Highlights
-
•
Comprehension and risk perceptions of modified-risk messages for snus were tested.
-
•
Modified-risk information communicated reduced risk of snus compared to cigarettes.
-
•
The modified-risk information was understood by majorities of respondents.
-
•
Most perceived snus to have considerable risk, although less risk than cigarettes.
-
•
Providing modified-risk information about snus could facilitate smokers switching.
Keywords: Comprehension, Risk perceptions, Modified-risk, Snus
Abstract
Introduction
Snus, a low nitrosamine smokeless tobacco product, presents less risks to health than cigarettes. Effectively communicating such risk information could facilitate smokers switching completely to snus, thereby benefiting public health.
Methods
This study assessed comprehension and perceptions of modified-risk information regarding snus. Adult cigarette smokers, former tobacco users, and never tobacco users (N = 3,922) from a US internet panel viewed an advertisement stating that smokers who switched completely to snus could greatly reduce risk of lung cancer, respiratory disease, heart disease, and oral cancer. Respondents answered questions regarding the modified-risk information and rated perceived risks of snus relative to cigarettes and other smokeless tobacco products.
Results
Across the four diseases mentioned in the advertisement, most respondents (49.7%–68.6%, across tobacco user groups) understood that snus presents less risk than cigarettes but is not completely safe. Some indicated snus presents the same risk as cigarettes; this was highest for oral cancer (33.7%–42.02%) and lowest for lung cancer (15.4%–23.1%) and respiratory disease (15.6%–23.4%). Majorities understood snus is addictive (77.7%–87.9%), quitting all tobacco is the best option for smokers (83.6%–93.1%), and non-users of tobacco should not use snus (80.4%–87.8%). Only 2.1%–5.8% indicated smokers would receive a health benefit if they continued to smoke while using snus.
Conclusions
The modified-risk information, conveying that snus presents less risk than cigarettes but is not completely safe, was understood by majorities of respondents. Differential risk beliefs across diseases suggest responses were shaped not only by the modified-risk information, but also by intuitions and pre-existing beliefs about tobacco products.
1. Introduction
A continuum of risk exists for tobacco products, with non-combustible products such as smokeless tobacco (SLT)1 posing less risks to health than combustible cigarettes (Levy et al., 2004, Nutt et al., 2014, Zeller, 2013). Using snus, an SLT product with low levels of tobacco-specific nitrosamines, poses less health risks than smoking, and complete switching from cigarettes to snus is associated with demonstrated decreases in morbidity and mortality due to lung cancer, respiratory disease, heart disease, and oral cancer compared to continued smoking (Lee, 2013, Levy et al., 2004, US Department of Health and Human Services, 2014).
The lower risks of SLT and snus are, however, not well understood by the general public, with multiple studies indicating that most people incorrectly perceive SLT and snus to be as harmful or more harmful than cigarettes (Czoli et al., 2017, Feirman et al., 2018, Fong et al., 2019, Kaufman, Mays, Koblitz, & Portnoy, 2014, Kiviniemi & Kozlowski, 2015, Wackowski et al., 2019). Misperceptions about SLT and snus are likely influenced by intuitive theories of how particular health harms arise. Compared to cigarettes, SLT and snus are often viewed as being more likely to cause oral cancer, equally likely to cause heart disease, and less likely to cause lung cancer (Choi, Fabian, Mottey, Corbett, & Forster, 2012, Lund and Scheffels, 2014, Pepper, Emery, Ribisl, Rini, & Brewer, 2015, Wray et al., 2012), presumably because SLT comes in contact with the mouth, and not the lungs. People who correctly believe SLT and snus are less harmful than cigarettes are more likely to use those products (Bernat et al., 2017, Fong et al., 2019, Kaufman, Mays, Koblitz, & Portnoy, 2014, Wackowski & Delnevo, 2016), suggesting that such misperceptions—regardless of the source—may prevent smokers from switching to SLT and snus.
Education about the relative harms associated with different tobacco products has the potential to correct misperceptions (Borland, Li, & Cummings, 2012). Communicating relative risk information to consumers can improve understanding and support changes in tobacco use that are expected to reduce health risks (Wackowski, O'Connor, et al., 2016b), such as switching completely to snus in lieu of continuing to smoke.
The Family Smoking Prevention and Tobacco Control Act (2009), which gave the US Food and Drug Administration (FDA) regulatory authority over tobacco products, provided that tobacco companies could apply for authorization to communicate accurate relative risk information to the public, through the modified-risk tobacco product application (US Department of Health and Human Services, 2012). As part of such an MRTPA, a modified-risk communication or advertisement is proposed, and consumer risk perceptions are assessed following exposure to the communication. It must be demonstrated that consumers—regardless of their experience with tobacco—understand key concepts in the communication that bear on the MRTPA’s potential impact on public health; for example, that quitting is the best option for cigarette smokers, that a modified-risk tobacco product (MRTP) is less risky than cigarette smoking but not completely safe, and that non-users of tobacco should not start using tobacco. The latter two concepts exemplify messages that need to be understood by non-tobacco users, as well as by current tobacco users.
As part of the evidence submitted to the FDA in support of an MRTPA for Camel Snus, the current study assessed comprehension and risk perceptions among US adults—including current cigarette smokers (who could benefit from switching to snus) and non-users of tobacco (i.e., former and never tobacco users, who could be harmed by initiating snus)—following exposure to an advertisement that included modified-risk information. The objectives were to assess comprehension of the MRTP messages and compare risk perceptions both across tobacco products and for diseases mentioned in the advertisement. While differences across tobacco user groups were not the focus of the analyses, the sample did include respondents with diverse tobacco use status to ensure representation of the entire population.
2. Methods
2.1. Sample
Participants were US adults drawn from the Research Now2 national consumer panel, a demographically diverse online panel of three million individuals. Adults ages 18 and older who were legally eligible to purchase tobacco where they lived were surveyed in June and July of 2015. Quota sampling was implemented to ensure representation across key demographic groups (i.e., gender, age, race/ethnicity, education level, and geographic region) in each of three distinct tobacco user groups—current cigarette smokers (n = 896), former tobacco users (n = 1,526), and never tobacco users (n = 1,500). The data were weighted to match the US population on those demographic variables using the Annual Social and Economic Supplement to the Current Population Survey (March 2014) and the Tobacco Use Supplement to the Current Population Survey (TUS-CPS; January 2011).
Assessment of tobacco use history included not only cigarettes, but the full range of tobacco products. Current cigarette smokers were defined as those who smoked at least 100 cigarettes in their lifetime (Bondy, Victor, & Diemert, 2009) and smoked cigarettes “every day” or “some days” at the time of the survey. Former tobacco users had been established users of one or more tobacco products (i.e., used at least 100 times in their lifetime) but did not use any tobacco at the time of the survey. Never tobacco users reported never using any tobacco product, even once or twice.
