Abstract
Permanent His Bundle Pacing (HBP) has recently gained popularity. However, implanting physicians and those who perform the device checks must invest in additional education in order to accurately program these devices, identify changes in morphology and perform troubleshooting to help achieve the best outcomes for the patients. This paper reviews key aspects of HBP and provides the educational tools for successful HBP follow-up and troubleshooting.
Keywords: His bundle pacing, Left bundle branch pacing, Conduction system pacing, Follow-up, Programming, Troubleshooting
1. Introduction
Conventional right ventricle (RV) pacing can cause electrical and mechanical dyssynchrony, resulting in pacing-induced cardiomyopathy, atrial fibrillation, heart failure hospitalizations, and even mortality [[1], [2], [3]]. Permanent His-bundle pacing (HBP) was first performed by Deshmukh et al. but did not gain popularity in the electrophysiology community until recently [4,5]. HBP offers an alternative mode of pacing that mimics native conduction via the His-Purkinje system. Challenges related to implanting HBP devices include the implantation learning curve, lack of robust HBP implantation tools, and absence of HBP specific device algorithms. In addition, there is a potential for increase in procedure duration, fluoroscopy duration, and an increase in threshold leading to shorter battery longevity. Despite these challenges, more recent data suggest an improved success rate of >90% with experience [6]. Implanting physicians and those who perform the device checks must invest in additional education in order to accurately program these devices, identify changes in morphology and perform troubleshooting to help achieve the best results for the patient. This in turn can help improve patient outcomes [6].
2. Anatomy
Based on the autopsy paper by Kawashima et al. [7], the anatomical course of the His-bundle (HB) was categorized into three types: I, II, and III, each of which presents unique morphology transitions. These anatomical variations are important to remember to help better understand the paced morphology at the HB.
-
•
Type I is the most common HB anatomy and occurs in about 47% of people. With Type I the HB runs along the lower border of the membranous part of the interventricular septum and is covered with a thin layer of myocardial fibers. Because the HB is covered with a thin layer of myocardial fibers, patients with Type I anatomy present with nonselective His bundle pacing (NS-HBP) at higher outputs due to capture of local ventricular myocardium in addition to the HB. At lower outputs, the morphology transitions to selective His bundle pacing (S-HBP) due to loss of the local ventricular myocardial capture and capture of the HB only.
-
•
Type II HB anatomy was found in about 32% of people. In this variation, the HB runs within the interventricular muscle, which can be challenging to recruit at implant. However, once the lead is successfully placed, there is NS-HBP at a higher output with transition to RV pacing only at low outputs. Patients with Type II HB anatomy will never have S-HBP, regardless of placement of the lead or the experience of the implanting physician.
-
•
Type III HB anatomy was noted in 21% of people. The anatomy of Type III His is known as the naked AV bundle because there are no surrounding myocardial fibers, which results in S-HBP at all outputs until loss of capture.
3. Terminology
3.1. Non-selective HBP (NS-HBP) vs Selective HBP (S-HBP)
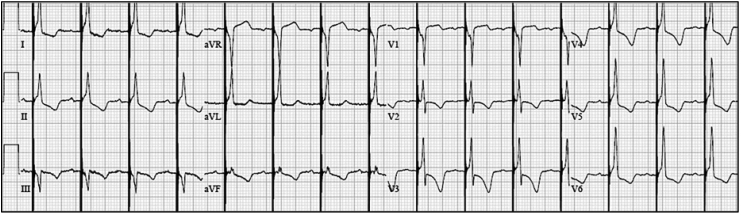
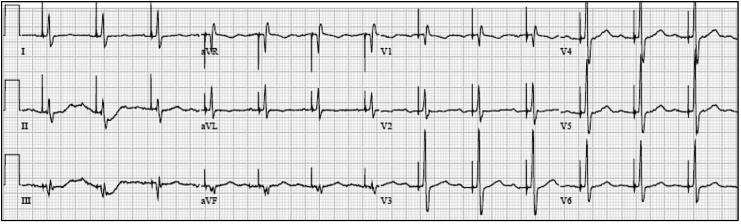
The paced morphology with a lead in the HB location can be non-selective His bundle capture (NS-HBP), selective His bundle capture (S-HBP) or RV septal capture alone. NS-HBP is capture of the surrounding ventricular myocardium and capture of the HB and is identified by presence of a pseudo delta wave, or “slur,” leading into a narrow QRS complex (Fig. 1). S-HBP is capture of the HB only and is identified by an isoelectric, or “flat straight line,” preceding the QRS complex (Fig. 2). These definitions are further described in a recent consensus document published for the standardization of nomenclature [8]. A study comparing patients with NS-HBP vs patients with S-HBP found no difference in clinical outcomes of death or heart failure hospitalization [9].
