Abstract
In many animals, catabolic and anabolic periods are temporally separated. Migratory birds alternate energy expenditure during flight with energy accumulation during stopover. The size of the energy stores at stopover affects the decision to resume migration and thus the temporal organization of migration. We now provide data suggesting that it is not only the size of the energy stores per se that may influence migration scheduling, but also the physiological consequences of flying. In two subspecies of the northern wheatear Oenanthe oenanthe, a long-distance migrant, estimated energy stores at a stopover during autumn migration were positively related with both constitutive innate and acquired immune function, and negatively related with oxidative damage to lipids. In other words, migrants’ physiological condition was associated with their energetic condition. Although time spent at stopover before sampling may have contributed to this relationship, our results suggest that migrants have to trade-off the depletion of energy stores during flight with incurring physiological costs. This will affect migrants’ decisions when to start and when to terminate a migratory flight. The physiological costs associated with the depletion of energy stores may also help explaining why migrants often arrive at and depart from stopover sites with larger energy stores than expected. We propose that studies on the role of energy stores as drivers of the temporal organization of (avian) migration need to consider physiological condition, such as immunological and oxidative states.
Keywords: migration, oxidative stress, eco-immunology, immunity, fat stores, physiology
Avoiding disease and minimizing physiological damage contribute to the optimization of survival and reproductive success. Hence, pivotal physiological processes such as maintaining immune defense and avoiding oxidative damage are thought to underlie many life-history traits (Lochmiller and Deerenberg 2000; Monaghan et al. 2009; Isaksson et al. 2011). The immune system of vertebrates consists of an innate and an acquired component, which both operate at a baseline (constitutive) level and can be prompted to higher levels of activity in response to an immune challenge (Roitt et al. 1998). The production and/or ingestion of antioxidants can mitigate the damaging actions of pro-oxidants, which are the by-product of aerobic metabolism, and thus an inevitable cost of life (Isaksson et al. 2011, and references therein). However, both physiological systems, immune function and oxidative balance, may be costly and trade-offs with other physiological processes or behavior may exist, especially when (and if) these require a relatively large fraction of the resources (e.g. macro- or micronutrients) available to the animal (Klasing 2004; Hasselquist and Nilsson 2012; Wone et al. 2014; Schwenke et al. 2016).
Migratory endurance flight is an energetically and physiologically demanding activity, and field studies as well as experiments on captive animals (mostly birds) have indicated that flight can negatively impact physiological state. First, it may impact constitutive immune function. Buehler et al. (2010a) found that red knots Calidris canutus that had just landed at a stopover site had lower constitutive immune function than conspecifics longer present, which may suggest that migratory flight is associated with reduced constitutive immune function. More direct evidence for this idea comes from studies on European starlings Sturnus vulgaris and western sandpipers Calidris mauri, in which several indices of constitutive immune function were shown to be lower in individuals flying in a wind-tunnel than in non-flying individuals (Nebel et al. 2012, 2013). Second, it may impact oxidative state, i.e., the balance between pro-oxidants and antioxidants. In zebra finches, Taeniopygia guttata several weeks of flight exercise, totaling 270 km of flying, increases oxidative damage to lipids (Skrip et al. 2016), corroborating the observation that homing pigeons Columba livia flying some 200 km showed more oxidative damage than conspecifics flying around 60 km (Costantini et al. 2008). Jenni-Eiermann et al. (2014) found that European robins Erithacus rubecula caught out of nocturnal migratory flight had higher oxidative damage to proteins than birds resting during the day, despite that the flying birds also had higher enzymatic antioxidant capacity. Finally, migrating Nathusius bats Pipistrelli natusii caught out of flight had higher oxidative damage than conspecifics resting for 18 or 24 h after having been caught (Costantini et al. 2019). Taken together, the above studies indicate that migratory flight may compromise constitutive immune function and increase oxidative damage (but see Bairlein et al. 2015). Yet, studies investigating both physiological systems within one migratory species are, to our best knowledge, lacking.
To fuel migratory flight birds store large quantities of energy, mainly in the form of fat (95%), and some as protein (Jenni and Jenni-Eiermann 1998). As most migrants cannot reach their final destination in one direct flight, they have to land and replenish their energy stores during so-called stopovers. Energy stores of migrants at stopover normally vary widely within a given species (e.g., Salewski and Schaub 2007), and some individuals may even arrive with fully depleted stores. The energetic condition of a migrant at stopover depends on the energy stores at departure on the flight preceding the stopover, the energetic demands of that flight, and the amount of energy accumulated (or lost) after landing at the stopover site. Although the relative contributions of these three factors to migrants’ energy stores at stopover are unknown, part of the variation in energy stores among migrants of a given species will be attributable to variation in the energetic demands of the flight preceding the stopover, especially when birds are caught relatively soon after their arrival. Hence, the physiological condition of migrants at stopover may be expected to depend, in part, on their energy stores. This expectation does not only follow from flight draining energy stores, but also from the idea that when accumulating energy at stopover, migrants possibly recover their physiological condition. Owen and Moore (2008) found that in migrants temporarily caged at stopover, immune response to phytohemagglutinin was positively related to change in mass, suggesting that immune function may improve during stopover. At the population level, Skrip et al. (2015) found that oxidative damage to lipids decreased with the time migrants had spent at stopover before being caught and sampled, i.e., with the time birds were (potentially) refueling.
