Abstract
Background
The optimal induction treatment in potentially-resectable stage IIIA-N2 NSCLC remains undefined.
Aim
To compare neoadjuvant high-dose chemoradiotherapy (CRT) to neoadjuvant chemotherapy (CHT) in patients with resectable, stage IIIA-N2 non-small-cell lung cancer (NSCLC).
Methods
Retrospective, multicentre study of 99 patients diagnosed with stage cT1-T3N2M0 NSCLC who underwent neoadjuvant treatment (high-dose CRT or CHT) followed by surgery between January 2005 and December 2014.
Results
47 patients (47.5%) underwent CRT and 52 (52.5%) CHT, with a median follow-up of 41 months. Surgery consisted of lobectomy (87.2% and 82.7%, in the CRT and CHT groups, respectively) or pneumonectomy (12.8% vs. 17.3%). Nodal downstaging (to N1/N0) and Pathologic complete response (pCR; pT0pN0) rates were significantly higher in the CRT group (89.4% vs. 57.7% and 46.8% vs. 7.7%, respectively; p < 0.001)). Locoregional recurrence was significantly lower in the CRT group (8.5% vs. 13.5%; p = 0.047) but distant recurrence rates were similar in the two groups. Median PFS was 45 months (CHT) vs. “not reached” (CRT). Median OS was similar: 61 vs. 56 months (p = 0.803). No differences in grade ≥3 toxicity were observed. On the Cox regression analysis, advanced pT stage was associated with worse OS and PFS (p < 0.001) and persistent N2 disease (p = 0.002) was associated with worse PFS.
Conclusions
Compared to neoadjuvant chemotherapy alone, a higher proportion of patients treated with preoperative CRT achieved nodal downstaging and pCR with better locoregional control. However, there were no differences in survival. More studies are needed to know the optimal treatment of these patients.
Keywords: Non-small cell lung cancer (NSCLC), N2 disease, Neoadjuvant therapy, High dose radiation
1. Background and aim
Lung cancer is the leading cause of cancer-related death in the world.1 Non-small cell lung cancer (NSCLC) accounts for approximately 80% of all lung cancer cases, with up to a quarter of patients diagnosed with locally advanced disease. The main treatment options in patients with stage IIIA-N2 NSCLC include chemotherapy, radiotherapy, and surgery. However, in routine clinical practice, the optimal combination of these treatment modalities in this heterogeneous group of patients is both controversial and challenging.
The results of the Intergroup randomized phase III trial showed that, in selected patients with stage IIIA-N2 disease, induction therapy followed by surgery yielded better disease-free survival (DFS) outcomes than standard chemoradiotherapy (CRT).2 However, in that trial, overall survival (OS) among the surgical patients versus the CRT group was better only in the lobectomy subgroup (but not pneumonectomy). At present, the optimal neoadjuvant treatment scheme remains undefined. Studies comparing induction therapies—chemotherapy (CHT) alone versus CRT—have reported higher rates of pathological downstaging and pathologic complete response (pCR) in patients who receive CRT,3, 4, 5, 6 but these improvement did not translate into better OS or DFS outcomes; moreover, CRT was associated with greater perioperative morbidity and mortality. Notably, however, the studies performed to date are highly heterogeneous, applying a wide range of different radiotherapy treatment schemes (dose and fractionation) as well as different combinations of chemotherapy and radiotherapy.
The standard preoperative radiation dose, established in the clinical trial conducted by Albain et al., is 45 Gy.2,7 Although studies comparing different radiotherapy doses for neoadjuvant CRT have reported that dose escalation improves pCR, this benefit did not yield a survival advantage.8, 9, 10 However, to our knowledge, none of the aforementioned studies have compared high-dose (>/=60 Gy) CRT to CHT alone as a neoadjuvant strategy.
In this context, we conducted the present multi-institutional, retrospective study to compare the outcomes among patients in Spain diagnosed with resectable stage IIIA-N2 NSCLC and treated with either neoadjuvant CRT with high-dose radiation or neoadjuvant CHT alone, in both cases followed by surgery.
2. Material and methods
2.1. Study population
This multi-institutional, retrospective study was carried out in 14 Spanish hospitals. The study was supported by the Radiation Oncology Clinical Research Group (GICOR) and the Oncologic Group for the Study of Lung Cancer-Spanish Society of Radiation Oncology (GOECP-SEOR).
Inclusion criteria were as follows: 1) diagnosis of potentially-resectable stage IIIA-N2 (cT1-3 N2 M0) NSCLC between the years 2005 and 2014, 2) radical-intent treatment involving neoadjuvant CHT alone plus surgery or neoadjuvant high-dose CRT plus surgery. Exclusion criteria were: 1) T4 tumor with mediastinal invasion, 2) presence of bulky nodal disease with loss of the tissue plane separating the mediastinal nodes from the trachea, 3) invasion of the large vessels and/or heart, and 4) presence of contralateral pulmonary nodules.
2.2. Staging and treatment scheme
Staging was performed by computed tomography (CT), positron-emission tomography (PET)-CT, and/or bone scan. Magnetic resonance imaging (MRI) and/or CT were performed to evaluate the brain. Baseline mediastinal involvement was evaluated by PET/CT or CT. When feasible, mediastinal disease was confirmed histologically by mediastinoscopy, endobronchial ultrasound (EBUS), or endoscopic ultrasound (EUS). Staging was performed in accordance with the 7th edition of the TNM Classification of Malignant Tumors.
Response to neoadjuvant treatment was assessed by CT or PET-CT imaging using RECIST (Response Evaluation Criteria in Solid Tumors) criteria. In some cases, in accordance with the institutional protocols of the treating centre, histological reassessment of the mediastinal nodes was also performed (EUS, EBUS or mediastinoscopy).
All patients received neoadjuvant treatment involving either CRT with high-dose radiotherapy (media dose: 62.6 Gy ± 3 (60–66.6 Gy at 1.8−2 Gy/fraction)) or CHT (three cycles of platinum-based doublets) followed by surgery. Surgery consisted of either lobectomy or pneumonectomy with homolateral and mediastinal hilar lymphadenectomy. Pathologic complete response was defined as the absence of viable tumor in the surgical specimen. Downstaging was defined as a reduction of at least one N-stage level from the preoperative (clinical) stage to the postoperative pathological stage.