Fig. 1.
Nonselective His bundle pacing (NS-HBP) is capture of ventricular myocardium and capture of the HB. NS-HBP is identified by presence of a pseudo delta wave, or “slur,” leading into a narrow QRS complex.
Fig. 2.
Selective His bundle pacing (S-HBP) is capture of the HB only. S-HBP is identified by an isoelectric, or “flat straight line,” preceding the QRS complex.
3.2. Bundle branch block recruitment/narrowing
HBP can recruit a bundle branch block (BBB) pattern by implanting the lead distal to the site of intra-hisian disease in 80–90% cases [[10], [11], [12]]. It is important to identify the HB capture threshold where the BBB recruitment is lost. The output should be programmed at least 1 V above loss of recruitment of the BBB.
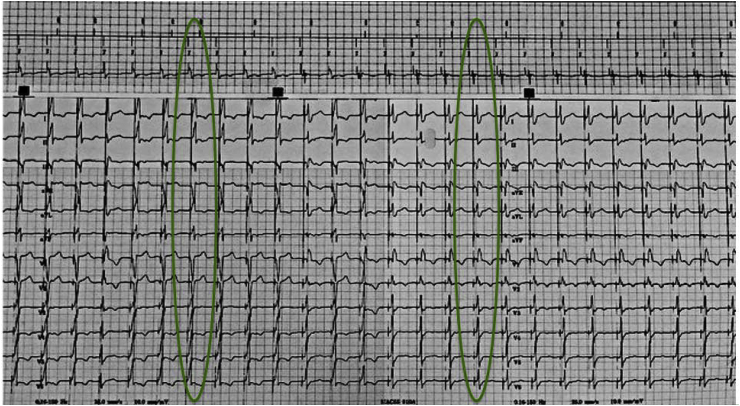
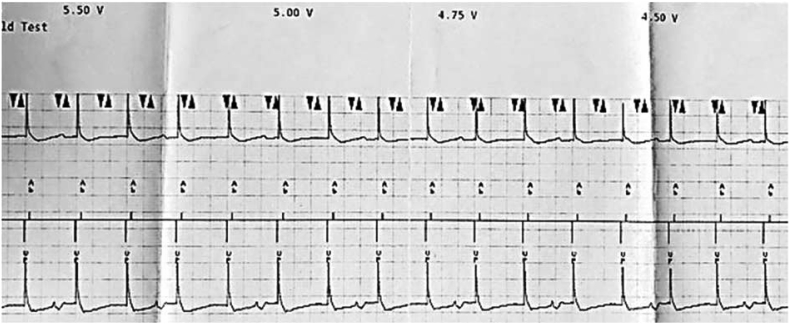
Understanding NS-HBP and S-HBP as well as recruitment of BBB and loss of recruitment of BBB is important. In Fig. 3, the first highlighted area shows NS-HBP as evident by the pseudo delta wave, or “slur,” leading into a narrow QRS complex as well as bundle branch recruitment. As we decrement on the pacing output, the second highlighted area in Fig. 3 shows a transition in morphology to S-HBP identified by the isoelectric, or “flat straight line,” preceding the QRS complex with loss of BBB recruitment. If the implanter is able to recruit the BBB during the implant, the person programming the device must program the output where there is BBB recruitment. Therefore, in this example, the output must be programmed where NS-HBP and BBB recruitment are noted. The programmer strip alone does not give enough information to identify BBB recruitment and loss of BBB recruitment. The 12-lead rhythm strip is necessary to clearly identifying these morphology changes.
