Abstract
Background
Patients with outflow tract ventricular tachycardia (OTVT) with normal echocardiogram are labeled as idiopathic VT (IVT). However, a subset of these patients is subsequently diagnosed with underlying cardiac sarcoidosis (CS). Objective:Whether electrocardiogram (ECG) abnormalities in sinus rhythm (SR) can differentiate underlying CS from IVT.
Methods
We retrospectively analyzed the SR-ECGs of 42 patients with OTVT/premature ventricular complexes (PVC) and normal echocardiography. All underwent advanced imaging with cardiac magnetic resonance (CMR)/18FDG PET-CT for screening of CS. Twenty-two patients had significant abnormalities in cardiac imaging and subsequently had biopsy-proven CS (Cases). Twenty patients had normal imaging and were categorized as IVT (Controls). SR-ECGs of all patients were analyzed by 2 independent, blinded observers.
Results
Baseline characteristics were comparable. Among the ECG features analyzed – fascicular (FB) or bundle branch block (BBB) was seen in 9/22 Cases vs. 1/20 controls (p = 0.01). Among patients without FB or BBB, fragmented QRS (fQRS) was present in 9/13 cases but in none of the controls (p < 0.001). Low voltage QRS was more often seen among cases as compared to controls (10/22 vs. 3/20 p = 0.03). A stepwise algorithm based on these 3 sets of ECG findings helped to diagnose CS among patients presenting with OTVT/PVC with sensitivity of 91%, specificity of 75%, a PPV of 80%, and a NPV of 88%.
Conclusions
In patients presenting with OTVT/PVC: FB/BBB, fQRS, and low QRS voltage on the baseline ECG were more often observed among patients with underlying CS as compared to true IVT. These findings may help to distinguish underlying CS among Cases presenting with OTVT/PVC.
Keywords: Cardiac sarcoidosis, Outflow tract ventricular tachycardia, Fascicular and bundle branch block, 12 lead ECG, QRS voltage
1. Introduction
Sarcoidosis is a multisystem disease characterized by the presence of non-caseating granulomas in the involved organs. Clinical cardiac involvement in sarcoidosis occurs 5% of patients and histopathological involvement is noted in 20–30% [1,2]. Cardiac involvement is the second leading cause of death and accounts for up to 25% of disease-associated mortality. Most of the morbidity and mortality is due to progressive heart failure and/or cardiac arrhythmias [3,4].
Patients presenting with monomorphic VT or frequent ventricular ectopy who have structurally normal hearts are labeled as having “idiopathic” arrhythmias, and the right ventricular outflow tract (RVOT) is a common site of origin of such arrhythmias. We have observed some patients with CS presenting with OTVT. Clearly, it is important diagnose underlying CS, as this has important therapeutic and prognostic implications [4].
IVT is defined as VT that occurs in patients without structural heart disease on routine cardiac imaging [5]. Abnormal echocardiographic findings like reduced left ventricular ejection fraction, wall motion abnormalities, areas of thinning and enlargement clearly suggest underlying structural heart disease. However, the reverse is not true, as patients with underlying structural heart disease with subtle abnormalities may have grossly normal echocardiograms. Patients with cardiac sarcoidosis often present with normal echocardiography, especially in the early stages of the disease. A multicenter registry documented echocardiographic abnormalities in only a third of such Cases [6]. Advanced cardiac imaging with CMR/18FDG PET-CT (18-fluoro-2-deoxyglucose positron emission computed tomography) is useful in such situations (Figure Appendix 1).
1.1. Objective
To evaluate whether ECG abnormalities in sinus rhythm (SR) among patients presenting with OTVT can differentiate underlying occult CS from IVT.
2. Methods
We performed a retrospective case-control study at a tertiary care center in Southern India.
Cases: We enrolled patients from the Granulomatous Myocarditis (GM) Registry maintained at our institute with the approval of the Institutional Ethics Committee. All patients with idiopathic OTVT underwent cardiac imaging (Appendix -1AB) as part of the routine protocol [7,8]. Subjects with abnormal imaging findings (CMR and/or 18FDG PET-CT) were further subjected to endomyocardial/lymph node biopsy. Those with biopsy proven CS were treated with disease specific therapy, and if needed, radio frequency catheter ablation. Patients fulfilling the clinical diagnostic criteria for CS based on Heart Rhythm Society Consensus statement [9] were included as Cases in our study. We selected 22 patients who presented with outflow tract VT or frequent PVCs with normal left ventricular function. Twelve lead ECGs taken prior to VT or at least 3 days after achieving SR were analyzed (to exclude T wave memory repolarization abnormalities).