2.3. Follow-up
Acute and late toxicity were evaluated according to the Common Toxicity Criteria for Adverse Events (CTCAE), version 4.0. Follow-up was performed weekly during the treatment phase and then every 3–6 months for the first 2 years, every 6–12 months for the following three years, and annually thereafter. The date of recurrence was defined as the date that abnormal findings were detected on follow-up imaging. Locoregional recurrences were defined as recurrences in the ipsilateral lung and/or nodal regions (hilar, mediastinal or supraclavicular fossa). Distant relapse was defined as a recurrence in other locations (AJCC staging criteria, 7th edition).
2.4. Statistical analysis
The patients’ baseline characteristics were compared using the Chi-square or Fisher’s exact test for categorical variables and Student’s t test or the U Mann-Whitney test (depending on the parametric or non-parametric distribution) for quantitative variables. OS and DFS were both calculated from the date of pathological diagnosis until death or last follow-up (OS) or until the first recurrence, death from any cause, or last follow-up (DFS). OS and DFS curves were estimated by the Kaplan-Meier method. The log-rank test was used to compare survival curves between the groups. Statistical significance was set at p < 0.05. All statistical analyses were performed with the SPSS statistical software program, v. 22.0 (SPSS-IBM; Armonk, NY; USA).
3. Results
3.1. Patient characteristics
A total of 99 patients diagnosed with stage IIIA-N2 NSCLC were included in the study. Table 1 shows the clinical and sociodemographic characteristics of the sample. At diagnosis, the mean age was 61 years (range, 53–69) and 81.8% of the patients were male. The ECOG (Eastern Cooperative Oncology Group) performance status was excellent (0–1) in nearly all (99%) of the patients. Histological confirmation of mediastinal involvement was performed prior to treatment in 57.6% of cases (51.9% and 63.8%, respectively, of the CHT and CRT groups). At diagnosis, a single mediastinal node station was involved in 59.6% (n = 59) of cases. Three patients (3%) had potentially resectable bulky lymph node disease (≥3 cm). Distribution by clinical T stage was as follows: cT1 (n = 28; 28.6%), cT2 (n = 36; 36.7%), and cT3 (n = 34; 34.7%). Slightly more than half of the tumours (n = 55; 56.1%) were adenocarcinomas.
Table 1.
Baseline clinical and sociodemographic characteristics of the study population.
| Type of neoadjuvant treatment | ||||
|---|---|---|---|---|
| Total n = 99 (%) | CHT n = 52 (%) | CRT n = 47 (%) | ||
| Age at diagnosis | ||||
| >60 years, n(%) | 59 (59.6) | 33 (63.5) | 26 (55.3) | 0.410 |
| <=60 years, n(%) | 40 (40.4) | 19 (36.5) | 21 (44.7) | |
| Sex | ||||
| Male, n(%) | 81 (81.8) | 44 (84.6) | 37 (78.7) | 0.448 |
| Female, n(%) | 18 (18.2) | 8 (15.4) | 10 (21.3) | |
| ECOG performance status | ||||
| 0–1, n(%) | 98 (99.0) | 51 (98,1) | 47 (100.0) | 1.000 |
| 2, n(%) | 1 (1.0) | 1 (1.9) | 0 (0.0) | |
| Smoking status | ||||
| No smoke at diagnosis, n(%) | 49 (49.5) | 31 (59.6) | 18 (38.3) | 0.034 |
| Smoke at diagnosis, n(%) | 50 (50.5) | 21 (40.4) | 29 (61.7) | |
| T Stage (TNM 7th edition) | ||||
| cT1, n(%) | 28 (28.6) | 16 (30.8) | 12 (26.1) | 0.432 |
| cT2, n(%) | 36 (36.7) | 21 (40.4) | 15 (32.6) | |
| cT3, n(%) | 34 (34.7) | 15 (28.8) | 19 (41.3) | |
| Nodal station | ||||
| 1, n(%) | 59 (59.6) | 31 (59.6) | 28 (59.6) | 0.997 |
| >1, n(%) | 40 (40.4) | 21 (40.4) | 19 (40.4) | |
| Size of lymph nodes | ||||
| <3 cm, n(%) | 96 (97.0) | 50 (96.2) | 46 (97.9) | 1.000 |
| >=3 cm, n(%) | 3 (3.0) | 2 (3.8) | 1 (2.1) | |
| Histology | ||||
| Adenocarcinoma and others, n(%) | 55 (56.1) | 27 (51.9) | 28 (60.9) | 0.373 |
| Squamous, n(%) | 43 (43.9) | 25 (48.1) | 18 (39.1) | |
| Histopathologically-confirmed mediastinal lymph nodes | ||||
| No, n(%) | 42 (42.4) | 25 (48.1) | 17 (36.2) | 0.231 |
| Yes, n(%) | 57 (57.6) | 27 (51.9) | 30 (63.8) | |
| Type of surgery | ||||
| Pneumonectomy, n(%) | 15 (15.2) | 9 (17.3) | 6 (12.8) | 0.529 |
| Lobectomy, n(%) | 84 (84.8) | 43 (82.7) | 41 (87.2) | |
| Adjuvant chemotherapy | ||||
| No, n(%) | 76 (76.8) | 36 (69.2) | 40 (85.1) | 0.062 |
| Yes, n(%) | 23 (23.2) | 16 (30.8) | 7 (14.9) | |
| Adjuvant radiotherapy | ||||
| No, n(%) | 68 (68.7) | 22 (42.3) | 46 (97.9) | 0.000 |
| Yes, n(%) | 31 (31.3) | 30 (57.7) | 1 (2.1) | |
| RT treatment interruptions (neoadjuvant/adjuvant)a | ||||
| No interruption, n(%) | 64 (87.7) | 21 (80.8) | 43 (91.5) | 0.266 |
| Interruption <1week, n(%) | 9 (12.3) | 5 (19.2) | 4 (8.5) |
RT, radiotherapy; CHT, chemotherapy; CRT, chemoradiotherapy; ECOG, Eastern Cooperative Oncology Group.