Fig. 3.
Identifying BBB recruitment. A transition from NS-HBP to S-HBP is noted on the programmer strip. However, the programmer strip does not show BBB recruitment and loss of BBB recruitment. A 12-lead rhythm strip is necessary to identify the loss of BBB recruitment. In this example BBB recruitment is lost when the output is decremented down and the morphology transitions to S-HBP.
4. Programming
4.1. Lead output
This is the programming practice at our institution. At implant, all HB lead output is generally programmed at 5 V at 1 ms regardless of the threshold. The output remains at 5 V at 1 ms for the first three months. If at the three-month check: (1) the HB threshold fluctuations have remained <1 V; (2) the patient is not dependent; and (3) the Hisian threshold or BBB recruitment threshold is less than 1 V at 1 ms, the threshold can be checked at 0.4 ms pulse width. In order to promote battery longevity, if the threshold is less than 1.5 V at 0.4 ms, the pulse width is decreased to 0.4 ms. If the patient has a narrow QRS, program the His lead output 1 V above HB capture threshold. If there is correction of BBB, program the His lead output 1 V above BBB correction. At minimum, the HB lead output should be programmed to 2 V at 0.4 ms. Capture management is unable to appropriately detect or differentiate an evoked response during HB capture. Therefore, capture management for the His lead is programmed to monitor only or off.
4.2. Device mode and AV delays
All His bundle devices should be programmed in a DDD(R) mode. When sinus node dysfunction is the reason for implant, the AV delays should be prolonged and rate response should be turned on. If AV block is the reason for implant, the AV delays should be shortened. On average, the paced AV delay is programmed approximately 130 ms and the sensed AV delay is approximately 100 ms. The AV delays for AV block are shortened to accommodate for the HV interval (usually 35–55 ms) and may vary from patient to patient based on measurements that are taken at implant. Additionally, caution should be exercised when programming devices with the HB lead in the atrial port. Programming a long AV delay (≥180 ms), AAI(R)≤>DDD(R) mode or atrioventricular hysteresis should be avoided as they pose the risk of HBP on the T wave in case of intermittent loss of HB capture. DDD mode is preferred, as ventricular based timing modes such as DVI, DDI can pose the risk of pacing above the programmed limit when the HB lead is in the atrial port. A recent paper by Burri et al. summarizes these programming options in more detail [13].
4.3. Value of 12-lead ECGs
At minimum, 12-leads are necessary at implant, post-op day #1, the four-week device clinic check, the three-month post implant check, and as needed. If there is any doubt in morphology changes during future follow up, a 12-lead rhythm strip should be done [8].
5. Sensing
5.1. Unipolar vs bipolar
Adequate R waves for a lead in the His bundle location are >1 mV, unless the patient is dependent. Generally, R-waves in the HB region in the unipolar configuration often are larger, when compared to bipolar R-waves, while the local atrial signal is smaller. However, programming the His lead in a unipolar sensing configuration can lead to oversensing issues due to myopotentials and/or noise. In addition to smaller R-waves, local atrial EGM might be larger in the bipolar configuration which can lead to oversensing of the atrial EGM on the His lead. Therefore, it is important to assess sensing in both unipolar and bipolar configuration at time of implant before permanently accepting the implanted site for the lead.