Summary of inclusion criteria for Cases:
-
1.
Patients presenting with outflow tract VT.
-
2.
Age >18 years.
-
3.
Tissue proven diagnosis of CS with non-caseating granuloma (node/endomyocardial biopsy)
-
4.
Normal echocardiographic parameters with preserved LV function (LVEF>50%)
Summary of exclusion Criteria for Cases:
-
1.
Other causes of granulomatous myocarditis-e.g. Myocardial tuberculosis (TB) [positive for TB polymerase chain reaction (PCR), caseating granuloma on biopsy, Acid-fast bacilli (AFB) culture, pulmonary TB]
-
2.
If the only available ECG was within 3 days of restoring sinus rhythm
-
3.
Prior PVC/OTVT ablation
-
4.
Known structural heart diseases disease like ARVC
Controls: Twenty patients with idiopathic OTVT/PVCs and normal cardiac imaging undergoing electrophysiological study and radiofrequency catheter ablation comprised the control group.
Thus, our study included a total of 22 Cases with proven CS with OTVT/PVCs presenting with normal LV function and 20 patients with OTVT with structurally normal hearts by extensive cardiac imaging who were included as Controls. The SR-ECG taken 3 days after/prior to the index arrhythmia was analyzed.
2.1. ECG characteristics
Sinus rhythm ECGs was evaluated by 2 blinded observers. The following ECG parameters were assessed.
-
1.
Intraventricular conduction defects: Presence of complete or incomplete BBB (bundle branch block (right- RBBB or left- LBBB), left anterior or posterior fascicular blocks (LAFB or LPFB), and non-specific intraventricular conduction delays (IVCD) [14], [15].
-
2.
QRS fragmentation: There are various published definitions for fQRS; we followed that of Das et al. [14,16,17]. QRS complexes with the presence of an additional R wave (R′), notching in the nadir of the R wave or the S wave, or the presence of >1 R’ (fragmentation) in 2 contiguous leads, were classified as fQRS. Typical patterns of complete and incomplete BBB were excluded from fQRS analysis as they themselves can produce notching. We considered fQRS to be present if any single ECG lead demonstrated fragmentation criteria.
-
3.
Low QRS voltage [18,19]: QRS Voltage less than 5 mV in limb leads and less than 10 mV in the precordial leads was considered as low voltage. Presence of low voltage in any 3 contiguous leads was required to define low voltage QRS.
2.2. Statistical analysis
Statistical analysis was performed using SPSS™. Continuous variables were represented as means ± SD. Comparison between the Cases and controls was performed by chi-square test for dichotomous variables and the Student T test was used for continuous variables.
3. Results
Baseline characteristics (Age/sex/diabetes mellites/Hypertension/LVEF) of Cases and controls were comparable, as shown in Table 1.
Table 1.
Baseline characteristics of Cases and controls were comparable as described below.
| Cases (n = 22) | Controls (n = 20) | |
|---|---|---|
| Age (mean ± SD) | 40 ± 11 | 45 ± 13 |
| Sex (male) | 18 | 8 |
| LVEF (mean ± SD) | 56 ± 8 | 59 ± 10 |
| VT/PVC morphology | RBBB∗-5, LBBB#-17 | RBBB-2, LBBB-18 |
| Diabetes Mellitus | 2 | 2 |
| Hypertension | 5 | 4 |
(∗RBBB- right bundle branch block. #LBBB- left bundle branch block).
The following ECG findings were significantly different between Cases and Controls:
-
1.