Estimating energetic condition of migrants at stopover is standard practice. However, studies testing the expectation that the physiology of migrants is related to their energetic condition are scarce, and results are mixed. For example, where Owen and Moore (2008) did find relationships between migrants’ energy stores and absolute leukocyte counts, Cornelius et al. (2014) found little evidence for such relationships. Similarly, estimates of energy stores have been found to be negatively (Jenni-Eiermann et al. 2014), positively (Skrip et al. 2015), or not (Costantini et al. 2007) correlated with oxidative damage. Furthermore, to our best knowledge, no study has measured the relationships between energy stores, immune function and oxidative damage in the same migrant species.
In the current study, we related energy stores of northern wheatears (Oenanthe oenanthe, wheatear hereafter) at stopover to their immunological and oxidative state. We measured two parameters of constitutive innate immune function [microbial killing capacity against Escherichia coli (BKA), and haptoglobin-like activity (Hp)], and one parameter of constitutive acquired immune function (total immunoglobulins, IgY). To assess the birds’ oxidative state, we measured total non-enzymatic antioxidant capacity (AOX) and malondialdehyde (MDA) level. The measure of non-enzymatic AOX is a reliable and general (non-specific) marker of antioxidant capacity of plasma, thus it provides a good overall marker of protection against reactive oxygen species (ROS) compared to specific assays of individual antioxidants. MDA is a frequently used biomarker of current and rapid oxidative damage to lipids, thus in this context more informative and reliable as an indicator of ROS exposure and AOX efficiency, than for example DNA damages that requires long-term exposure to ROS before accumulation can be detected. Due to the negative effect of flight on physiological state, we expected that individuals with depleted energy stores have lower constitutive immune function, lower AOX and higher MDA level than individuals still carrying considerable energy stores.
At our study stopover site, the North Sea island of Helgoland, two wheatear subspecies co-occur during migration: O. o. leucorhoa (leucorhoa wheatears hereafter) and O. o. oenanthe (oenanthe wheatears hereafter). Leucorhoa wheatears breed in Iceland, Greenland, the Faroe Islands, and Northeastern Canada (Del Hoyo et al. 2005). Oenanthe wheatears breed throughout Northern and central Europe, North Asia-Eastern Siberia, and the Northwestern parts of North America (Del Hoyo et al. 2005), but oenanthe wheatears trapped on Helgoland are mainly of Scandinavian origin (Delingat et al. 2011). The wheatear subspecies winter sympatrically in Western Africa (Bairlein et al. 2012; Schmaljohann et al. 2012a). Although we do not know the exact breeding areas of the individual wheatears in our study, in general leucorhoa wheatears make longer migrations and, importantly, face a lengthy sea crossing before they reach Helgoland in autumn, which oenanthe wheatears do not (Dierschke and Delingat 2001; Müller et al. 2018, Figure 1). Hence, selection for features important for migration likely is stronger for leucorhoa wheatears. This is supported by the observations that leucorhoa wheatears have longer and pointier wings (Schmaljohann et al. 2015), increasing air speed, and that they can replenish energy stores much faster than oenanthe wheatears (Corman et al. 2014; Eikenaar et al. 2015; Eikenaar 2017). Possibly, leucorhoa wheatears are also physiologically better adapted to deal with the challenges of migration and/or invest more energy into maintaining immune function and oxidative balance. If so, we would expect leucorhoa wheatears to be in a better physiological state (higher BKA, Hp and IgY, and lower MDA level) at stopover than oenanthe wheatears. However, the proximate negative effect that flight has on birds’ physiology (see above) could counter this ultimate effect; in autumn, leucorhoa wheatears need more and/or longer flights to reach Helgoland than oenanthe wheatears. Whether the subspecies at stopover differ in their physiological state thus depends on the strengths of the opposing effects that migratory flight may have on the wheatears’ physiology.
Figure 1.

Hypothetical migration routes of Northern Wheatears passing Helgoland during autumn. Solid arrows depict the assumed minimum migration distance from the subspecies-specific breeding areas (blue) and to the shared wintering areas (orange). Map represents an orthographic projection with Helgoland as the projection center. Northern Wheatear distribution data was provided by BirdLife International and Handbook of the Birds of the World (2016). Adapted from Müller et al. (2018).
Materials and Methods
Field methods
Wheatears are small (∼25g), insectivorous, nocturnal, long-distance migratory birds. Data were collected on Helgoland (54°11’N, 07°55’E), a small (1 km2) island ∼50 km off the German North Sea coastline. In 2014 and 2016, during the peak of wheatear autumn migration on Helgoland (end of August to end of September), the birds were caught using mealworm-baited spring traps. By maintaining a high trapping effort throughout the two study periods, we aimed at sampling the birds soon after their arrival. All birds were trapped between 9 am and 7 pm. Traps were monitored continuously, and when a bird was caught it was blood-sampled (∼100 μl) from a wing vein within 10 min from triggering the trap. The blood was collected using heparinized micro-capillaries. The plasma was separated within 4 h of capture and frozen at −20°C during the field season and afterwards at −80°C until assaying. The plasma samples collected in 2014 were used to measure parameters of the oxidative balance, and the 2016 samples were used to measure indices of constitutive immune function. Red blood cells were stored on 80% ethanol at room temperature for molecular sexing. After blood-sampling, birds were aged (1st year or adult) according to Svensson (1992), ringed, and their body mass was measured to the nearest 0.1 g using an electronic balance. Wing length (maximum chord) was used to separate the subspecies; males and females with wing length exceeding 102 and 97 mm, respectively, were treated as belonging to the leucorhoa subspecies, and males and females with wing length below 99 and 96 mm, respectively, were treated as belonging to the oenanthe subspecies (Svensson 1992). Fifty-eight birds that could not be assigned to subspecies on wing length were not considered in this study. Wing length was also used to estimate birds’ lean body mass (LBM), employing a linear regression based on 220 “lean” [visible, subcutaneous fat score <2 on a scale of 0–8 (Kaiser 1993) and flight muscle score <2 on a scale of 0–3 (Bairlein 1994)] northern wheatears caught on Helgoland in previous years: LBM [g] = 0.29 g mm−1 × wing length [mm] – 6.85 g (linear regression: n = 220, F1,218 = 95.07, adj-R2 = 0.30, P < 0.001, after Schmaljohann and Naef-Daenzer 2011). The estimates of LBM were used to calculate energy stores: fuel load = (body mass [g] – LBM [g])/LBM [g]. Fuel load thus represents the amount of fuel (both fat tissue and proteins) a bird carries relative to its lean body mass. To exemplify, a wheatear with a fuel load of 0.1 carries a fuel mass equivalent to 10% of its estimated LBM. Negative fuel loads may occur when tissue not included in fat and muscle scores, e.g., non-visible (endogenous) fat and/or protein from other muscles than the flight muscle, is being catabolized. All field procedures were approved by the Ministry of Energy, Agriculture, the Environment, Nature and Digitalization, Schleswig-Holstein, Germany (project V242-7224.123-11).