In accordance with the Code of Federal Regulations 45 Part 46.101.b, which dictates that survey research that is anonymous or does not solicit subject-identified sensitive information that could harm participants is considered exempt (US Department of Health and Human Services, 2017), the study was not reviewed by an institutional review board.
2.2. Procedures
Panelists who responded to online invitations were assessed for demographic characteristics and tobacco use history. Participants were then shown an advertisement for Camel Snus that included modified-risk information, general information about the product and its use, and balancing information intended to communicate that less risk does not mean no risk and to caution against use by unintended populations (Supplemental Fig. 1). The advertisement included three color images that appeared one above the other on the same screen. The bottom fifth of each image included one of four government-mandated warning labels for SLT (US Food and Drug Administration, 2018a), which were randomly rotated. See Supplemental Table 1 for the information in the advertisement.
2.3. Measures
Following exposure to the advertisement, respondents were asked a series of questions (Supplemental Table 2) largely adapted from published literature (Haddock et al., 2004, O'Connor et al., 2005, Peiper et al., 2010). The first four questions assessed comprehension of the modified-risk information. This was followed by questions on risk perceptions, which included both direct and indirect approaches to assess the absolute and relative risks of snus, as some research suggests that these different approaches may produce dissimilar results (Popova & Ling, 2013, Wackowski et al., 2016). First, participants were asked a direct comparison question (Popova & Ling, 2013, Wackowski et al., 2016) about the health risks of snus relative to cigarettes; this question asked respondents to characterize the risk associated with snus as (a) the same risk as continuing to smoke, (b) less risk than continuing to smoke, (c) no health risk at all, or (d) I don’t know. These assessments were made separately for the four diseases (lung cancer, respiratory disease, heart disease, and oral cancer) mentioned in the advertisement. Respondents were instructed to answer these initial risk perception questions based on what the advertisement communicated. The next questions asked for quantitative ratings of the absolute risks for snus, cigarettes, and other SLT products, respectively, on a 1–7 scale, for each of the four diseases, as well as for “generally poorer health” and “addictiveness,” based on respondents’ beliefs, allowing for an indirect comparison of relative risk (Wackowski et al., 2016). A subsequent question asked whether snus reduces the risk of other smoking-related diseases not mentioned in the advertisement (yes/no), and the last two questions asked respondents to identify the true statement (from two oppositely worded statements) about (1) the safety of snus compared to nicotine replacement therapy (NRT) and (2) the safety of snus compared to quitting tobacco entirely. Following completion of the questions, the Newest Vital Sign health literacy test (Weiss et al., 2005) was administered.
Comprehension and direct comparison questions appeared directly below the advertisement (on the same screen), so that respondents could scroll between the questions and the advertisement. This follows the practice recommended by the FDA for assessing comprehension of consumer drug labels (US Department of Health and Human Services, 2010), which focuses on documenting what consumers understand upon viewing the label, rather than what they recall or how the label changed their beliefs.
2.4. Data analyses
The analyses are primarily descriptive. Comparisons focus on differences in risk perceptions of different tobacco products and diseases, rather than differences among the tobacco user groups; however, broad trends across the three tobacco user groups are described. Where comparisons were made, tests of significance were done using an alpha level of p < 0.05. Analyses were conducted using SAS 9.4. Percentages are weighted, and Ns represent unweighted counts.
3. Results
3.1. Sample demographics
Table 1 presents the weighted demographic characteristics of the sample. Overall, 65.6% of respondents were non-Hispanic White and 11.6% were non-Hispanic Black; 35.3% of the sample had limited health literacy. Among current cigarette smokers, 78.7% smoked every day and 21.3% smoked some days; 7.3% reported dual/poly use of cigarettes, snus, and/or SLT. Among former tobacco users, 91.2% reported past use of cigarettes, 3.7% had used snus, and 13.7% had used SLT.
Table 1.
Demographic characteristics of the sample.
| Total Sample (N = 3,922) | Current Cigarette Smokers (n = 896) | Former Tobacco Users (n = 1,526) | Never Tobacco Users (n = 1,500) | |
|---|---|---|---|---|
| Gender | ||||
| Male | 46.8% | 54.0% | 56.0% | 40.4% |
| Female | 53.2% | 46.0% | 44.0% | 59.6% |
| Age (years) | ||||
| 18–24 | 7.3% | 5.5% | 2.8% | 10.1% |
| 25–30 | 15.7% | 16.4% | 9.9% | 18.7% |
| 31–50 | 33.6% | 38.1% | 31.7% | 33.8% |
| 51 and older | 43.4% | 40.0% | 55.6% | 37.4% |
| Race/Ethnicity | ||||
| Non-Hispanic White | 65.6% | 76.4% | 73.8% | 59.1% |
| Hispanic or Latino | 14.9% | 7.4% | 11.3% | 18.4% |
| Non-Hispanic Black | 11.6% | 11.4% | 8.8% | 13.1% |
| Non-Hispanic Asian or other race | 7.9% | 4.8% | 6.1% | 9.4% |
| Education | ||||
| High school or less | 41.7% | 62.2% | 37.3% | 40.2% |
| Some college | 28.9% | 31.2% | 28.5% | 28.6% |
| Bachelor’s degree or more | 29.4% | 6.6% | 34.2% | 31.2% |
| Health Literacy | ||||
| Adequate literacy | 64.7% | 56.7% | 70.3% | 63.2% |
| Limited literacy | 35.3% | 43.3% | 29.7% | 36.8% |
| Geographic Region | ||||
| Northeast | 18.3% | 15.3% | 18.0% | 19.0% |
| Midwest | 21.2% | 25.5% | 21.8% | 20.0% |
| South | 36.9% | 41.6% | 36.0% | 36.6% |
| West | 23.6% | 17.7% | 24.2% | 24.4% |
3.2. Assessment of absolute risk perceptions, and indirect assessment of relative risks
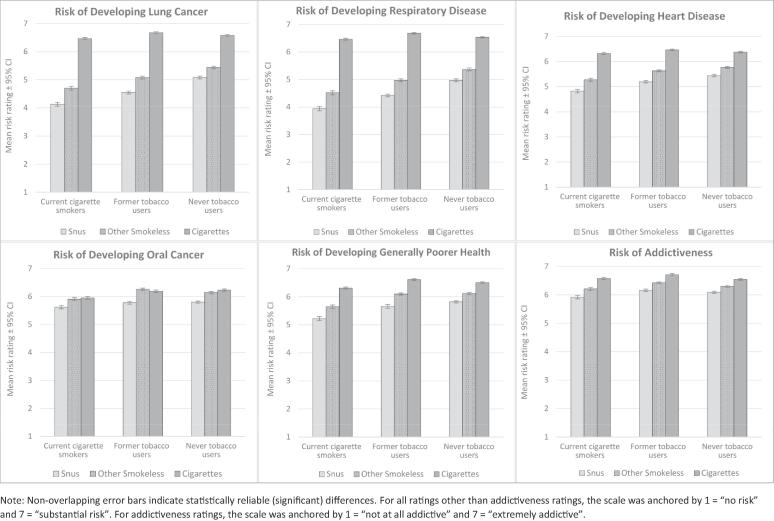
Fig. 1 displays the tobacco user groups’ ratings—based on respondents’ beliefs—of the impact of snus, other SLT products, and cigarettes on the risk of developing the four diseases mentioned in the advertisement (i.e., lung cancer, respiratory disease, heart disease, and oral cancer). The mean risk ratings assigned by current cigarette smokers, and former and never tobacco users for each disease were similar. Across each of these groups, mean risk ratings for cigarettes were always the highest and generally near the top of the scale (designated as “substantial risk”); risk ratings for snus for each of the four diseases were always significantly lower than those for cigarettes and other SLT (p’s < 0.0001; see Supplemental Table 3); and all risk ratings were approximately at or above the midpoint of the 1–7 scale. Even the lowest mean risk rating for snus for any disease (respiratory disease risk rating of current smokers, 3.9) reflected an expectation of substantial risk. The risk ratings for oral cancer were consistently the highest (p’s < 0.0001 [see Supplemental Table 4] compared to other diseases; range = 5.6–5.8).