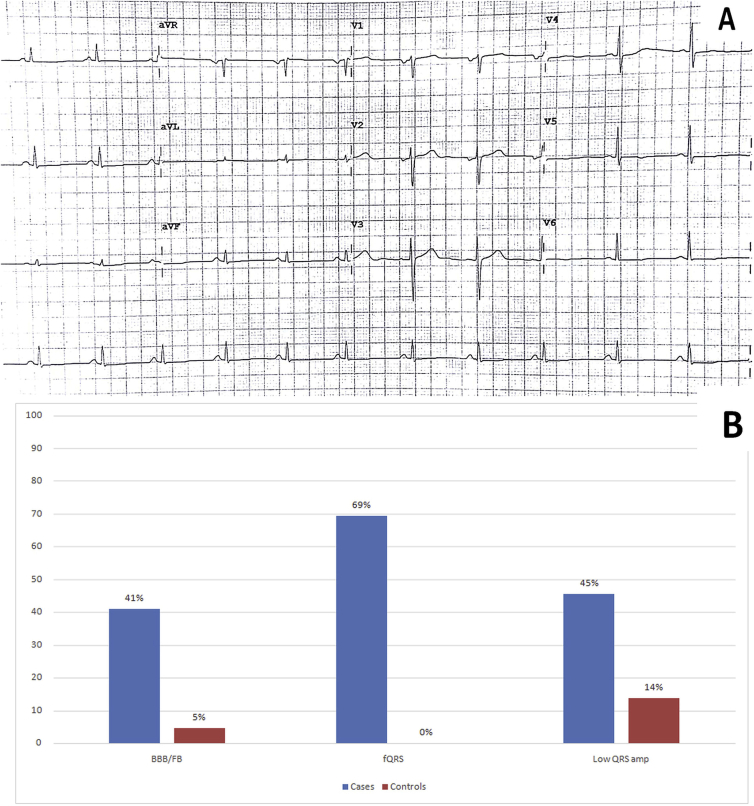
BBB/FB: Conduction abnormalities were noted among 9/22 Cases. One patient had complete RBBB, one had incomplete RBBB, 2 had bifascicular block (BFB), 4 had LAFB, 1 had IVCD. None had LPFB or complete LBBB in this cohort (Figure 1A and B). Among Controls, only 1 patient had incomplete RBBB. Thus, presence of conduction abnormalities was more often observed among Cases (p = 0.01).
-
2.
Fragmented QRS (fQRS): Nine Cases had fQRS complexes (Figure 2A and B). The maximum occurrence of fragmentation was seen in lead 3, followed by lead 2 and V1. In contrast, none of the Controls had fQRS complexes (p < 0.001).
-
5.
Low QRS voltage: Among 22 Cases, 10 had low QRS voltage. The most common lead showing low voltage was aVF, followed by lead 2 (Figure 3A). In contrast, only 3 Controls had low QRS voltage (p = 0.03).
Fig. 1.
A: Complete right bundle branch block (RBBB) noted in the ECG in sinus rhythm. B: IVCD (Intraventricular conduction defect)/(Left anterior fascicular block) LAFB noted in Sinus rhythm ECG.
Fig. 2.
A: Significant fragmentation found consistently in lead 3, lead V2 and V3.(v2,v3-zoomed in inset). B: Fragmented QRS (fQRS) most prominently seen in aVL, V1 and V2 (v1, v2 -Zoomed to inset).
Fig. 3.
A: Leads3, avL and avF having low voltage QRS voltage in after reverting to sinus rhythm. B: Bar diagram showing prevalence of ECG features in group A and B.
Table 2 shows the sensitivity, specificity, positive and negative predictive values for each of the ECG findings in recognizing Cases.
Table 2.
Sensitivity (SN), Specificity (SP), Positive predictive value (PPV), Negative predictive value (NPV) of individual and summed ECG features.
| ECG characteristics | SN(%) | SP(%) | PPV(%) | NPV(%) |
|---|---|---|---|---|
| BBB/FB | 41 | 95 | 90 | 59.4 |
| Fragmented QRS | 69.3 | 100 | 100 | 86 |
| Low QRS voltage | 45.4 | 85 | 76.9 | 58.6 |
| Any one of the above three present | 90.9 | 75 | 80 | 88.2 |
4. Discussion
The main findings of our study are that ECG abnormalities are detected in a large proportion of relatively young patients presenting with what may initially be labeled as idiopathic OTVT/PVCs, but who are subsequently recognized as having CS. CS is known to masquerade as idiopathic VT, especially early in the course of the disease when echocardiography is normal [6,21]. Such patients are often treated with anti-arrhythmic drugs or undergo catheter ablation, and the underlying smoldering inflammation may not be addressed. Such patients may have VT recurrence during follow up, and some develop progressive systolic dysfunction due to persistent inflammation and/or scarring. Thus, the initial focus on ablating the ventricular arrhythmia may delay making the correct diagnosis of CS, delay initiation of treatment, and may result in increased morbidity and mortality [21].