Laboratory work
We measured two parameters of constitutive innate immune function, an individual’s first line of defense. First, microbial killing capacity against E. coli (BKA) was determined following the method described by French and Neuman-Lee (2012) with a few modifications (see Eikenaar and Hegemann 2016). Specifically, we used a dilution of 3 µl plasma mixed in 4 µl of 105E. coli solution. We measured bacteria growth at 600 nm using a microplate reader. Second, haptoglobin-like activity (mg/ml) was quantified using a commercially available colorimetric assay kit (TP801; Tri-Delta Diagnostics, NJ, USA), which quantifies the heme-binding capacity of plasma. We followed the “manual method” instructions provided by the kit manufacturer with a few minor modifications following Matson et al. (2012). Furthermore, we measured one parameter of constitutive acquired immune function. The total level of antibodies in plasma (total immunoglobulins, IgY) was quantified by means of an enzyme-linked immunosorbent assay (ELISA) following Sköld-Chiriac et al. (2014).
One of the major damages that occur as a result of reactive oxygen species (ROS)-induced oxidative stress is lipid peroxidation (Costantini 2014). Malondialdehyde (MDA), a secondary product of peroxidation of polyunsaturated fatty acids (Gardner 1979), is the most frequently used biomarker of overall lipid peroxidation level. MDA concentration was measured following Eikenaar et al. (2016) by coupled gas chromatography and electron ionization mass spectrometry (GC/EI/MS) analysis after derivatization with O-(2, 3, 4, 5, 6-pentafluorbenzyl) hydroxylamine hydrochloride (PFBHA⋅HCl). Total non-enzymatic antioxidant capacity (AOX) was measured using the ferric reducing antioxidant power (FRAP) assay, which gives the overall reducing potential, i.e., the non-enzymatic antioxidant potential of the sample (Benzie and Strain 1996). Uric acid concentration was assessed using a commercial kit from SPINREACT (Sant Esteve de Bas, Spain). Details of all laboratory work can be found in the Supplementary Appendix.
Data analysis
For each physiological parameter of interest, we ran general linear models (GLM), using SPSS 23.0 (IBM, New York), containing fuel load and subspecies as independent variables. In birds, immune parameters may differ between the sexes (e.g., Pap et al. 2010; Arriero et al. 2015) and hence sex was entered as a fixed factor in the models on immune parameters. Markers of the oxidative balance may show diurnal variation and differ between the sexes (e.g., Jenni-Eiermann et al. 2014; Skrip et al. 2016; Eikenaar et al. 2017). Therefore, time of capture and sex were entered as explanatory variables in the models on AOX and MDA level. Uric acid is an antioxidant formed in the bird’s body by metabolism of proteins. Consequently, uric acid concentrations may not reflect regulated antioxidant defense, but rather indicate catabolism of amino acids (Cohen et al. 2007, but see Eikenaar et al. 2016). As the FRAP assay, used to measure AOX, is strongly affected by uric acid concentration (Benzie and Strain 1996), the model on AOX was run without and with uric acid concentration as a covariate.
Age was not entered in our models for the following reason. In both study years, only about a quarter of the sampled wheatears were adults and among adult birds there was a strong bias towards leucorhoa wheatears. For example, in our MDA dataset, of the 10 adults, 8 were leucorhoa wheatears and only 2 were oenanthe wheatears (for 1st year birds corresponding numbers were 14 and 15, respectively). The two subspecies were much more equally represented in both datasets than the age classes. With these distributions of age and subspecies groups in our datasets, the risk that an observed age effect was actually due to a subspecies effect was much larger than the risk that an observed subspecies effect was in reality due to an age effect. Therefore, we decided to enter subspecies, but not age, as a factor into all models. In case a subspecies effect was observed, we repeated the analysis on 1st year birds only (see “Results” section).
In all analyses, model selection was done using stepwise backward elimination of non-significant terms (P > 0.05) in order of least significance. Using an AIC model selection approach gave qualitatively similar results (Supplementary Table S1). To normalize residuals, BKA, MDA level, and AOX were log transformed prior to analyses. Two small negative values of BKA were set to 1 to allow log transformation of also these BKA values. Limited plasma volumes resulted in variation in sample sizes for the parameters of immune function and for the markers of the oxidative balance. Descriptive results are expressed as mean ± SD.