Fig. 1.
Ratings (1–7 scale) of the health risks of snus, other smokeless tobacco products, and cigarettes across the three tobacco user groups.
As seen in Fig. 1, the patterns noted above for risk perceptions of the three products (i.e., snus, SLT, and cigarettes) were similar across the three tobacco user groups for each disease. Within each of the groups, and for each of the risks, all between product comparisons were highly significant (p’s < 0.0001; see Supplemental Table 5). The one exception was in evaluation of oral cancer risk of SLT compared to cigarettes, where current cigarette smokers evaluated those risks as similar (p = 0.50), former tobacco users thought SLT carried more risk than smoking (p < 0.05), and never tobacco users thought SLT carried less risk (p < 0.003), but these variations were small. As seen in Fig. 1, the patterns across products were highly similar across tobacco user groups for the other diseases and risks.
3.3. Direct assessment of relative risk perceptions
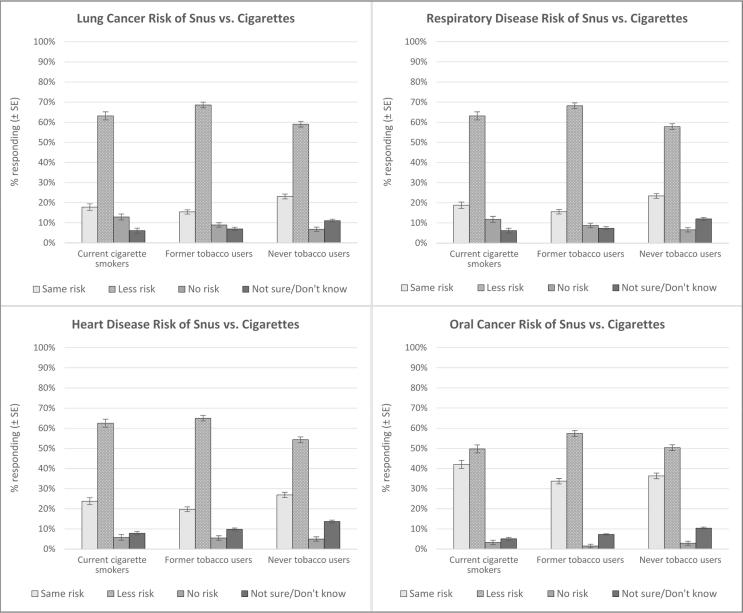
Respondents were asked to characterize the health risks presented by snus as the same as cigarettes, less than cigarettes, or having no risk at all—separately for each of the four diseases—based on information communicated in the advertisement (Fig. 2). Approximately 60% of respondents in each of the three tobacco user groups indicated that, compared to cigarettes, snus presented less risk of lung cancer, respiratory disease, and heart disease; the percentages that offered the same assessment of less risk were significantly lower for oral cancer (p’s < 0.0001; see Supplemental Table 6). The modified-risk information was not recognized or accepted by some respondents, with 15.4%–26.9% in each of the three groups reporting that snus and cigarettes presented the same risk for lung cancer, respiratory disease, and heart disease. The risk reduction for snus was most often doubted for oral cancer, with 33.7%–42.0% indicating that snus presented the same risk for oral cancer as cigarettes.
Fig. 2.
Perceptions of the health risks of snus relative to continuing to smoke cigarettes across the three tobacco user groups.
For each disease, <13% in each tobacco user group reported that snus presented no risk at all (this figure was significantly lower for oral cancer [range = 1.5%–3.3%] across the three groups; p’s < 0.0004 compared to the other three diseases [see Supplemental Table 7]). Similarly, for each disease respectively, approximately 10% did not know how to characterize the risk of snus compared to cigarettes. Respondents giving “don’t know” answers were most often never tobacco users, less often former tobacco users, and least often current cigarette smokers, suggesting such answers increased with decreasing engagement with tobacco products.
3.3.1. Differential risk perception ratings by disease
The advertisement mentioned reduced risk for four diseases, and although the advertisement did not explicitly distinguish the risk reductions by disease, respondents appeared to do so in their risk ratings. In the indirect assessment of risk (Fig. 1), respondents in each of the three tobacco user groups consistently rated the risk of oral cancer with snus higher (p’s < 0.0001; range of risk ratings = 5.6–5.8; see Supplemental Table 4) than that of the respiratory conditions (lung cancer = 4.1–5.1; respiratory disease = 3.9–5.0), with heart disease intermediate (range = 4.8–5.4). While respondents rated the risk of snus lower than that of cigarettes and other SLT for all four diseases (all p’s < 0.0001), the comparison was much narrower for oral cancer than for the other three diseases. In each of the three tobacco user groups, the highest risk ratings were given for cigarette smoking for all diseases except oral cancer, where other SLT was rated similarly to cigarette smoking (Fig. 1).
Respondents also differentially assessed the risk of oral cancer with snus as greater than the risk of the other three diseases in the direct measure of relative risk (Fig. 2 provides risk estimates by tobacco user group, from which overall estimates are determined). Across all three tobacco user groups, 36.1% believed the risk of oral cancer with snus use was the same as that associated with continuing to smoke cigarettes; this was significantly higher than risks assigned for the other three diseases (20.1% for lung cancer, 20.4% for respiratory disease, and 24.3% for heart disease; p’s < 0.0001 [see Supplemental Table 8]). Further, about 8.0% believed that snus presented no risk at all for lung cancer and respiratory disease, respectively, while only 2.5% perceived no risk for oral cancer (p’s < 0.0001; see Supplemental Table 9).