We found several important diagnostic ECG clues that increase the clinical suspicion for underlying CS. Because CS has a predilection for involvement of the basal septum, the conduction system gets involved relatively early, resulting in fascicular blocks or BBB. A study by Schuller et al. pointed at the occurrence of BBB in patients with pulmonary sarcoid as an indicator of cardiac involvement [14], and our results are concordant with those observations. The implication is that in patients presenting with OTVT/PVCs, evidence of conduction abnormalities on baseline ECG should be considered as surrogate of abnormal substrate/scar, especially in relatively younger patients.
fQRS is a marker of slow conduction and scarring. We found fQRS to be a consistent ECG finding in Cases of CS, whereas its incidence was significantly lower (p < 0.001) in Controls with idiopathic VT. This finding has been reported previously [14,22], and based on our results, this finding should raise the suspicion of CS masquerading as idiopathic OTVT/PVCs. In our study, the commonest leads manifesting fQRS were the inferior leads and lead V1. The presence of overt conduction system disease is indicative of granulomatous disease in patients with OTVT/PVCs, obviating the need to look for fQRS. In the absence of prior myocardial infarction, fQRS and conduction abnormalities in the setting of OTVT should prompt further evaluation to exclude CS.
Another interesting finding was low QRS voltage. Ten of the 22 Cases had low QRS voltage in 3 leads or more, whereas only 3 Controls had such a finding. This may be attributable to myocardial edema or scarring in CS. Some of these patients with CS indeed showed myocardial edema on cardiac MRI, supporting this hypothesis. A case report by Virmani et al. [23], [24] suggested that low voltage QRS complexes can be makers of CS.
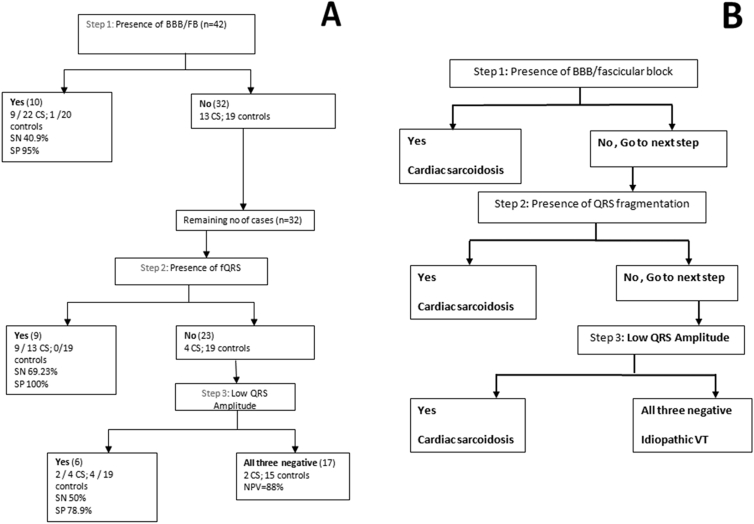
Based on our observations, we suggest the following, easy-to-use stepwise algorithm (Figure 4A and B) to allow the recognition of CS from the baseline or follow up ECG (obtained before or at least 3 days after restoration of sinus rhythm). We selected 3 objective criteria which are non-continuous, simple and easy to apply, and demonstrated good specificity.
Fig. 4.
AB: Algorithm for identifying occult CS in Cases of OTVT/PVC, with normal echocardiography. A: Derivation of algorithm. B: Clinically applicable summarized algorithm.
This algorithm was based on the following calculation:
Step 1: Presence of any fascicular block/BBB: if present: CS is likely. This has specificity of 95% with a sensitivity of 40.9%. As the sensitivity is not very high, absence of this does not rule out CS, hence proceed to step 2.
Step 2: Presence of fQRS (in absence of BBB): if present in SR ECG, CS is highly likely. This was had specificity and PPV of 100%. However, the sensitivity was 69.3% among the subjects. Thus, even when this feature is absent, it may not rule out CS, hence proceed to step 3.
Step 3: Low QRS voltage any 3 leads (less than 0.5 mv for limb and 1 mv for precordial leads): more likely to be CS. This has specificity (78.9%) among rest of the cohort.
Overall, taking these 3 simple ECG characteristics together into consideration, if any one ECG feature is present, the following results are obtained: Sensitivity = 90.91% (Specificity = 75%), and PPV = 80% (Negative predictive value = 88.24% for ruling out CS).
4.1. Limitations
-
1.
This is a retrospective study and has its inherent limitations. However, blinding of the observers was the strength of study.
-
2.
Our sample size was small, and these findings need to be validated in a larger prospective study.