Bold values signifies statistically significant results.
Unknown data of interruptions in 4 patients who received PORT.
3.2. Treatment characteristics (neoadjuvant and postoperative treatment, and surgical type)
The neoadjuvant treatment consisted of CRT in 47 cases (47.5%) and CHT in 52 cases (52.5%). Most patients underwent lobectomy (87.2% and 82.7%, respectively, in the CRT and CHT groups), with the remaining patients (12.8% and 17.3%, respectively) undergoing pneumonectomy (p = 0.529). Postoperative radiotherapy (PORT) was administered in 57.7% of patients in the CHT group and in one patient in the CRT group. Treatment interruptions were observed in 9 patients (12.3%), with the interruption lasting < one week in all cases. Adjuvant CHT was administered in 14.9% and 30.8%, respectively, of the CRT and CHT groups (p = 0.062, Table 1). Propensity score matching was not performed because the two groups were equally balanced in terms of the number of patients.
3.3. Pathologic outcomes and recurrence patterns
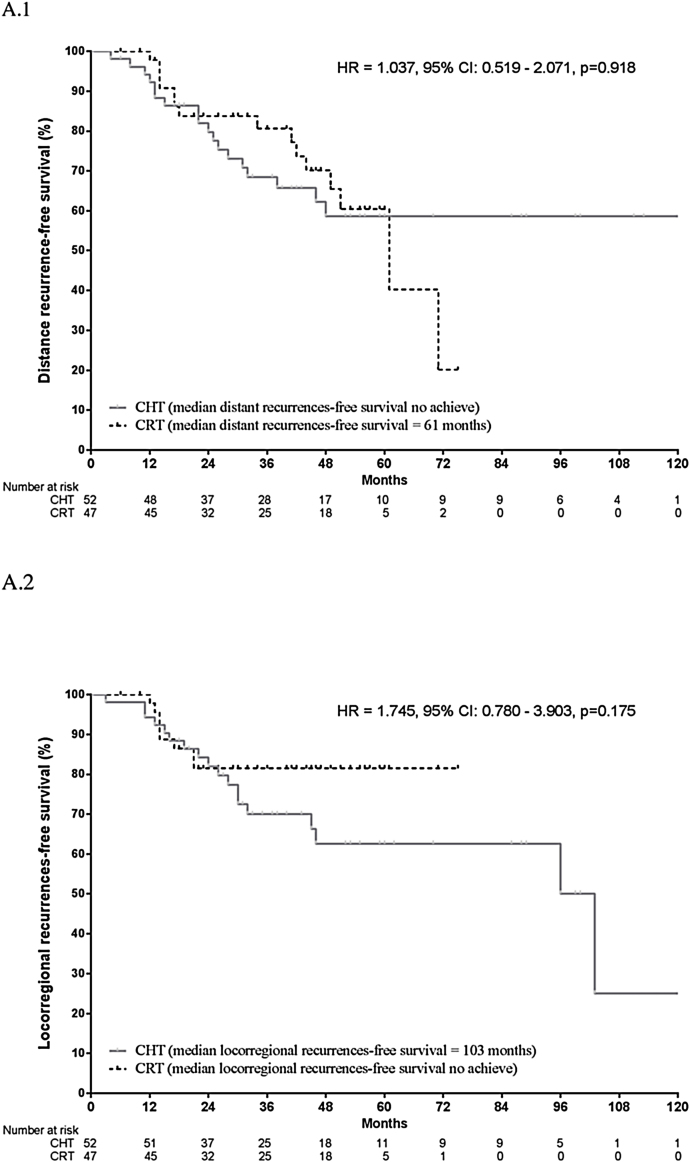
Nodal downstaging was observed in 89.4% of the CRT group and 57.7% of the CHT group, with pCR (pT0pN0) achieved in 46.8% and 7.7%, respectively. Nodal downstaging and pCR rates were significantly higher in the CRT group (p < 0.001; Table 2). The locoregional recurrence rate was significantly lower in the CRT group (17% vs. 34.6%, p = 0.047). No significant between-group differences were observed in distant recurrence rates: 31.9% (CRT) vs. 34.6% (CHT) (p = 0.776; Fig. 1). There were no significant between-group differences in DFS on the multivariate analysis.
Table 2.
Recurrence patterns and pathological outcomes.
| Type of neoadjuvant treatment | ||||
|---|---|---|---|---|
| Total n = 99 (%) | CHT n = 52 (%) | CRT n = 47 (%) | p value | |
| Median follow up, months (SD) | 41 (27) | 41.5 (30.0) | 41 (22.0) | 0.379 |
| Median time to recurrence, months (SD) | 30 (34.0) | 31 (33.8) | 29 (32.0) | 0.385 |
| Median time to distance recurrence, months (SD | 33 (32.0) | 35 (32.5) | 32 (33.0) | 0.320 |
| Pathological complete response (pT0 pN0) | ||||
| No, n(%) | 73 ( 73.7) | 48 (92.3) | 25 (53.2) | 0.000 |
| Yes, n(%) | 26 (26.3) | 4 (7.7) | 22 (46.8) | |
| Nodal downstaging | ||||
| No, n(%) | 27 (27.3) | 22 (42.3) | 5 (10.6) | 0.000 |
| Yes, n(%) | 72 (72.7) | 30 (57.7) | 42 (89.4) | |
| Locoregional recurrence | ||||
| No, n(%) | 73 (73.7) | 34 (65.4) | 39 (83.0) | 0.047 |
| Yes, n(%) | 26 (26.3) | 18 (34.6) | 8 (17.0) | |
| Distance recurrence | ||||
| No, n(%) | 66 (66.7) | 34 (65.4) | 32 (68.1) | 0.776 |
| Yes, n(%) | 33 (33.3) | 18 (34.6) | 15 (31.9) | |
| Locoregional and distance recurrence | ||||
| No, n(%) | 88 (88.9) | 45 (86.5) | 43 (91.5) | 0.434 |
| Yes, n(%) | 11 (11.1) | 7 (13.5) | 4 (8.5) | |
| PFS (5 years/median) | 42.6 / 45 | 55.5 / undef. | 0.092 | |
| OS (5 years/median) | 41.3 / 56 | 54.1 / 61 | 0.163 |
CHT, chemotherapy; CRT, chemoradiotherapy; PFS, progression-free survival; OS, overall survival; undef, undefined.