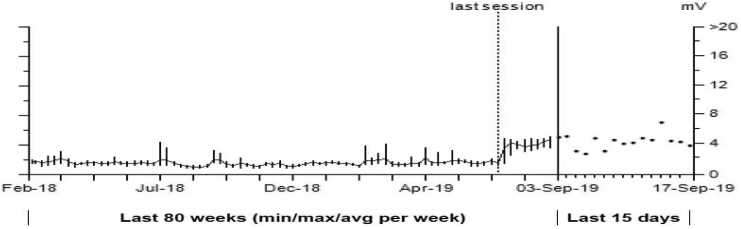
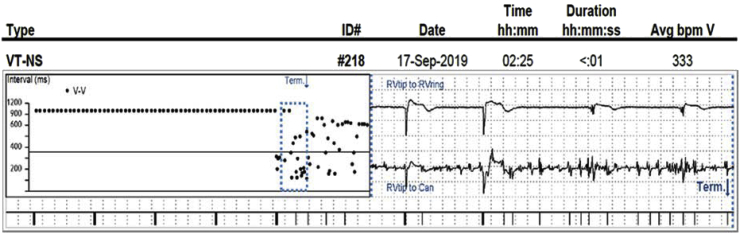
Fig. 4A, Fig. 4B shows the R wave trend for a patient with a lead in the HB location, which has been stable for two years. The graph shows the R waves doubled starting at the “Last Session,” which is an abnormal finding given the patient’s historical lead stability. However, during a recent remote transmission it was noted that during the “Last session,” which was at an outside hospital, the sensing polarity was changed from bipolar to unipolar. Since this programming change was made, the patient has experienced 3902 short V–V intervals and 163 non-sustained episodes, all resulting from the change in sensing polarity from bipolar to unipolar. Fig. 4B depicts one of the non-sustained episodes. The unipolar EGM has constant noise being sensed, while the bipolar EGM shows appropriate sensing. Programming the His lead in a unipolar sensing configuration should always be avoided, especially if the patient is dependent. When performing sensing tests, although the R-wave may be larger in the unipolar configuration, it may not be the best strategy for a given patient.
Fig. 4A.
Bipolar vs unipolar sensing. Starting at the “last session” the R waves doubled on a stable chronic lead in the HB location. The R waves doubled due to a change from bipolar sensing to unipolar sensing.
Fig. 4B.
Although the R waves are often larger in the unipolar sensing configuration, programming unipolar sensing is not advised due to myopotentials and/or noise that can be assessed. Here we have a VT-NS episode that demonstrates constant noise on the unipolar EGM and the noise is not seen on the bipolar EGM.
5.2. Implantable pulse generator considerations
The implanting physician must be mindful of the programming limitation of certain devices. For example, on the Adapta device (Medtronic Inc., Minneapolis, MN) sensing can only be programmed as low as 1 mV. Because R-waves in the HB location can be as low as >1 mV, the Adapta device might result in undersensing. By contrast, the Advisa and Azure (Medtronic Inc., Minneapolis, MN) devices allow programming the sensitivity to as low as 0.45 mV.
6. Impedances
A lead in the HB location typically demonstrates a pacing impedance in the range of 400–600 Ω. A sudden decline in impedance may be an early indicator of poor contact of the His lead with the local myocardium. If a sudden drop in impedance is observed on a remote transmission, the patient should have a full device interrogation in person with a particular focus on the threshold and the threshold trend.
7. Capture thresholds
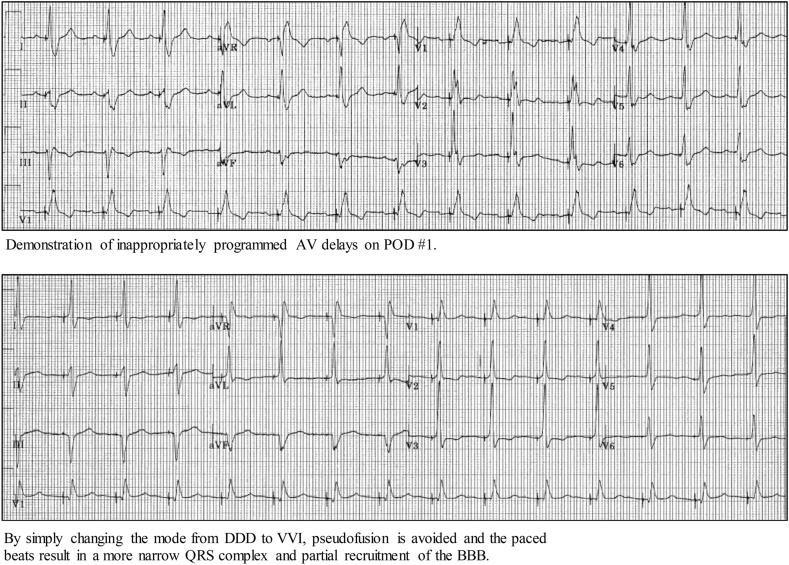
Prior to threshold testing the patient should be connected to a 12-lead ECG machine. A presenting rhythm strip is used to identify what the device is seeing and what the device is doing. As long as the patient is not dependent, obtain an underlying rhythm strip to identify if the patient has an underlying bundle branch block. On the programmer screen have the near-field and far-field EGMs visible. When running HBP threshold tests always start at 5 V at 1 ms in order to clearly identify the changes in morphology while decrementing the pacing output. In addition, the threshold test should be run in a VVI mode (or an AAI mode if the His lead is in the atrial port). We recommend avoiding running the threshold test in a DDD mode because an inappropriately programmed AV delay might result in pseudofusion, as shown in Fig. 5. While running the threshold test, run both the 12-lead rhythm strip continuously as well as the programmer strip with the near-field and far-field EGMs. Threshold tests should be done in both bipolar and unipolar pacing configurations.