Results
Energy stores (estimated fuel load) at capture in 2014 were: leucorhoa: 0.083 ± 0.09 (n = 31) and oenanthe: 0.081 ± 0.11 (n = 32), and in 2016 were: leucorhoa: 0.045 ± 0.10 (n = 73) and oenanthe: 0.033 ± 0.091 (n = 42). Energy stores were larger in 2014 than in 2016, but did not differ between the subspecies (GLM with fixed effects of year: β ± SE = 0.042 ± 0.016, t = 2.69, P = 0.008, and subspecies: β ± SE = 0.008 ± 0.015, t = 0.52, P = 0.61).
Immunological state
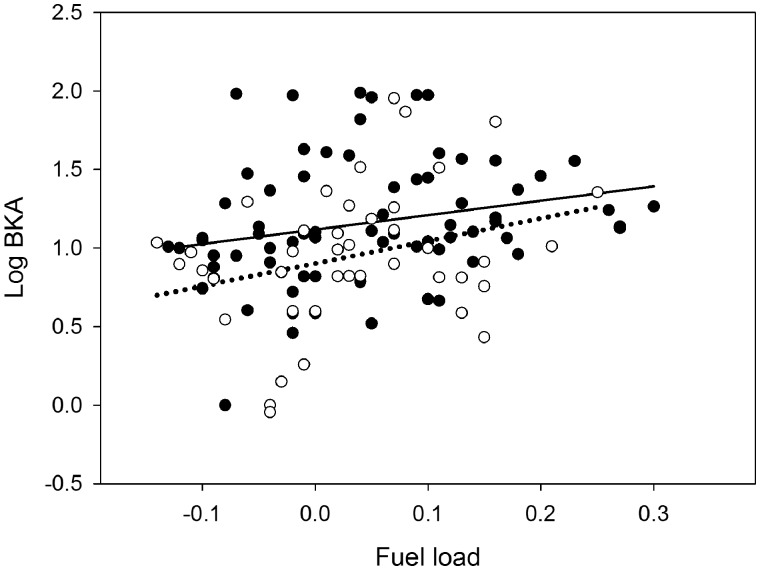
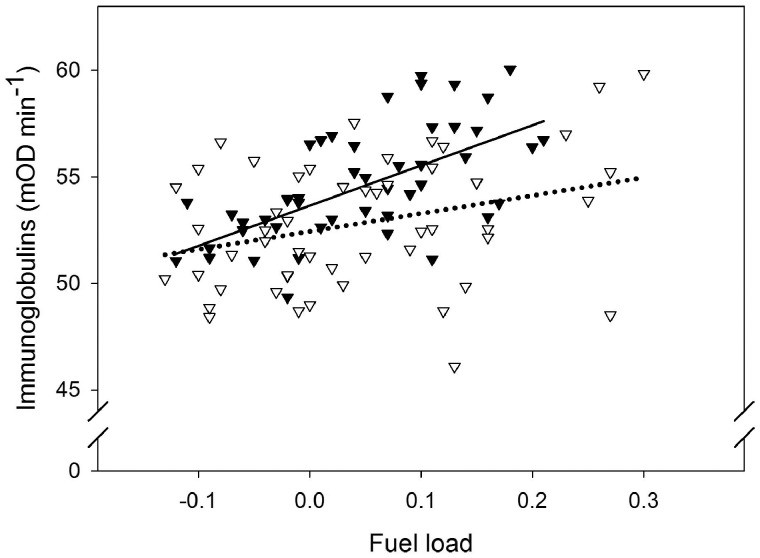
Microbial killing capacity against E. coli (BKA) was positively related with energy stores (Table 1, Figure 2). BKA further differed between the subspecies, with leucorhoa wheatears having higher microbial killing capacity than oenanthe wheatears (Table 1, Figure 2). Sex did not affect BKA (Table 1). Restricting the analyses to 1st year birds did not change the patterns (see Supplementary Table S2). Similar to BKA, total immunoglobulins (IgY) was positively related with energy stores (Table 1, Figure 3). Sex affected IgY with females having higher total immunoglobulins than males (Table 1, Figure 3). The two subspecies did not differ in IgY levels (Table 1). Variation in haptoglobin-like activity (Hp) was not explained by energy stores, subspecies or sex (Table 1, Supplementary Figure S1). Correcting Hp level for plasma redness by adding the 405 nm or 450 nm “pre-scan value” as a covariate (as in Matson et al. 2012) did not qualitatively change this result.
Table 1.
The effects of estimated fuel load (energy stores), subspecies, and sex on three parameters of immune function in Northern wheatears BKA, microbial killing capacity, HP, haptoglobin-like activity; IgY, total immunoglobulins. Variable statistics are given as in the step prior to removal from the model. The final models are in italic.
| β ± SE | t | P | df | |
|---|---|---|---|---|
| BKA model (n = 115) | ||||
| Fuel load | 1.073 ± 0.382 | 2.81 | 0.006 | 1 |
| Subspecies | 0.197 ± 0.078 | 2.52 | 0.013 | 1 |
| Sex | 0.065 ± 0.076 | 0.86 | 0.39 | 1 |
| Hp model (n = 115) | ||||
| Fuel load | −0.041 ± 0.064 | −0.65 | 0.52 | 1 |
| Subspecies | 0.016 ± 0.013 | 1.27 | 0.21 | 1 |
| Sex | −0.005 ± 0.013 | −0.36 | 0.72 | 1 |
| IgY model (n = 102) | ||||
| Fuel load | 12.20 ± 2.60 | 4.71 | <0.001 | 1 |
| Subspecies | −0.368 ± 0.537 | −0.69 | 0.50 | 1 |
| Sex | 1.668 ± 0.515 | 3.24 | 0.002 | 1 |
Reference categories were oenanthe for subspecies and male for sex.