3.4. Risk perceptions for diseases not included in the modified-risk advertisement
To assess whether respondents generalized the modified-risk information to other diseases, respondents were asked about the risk reduction potential of snus for diseases “not mentioned in the advertisement.” Across the three tobacco user groups, 14.5%–22.2% reported that snus reduces the risk of other smoking-related diseases not discussed in the advertisement, 32.1%–38.4% disagreed, but a plurality (45.7%–52.0%) were unsure (Table 2).
Table 2.
Comprehension of the information about using Camel Snus for current cigarette smokers (n = 896), and former (n = 1,526) and never (n = 1,500) tobacco users.
| “Does Camel Snus reduce the risk of other smoking-related diseases that are not discussed in the ad?” | |||
| Yes | No | Don’t know/Not sure | |
| % (95% CI) |
% (95% CI) |
% (95% CI) |
|
| Current cigarette smokers | 22.2% (18.8%–25.6%) |
32.1% (28.4%–35.8%) |
45.7% (41.8%–49.7%) |
| Former tobacco users | 17.1% (14.9%–19.3%) |
30.9% (28.3%–33.5%) |
52.0% (49.2%–54.8%) |
| Never tobacco users | 14.5% (12.5%–16.5%) |
38.4% (35.6%–41.1%) |
47.1% (44.3%–49.9%) |
| “Is Camel Snus, which contains nicotine, addictive?” | |||
| Yes | No | Don’t know/Not sure | |
| % (95% CI) |
% (95% CI) |
% (95% CI) |
|
| Current cigarette smokers | 85.6% (82.9%–88.4%) |
3.6% (2.2%–5.0%) |
10.8% (8.3%–13.3%) |
| Former tobacco users | 87.9% (86.0%–89.9%) |
2.7% (1.8%–3.7%) |
9.3% (7.6%–11.0%) |
| Never tobacco users | 77.7% (75.3%–80.1%) |
8.3% (6.6%–9.9%) |
14.0% (12.0%–16.0%) |
| “Is quitting the best choice for a smoker who is concerned about the health risks from smoking?” | |||
| Yes | No | Don’t know/Not sure | |
| % (95% CI) |
% (95% CI) |
% (95% CI) |
|
| Current cigarette smokers | 90.9% (88.6%–93.3%) |
3.7% (2.3%–5.2%) |
5.4% (3.5%–7.2%) |
| Former tobacco users | 93.1% (91.6%–94.6%) |
3.8% (2.7%–4.9%) |
3.2% (2.1%–4.2%) |
| Never tobacco users | 83.6% (81.5%–85.8%) |
8.6% (7.0%–10.2%) |
7.8% (6.2%–9.4%) |
| “Should adults who do not use or who have quit using tobacco products start using Camel Snus?” | |||
| Yes | No | Don’t know/Not sure | |
| % (95% CI) |
% (95% CI) |
% (95% CI) |
|
| Current cigarette smokers | 7.6% (5.4%–9.8%) |
80.4% (77.1%–83.7%) |
12.0% (9.3%–14.7%) |
| Former tobacco users | 3.9% (2.8%–5.0%) |
87.8% (85.9%–89.8%) |
8.3% (6.6%–9.9%) |
| Never tobacco users | 4.6% (3.4%–5.8%) |
82.7% (80.5%–84.9%) |
12.7% (10.8%–14.6%) |
Respondents were also asked—based on their beliefs—to rate (1–7 scale) the impact of snus, cigarettes, and other SLT on the risk of developing “generally poorer health.” Fig. 1 shows that for each of the three tobacco user groups, respectively, the mean risk ratings for snus were lower than those for cigarettes and other SLT (p’s < 0.0001; see Supplemental Table 10). The risk ratings indicate respondents thought snus carried greater risk for generally poorer health than for lung cancer, respiratory disease, and heart disease (p’s < 0.0001; see Supplemental Table 11); the risk was seen as closest to that for oral cancer, though generally slightly lower.
3.5. Perceptions of the addictiveness of snus, cigarettes, and other smokeless tobacco
Respondents’ risk ratings for the addictiveness of snus were very high, ranging from 5.92 to 6.16 on the 7-point scale, which was higher than the risk ratings for the four diseases (p’s < 0.0001; Fig. 1 [see Supplemental Table 12]). In all three tobacco user groups, snus was perceived—based on respondents’ beliefs—as less addictive than cigarettes and other SLT products; the product differences were small but reliable (p’s < 0.0001; see Supplemental Table 13).
Addictiveness of snus was also assessed by asking “Is Camel Snus, which contains nicotine, addictive?”, mirroring statements made in the modified-risk advertising. Understanding that snus is addictive is important for all three tobacco user groups, and majorities in each group (77.7%–87.9%) responded that snus is addictive, though never tobacco users were most likely to answer incorrectly (8.3%) or to say they did not know if snus is addictive (14.0%; Table 2).
3.6. Comprehension of information about reducing health risks
There was good comprehension in the specific tobacco user group(s) for whom particular information is most germane. As shown in Table 3, a majority of current cigarette smokers (80.9%) understood that smokers should “stop smoking completely and use Camel Snus instead” to receive a health benefit, while few indicated that snus should be used while continuing to smoke (5.8%). Similarly, a large majority of current cigarette smokers (90.9%) correctly understood that “quitting is the best choice for a smoker who is concerned about health risks from smoking”, with small proportions indicating the wrong answer (3.7%) or unsure of the correct response (5.4%) (Table 2). Finally, 70.0% of current cigarette smokers and 73.9% of former tobacco users correctly responded that “Camel Snus is NOT a safer alternative than quitting tobacco entirely”; 20.1% and 13.6%, respectively, answered incorrectly (Table 4).
Table 3.
Comprehension of the information about receiving a health benefit with Camel Snus for current cigarette smokers (n = 896), and former (n = 1,526) and never (n = 1,500) tobacco users.
| “According to the ad, what do smokers need to do in order to receive a health benefit from using Camel Snus?” | |||
|---|---|---|---|
| Stop smoking completely and use Camel Snus instead | Continue to smoke but use Camel Snus as well | Don’t know/Not sure | |
| % (95% CI) |
% (95% CI) |
% (95% CI) |
|
| Current cigarette smokers | 80.9% (77.8%–84.1%) |
5.8% (3.8%–7.8%) |
13.3% (10.6%–15.9%) |
| Former tobacco users | 85.2% (83.2%–87.3%) |
2.1% (1.4%–2.9%) |
12.6% (10.7%–14.5%) |
| Never tobacco users | 74.7% (72.2%–77.2%) |
3.5% (2.4%–4.6%) |
21.8% (19.5%–24.2%) |
Table 4.