Fig. 5.
Demonstration of inappropriately programmed AV delays on POD #1. By simply changing the mode from DDD to VVI, pseudofusion is avoided and the paced beats result in a more narrow QRS complex and partial recruitment of the BBB.
In a recent paper, we demonstrated the value of utilizing near-field and far-field EGMs to assess the transitions between NS-HBP, S-HBP and RV septal capture [14]. An initial negative deflection with a short time-to-peak on the near-field EGM suggests local myocardial capture, NS-HBP. A positive deflection with a longer time-to-peak suggests HB capture, S-HBP.
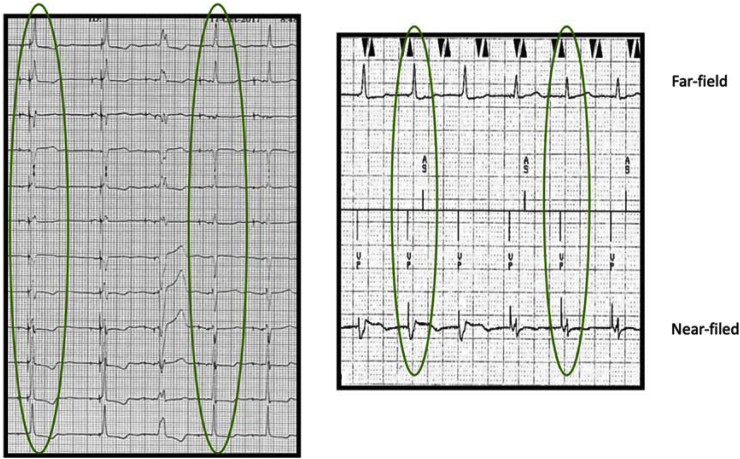
To correctly identify the changes in morphology, we recommend using all available information: 12-lead rhythm strip, device near-field EGMs, and device far-field EGMs. The first highlighted area in Fig. 6 on the 12-lead rhythm strip is NS-HBP because of the pseudo delta wave, or “slur,” leading into a narrow QRS complex. The first highlighted area on the device strip near-field EGM shows an initial negative deflection which is indicative of NS-HBP. The second highlighted area on the 12-lead rhythm strip is S-HBP because of the isoelectric, or “flat straight line,” preceding the QRS complex. On the device strip near-field EGM, the second highlighted area shows an initial positive deflection and time to peak is slightly longer which is indicative of S-HBP. The device EGMs show clear identification of changes in morphology. However, 12-lead rhythm strips are still necessary.
Fig. 6.
HBP morphology transitions while decrementing the pacing output on a 12-lead rhythm strip and the device electrograms (EGMs). The first highlighted area on the 12-lead rhythm strip is NS-HBP; evident by the pseudo delta wave, or “slur,” leading into a narrow QRS complex. The second highlighted area on the 12-lead rhythm strip is S-HBP; evident by the isoelectric, or “flat straight line,” preceding the QRS complex. On the device electrogram, NS-HBP is noted in the first highlighted area— a negative evoked response, a short stim-peak, and a wider far-field QRSd. The morphology transitions to S-HBP— a positive evoked response, the stim-peak is longer, and the far-field QRSd is narrower.