Figure 2.
The bivariate relationship between estimated fuel load (indicative of energy stores) and microbial killing capacity against E. coli (BKA) in two subspecies of northern wheatears sampled during autumn migration. Filled circles and the solid trend line represent the leucorhoa subspecies, and open circles and the dashed trend line represent the oenanthe subspecies. N = 115. Both regression lines are significant (see Table 1 for statistics).
Figure 3.
The bivariate relationship between estimated fuel load (indicative of energy stores) and total immunoglobulins in female (filled triangles and the solid trend line) and male (open triangles and the dashed trend line) northern wheatears sampled during autumn migration. N = 102. Both regression lines are significant (see Table 1 for statistics).
Oxidative state
There was a strong negative relationship between fuel load and malondialdehyde (MDA) level (Figure 4, Table 2). Leucorhoa wheatears had lower MDA level, i.e., incurred less oxidative damage than oenanthe wheatears (Figure 4, Table 2). Additionally, females had lower MDA level than males (Table 2). Time of capture did not affect MDA level (Table 2). When analyzing 1st year birds only, the strong negative relationship between fuel load and MDA level persisted, whereas the effects of subspecies and sex were no longer significant (see Supplementary Table S3). Time of capture was the only variable explaining some of the variation in total non-enzymatic antioxidant capacity (AOX), with a diurnal increase in AOX (Table 2). This pattern, however, disappeared when uric acid level was added to the model as a covariate (Table 2), indicating that the total level of antioxidants other than uric acid did not change (linearly) over the day.
Figure 4.
The bivariate relationship between estimated fuel load (indicative of energy stores) and malondialdehyde level (MDA) in two subspecies of northern wheatears sampled during autumn migration. Filled circles and the solid trend line represent the leucorhoa subspecies, and open circles and the dashed trend line represent the oenanthe subspecies. N = 39. Both regression lines are significant (see Table 2 for statistics).
Table 2.
The effects of estimated fuel load (energy stores), subspecies, time of capture, and sex on Northern wheatears’ malondialdehyde level (MDA), and total non-enzymatic antioxidant capacity (AOX) in models without and with uric acid concentration (UA) level as a covariate. Variable statistics are given as in the step prior to removal from the model. The final models are in italic.
| β ± SE | t | P | df | |
|---|---|---|---|---|
| MDA model (n = 39) | ||||
| Fuel load | −2.255 ± 0.534 | −4.43 | <0.001 | 1 |
| Subspecies | −0.236 ± 0.108 | −2.19 | 0.035 | 1 |
| Time of capture | −0.052 ± 0.407 | −0.13 | 0.90 | 1 |
| Sex | −0.236 ± 0.112 | −2.11 | 0.042 | 1 |
| AOX model without UA (n = 63) | ||||
| Fuel load | 0.147 ± 0.237 | 0.62 | 0.54 | 1 |
| Subspecies | 0.030 ± 0.044 | 0.69 | 0.50 | 1 |
| Time of capture | 0.334 ± 0.159 | 2.10 | 0.040 | 1 |
| Sex | 0.054 ± 0.043 | 1.25 | 0.22 | 1 |
| AOX model with UA (n = 59) | ||||
| Fuel load | −0.083 ± 0.195 | −0.43 | 0.67 | 1 |
| Subspecies | −0.006 ± 0.038 | −0.15 | 0.88 | 1 |
| Time of capture | 0.178 ± 0.137 | 1.30 | 0.20 | 1 |
| Sex | −0.013 ± 0.038 | −0.36 | 0.72 | 1 |
| UA concentration | 0.003 ± 0.001 | 6.0 | <0.001 | 1 |
Reference categories were oenanthe for subspecies and male for sex.
Discussion
We found that estimated energy stores in migrating northern wheatears were positively correlated with parameters of constitutive innate and acquired immune function, and negatively correlated with malondialdehyde level, a commonly used marker of oxidative damage to lipids. Thus, wheatears’ energetic condition was associated with their physiological condition. Non-enzymatic antioxidant capacity was not correlated with energy stores, which could mean that the birds with low energy stores had a higher ROS production that could not be defeated by the non-enzymatic or the enzymatic (data not measured here) antioxidant defenses. Alternatively, there was no increase in ROS, but instead a decreased investment in enzymatic antioxidant defense when energy stores were low (but see Jenni-Eiermann et al. 2014).
Our results are most likely explained by the effect that flying has on birds’ immunological and oxidative state (Costantini et al. 2008; Nebel et al. 2012, 2013; Jenni-Eiermann et al. 2014; Skrip et al. 2016, but see Bairlein et al. 2015). This effect may be a direct or indirect consequence of the high physical activity during migratory flight. The existence of a direct effect seems plausible certainly for oxidative state as intense physical activity, such as flight, is thought to disrupt the oxidative balance in favor of damaging pro-oxidants (Costantini et al. 2008; Powers and Jackson 2008). The lack of a corresponding increase in antioxidant protection (in the current study total non-enzymatic antioxidant capacity) could either be due to limited energy stores after flying, or a constraint in the rate of enzymatic activity. Measuring both enzymatic and non-enzymatic components of oxidative state could enhance the interpretation of results in future studies. Regarding the immune system, Nebel et al. (2012) suggested that the reduction in immune function observed as a result of flying in a wind tunnel may be explained by an energetic trade-off. For our results this would imply that the effect of migratory flight on the immune system is indirect and operates through energy stores, i.e., it would be the result of a reduction in investment of energy in immune defense (and maintaining oxidative balance) when energy stores are depleted during flight. However, whether an energetic trade-off explains our findings is questionable because the energetic costs of maintaining immune responsiveness and oxidative balance are thought to be rather low (Klasing 2004; Hasselquist and Nilsson 2012). To disentangle direct and indirect effects, one could measure the immunological and oxidative state of fat and lean migrants after flying for the same amount of time in a wind-tunnel; if indirect effects play a role these should be most apparent in individuals that, after flying, have fully depleted their energy stores. It is also possible that not energy (i.e., macronutrients like fat), but other currencies, such as micronutrients explain (or contribute to) the relationships observed between energy stores and physiological state. Perhaps very lean birds (negative fuel loads) lack micronutrients needed to maintain, for example, macrophage function (Erickson et al. 2000).