Comprehension of the information about Camel Snus as a safer alternative for current cigarette smokers (n = 896), and former (n = 1,526) and never (n = 1,500) tobacco users for the true/false questions.
| “Which of the following statements is true?” | |||
| Camel Snus is a safer alternative than quitting tobacco entirely. | Camel Snus is NOT a safer alternative than quitting tobacco entirely. |
Don’t know / Not sure |
|
| % (95% CI) |
% (95% CI) |
% (95% CI) |
|
| Current cigarette smokers | 20.1% (17.0%–23.2%) |
70.0% (66.5%–73.6%) |
9.8% (7.5%–12.2%) |
| Former tobacco users | 13.6% (11.5%–15.6%) |
73.9% (71.3%–76.5%) |
12.5% (10.6%–14.5%) |
| Never tobacco users | 12.0% (10.1%–13.8%) |
69.1% (66.5%–71.7%) |
18.9% (16.7%–21.2%) |
| “Which of the following statements is true?” | |||
| Camel Snus is a safer alternative than products that used to quit tobacco such as gum, patches, and lozenges. | Camel Snus is NOT a safer alternative than products that used to quit tobacco such as gum, patches, and lozenges. | Don’t know/Not sure | |
| % (95% CI) |
% (95% CI) |
% (95% CI) |
|
| Current cigarette smokers | 15.4% (12.5%–18.2%) |
67.4% (63.8%–71.1%) |
17.2% (14.3%–20.1%) |
| Former tobacco users | 10.5% (8.7%–12.3%) |
66.0% (63.3%–68.7%) |
23.5% (21.1%–25.9%) |
| Never tobacco users | 10.4% (8.7%–12.1%) |
61.9% (59.2%–64.6%) |
27.7% (25.2%–30.2%) |
3.7. Comprehension of other information in the modified-risk advertisement
A majority of current cigarette smokers (67.4%) correctly endorsed the statement “Camel Snus is NOT a safer alternative than products that are used to quit tobacco such as gum, patches, and lozenges”, while approximately equal proportions gave an incorrect answer (15.4%) or indicated they did not know the answer (17.2%) (Table 4).
Respondents were asked, “Should adults who do not use or who have quit using tobacco products start using Camel Snus?”. Large majorities of never and former tobacco users (the two groups for whom this information is most relevant) responded correctly (82.7% and 87.8%, respectively), while few answered incorrectly (4.6% and 3.9%) (Table 2).
3.8. Comprehension among respondents with limited health literacy
Results were examined by health literacy status for the six comprehension questions (data not shown). Compared to those with adequate health literacy, respondents with limited health literacy typically showed lower comprehension of the information and were consistently more likely to answer, “don't know” (odds ratios ranging from 1.8 to 5.1; p’s < 0.0001 [see Supplemental Table 14]). Limited health literacy respondents answered “don’t know” 24.2% of the time versus 9.6% of the time for respondents with adequate health literacy (p < 0.0001; see Supplemental Table 15). Across questions, limited health literacy respondents were more likely to respond, “don't know” (averaging 24.2% of the time) than to respond incorrectly (12.0%).
4. Discussion
This study assessed comprehension and risk perceptions after exposure to modified-risk information about Camel Snus, a low nitrosamine SLT product that presents less risk of disease than cigarettes. Although, as expected, comprehension scores were not perfect, strong majorities of current cigarette smokers, and former and never tobacco users understood the various modified-risk and balancing information. The information that smokers who switch completely from cigarettes to snus may greatly reduce their risk of lung cancer, respiratory disease, heart disease, and oral cancer was understood by a majority of respondents in each tobacco user group, with average risk ratings being lower for snus relative to cigarettes and other SLT products (Fig. 1). The three tobacco user groups showed very similar patterns of responses across the different tobacco products and diseases.
Absolute risk ratings for snus consistently averaged above the midpoint of the 7-point scale, implying perception of considerable risk. Respondents understood that snus presents less risk than cigarettes, but still presents some risk and is not completely safe. Very few considered snus to be without risk (Fig. 2).
Respondents generally underestimated the degree of risk reduction that smokers might gain from switching completely to snus. Experts have assessed that snus use presents about 90% less risk than cigarette smoking (Levy et al., 2004). However, respondents' absolute risk ratings implied very modest reductions compared to cigarette smoking, thus understating the actual risk reduction, particularly for lung cancer and respiratory disease. Given the explicit statement in the modified-risk information that switching completely from cigarettes to snus reduces the risk of the four diseases, it was striking that, across the four diseases, between one-fifth and one-third of respondents in the various tobacco users groups believed that—based on the information provided—snus presented the same risk as continuing to smoke. Respondents likely formulated their responses not only on what they read and understood from the advertisement, but also on their pre-existing beliefs regarding risks of tobacco products, as many people believe SLT products are as harmful as smoking (Fong et al., 2019, Kaufman, Mays, Koblitz, & Portnoy, 2014, Kiviniemi & Kozlowski, 2015, Liu et al., 2015, Regan et al., 2012, Wackowski et al., 2019, Wray et al., 2012).
The results also suggested that respondents made distinctions among the four diseases, even though the modified-risk information claimed risk reduction for each disease without distinguishing among them or providing comparative or quantitative information. Intuitively, people believe that because SLT comes in contact with the mouth, its effects on oral cancer must be greater than on respiratory disease (Choi, Fabian, Mottey, Corbett, & Forster, 2012, Pepper, Emery, Ribisl, Rini, & Brewer, 2015). Conversely, people think of smoking as affecting the lungs, neglecting the fact that smoke passes through the mouth, making cigarette smoking a high risk for oral cancer (US Department of Health and Human Services, 2014). The pattern of results suggests that respondents applied their own beliefs and insufficient understanding of disease, and not just their understanding of the information provided, to assess absolute risks and relative risks.
Thus, the results are consistent with documented public misperceptions about SLT. Given these views, the skepticism with which reduced-risk information is received (Borland, Li, & Cummings, 2012, Fix et al., 2017), and the fact that the source of the information in this study was an advertisement from a tobacco company (which consumers believe to be less credible than health professionals and other trusted sources of health information [Byrne et al., 2012, Owusu et al., 2019]), it is understandable that some respondents continued to believe that snus was as harmful as cigarettes. Modified-risk information may need repetition and endorsement from multiple authoritative sources to become more persuasive and believable to consumers—and to overcome widely held misperceptions—in order to change beliefs and to support changes in tobacco use behaviors.
Consumers hold similar misperceptions about NRT, believing it to be unsafe (Bansal, Cummings, Hyland, & Giovino, 2004, Ferguson et al., 2011, Heavner, Rosenberg, & Phillips, 2009, Shiffman et al., 2008). Such misperceptions may have influenced respondents' uncertainty about whether snus is safer than those medications.
