO report on neglected tropical diseases. Geneva. [Google Scholar]
- 2.Chappuis F, Sundar S, Hailu A, et al. Visceral leishmaniasis: What are the needs for diagnosis, treatment and control? Nat Rev Microbiol. 2007;5(11):873–82. [DOI] [PubMed] [Google Scholar]
- 3.De Ruiter CM, Van Der Veer C, Leeflang MMG, et al. Molecular tools for diagnosis of visceral leishmaniasis: Systematic review and meta-analysis of diagnostic test accuracy. J Clin Microbiol. 2014; 52(9):3147–3155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Notomi T, Okayama H, Masubuchi H, et al. Loop-mediated isothermal amplification of DNA. Nucleic Acids Res. 2000; 28(12):E63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ghasemian M, Gharavi MJ, Akhlaghi L, et al. Development and assessment of loop-mediated isothermal amplification (LAMP) assay for the diagnosis of human visceral leishmaniasis in Iran. Iran J Parasitol. 2014;9(1):50–59. [PMC free article] [PubMed] [Google Scholar]
- 6.Gualda KP, Marcussi LM, Neitzke-Abreu HC, et al. New primers for detection of Leishmania Infantum using polymerase chain reaction. Rev Inst Med Trop Sao Paulo. 2015;57(5):377–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Takagi H, Itoh M, Islam MZ, et al. Sensitive, specific, and rapid detection of Leishmania donovani DNA by loop-mediated isothermal amplification. Am J Trop Med Hyg. 2009;81(4):578–582. [DOI] [PubMed] [Google Scholar]
- 8.Khan MGM, Bhaskar KRH, Salam MA, et al. Diagnostic accuracy of loop-mediated isothermal amplification (LAMP) for detection of Leishmania DNA in buffy coat from visceral leishmaniasis patients. Parasit Vectors. 2012; 5:280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Verma S, Avishek K, Sharma V, et al. Application of loop-mediated isothermal amplification assay for the sensitive and rapid diagnosis of visceral leishmaniasis and post-kala-azar dermal leishmaniasis. Diagn Microbiol Infect Dis. 2013; 75(4):390–5. [DOI] [PubMed] [Google Scholar]