Next to flight, variation in time at stopover before sampling may affect oxidative damage. A cross-sectional study on garden warblers Sylvia borin found that oxidative damage to lipids decreased with the time spent at stopover before sampling (Skrip et al. 2015). This could not only suggest that migrants recover from the damaging effect of flight, but it may also suggest that migrants prepare physiologically for the next flight bout (Skrip et al. 2015). Although we maintained a high trapping effort throughout the study period and mean autumn stopover duration of wheatears on Helgoland is only two days (F Packmor, unpublished data), there likely was some variation in the time that individual wheatears already spent at our stopover study site before we caught and sampled them. Hence, if migrants at stopover indeed prepare physiologically for the next flight bout, such variation may have contributed to the relationship we observed between energy stores and oxidative damage to lipids.
In theory, variation in genetic quality among individual wheatears could also be the driver behind the relationships between energy stores and physiological condition. This, however, seems very unlikely, as in this scenario some (low quality) individuals would have to simultaneously do poorly in multiple physiological processes critical to survival and migration: immune defense, maintaining oxidative balance, and energy metabolism (Roitt et al. 1998; Bize et al. 2008). Although there will be variation in each of these processes among individuals of any bird population, we would expect extremely high selection against individuals doing poorly in all three processes, especially during migration characterized by extreme energetic demands (Butler and Woakes 1990; Schmaljohann et al. 2012b).
We acknowledge that immune function and oxidative balance were measured in individuals caught in two different years. Hence we cannot conclude that wheatears with low energy stores have both low constitutive immune function and suffer much oxidative damage to lipids. This does not, however, affect our finding that migratory flight comes with physiological costs.
Physiological costs to depletion of energy stores
The negative correlations between migrants’ energy stores and physiological condition observed in our study suggest that for birds the depletion of energy stores during migratory flight comes with physiological costs. The high plasmatic oxidative damage to lipids in wheatears with low energy stores likely means that also lipids in cell membranes are affected. There, oxidative damage affects the structure and function of the cell membranes, for example, the inactivation of membrane-bound receptors and enzymes, and MDA itself may inactivate proteins (Birben et al. 2012). Also, replacement of damaged lipids comes at a cost, as this will take away resources from other (physiological) functions. With our study we corroborate the finding that migrating European robins that had catabolized much of the proteins stored in their flight muscles suffered from increased oxidative damage to proteins (Jenni-Eiermann et al. 2014). To get a more complete picture of the association between energy stores and oxidative damage in migrants, future studies could measure markers of damage to both proteins and lipids, such as protein carbonyls and MDA level, respectively.
Haptoglobin levels increase (up to 2.0 mg/ml) during inflammation (Thomas 2000; Buehler et al. 2009; Matson et al. 2012), and likely only birds with very high levels are undergoing an infection (Hegemann et al. 2018).The low Hp levels observed in our study (Supplementary Figure S1) thus suggest that none of our birds was in an acute phase response (inflammation). For birds not undergoing an acute infection, relatively high levels of IgY and BKA may indicate a well-functioning constitutive immune defense. Constitutive acquired immune function (as measured by IgY) reflects the investment into immune function over longer time scales and hence is related to phenotypic quality (Hasselquist et al. 2001), and high immunoglobulin levels are thought to reflect long-term investment in immune function rather than current infections (Garvin et al. 2008; Dunn et al. 2010). High microbial killing capacity (BKA) was found to be positively associated with survival probability during an epidemic outbreak (Wilcoxen et al. 2010), suggesting that high BKA is beneficial to fight infections. Hence, we assume that lean wheatears with low BKA and low IgY potentially face increased risk of infections by harmful viruses, bacteria, and other pathogens. This risk may be especially high for migrating birds because they travel through a variety of habitats, in which they may encounter more or different pathogens (Buehler et al. 2010b). A consequence of reduced immuno-competence and hence potentially increased infection risk are the energetic and autoimmune costs of an immune response following infection (Hegemann et al. 2012; Råberg et al. 1998) or even decreased probability of survival (Hegemann et al. 2013).
In our study system, we are unable to follow individuals beyond their stay on Helgoland. Hence, we cannot quantify the physiological costs incurred when depleting energy stores during migratory flight in evolutionary terms. Still, the observation that, at equal energy stores, leucorhoa wheatears had higher BKA and lower MDA levels than oenanthe wheatears could suggest that migrants face a selective pressure to maintain a proper immune defense and to avoid oxidative damage. A major difference in the life histories of leucorhoa and oenanthe wheatears are the challenges the birds face during migration, i.e., the overall distance and nature of their migration route (Müller et al. 2018; Figure 1). This difference has likely resulted in the subspecies difference in features important for fast and efficient migration, such as wing shape (Schmaljohann et al. 2015) and the rate of energy replenishment (Corman et al. 2014; Eikenaar et al. 2015). We therefore hypothesize that the subspecies difference in BKA and MDA level is a consequence of their different migratory challenges. However, we cannot exclude alternative explanations, such as subspecies-specific parasite pressures at the breeding grounds (Hegemann et al. 2015; Horrocks et al. 2015) or at earlier stopover sites (Buehler et al. 2010b).