Some respondents (29.3%) in this study did not provide the correct answer or did not know that switching to snus is not a safer alternative to quitting tobacco. This may have been because the statement to which they were responding was a negation, which may have made answering a true/false question confusing. Notably, the findings from this study demonstrate that even after exposure to the modified-risk information, large majorities understood that snus should not be used by those who are not already using tobacco. Respondents also understood the statements that quitting smoking is the best choice for smokers, and that snus is addictive.
Generally, comprehension of the modified-risk and balancing information was good across the three tobacco user groups, and there was good comprehension in the groups for whom particular information is most relevant (e.g., current smokers understood that quitting is the best option). Respondents with limited health literacy typically showed lower comprehension, being particularly likely to give “don’t know” responses. This is consistent with other studies that have repeatedly demonstrated an association between limited health literacy and lower comprehension of consumer communications, including prescription and over-the-counter drug labels (Davis et al., 2006, Raymond et al., 2002, Wolf et al., 2006) and FDA risk communications (McCormack, Craig Lefebvre, Bann, Taylor, & Rausch, 2016, Shiffman et al., 2011). The advertisement communicated a substantial amount of information, which can complicate communications, particularly in a single, brief exposure. Repeated and prolonged exposure, or expression of the modified-risk information in different ways from different sources may improve comprehension among those with limited health literacy.
The results of this study indicate that modified-risk and balancing information can be effectively communicated, without promoting misconceptions such as a belief that snus is completely safe. This suggests that such information could help motivate cigarette smokers to switch to snus, while avoiding attracting non-users of tobacco. Indeed, a companion study also exposed a range of US adults to this modified-risk information and found that interest in snus, and projected use of snus, was greatest among current smokers who could benefit by switching to snus, with low rates of likely use among those who might be harmed by adopting snus (Gerlach, Shiffman, Battista, Polster, & Curtin, 2019).
4.1. Study strengths
This study had considerable strengths. The sample was large, diverse, weighted to match the demographic characteristics of US adults, and included individuals with a range of tobacco use states. The study used questions drawn from the published literature, and evaluated perceived risks using both direct and indirect assessments, with consistent, convergent results. In addition, the study's findings were replicated in two very similar executions of this study (US Food and Drug Administration, 2018b).
4.2. Study limitations
This study also had limitations, including the fact that the sample was drawn from an online panel, and thus may not be fully representative of the US population. The advertisement was evaluated online, as an on-screen display in a research context; such methods are often used to evaluate communications (Sullivan & O’Donoghue, 2015), and there is little reason to think results would not generalize to other media. The current study assessed a particular set of modified-risk information; other information might perform differently. However, two studies testing slightly different modified-risk information with the same methods yielded very similar results (US Food and Drug Administration, 2018b), suggesting that the findings are relatively robust to such variations in the information.
The study measured the effects of a single exposure of the modified-risk advertising, as opposed to the effects of multiple advertising exposures over time in the real world. It is possible that repeated exposure over time would lead to improved understanding of the absolute and relative health risks of snus and cigarettes (Borland et al., 2012). The advertisement communicated a great deal of presumably new information about snus and its risk-reduction potential relative to continued smoking. Nonetheless, the results indicate good comprehension of the modified-risk information.
4.3. Conclusions
Across a broad sample that included representatives of three different tobacco user groups, respondents demonstrated good understanding and application of the modified-risk information and did not develop misperceptions that snus is completely safe. Balanced information about reduced risk may support smokers taking action to reduce the harm from cigarette smoking.
Role of Funding Sources
This work was supported by RAI Services Company.
Funding for this study was provided by RAI Services Company. The study was conducted for a regulatory submission to the U.S. Food and Drug Administration; as such, the study sponsor has a contributing role in the study design, interpretation of the data, writing the manuscript, and decision to submit the paper for publication. The analysis was conducted by PinneyAssociates, who had complete access to the data.
Contributors
MP and GC designed the study; MP was responsible for data collection; MP, MS, and SS had contributing roles during the analysis of data; all authors had a role in interpreting the data, writing the manuscript, and approving the paper for publication.
Credit authorship contribution statement
Janine L. Pillitteri: Writing - original draft, Writing - review & editing. Saul Shiffman: Validation, Formal analysis, Writing - review & editing. Mark A. Sembower: Formal analysis, Visualization. Michael R. Polster: Methodology, Investigation, Data curation. Geoffrey M. Curtin: Conceptualization, Supervision, Writing - review & editing.
Declaration of Competing Interest
Dr. Curtin is employed by RAI Services Company, a wholly owned subsidiary of Reynolds American Inc., whose operating companies market smokeless tobacco products including Camel Snus. Drs. Shiffman and Pillitteri and Mr. Sembower are employees of PinneyAssociates, Inc. PinneyAssociates provides consulting services on smoking cessation and tobacco harm reduction (including smokeless tobacco and vapor products, but not combustible cigarettes) to RAI Services Company, a subsidiary of Reynolds American Inc., now a subsidiary of British American Tobacco. Dr. Shiffman also owns an interest in intellectual property for a novel nicotine medication that has neither been developed nor commercialized. Dr. Polster is employed by NAXION, which provides consulting and research services to RAI Services Company.
Footnotes
AbbreviationsFDA: Food and Drug Administration; MRTP: modified-risk tobacco product; MRTPA: modified-risk tobacco product application; NRT: nicotine replacement therapy; SLT: smokeless tobacco; US: United States
In 2017, Research Now merged with Survey Sampling International (SSI) to form Research Now SSI, which was renamed Dynata in 2019.
Supplementary data to this article can be found online at https://doi.org/10.1016/j.abrep.2020.100254.
Contributor Information
Janine L. Pillitteri, Email: jpillitt@comcast.net.
Saul Shiffman, Email: shiffman@pinneyassociates.com.
Mark A. Sembower, Email: msembower@pinneyassociates.com.
Michael R. Polster, Email: mpolster@naxionthinking.com.
Geoffrey M. Curtin, Email: curting@rjrt.com.