Bold values signifies statistically significant results.
Fig. 1.
Distance recurrence (A.1) and Locoregional recurrence (A.2) for the full cohort according to treatment group.
3.4. Follow up and survival outcomes
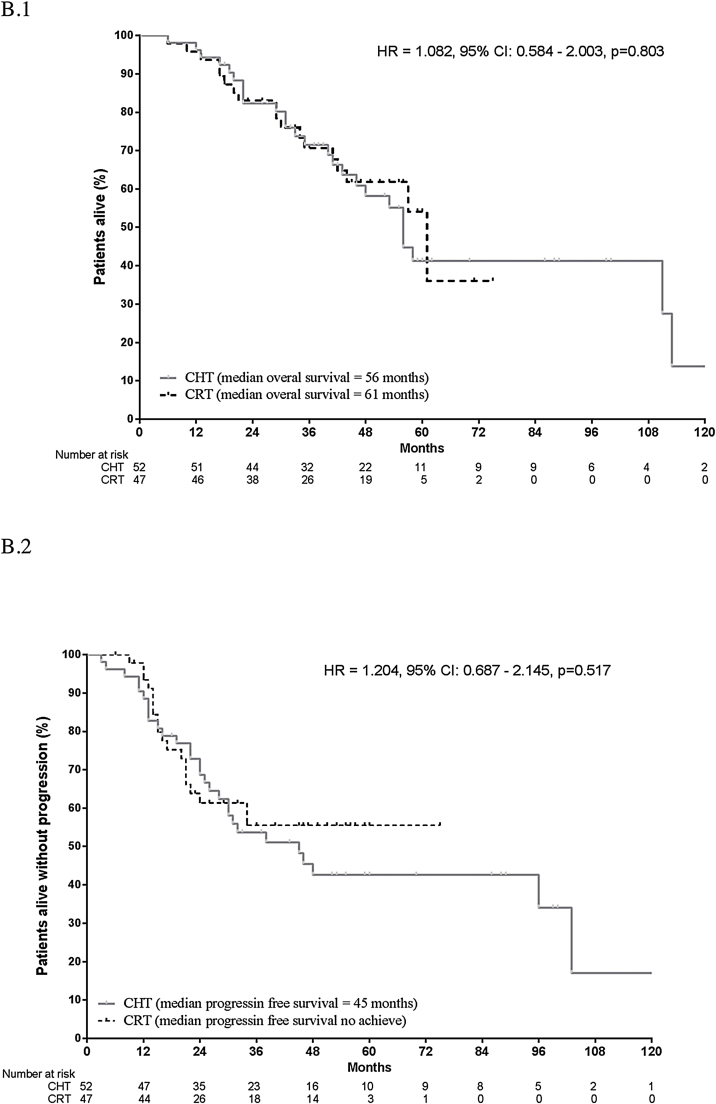
Median follow-up was 41 months in the CRT group and 41.5 months in the CHT group (Table 2). Median OS was similar in both groups (61 vs. 56 months, log-rank p = 0.803). Median PFS was 45 months in the CHT group and “not reached” in the CRT group (Fig. 2). There were no significant differences in survival outcomes between the CRT and CHT groups: 5-year PFS: 55.5% vs. 42.6% (log-rank, p = 0.092) and 5-year OS: 54.1% vs. 41.3% (log-rank, p = 0.163).
Fig. 2.
Overall survival (B.1) and Progression free survival (B.2) for the full cohort according to treatment group.
On the univariate analysis, advanced ypT stage (ypT3-4) was associated with worse OS (p < 0.001). However, neither pCR nor nodal downstaging were significant prognostic factors for overall survival. By contrast, advanced ypT stage (ypT3-4) (p < 0.001), persistent nodal involvement (ypN1-2) (p = 0.001), and absence of downstaging (p < 0.001) were all independently associated with worse PFS (Table 3).
Table 3.
Univariate analysis showing the association between the patients’ baseline clinical, sociodemographic, and treatment-related characteristics with PFS and OS (n = 99).
| OS | ||||
|---|---|---|---|---|
| Variable | HR (95% CI) | p | HR (95% CI) | p |
| Age, years | ||||
| <60 (n = 40) [ref: > 60 (n = 59)] | 0.596 (0.315–1.128) | 0.112 | 0.682 (0.377–1.233) | 0.205 |
| Sex | ||||
| women (n = 18) [ref: men (n = 81)] | 0.424 (0.164–1.095) | 0.076 | 0.808 (0.389–1.678) | 0.567 |
| ECOG | ||||
| 2 (n = 1) [ref: 0–1 (n = 98)] | – | – | 2.766 (0.377–20.314) | 0.317 |
| Smoking status | ||||
| Former (n = 45) [ref: never (n = 4)] | 1.289 (0.302–5.5) | 0.731 | 0.673 (0.203–2.235) | 0.518 |
| Current (n = 50) | 1.054 (0.243–4.568) | 0.944 | 0.494 (0.146–1.664) | 0.255 |
| T stage (n = 98) | ||||
| cT2 (n = 36) [ref: cT1 (n = 28)] | 1.178 (0.548–2.534) | 0.675 | 1.295 (0.614–2.732) | 0.497 |
| cT3 (n = 34) | 1.179 (0.538–2.585) | 0.681 | 1.68 (0.789–3.576) | 0.178 |
| Number of positive lymph nodal stations | ||||
| >1 (n = 40) [ref: 1 (n = 59)] | 1.172 (0.642–2.142) | 0.605 | 1.176 (0.662–2.089) | 0.581 |
| Mediastinal lymph node size | ||||
| ≥3 cm (n = 3) [ref: < 3 cm (n = 96)] | 1.183 (0.285–4.904) | 0.817 | 1.339 (0.324–5.534) | 0.687 |
| Histology (n = 98) | ||||
| Squamous (n = 43) [ref: Adenocarcinoma & others (n = 55)] | 1.266 (0.695–2.304) | 0.441 | 1.074 (0.603–1.913) | 0.808 |
| Histopathological mediastinum confirmation before neoadjuvant treatment | ||||
| Yes (n = 57) [ref: No (n = 42)] | 1.746 (0.917–3.322) | 0.090 | 1.359 (0.749–2.468) | 0.313 |
| Neoadjuvant treatment | ||||
| CRT (n = 47) [ref: CHT (n = 52)] | 0.925 (0.5–1.711) | 0.804 | 0.825 (0.459–1.484) | 0.521 |
| Radiological re-evaluation prior to resection (RECIST criteria) | ||||
| No response (SD, DP) (n = 4) [ref: Response (CR, PR) (n = 95)] | 1.476 (0.355–6.127) | 0.592 | 2,001 (0.62–6.461) | 0.246 |
| Pathological mediastinal evaluation (n = 40) | ||||
| Positive (n = 5) [ref: Negative (n = 35)] | 1.617 (0.356–7.345) | 0.534 | 2.077 (0.585–7.374) | 0.258 |
| Interval to surgery after completion of induction treatment (n = 98) | ||||
| >3 to ≤6 weeks, (n = 22) [ref:0-3 wk (n = 1)] | 0.397 (0.049–3.186) | 0.384 | 0.438 (0.056–3.432) | 0.432 |