Revisiting the role of energy stores in migration
Our novel finding that the depletion of energy stores is negatively associated with both constitutive immune function and oxidative balance may have important implications for our understanding of how energy stores affect the way birds organize their migrations. Energy stores have played a central role in migration research for decades. For example, many studies have related energy stores to stopover departure decisions and found increased departure likelihood with larger energy stores, although other studies did not find such an effect (reviewed by Schmaljohann and Eikenaar 2017). Our results now indicate that next to the size of the energy stores per se, also the physiological consequences of flight, and thus of draining energy stores may affect how birds organize their migration. As previously proposed by Jenni-Eiermann et al. (2014), our results suggest that, to minimize physiological costs, migrants should, if possible, avoid lengthy flights. Furthermore, we propose that the excess in energy stores that migrants often carry at stopover sites (Bairlein 1985; Safriel and Lavee 1988; Bolshakov et al. 2003; Salewski et al. 2010; Schmaljohann et al. 2013; this study), is not only a safety margin for unexpected environmental conditions (Sandberg and Moore 1996; Schaub and Jenni 2001; Schmaljohann and Eikenaar 2017), but also a strategy to minimize immunological and oxidative costs associated with the (full) depletion of energy stores. Widening the spectrum of species and markers of immunological and oxidative state as well as collecting longitudinal data are now required to test the generality of our results and to better understand how energy stores affect migration scheduling.
Supplementary Material
Acknowledgments
We thank Jochen Dierschke and Klaus Müller for logistical support on Helgoland, Thomas Klinner for field work, and Amparo Herrera-Dueñas for laboratory work. Two anonymous reviewers provided useful comments to an earlier version of this article. The study was supported with grants from the Deutsch Forschungsgemeinschaft (DFG) awarded to C.E. (EI 1048/3-1) and to Heiko Schmaljohann (SCHM 2647/3-1), the Swedish Research Council C0361301 (to C.I.) and Marie Curie Career Integration Grant FP7-CIG ID: 322217 (to C.I.). A.H. is associated with the Centre for Animal Movement Research (CAnMove) which is financed by a Linnaeus grant (349-2007-8690) from the Swedish Research Council and Lund University.
Author contributions
C.E. conceived the idea, C.E., F.P., and I.K. conducted fieldwork, A.H. and C.I. performed laboratory analyses, and all authors together wrote the article.
Conflict of interest
The authors declare that they have no conflict of interest.
Animal welfare statement
All applicable institutional and/or national guidelines for the care and use of animals were followed.
References
- Arriero E, Müller I, Juvaste R, Martínez FJ, Bertolero A, 2015. Variation in immune parameters and disease prevalence among lesser black-backed gulls Larus fuscus sp. with different migratory strategies. PLoS ONE 10:e0118279.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bairlein F, 1985. Body weights and fat deposition of Palearctic passerine migrants in the central Sahara. Oecologia 66:141–146. [DOI] [PubMed] [Google Scholar]
- Bairlein F, 1994. Manual of Field Methods: European-African Songbird Migration Network. Wilhelmshaven: Institut für Vogelforschung. [Google Scholar]
- Bairlein F, Norris DR, Nagel R, Bulte M, Voigt CC. et al. 2012. Cross-hemisphere migration of a 25g songbird. Biol Lett 8:505–507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bairlein F, Fritz J, Scope A, Schwendenwein I, Stanclova G. et al. 2015. Energy expenditure and metabolic changes of free-flying migrating northern bald ibis. PLoS ONE 10:e0134433.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benzie IFF, Strain JJ, 1996. The ferric reducing ability of plasma, FRAP, as a measure of antioxidant power: the FRAP assay. Analyt Biochem 239:70–76. [DOI] [PubMed] [Google Scholar]
- Birben E, Sahiner UM, Sackesen C, Erzurum S, Kalayci O, 2012. Oxidative stress and antioxidant defense. WAO J 5:9–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BirdLife International and Handbook of the Birds of the World, 2016. Bird species distribution maps of the world. Version 6.0. Available at http://datazone.birdlife.org/species/requestdis
- Bize P, Devevey G, Monaghan P, Doligez B, Christe P, 2008. Fecundity and survival in relation to resistance to oxidative stress in a free-living bird. Ecology 89:2584–2593. [DOI] [PubMed] [Google Scholar]
- Bolshakov C, Bulyuk V, Chernetsov N, 2003. Spring nocturnal migration of reed warblers Acrocephalus scirpaceus: departure, landing and body condition. Ibis 145:106–112. [Google Scholar]
- Buehler DM, Encinas-Viso F, Petit M, Vezina F, Tieleman BI. et al. 2009. Limited access to food and physiological trade-offs in a long-distance migrant shorebird. II. Constitutive immune function and the acute-phase response. Physiol Biochem Zool 82:561–571. [DOI] [PubMed] [Google Scholar]
- Buehler DM, Tieleman BI, Piersma T, 2010a. Indices of immune function are lower in red knots Calidris canutus recovering protein than in those storing fat during stopover in Delaware Bay. Auk 127:394–401. [Google Scholar]