Appendix A. Supplementary material
The following are the Supplementary data to this article:
References
- Bansal M.A., Cummings K.M., Hyland A., Giovino G.A. Stop-smoking medications: who uses them, who misuses them, and who is misinformed about them? Nicotine & Tobacco Research. 2004;6(Suppl 3):S303–310. doi: 10.1080/14622200412331320707. [DOI] [PubMed] [Google Scholar]
- Bernat J.K., Ferrer R.A., Margolis K.A., Blake K.D. US adult tobacco users’ absolute harm perceptions of traditional and alternative tobacco products, information-seeking behaviors, and (mis)beliefs about chemicals in tobacco products. Addictive Behaviors. 2017;71:38–45. doi: 10.1016/j.addbeh.2017.02.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bondy S.J., Victor J.C., Diemert L.M. Origin and use of the 100 cigarette criterion in tobacco surveys. Tobacco Control. 2009;18(4):317–323. doi: 10.1136/tc.2008.027276. [DOI] [PubMed] [Google Scholar]
- Borland R., Li L., Cummings K.M. Effects of a Fact Sheet on beliefs about the harmfulness of alternative nicotine delivery systems compared with cigarettes. Harm Reduction Journal. 2012;9:19. doi: 10.1186/1477-7517-9-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byrne S., Guillory J.E., Mathios A.D., Avery R.J., Hart P.S. The unintended consequences of disclosure: Effect of manipulating sponsor identification on the perceived credibility and effectiveness of smoking cessation advertisements. Journal of Health Communication. 2012;17(10):1119–1137. doi: 10.1080/10810730.2012.665425. [DOI] [PubMed] [Google Scholar]
- Choi K., Fabian L., Mottey N., Corbett A., Forster J. Young adults’ favorable perceptions of snus, dissolvable tobacco products, and electronic cigarettes: Findings from a focus group study. American Journal of Public Health. 2012;102(11):2088–2093. doi: 10.2105/AJPH.2011.300525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Czoli C.D., Fong G.T., Mays D., Hammond D. How do consumers perceive differences in risk across nicotine products? A review of relative risk perceptions across smokeless tobacco, e-cigarettes, nicotine replacement therapy and combustible cigarettes. Tobacco Control. 2017;26(e1):e49–e58. doi: 10.1136/tobaccocontrol-2016-053060. [DOI] [PubMed] [Google Scholar]
- Davis T.C., Wolf M.S., Bass P.F., Middlebrooks M., Kennen E., Baker D.W. Low literacy impairs comprehension of prescription drug warning labels. Journal of General Internal Medicine. 2006;21(8):847–851. doi: 10.1111/j.1525-1497.2006.00529.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Family Smoking Prevention and Tobacco Control Act. (2009). Title 21 U.S.C. 301. Public Law 111-31. Available at: https://www.govinfo.gov/content/pkg/PLAW-111publ31/html/PLAW-111publ31.htm.
- Feirman S.P., Donaldson E.A., Parascandola M., Snyder K., Tworek C. Monitoring harm perceptions of smokeless tobacco products among US adults: Health Information National Trends Survey 2012, 2014, 2015. Addictive Behaviors. 2018;77:7–15. doi: 10.1016/j.addbeh.2017.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferguson S.G., Gitchell J.G., Shiffman S., Sembower M.A., Rohay J.M., Allen J. Providing accurate safety information may increase a smoker's willingness to use nicotine replacement therapy as part of a quit attempt. Addictive Behaviors. 2011;36(7):713–716. doi: 10.1016/j.addbeh.2011.02.002. [DOI] [PubMed] [Google Scholar]
- Fix B.V., Adkison S.E., O’Connor R.J., Bansal-Travers M., Cummings K.M., Rees V.W. Evaluation of modified risk claim advertising formats for Camel Snus. Health Education Journal. 2017;76:971–985. doi: 10.1177/0017896917729723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fong G.T., Elton-Marshall T., Driezen P., Kaufman A.R., Cummings K.M., Choi K. US adult perceptions of the harmfulness of tobacco products: Descriptive findings from the 2013–14 baseline wave 1 of the PATH study. Addictive Behaviors. 2019;91:180–187. doi: 10.1016/j.addbeh.2018.11.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerlach K., Shiffman S., Battista D., Polster M., Curtin G. Assessing likelihood of product use for snus modified-risk information among adult current cigarette smokers, former tobacco users, and never tobacco users. Addictive Behaviour Reports. 2019;10 doi: 10.1016/j.abrep.2019.100208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haddock C.K., Lando H., Klesges R.C., Peterson A.L., Scarinci I.C. Modified tobacco use and lifestyle change in risk-reducing beliefs about smoking. American Journal of Preventive Medicine. 2004;27(1):35–41. doi: 10.1016/j.amepre.2004.03.010. [DOI] [PubMed] [Google Scholar]
- Heavner K.K., Rosenberg Z., Phillips C.V. Survey of smokers’ reasons for not switching to safer sources of nicotine and their willingness to do so in the future. Harm Reduction Journal. 2009;6:14. doi: 10.1186/1477-7517-6-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufman A.R., Mays D., Koblitz A.R., Portnoy D.B. Judgments, awareness, and the use of snus among adults in the United States. Nicotine & Tobacco Research. 2014;16(10):1404–1408. doi: 10.1093/ntr/ntu116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiviniemi M.T., Kozlowski L.T. Deficiencies in public understanding about tobacco harm reduction: Results from a United States national survey. Harm Reduction Journal. 2015;12:21. doi: 10.1186/s12954-015-0055-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee P.N. The effect on health of switching from cigarettes to snus - a review. Regulatory Toxicology and Pharmacology. 2013;66(1):1–5. doi: 10.1016/j.yrtph.2013.02.010. [DOI] [PubMed] [Google Scholar]
- Levy D.T., Mumford E.A., Cummings K.M., Gilpin E.A., Giovino G., Hyland A. The relative risks of a low-nitrosamine smokeless tobacco product compared with smoking cigarettes: Estimates of a panel of experts. Cancer Epidemiology, Biomarkers & Prevention. 2004;13(12):2035–2042. [PubMed] [Google Scholar]
- Liu S.T., Nemeth J.M., Klein E.G., Ferketich A.K., Kwan M.P., Wewers M.E. Risk perceptions of smokeless tobacco among adolescent and adult users and nonusers. Journal of Health Communication. 2015;20(5):599–606. doi: 10.1080/10810730.2015.1012237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lund I., Scheffels J. Perceptions of relative risk of disease and addiction from cigarettes and snus. Psychology of Addictive Behaviors. 2014;28(2):367–375. doi: 10.1037/a0032657. [DOI] [PubMed] [Google Scholar]
- McCormack L., Craig Lefebvre R., Bann C., Taylor O., Rausch P. Consumer understanding, preferences, and responses to different versions of drug safety messages in the United States: A randomized controlled trial. Drug Safety. 2016;39(2):171–184. doi: 10.1007/s40264-015-0358-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nutt D.J., Phillips L.D., Balfour D., Curran H.V., Dockrell M., Foulds J. Estimating the harms of nicotine-containing products using the MCDA approach. European Addiction Research. 2014;20:218–225. doi: 10.1159/000360220. [DOI] [PubMed] [Google Scholar]