| >6 to ≤9 weeks, (n = 32) | 0.336 (0.043–2.620) | 0.298 | 0.342 (0.044–2.647) | 0.304 |
| >9 to ≤12 weeks, (n = 16) | 0.591 (0.074–4.722) | 0.620 | 0.535 (0.067–4.258) | 0.555 |
| >12 weeks, (n = 27) | 0.422 (0.055–3.269) | 0.409 | 0.418 (0.054–3.223) | 0.402 |
| Pathologic stage ypT | ||||
| ypT3 to ypT4(n = 12) [ref: ypT0 to ypT2 (n = 87)] | 4.233 (2.06–8.698) | 0.000 | 4.964 (2.466–9.989) | 0.000 |
| Pathologic stage ypN | ||||
| ypN1-3 (n = 30) [ref: ypN0 (n = 69)] | 1.759 (0.958–3.231) | 0.068 | 2.701 (1.518–4.808) | 0.001 |
| Margin status (n = 98) | ||||
| R1/R2 (n = 4) [ref: R0 (n = 94)] | 1.107 (0.151–8.108) | 0.920 | 2.002 (0.484–8.282) | 0.338 |
| Surgery type | ||||
| Lobectomy (n = 84) [ref: Pneumonectomy (n = 15)] | 0.741 (0.328–1.67) | 0.469 | 0.733 (0.342–1.573) | 0.426 |
| Downstaging | ||||
| Yes (n = 72) [ref: No (n = 27)] | 0.566 (0.306–1.047) | 0.070 | 0.353 (0.198 - 0.631) | 0.000 |
| Pathologic complete response (pT0pN0) | ||||
| Yes (n = 26) [ref: No (n = 73)] | 0.47 (0.197–1.12) | 0.088 | 0.519 (0.242–1.113) | 0.092 |
OS, overall survival; PFS, progression-free survival; HR, hazard ratio.
Bold values signifies statistically significant results.
On the Cox multivariate analysis, advanced ypT stage (p < 0.001) and persistent nodal involvement after neoadjuvant therapy (ypN1-2) (p = 0.002) remained independent prognostic factors for worse PFS.
3.5. Treatment-related toxicity and mortality
No significant between-group differences were observed in ≥ grade 3 toxicity (Table 4), with the exception of one death in the CRT group attributed to acute respiratory distress syndrome (ARDS) after left pneumonectomy. No deaths were observed in the CHT group.
Table 4.
Treatment-related toxicity for the full cohort.
| Type of neoadjuvant treatment | ||||
|---|---|---|---|---|
| Total n = 99 (%) | CHT n = 52 (%) | CRT n = 47 (%) | p value | |
| Haematological toxicity (anemia, neutropenia, thrombopenia) | ||||
| <grade 3, n (%) | 88 (97.8) | 42 (97.7) | 46 (97.9) | 1.000 |
| >= grade 3, n (%) | 2 (2.2) | 1 (2.3) | 1 (2.1) | |
| Gastrointestinal toxicity (mucositis, esophagitis) | ||||
| <grade 3, n (%) | 89 (96.7) | 44 (97.8) | 45 (95.7) | 1.000 |
| >= grade 3, n (%) | 3 (3.3) | 1 (2.2) | 2 (4.3) | |
| Gastrointestinal toxicity (nausea, vomiting) | ||||
| <grade 3, n (%) | 92 (100.0) | 45 (100.0) | 47 (100.0) | – |
| >=grade 3, n (%) | 0 (0.0) | 0 (0.0) | 0 (0.0) | |
| Thoracic toxicity (chest pain) | ||||
| <grade 3, n (%) | 92 (100.0) | 45 (100.0) | 47 (100.0) | – |
| >=grade 3, n (%) | 0 (0.0) | 0 (0.0) | 0 (0.0) | |
| Thoracic toxicity (pneumonitis) | ||||
| <grade 3, n (%) | 92 (100.0) | 45 (100.0) | 47 (100.0) | – |
| >=grade 3, n (%) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
CHT, chemotherapy; CRT, chemoradiotherapy.
4. Discussion
To our knowledge, this is the first study to compare neoadjuvant high-dose (>/=60 Gy) concomitant CRT to CHT alone followed by surgery in patients with potentially resectable stage IIIA-N2 NSCLC. As our results show, compared to neoadjuvant CHT alone, high-dose CRT resulted in better nodal downstaging rates (89.4% vs. 57.7%; p < 0.001), better pCR rates (46.8% vs. 7.7%; p < 0.001) and improved local control (locoregional recurrence rates: 8.5% vs. 13.5%; p = 0.047). However, consistent with previous studies, there were no significant differences in survival outcomes (OS or DFS) between the two groups.