-
3.
These findings apply only to patients presenting with OTVT/PVCs, and idiopathic VTs of other morphologies may be associated with other ECG findings.
-
4.
These ECG findings indicate abnormal myocardial substrate and are probably not specific for CS; they may be observed in other conditions like ARVC, if the RVOT is involved. Moreover, other entities like idiopathic DCM, Lamin A/C cardiomyopathy, giant cell myocarditis etc. may present with VT with preserved EF, where PET may be normal. However, we limited our study only to granulomatous myocarditis, which is probably more prevalent.
5. Conclusion
Cardiac sarcoidosis can present as “idiopathic” OTVT/PVCs. We report overt conduction system disease, fQRS and low voltage complexes being more often observed among patients with underlying CS as compared to true Idiopathic OTVTs. A stepwise algorithmic approach, as suggested above, would help in identifying underlying CS among subjects presenting with OTVT. A high index of suspicion for CS and low threshold for evaluation by advanced imaging (CMR/cardiac PET-CT) might help to diagnose underlying CS early and avert its complications.
Declaration of competing interest
None.
Acknowledgment
We convey our regards to Mr. Sudhir Kumar for statistical analysis, Mrs. Venkatlaxmi Lavishetty for helping us in writing the manuscript and Ms. Kalpana for assisting in data collection. We also extend our regards to Dr. Vihang Shah for helping us in preparing the images.
Footnotes
Peer review under responsibility of Indian Heart Rhythm Society.
Appendix.
Appendix 1.
A: Cardiac PET-CT scan showing prominent uptake in the infero-basal LV myocardium and interventricular septum.
B: Cardiac MRI showing late gadolinium uptake suggestive of septal scar in the same patient.
References
- 1.Fleming H.A. Sarcoid heart disease. Br Heart J. 1974 Jan;36(1):54–68. doi: 10.1136/hrt.36.1.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sekiguchi M., Hiroe M., Take M., Hirosawa K. Clinical and histopathological profile of sarcoidosis of the heart and acute idiopathic myocarditis. Concepts through a study employing endomyocardial biopsy. II. Myocarditis. Jpn Circ J. 1980 Apr;44(4):264–273. doi: 10.1253/jcj.44.264. [DOI] [PubMed] [Google Scholar]
- 3.Lynch J.P., 3rd, Hwang J., Bradfield J., Fishbein M., Shivkumar K., Tung R. Cardiac involvement in sarcoidosis: evolving concepts in diagnosis and treatment. Semin Respir Crit Care Med. 2014 Jun;35(3):372–390. doi: 10.1055/s-0034-1376889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Furushima H., Chinushi M., Sugiura H., Kasai H., Washizuka T., Aizawa Y. Ventricular tachyarrhythmia associated with cardiac sarcoidosis: its mechanisms and outcome. Clin Cardiol. 2004 Apr;27(4):217–222. doi: 10.1002/clc.4960270409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lerman B.B., Stein K.M., Markowitz S.M., Mittal S., Slotwiner D.J. Ventricular arrhythmias in normal hearts. Cardiol Clin. 2000 May;18(2):265–291. doi: 10.1016/s0733-8651(05)70141-7. [vii] [DOI] [PubMed] [Google Scholar]
- 6.Jefic D., Joel B., Good E., Morady F., Rosman H., Knight B. Role of radiofrequency catheter ablation of ventricular tachycardia in cardiac sarcoidosis: report from a multicenter registry. Heart Rhythm. 2009 Feb;6(2):189–195. doi: 10.1016/j.hrthm.2008.10.039. [DOI] [PubMed] [Google Scholar]
- 7.Yalagudri S., Zin Thu N., Devidutta S., Saggu D., Thachil A., Chennapragada S. Tailored approach for management of ventricular tachycardia in cardiac sarcoidosis. J Cardiovasc Electrophysiol. 2017 Aug;28(8):893–902. doi: 10.1111/jce.13228. [DOI] [PubMed] [Google Scholar]