- O'Connor R.J., Hyland A., Giovino G.A., Fong G.T., Cummings K.M. Smoker awareness of and beliefs about supposedly less-harmful tobacco products. American Journal of Preventive Medicine. 2005;29(2):85–90. doi: 10.1016/j.amepre.2005.04.013. [DOI] [PubMed] [Google Scholar]
- Owusu D., Weaver S.R., Yang B., Ashley D.L., Popova L. Trends in trust in the sources of health information on e-cigarettes among US adults, 2015–2017. American Journal of Public Health. 2019;109:145–147. doi: 10.2105/AJPH.2018.304754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peiper N., Stone R., van Zyl R., Rodu B. University faculty perceptions of the health risks related to cigarettes and smokeless tobacco. Drug and Alcohol Review. 2010;29(2):121–130. doi: 10.1111/j.1465-3362.2009.00143.x. [DOI] [PubMed] [Google Scholar]
- Pepper J.K., Emery S.L., Ribisl K.M., Rini C.M., Brewer N.T. How risky is it to use e-cigarettes? Smokers’ beliefs about their health risks from using novel and traditional tobacco products. Journal of Behavioral Medicine. 2015;38(2):318–326. doi: 10.1007/s10865-014-9605-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Popova L., Ling P.M. Perceptions of relative risk of snus and cigarettes among US smokers. American Journal of Public Health. 2013;103(11):e21–e23. doi: 10.2105/AJPH.2013.301547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raymond E.G., Dalebout S.M., Camp S.I. Comprehension of a prototype over-the-counter label for an emergency contraceptive pill product. Obstetrics and Gynecology. 2002;100(2):342–349. doi: 10.1016/s0029-7844(02)02086-0. [DOI] [PubMed] [Google Scholar]
- Regan A.K., Dube S.R., Arrazola R. Smokeless and flavored tobacco products in the US: 2009 Styles survey results. American Journal of Preventive Medicine. 2012;42(1):29–36. doi: 10.1016/j.amepre.2011.08.019. [DOI] [PubMed] [Google Scholar]
- Shiffman S., Ferguson S.G., Rohay J., Gitchell J.G. Perceived safety and efficacy of nicotine replacement therapies among US smokers and ex-smokers: Relationship with use and compliance. Addiction. 2008;103(8):1371–1378. doi: 10.1111/j.1360-0443.2008.02268.x. [DOI] [PubMed] [Google Scholar]
- Shiffman S., Gerlach K.K., Sembower M.A., Rohay J.M. Consumer understanding of prescription drug information: An illustration using an antidepressant medication. Annals of Pharmacotherapy. 2011;45(4):452–458. doi: 10.1345/aph.1P477. [DOI] [PubMed] [Google Scholar]
- Sullivan H.W., O’Donoghue A.C., Aikin K.J. Communicating benefit and risk information in direct- to-consumer print advertisements – A randomized study. Therapeutic Innovation & Regulatory Science. 2015;49(4):493–502. doi: 10.1177/2168479015572370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- US Department of Health and Human Services. (2010). Guidance for Industry. Label Comprehension Studies for Nonprescription Drug Products. Silver Spring, MD: US Department of Health and Human Services, Centers for Disease Control and Prevention, Food and Drug Administration, Center for Drug Evaluation and Research. Available at: https://www.fda.gov/media/75626/download.
- US Department of Health and Human Services. (2012). Guidance for Industry. Modified Risk Tobacco Product Applications. Draft Guidance. US Department of Health and Human Services, Food and Drug Administration, Center for Tobacco Products. Available at: https://www.fda.gov/downloads/TobaccoProducts/Labeling/RulesRegulationsGuidance/UCM297751.pdf.
- US Department of Health and Human Services. (2014). The Health Consequences of Smoking – 50 Years Of Progress: A Report of the Surgeon General. Atlanta, GA: U.S. Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health. Available at: https://www.ncbi.nlm.nih.gov/books/NBK179276/pdf/Bookshelf_NBK179276.pdf.
- US Department of Health and Human Services Federal Policy for the Protection of Human Subjects. Final rule. Federal Register. 2017;82:7149–7274. [PubMed] [Google Scholar]
- US Food and Drug Administration. (2018a). Smokeless Tobacco Labeling and Warning Statement Requirements. Available at: https://www.fda.gov/tobacco-products/labeling-and-warning-statements-tobacco-products/smokeless-tobacco-labeling-and-warning-statement-requirements.
- US Food and Drug Administration. (2018b). RJ Reynolds Tobacco Company Modified Risk Tobacco Product (MRTP) Applications. Camel Snus Modified Risk Advertising: Comprehension and Perceptions among Tobacco Users and Non-Users. Available at: https://digitalmedia.hhs.gov/tobacco/static/mrtpa/RJR/6_RESEARCH/7%20Section%206.2%20-%20Comprehension%20and%20Perception_Release%20in%20Full.pdf.
- Wackowski O.A., Bover Manderski M.T., Delnevo C.D. Comparison of direct and indirect measures of e-cigarette risk perceptions. Tobacco Regulatory Science. 2016;2(1):38–43. doi: 10.18001/TRS.2.1.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wackowski O.A., Delnevo C.D. Young adults’ risk perceptions of various tobacco products relative to cigarettes: Results from the National Young Adult Health Survey. Health Education & Behavior. 2016;43(3):328–336. doi: 10.1177/1090198115599988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wackowski O.A., O’Connor R.J., Strasser A.A., Hammond D., Villanti A.C., Delnevo C.D. Smokers’ and e-cigarette users’ perceptions of modified risk warnings for e-cigarettes. Preventive Medicine Reports. 2016;4:309–312. doi: 10.1016/j.pmedr.2016.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wackowski O.A., Ray A.E., Stapleton J.L. Smokers’ perceptions of risk and harm from snus relative to cigarettes. A latent profile analysis study. Addictive Behaviors. 2019;91:171–174. doi: 10.1016/j.addbeh.2018.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss B.D., Mays M.Z., Martz W., Merriam Castro K., DeWalt D.A., Pignone M.P. Quick assessment of literacy in primary care: The Newest Vital Sign. The Annals of Family Medicine. 2005;3(6):514–522. doi: 10.1370/afm.405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf M.S., Davis T.C., Tilson H.H., Bass P.F., 3rd, Parker R.M. Misunderstanding of prescription drug warning labels among patients with low literacy. American Journal of Health System Pharmacy. 2006;63(11):1048–1055. doi: 10.2146/ajhp050469. [DOI] [PubMed] [Google Scholar]
- Wray R.J., Jupka K., Berman S., Zellin S., Vijaykumar S. Young adults' perceptions about established and emerging tobacco products: Results from eight focus groups. Nicotine & Tobacco Research. 2012;14(2):184–190. doi: 10.1093/ntr/ntr168. [DOI] [PubMed] [Google Scholar]
- Zeller M. Reflections on the 'endgame' for tobacco control. Tobacco Control. 2013;22(Suppl 1):i40–i41. doi: 10.1136/tobaccocontrol-2012-050789. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.