The optimal treatment for patients with potentially resectable stage IIIA-N2 NSCLC is controversial. However, the findings from several randomized trials and retrospective studies indicate that trimodal therapy (radiotherapy, chemotherapy, and surgery) may improve survival in selected patients. While the precise characteristics of that patient subset remain to be defined, it would certainly include patients considered eligible for surgical salvage with lobectomy. The optimal induction treatment also remains undefined. The use of preoperative radiotherapy is controversial because randomized clinical trials have failed to find a survival benefit for neoadjuvant radiotherapy. However, it has been postulated that mediastinal nodal clearance (MNC) is the most important predictor of OS, with several studies reporting better pCR and MNC rates when radiotherapy is included in the neoadjuvant treatment regimen. In this regard, the present retrospective study was performed based on the premise that neoadjuvant high-dose radiotherapy combined with chemotherapy is an optimal treatment to achieve better local control in this patient population.
A recently published retrospective study found that MNC was the most important prognostic factor for survival in patients treated with neoadjuvant high-dose CRT.11 Several retrospective studies have confirmed the survival benefit in patients who achieved mediastinal downstaging after neoadjuvant CRT.6,12 It seems clear that radiotherapy plays a key role, in a dose-dependent manner, in achieving MNC. Studies that have compared neoadjuvant CRT administered at the standard radiation dose (45 Gy) to CHT alone have reported MNC rates of approximately 40% for CRT versus 64% for CHT.3,13,14 By contrast, a population-based study carried out by Sher et al. to assess dose escalated CRT found that patients receiving 54−66 Gy were significantly less likely to present residual nodal disease than those receiving lower doses (OR, 0.59).8 In that same clinical scenario, two other phase III trials by Cerfolio et al.10 and Bharadwaj et al.15 comparing high-dose to standard dose RT also found that high-dose CRT yielded significantly better MNC outcomes (83% vs. 74% and 75% vs. 42%, respectively) and pCR rates (50% vs. 15% and 28% vs. 10%, respectively).10,15 Retrospective studies report mediastinal downstage rates with high-dose neoadjuvant CRT ranging from 63% to 69%,11,16,17 which is substantially better than can be achieved with standard dose radiotherapy. In our series, the downstaging rate in the high-dose CRT group (89.4%) is consistent with previous reports and associated with a significantly lower locoregional recurrence rate compared to the CHT group (17% vs. 34.6%, respectively), despite the fact that half of patients in the CHT group received PORT. Several phase III randomized trials have compared the same two induction modalities (but with lower radiation doses), finding similar recurrence patterns in both treatment groups, but better locoregional control rates (<34%) in the neoadjuvant CRT arms.3,13,14 However, as in our study, better local control did not result in improved survival outcomes. Median survival in the phase III trial by Pless et al. comparing neoadjuvant CHT alone to neoadjuvant CRT was 27.1 vs. 26.2 months, despite a higher incidence of R0 resection in the CRT arm.14 In the trial carried out by Thomas et al.,3 the authors also found that survival outcomes were not improved in the CRT arm, despite a better local control. The meta analysis by Chen et al. reached the same conclusion (i.e., lack of survival benefit for CRT), despite significantly better outcomes in terms of tumor and mediastinal response and pCR rates.3,5 The median OS in the two treatment groups in our series was comparable to previous reports, but 5-year OS rates were slightly better in the high-dose CRT group. However, it is important to stress that the median DFS in the high-dose CRT group has not been reached yet; as a result, significant differences in DFS between the groups may become apparent with longer follow-up.
The reason why a better local control does not translate into improved survival outcomes may be multifactorial. Some authors suggest that the lack of improvement in OS may be due to postoperative toxicity or to a delay in surgery caused by the toxicity associated with neoadjuvant treatment. Data from randomized trials and retrospective series indicate that the postoperative mortality rate in these patients ranges from 2% to 9%,3,6,14,16,18,19 with three studies even reporting that this mortality rate may be as high as 3% in patients treated with high-dose neoadjuvant CRT,16,20,21 although these are the only studies to report this finding. However, the available evidence suggests that preoperative high-dose RT does not increase postoperative morbidity and mortality, except in patients who undergo pneumonectomy.2,22 Moreover, surgical series confirm the safety of high-dose neoadjuvant RT (>60 Gy) in patients who undergo lobectomy.9,10,20,21,23 In the meta-analysis by Chen and colleagues, the addition of RT to the neoadjuvant treatment regimen did lead to increased toxicity.5 In our series, we found no differences in morbidity or mortality between the two treatment groups. Moreover, we observed only one toxicity-related death (due to ARDS) after neoadjuvant CRT followed by left pneumonectomy. Therefore, our data also confirm the safety of trimodal therapy with high-dose neoadjuvant CRT followed by surgery in patients with stage IIIA-N2 NSCLC. This treatment modality is feasible and well tolerated with low morbidity and mortality.
In our series, the lack of improved survival in the CRT group can probably be attributed to the high incidence of distant metastasis (around 30% in both groups). Several studies have reported similar rates of distant metastasis,3,11,12 with some reporting rates of nearly 80%.20,24 Ultimately, the failure to achieve distant control in this patient population—despite the use of trimodal therapy in these patients counteracts the good locoregional control and may thus explain why there is no improvement in survival outcomes. In recent years, the emergence of new systemic therapies, such as tyrosine kinase inhibitors (TKI), has improved outcomes in patients with locally-advanced NSCLC who present driver mutations. Indeed, the emerging data demonstrating the positive impact of TKIs and immunotherapy on DFS in patients with advanced, locally unresectable NSCLC25 suggests a change in the therapeutic approach in patients with resectable, stage IIIA-N2 NSCLC. These new medical therapies improve distant control, which is likely to lead to improved local control and thus better OS outcomes.
4.1. Study strengths and limitations
The main limitation of this study is the retrospective design, with the potential risk of selection bias. In addition, we only included patients who underwent surgery after neoadjuvant therapy and thus our findings cannot be generalized to all patients with stage IIIA-N2 NSCLC. Sample heterogeneity is another limitation, particularly the differences among patients with regard to histological confirmation of mediastinal node involvement after induction therapy and difference in the specific neoadjuvant treatment regimen. Finally, due to the relatively small sample size and limited follow-up (40 months), we were unable to detect differences in survival. However, the main strength of this study is that it is—to our knowledge—the first to compare high-dose (>/=60 Gy) neoadjuvant RT with concomitant chemotherapy to chemotherapy alone in patients who underwent surgical resection for stage IIIA-N2 NSCLC.