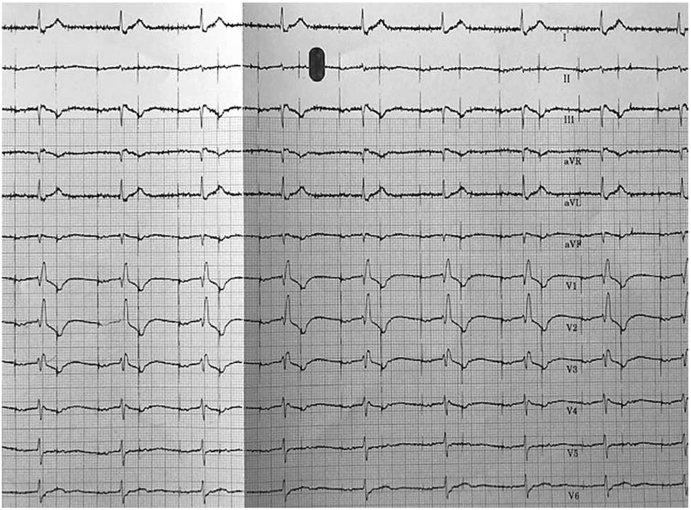
Similarly, the near-field EGM in Fig. 3 shows a transition from NS-HBP to S-HBP. However, without the 12-lead rhythm strip it is almost impossible to identify BBB recruitment and loss of BBB recruitment. Another example where the 12-lead rhythm strip is imperative, is when there is transition from NS-HBP to RV septal capture as shown in Fig. 7. If the 12-lead rhythm strip is not available, the transition to RV septal capture cannot be identified. As a result, the output may be programmed where there is RV septal capture and not NS-HBP.
Fig. 7.
There is no change in morphology on the device EGMs. However, the 12-lead rhythm strip demonstrates a transition from NS-HBP to loss of HB capture, evident by the widening of the QRS. In order to identify the transition from NS-HBP to RV septal capture, a 12-lead rhythm strip is essential.
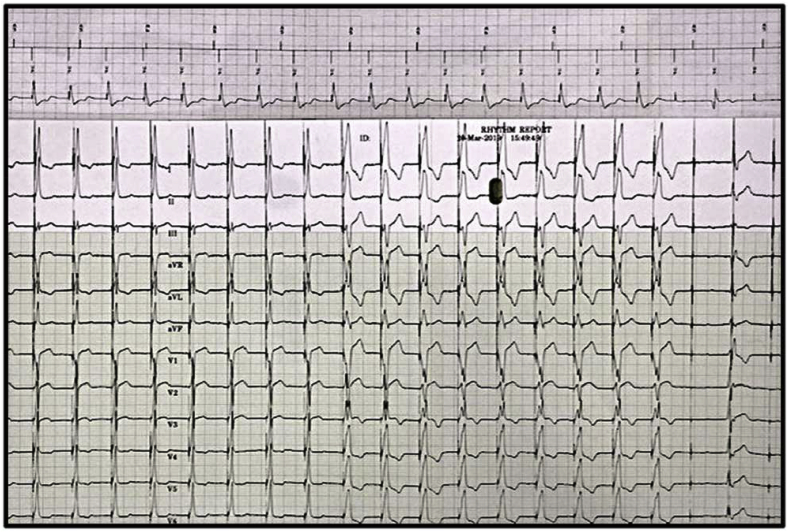
Assessing the 12-lead ECGs during implant can allow early identification of atrial capture by the His lead which can occur if the lead is placed too proximal. If only the device EGMs are used during implant this may be overlooked. Upon initial review of Fig. 8A, it may appear there is nothing wrong with the lead location. However, if Fig. 8B is assessed, the 12-lead shows capture of the atrium with the His lead resulting in 2:1 AV conduction. Atrial capture by the His lead must be identified at the time of implant since it is difficult to program around this during follow-up and the patient may require a lead revision.
Fig. 8A.