- 8.Tung R., Bauer B., Schelbert H., Lynch J.P., 3rd, Auerbach M., Gupta P. Incidence of abnormal positron emission tomography in patients with unexplained cardiomyopathy and ventricular arrhythmias: the potential role of occult inflammation in arrhythmogenesis. Heart Rhythm. 2015 Dec;12(12):2488–2498. doi: 10.1016/j.hrthm.2015.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Birnie D.H., Sauer W.H., Bogun F., Cooper J.M., Culver D.A., Duvernoy C.S. HRS expert consensus statement on the diagnosis and management of arrhythmias associated with cardiac sarcoidosis. Heart Rhythm. 2014 Jul;11(7):1305–1323. doi: 10.1016/j.hrthm.2014.03.043. [DOI] [PubMed] [Google Scholar]
- 14.Schuller J.L., Olson M.D., Zipse M.M., Schneider P.M., Aleong R.G., Wienberger H.D. Electrocardiographic characteristics in patients with pulmonary sarcoidosis indicating cardiac involvement. J Cardiovasc Electrophysiol. 2011 Nov;22(11):1243–1248. doi: 10.1111/j.1540-8167.2011.02099.x. [DOI] [PubMed] [Google Scholar]
- 15.Surawicz B., Childers R., Deal B.J., Gettes L.S., Bailey J.J., Gorgels A. AHA/ACCF/HRS recommendations for the standardization and interpretation of the electrocardiogram: part III: intraventricular conduction disturbances: a scientific statement from the American heart association electrocardiography and arrhythmias committee, council on clinical cardiology; the American college of cardiology foundation; and the heart rhythm society: endorsed by the international society for computerized electrocardiology. Circulation. 2009 Mar 17;119(10):235–e240. doi: 10.1161/CIRCULATIONAHA.108.191095. [DOI] [PubMed] [Google Scholar]
- 16.Das M.K., Suradi H., Maskoun W., Michael M.A., Shen C., Peng J. Fragmented wide QRS on a 12-lead ECG: a sign of myocardial scar and poor prognosis. Circ Arrhythm Electrophysiol. 2008 Oct;1(4):258–268. doi: 10.1161/CIRCEP.107.763284. [DOI] [PubMed] [Google Scholar]
- 17.Das M.K., Maskoun W., Shen C., Michael M.A., Suradi H., Desai M. Fragmented QRS on twelve-lead electrocardiogram predicts arrhythmic events in patients with ischemic and nonischemic cardiomyopathy. Heart Rhythm. 2010 Jan;7(1):74–80. doi: 10.1016/j.hrthm.2009.09.065. [DOI] [PubMed] [Google Scholar]
- 18.Kamath S.A., Meo Neto J. de P., Canham R.M., Uddin F., Toto K.H., Nelson L.L. Low voltage on the electrocardiogram is a marker of disease severity and a risk factor for adverse outcomes in patients with heart failure due to systolic dysfunction. Am Heart J. 2006 Aug;152(2):355–361. doi: 10.1016/j.ahj.2005.12.021. [DOI] [PubMed] [Google Scholar]
- 19.Kosuge M., Kimura K. Low QRS voltage and attenuation of the voltage of QRS complexes in takotsubo cardiomyopathy. J Electrocardiol. 2015 Feb;48(1):126. doi: 10.1016/j.jelectrocard.2014.09.004. [DOI] [PubMed] [Google Scholar]
- 21.Kron J., Sauer W., Mueller G., Schuller J., Bogun F., Sarsam S. Outcomes of patients with definite and suspected isolated cardiac sarcoidosis treated with an implantable cardiac defibrillator. J Interv Card Electrophysiol Int J Arrhythm Pacing. 2015 Jun;43(1):55–64. doi: 10.1007/s10840-015-9978-3. [DOI] [PubMed] [Google Scholar]
- 22.Roukoz H., Shah M., Masilamani L.J., Thachil A., Jayakumar P.K., Benditt D.G. fQRS as a marker of granulomatous disease in patients presenting with ventricular tachycardia and normal left ventricular ejection fraction. Indian Heart J. 2015 Jun;67(3):222–226. doi: 10.1016/j.ihj.2015.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Virmani R., Roberts W.C. Structure-function correlations in cardiovascular and pulmonary diseases (CPC). Disappearance of symptomatic coronary heart disease and death from a noncardiac condition. Clinical conference from the Pathology Branch, National Heart, Lung, and Blood Institute, National Institutes of Health, Bethesda. Chest. 1980 Jan;77(1):91–93. doi: 10.1378/chest.77.1.91. [DOI] [PubMed] [Google Scholar]
- 24.Virmani R., Bures J.C., Roberts W.C. Cardiac sarcoidosis; a major cause of sudden death in young individuals. Chest. 1980 Mar;77(3):423–428. doi: 10.1378/chest.77.3.423. [DOI] [PubMed] [Google Scholar]