5. Conclusion
The results of this study show that trimodal treatment with high-dose neoadjuvant CRT followed by surgery in selected patients with stage IIIA-N2 NSCLC is feasible and well-tolerated, with low morbidity and mortality. Nodal downstaging was achieved in nearly 90% of patients in the CRT group and a high proportion of these patients also achieved complete pathological response. Locoregional control was superior to neoadjuvant CHT alone. Although we did not observe any differences between the groups in terms of survival, it seems reasonable to believe that significant differences in PFS may emerge with longer follow-up. More studies are needed to know the optimal treatment of these patients.
Conflict of interest
None declared.
Financial disclosure
None declared.
Acknowledgments
The study was supported by the Radiation Oncology Clinical Research Group (GICOR) and the GOECP-SEOR (Oncologic Group for the Study of Lung Cancer-Spanish Society of Radiation Oncology). The author wish to thank Bradley Londres for translating and editing this manuscript.
Contributor Information
Sara Montemuiño, Email: sara.montemuino@salud.madrid.org.
Núria Rodriguez de Dios, Email: nrodriguez@parcdesalutmar.cat.
Margarita Martín, Email: margarita.martin@salud.madrid.org.
Begoña Taboada, Email: maria.begona.taboada.valladares@sergas.es.
Patricia Calvo-Crespo, Email: patricia.calvo.crespo@sergas.es.
María Pilar Samper-Ots, Email: pilar.samper@hospitalreyjuancarlos.es.
José Luis López-Guerra, Email: chanodetriana@yahoo.es.
M. López-Mata, Email: mlopezm76@gmail.com.
Josep Jové-Teixidó, Email: jjove@iconcologia.net.
Verónica Díaz-Díaz, Email: veronicaoncort@gmail.com.
Lourdes de Ingunza-Barón, Email: lourdes.ingunza@gmail.com.
Mauricio Murcia-Mejía, Email: mmurcia@hotmail.com.
Marisa Chust, Email: mchust@fivo.org.
Tamara García-Cañibano, Email: tgcanibano@salud.madrid.
María Luz Couselo, Email: maria.de.la.luz.couselo.paniagua@sergas.es.
María Mar Puertas, Email: mmpuertas@gmail.com.
Elia del Cerro, Email: elia.delcerro@quironsalud.es.
Javier Moradiellos, Email: javier.moradiellos@quironsalud.es.
Sergio Amor, Email: sergio.amor@quironsalud.es.
A. Varela, Email: andres.varela@quironsalud.es.
I.J. Thuissard, Email: ithuissard@gmail.com.
David Sanz-Rosa, Email: david.sanz@universidadeuropea.es.
Felipe Couñago, Email: felipe.counago@quironsalud.es.
References
- 1.Torre L.A., Siegel R.L., Jemal A. Lung Cancer statistics. Adv Exp Med Biol. 2016;893:1–19. doi: 10.1007/978-3-319-24223-1_1. [DOI] [PubMed] [Google Scholar]
- 2.Albain K.S., Swann R.S., Rusch V.W. Radiotherapy plus chemotherapy with or without surgical resection for stage III non-small-cell lung cancer: A phase III randomised controlled trial. Lancet Lond Engl. 2009;374(9687):379–386. doi: 10.1016/S0140-6736(09)60737-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Thomas M., Rübe C., Hoffknecht P. Effect of preoperative chemoradiation in addition to preoperative chemotherapy: A randomised trial in stage III non-small-cell lung cancer. Lancet Oncol. 2008;9(7):636–648. doi: 10.1016/S1470-2045(08)70156-6. [DOI] [PubMed] [Google Scholar]
- 4.Shah A.A., Berry M.F., Tzao C. Induction chemoradiation is not superior to induction chemotherapy alone in stage IIIA lung cancer. Ann Thorac Surg. 2012;93(6):1807–1812. doi: 10.1016/j.athoracsur.2012.03.018. [DOI] [PubMed] [Google Scholar]
- 5.Chen Y., Peng X., Zhou Y., Xia K., Zhuang W. Comparing the benefits of chemoradiotherapy and chemotherapy for resectable stage III A/N2 non-small cell lung cancer: A meta-analysis. World J Surg Oncol. 2018;16(1):8. doi: 10.1186/s12957-018-1313-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Krantz S.B., Mitzman B., Lutfi W. Neoadjuvant chemoradiation shows No survival advantage to chemotherapy alone in stage IIIA patients. Ann Thorac Surg. 2018;105(4):1008–1016. doi: 10.1016/j.athoracsur.2017.10.056. [DOI] [PubMed] [Google Scholar]
- 7.Albain K.S., Rusch V.W., Crowley J.J. Concurrent cisplatin/etoposide plus chest radiotherapy followed by surgery for stages IIIA (N2) and IIIB non-small-cell lung cancer: Mature results of Southwest Oncology Group phase II study 8805. J Clin Oncol Off J Am Soc Clin Oncol. 1995;13(8):1880–1892. doi: 10.1200/JCO.1995.13.8.1880. [DOI] [PubMed] [Google Scholar]
- 8.Sher D.J., Fidler M.J., Seder C.W., Liptay M.J., Koshy M. Relationship between radiation therapy dose and outcome in patients treated with neoadjuvant chemoradiation therapy and surgery for stage IIIA non-small cell lung Cancer: A population-based, comparative effectiveness analysis. Int J Radiat Oncol Biol Phys. 2015;92(2):307–316. doi: 10.1016/j.ijrobp.2015.01.032. [DOI] [PubMed] [Google Scholar]
- 9.Seder C.W., Allen M.S., Cassivi S.D. Stage IIIA non-small cell lung cancer: Morbidity and mortality of three distinct multimodality regimens. Ann Thorac Surg. 2013;95(5):1708–1716. doi: 10.1016/j.athoracsur.2013.02.041. [DOI] [PubMed] [Google Scholar]