Importance of 12-lead rhythm strips during follow-up. The device EGM appears to be appropriately capturing the HB during a VVI threshold test.
Fig. 8B.
However, the 12-lead rhythm strip during the same threshold test displays capture of the atrium with the His lead, resulting in 2:1 AV conduction.
Table 1 summarizes some of these issues with HBP and the troubleshooting options in follow-up.
Table 1.
Reproduced with permission from Lustgarten D, Sharma PS &, Vijayaraman P. Heart Rhythm 2019.
| HBP issues | Consequence | Best practices at implant | Troubleshooting options |
|---|---|---|---|
| Sensing Issues | |||
| Atrial oversensing | Ventricular safety pacing, Inhibition of pacing |
Avoid implantation at a site with large atrial electrogram | If R-waves larger than P-wave, decrease the sensitivity |
| Ventricular undersensing | R on T phenomenon | Check for PVC sensing during implant | Consider switch to different sensing polarity. |
| His Injury oversensing | High V-rate episodes Inhibition of pacing |
Monitor for His Injury current and resolution of His injury | Wait for injury to resolve and reassess |
| His potential oversensing | High V-rate episodes Inhibition of pacing |
Ensure larger R-waves at implant relative to His potential | Decrease sensitivity |
| Capture issues | |||
| Atrial Capture | Pacemaker syndrome | Check for atrial capture:
|
Program DDD with short AV delays (ensure atrium refractory near His lead) |
| Long programmed AV delays | Pseudofusion | Measure intrinsic AV conduction time and adjust for HV interval | Shorten Paced/sensed AV intervals if indication for implant was AV block |
| Autocapture On | Septal RV only capture | Avoid Autocapture On | Turn Autocapture off or monitor only |
| Unipolar programming Issues | |||
| Sensing: Myopotential oversensing | Inhibition of pacing | Avoid programming this in dependent patients | If bipolar R-waves are > 1 mV, switch to bipolar sensing |
| Capture: Pectoral stimulation | Patient discomfort | Look for pectoral stimulation at time of programming | Switch to bipolar pacing configuration |
| Bipolar programming Issues | |||
| Sensing: Larger A and smaller V signals | Atrial oversensing, ventricular undersensing | Look for amplitude of A signal on bipolar sensing |
|
| Capture: Higher capture threshold | Battery drain | Ensure threshold at implant is similar in unipolar and bipolar configuration | Switch to unipolar pacing |
8. Left bundle branch area pacing
As we have discussed, HBP is form of physiological pacing. With proper lead placement the implanter is able to mimic native conduction via the His-Purkinje system, prevent RV septal pacing, restore synchronization, and often times recruit an underlying BBB. However, HBP can result in higher thresholds, requires unique programming, and may present troubleshooting challenges. HBP is the primary strategy for conduction system pacing, but if the HB capture threshold is high or if the implanter is unable to obtain an adequate paced morphology, the implanter will often then attempt left bundle branch area pacing (LBBAP). In our experience, LBBAP results in a narrow paced QRS morphology (IRBBB pattern) with lower pacing thresholds and larger R waves. At implant, leads implanted at the LBB are programmed to 5 V @ 0.4 ms and this output is reduced to 2X safety margin (lowest output of 2.5 V @ 0.4 ms) at the 3-month follow-up. All LBBAP patients follow the same 12-lead rhythm strip frequency as our HBP patients for follow-up device checks. The 12-leads are vital in order to assess the changes in morphology and to identify a possible lead dislodgement resulting in RV septal capture alone or perforation into the LV cavity. As more LBBAP devices are implanted, we will continue to assess the threshold trends and the long-term stability of these leads and this might become a more promising strategy for conduction system pacing.
9. Conclusion
Programming and troubleshooting for HBP devices can be challenging. However, with this education, implanting physicians and those who perform HBP device follow up will have the necessary tools to program and successfully troubleshoot HBP devices.
Declaration of competing interest
JLH, VR, RGT: None.
PSS: honoraria- Medtronic, consultant- Medtronic, Abbott, Boston Scientific, Biotronik.
Footnotes
Peer review under responsibility of Indian Heart Rhythm Society.
References
- 1.Sweeney M.O. Adverse effect of ventricular pacing on heart failure and atrial fibrillation among patients with normal baseline QRS duration in a clinical trial of pacemaker therapy for sinus node dysfunction. Circulation. 2003;107:2932–2937. doi: 10.1161/01.Cir.0000072769.17295.B1. [DOI] [PubMed] [Google Scholar]
- 2.Khurshid S. Incidence and predictors of right ventricular pacing-induced cardiomyopathy. Heart Rhythm. 2014;11:1619–1625. doi: 10.1016/j.hrthm.2014.05.040. [DOI] [PubMed] [Google Scholar]
- 3.Kiehl E.L. Incidence and predictors of right ventricular pacing-induced cardiomyopathy in patients with complete atrioventricular block and preserved left ventricular systolic function. Heart Rhythm. 2016;13:2272–2278. doi: 10.1016/j.hrthm.2016.09.027. [DOI] [PubMed] [Google Scholar]
- 4.Deshmukh P., Casavant D.A., Romanyshyn M., Anderson K. Permanent, direct his-bundle pacing : a novel approach to cardiac pacing in patients with normal his-purkinje activation. Circulation. 2000;101:869–877. doi: 10.1161/01.cir.101.8.869. [DOI] [PubMed] [Google Scholar]
- 5.Sharma P.S. Permanent His-bundle pacing is feasible, safe, and superior to right ventricular pacing in routine clinical practice. Heart Rhythm. 2015;12:305–312. doi: 10.1016/j.hrthm.2014.10.021. [DOI] [PubMed] [Google Scholar]
- 6.Abdelrahman M. Clinical outcomes of His bundle pacing compared to right ventricular pacing. J Am Coll Cardiol. 2018;71:2319–2330. doi: 10.1016/j.jacc.2018.02.048. [DOI] [PubMed] [Google Scholar]
- 7.Kawashima T., Sasaki H. A macroscopic anatomical investigation of atrioventricular bundle locational variation relative to the membranous part of the ventricular septum in elderly human hearts. Surg Radiol Anat. 2005;27:206–213. doi: 10.1007/s00276-004-0302-7. [DOI] [PubMed] [Google Scholar]
- 8.Vijayaraman P. Permanent His bundle pacing: recommendations from a multicenter His bundle pacing collaborative working group for standardization of definitions, implant measurements, and follow-up. Heart Rhythm. 2018;15:460–468. doi: 10.1016/j.hrthm.2017.10.039. [DOI] [PubMed] [Google Scholar]
- 9.Beer, D. et al. Clinical outcomes of selective versus nonselective His bundle pacing. [DOI] [PubMed]
- 10.Sharma P.S., Ellison K., Patel H.N., Trohman R.G. Overcoming left bundle branch block by permanent His bundle pacing: evidence of longitudinal dissociation in the His via recordings from a permanent pacing lead. HeartRhythm case reports. 2017;3:499–502. doi: 10.1016/j.hrcr.2017.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sharma P.S. Permanent His-bundle pacing as an alternative to biventricular pacing for cardiac resynchronization therapy: a multicenter experience. Heart Rhythm. 2018;15:413–420. doi: 10.1016/j.hrthm.2017.10.014. [DOI] [PubMed] [Google Scholar]
- 12.Sharma P.S. Permanent His bundle pacing for cardiac resynchronization therapy in patients with heart failure and right bundle branch block. Circ Arrhythm Electrophysiol. 2018;11 doi: 10.1161/circep.118.006613. [DOI] [PubMed] [Google Scholar]
- 13.Burri H., Keene D., Whinnett Z., Zanon F., Vijayaraman P. Device programming for His bundle pacing. Circ Arrhythm Electrophysiol. 2019;12 doi: 10.1161/circep.118.006816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Saini A. Novel method for assessment of His bundle pacing morphology using near field and far field device electrograms. Circ Arrhythm Electrophysiol. 2019;12 doi: 10.1161/circep.118.006878. [DOI] [PubMed] [Google Scholar]