- 10.Cerfolio R.J., Bryant A.S., Spencer S.A., Bartolucci A.A. Pulmonary resection after high-dose and low-dose chest irradiation. Ann Thorac Surg. 2005;80(4):1224–1230. doi: 10.1016/j.athoracsur.2005.02.091. discussion 1230. [DOI] [PubMed] [Google Scholar]
- 11.Vyfhuis M.A.L., Burrows W.M., Bhooshan N. Implications of pathologic complete response beyond mediastinal nodal clearance with high-dose neoadjuvant chemoradiation therapy in locally advanced, non-small cell lung Cancer. Int J Radiat Oncol Biol Phys. 2018;101(2):445–452. doi: 10.1016/j.ijrobp.2018.02.003. [DOI] [PubMed] [Google Scholar]
- 12.Spicer J.D., Shewale J.B., Nelson D.B. Multimodality therapy for N2 non-small cell lung Cancer: An evolving paradigm. Ann Thorac Surg. 2019;107(1):277–284. doi: 10.1016/j.athoracsur.2018.07.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Katakami N., Tada H., Mitsudomi T. A phase 3 study of induction treatment with concurrent chemoradiotherapy versus chemotherapy before surgery in patients with pathologically confirmed N2 stage IIIA nonsmall cell lung cancer (WJTOG9903) Cancer. 2012;118(24):6126–6135. doi: 10.1002/cncr.26689. [DOI] [PubMed] [Google Scholar]
- 14.Pless M., Stupp R., Ris H.-B. Induction chemoradiation in stage IIIA/N2 non-small-cell lung cancer: A phase 3 randomised trial. Lancet Lond Engl. 2015;386(9998):1049–1056. doi: 10.1016/S0140-6736(15)60294-X. [DOI] [PubMed] [Google Scholar]
- 15.Bharadwaj S.C., Vallières E., Wilshire C.L. Higher versus standard preoperative radiation in the trimodality treatment of stage IIIa lung Cancer. Ann Thorac Surg. 2015;100(1):207–213. doi: 10.1016/j.athoracsur.2015.03.075. discussion 213-214. [DOI] [PubMed] [Google Scholar]
- 16.Suntharalingam M., Paulus R., Edelman M.J. Radiation therapy oncology group protocol 02-29: A phase II trial of neoadjuvant therapy with concurrent chemotherapy and full-dose radiation therapy followed by surgical resection and consolidative therapy for locally advanced non-small cell carcinoma of the lung. Int J Radiat Oncol Biol Phys. 2012;84(2):456–463. doi: 10.1016/j.ijrobp.2011.11.069. [DOI] [PubMed] [Google Scholar]
- 17.Couñago F., Montemuiño S., Martin M. Prognostic factors in neoadjuvant treatment followed by surgery in stage IIIA-N2 non-small cell lung cancer: A multi-institutional study by the Oncologic Group for the Study of Lung Cancer (Spanish Radiation Oncology Society) Clin Transl Oncol. 2019;21(6):735–744. doi: 10.1007/s12094-018-1976-3. Jun. [DOI] [PubMed] [Google Scholar]
- 18.Higgins K., Chino J.P., Marks L.B. Preoperative chemotherapy versus preoperative chemoradiotherapy for stage III (N2) non-small-cell lung cancer. Int J Radiat Oncol Biol Phys. 2009;75(5):1462–1467. doi: 10.1016/j.ijrobp.2009.01.069. [DOI] [PubMed] [Google Scholar]
- 19.Früh M., Betticher D.C., Stupp R. Multimodal treatment in operable stage III NSCLC: A pooled analysis on long-term results of three SAKK trials (SAKK 16/96, 16/00, and 16/01) J Thorac Oncol Off Publ Int Assoc Study Lung Cancer. 2019;14(1):115–123. doi: 10.1016/j.jtho.2018.09.011. [DOI] [PubMed] [Google Scholar]
- 20.Allen A.M., Shochat T., Flex D. High-dose radiotherapy as neoadjuvant treatment in non-small-cell lung Cancer. Oncology. 2018;95(1):13–19. doi: 10.1159/000487928. [DOI] [PubMed] [Google Scholar]
- 21.Cerfolio R.J., Bryant A.S., Jones V.L., Cerfolio R.M. Pulmonary resection after concurrent chemotherapy and high dose (60Gy) radiation for non-small cell lung cancer is safe and may provide increased survival. Eur J Cardio-Thorac Surg Off J Eur Assoc Cardio-Thorac Surg. 2009;35(4):718–723. doi: 10.1016/j.ejcts.2008.12.029. discussion 723. [DOI] [PubMed] [Google Scholar]
- 22.Couñago F., Rodriguez de Dios N., Montemuiño S. Neoadjuvant treatment followed by surgery versus definitive chemoradiation in stage IIIA-N2 non-small-cell lung cancer: A multi-institutional study by the oncologic group for the study of lung cancer (Spanish Radiation Oncology Society) Lung Cancer Amst Neth. 2018;118:119–127. doi: 10.1016/j.lungcan.2018.02.008. [DOI] [PubMed] [Google Scholar]
- 23.Bauman J.E., Mulligan M.S., Martins R.G., Kurland B.F., Eaton K.D., Wood D.E. Salvage lung resection after definitive radiation (&59 Gy) for non-small cell lung cancer: Surgical and oncologic outcomes. Ann Thorac Surg. 2008;86(5):1632–1638. doi: 10.1016/j.athoracsur.2008.07.042. discussion 1638-1639. [DOI] [PubMed] [Google Scholar]
- 24.Lee J., Kim H.K., Park B.J. Recurrence dynamics after trimodality therapy (Neoadjuvant concurrent chemoradiotherapy and surgery) in patients with stage IIIA (N2) lung cancer. Lung Cancer Amst Neth. 2018;115:89–96. doi: 10.1016/j.lungcan.2017.11.020. [DOI] [PubMed] [Google Scholar]
- 25.Antonia S.J., Villegas A., Daniel D. Durvalumab after chemoradiotherapy in stage III non-small-Cell lung Cancer. N Engl J Med. 2017;377(20):1919–1929. doi: 10.1056/NEJMoa1709937. [DOI] [PubMed] [Google Scholar]