- Buehler DM, Tieleman BI, Piersma T, 2010b. How do migratory species stay healthy over the annual cycle? A conceptual model for immune function and for resistance to disease. Integr Comp Biol 50:346–357. [DOI] [PubMed] [Google Scholar]
- Butler PJ, Woakes AJ, 1990. The physiology of bird flight In: Gwinner E, editor Bird Migration. Springer: Berlin: 300–318. [Google Scholar]
- Cohen A, Klasing K, Ricklefs R, 2007. Measuring circulating antioxidants in wild birds. Comp Biochem Physiol B 147:110–121. [DOI] [PubMed] [Google Scholar]
- Corman AM, Bairlein F, Schmaljohann H, 2014. The nature of the migration route shapes physiological traits and aerodynamic properties in a migratory songbird. Behav Ecol Sociobiol 68:391–402. [Google Scholar]
- Cornelius EA, Davis AK, Altizer SA, 2014. How important are hemoparasites to migratory songbirds? Evaluating physiological measures and infection status in three neotropical migrants during stopover. Physiol Biochem Zool 87:719–728. [DOI] [PubMed] [Google Scholar]
- Costantini D, Cardinale M, Carere C, 2007. Oxidative damage and anti-oxidant capacity in two migratory bird species at a stop-over site. Comp Biochem Physiol C 144:363–371. [DOI] [PubMed] [Google Scholar]
- Costantini D, Dell’Ariccia G, Lipp HP, 2008. Long flights and age affect oxidative status of homing pigeons Columba livia. J Exp Biol 211:377–381. [DOI] [PubMed] [Google Scholar]
- Costantini D, 2014. Oxidative Stress and Hormesis in Evolutionary Ecology and Physiology: A Marriage between Mechanistic and Evolutionary Approaches. Berlin: Springer. [Google Scholar]
- Costantini D, Lindecke O, Petersons G, Voigt CC, 2019. Migratory flight imposes oxidative stress in bats. Curr Zool, in press, 10.1093/cz/zoy039 [DOI] [PMC free article] [PubMed]
- Del Hoyo J, Ellliot A, Christie DA, 2005. Handbook of the Birds of the World, Vol. 10, Cuckoo-Shrikes to Thrushes. Barcelona: Lynx Edicions. [Google Scholar]
- Delingat J, Hobson KA, Dierschke V, Schmaljohann H, Bairlein F, 2011. Morphometrics and stable isotopes differentiate populations of northern wheatears Oenanthe oenanthe. J Ornithol 152:383–395. [Google Scholar]
- Dierschke V, Delingat J, 2001. Stopover behaviour and departure decisions of northern wheatears Oenanthe oenanthe facing different onward non-stop flight distances. Behav Ecol Sociobiol 50:535–545. [Google Scholar]
- Dunn PO, Garvin JC, Whittingham LA, Freeman-Gallant CR, Hasselquist D, 2010. Carotenoid and melanin-based ornaments signal similar aspects of male quality in two populations of the common yellowthroat. Funct Ecol 24:149–158. [Google Scholar]
- Eikenaar C, Tsvey A, Schmaljohann H, 2015. Faster spring migration in northern wheatears is not explained by an endogenous seasonal difference in refueling rates. J Avian Biol 46:616–621. [Google Scholar]
- Eikenaar C, Hegemann A, 2016. Migratory common blackbirds have lower innate immune function during autumn migration than resident conspecifics. Biol Lett 12:20160078.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eikenaar C, Jönsson J, Fritzsch A, Wan HL, Isaksson C, 2016. Migratory refueling affects non-enzymatic antioxidant capacity, but does not increase lipid peroxidation. Physiol Behav 158:26–32. [DOI] [PubMed] [Google Scholar]
- Eikenaar C, 2017. Endocrine regulation of fueling by hyperphagia in migratory birds. J Comp Physiol A 203:439–445. [DOI] [PubMed] [Google Scholar]
- Eikenaar C, Källstig E, Andersson MN, Herrera-Dueñas A, Isaksson C, 2017. Oxidative challenges of avian migration: a comparative field study on a partial migrant. Phys Biochem Zool 90:223–229. [DOI] [PubMed] [Google Scholar]
- Erickson KL, Median EA, Hubbard NE, 2000. Micronutrients and innate immunity. J Infect Dis 182:S5–S10. [DOI] [PubMed] [Google Scholar]
- French SS, Neuman-Lee LA, 2012. Improved ex vivo method for microbiocidal activity across vertebrate species. Biol Open 1:482–487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardner WH, 1979. Lipid hydroperoxide reactivity with proteins and amino acids: a review. J Agric Food Chem 27:220–229. [Google Scholar]
- Garvin JC, Dunn PO, Whittingham LA, Steeber DA, Hasselquist D, 2008. Do male ornaments signal immunity in the common yellowthroat?. Behav Ecol 19:54–60. [Google Scholar]
- Hasselquist D, Wasson MF, Winkler DW, 2001. Humoral immunocompetence correlates with date of egg-laying and reflects work load in female tree swallows. Behav Ecol 12:93–97. [Google Scholar]
- Hasselquist D, Nilsson J, 2012. Physiological mechanisms mediating costs of immune responses: what can we learn from studies of birds?. Anim Behav 83:1303–1312. [Google Scholar]
- Hegemann A, Matson KD, Versteegh MA, Tieleman BI, 2012. Wild skylarks seasonally modulate energy budgets but maintain energetically costly inflammatory immune responses throughout the annual cycle. PLoS ONE 7:e36358.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hegemann A, Matson KD, Flinks H, Tieleman BI, 2013. Offspring pay sooner, parents pay later: experimental manipulation of body mass reveals trade-offs between immune function, reproduction and survival. Front Zool 10:77.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hegemann A, Marra PP, Tieleman BI, 2015. Causes and consequences of partial migration in a passerine bird. Am Nat 186:531–546. [DOI] [PubMed] [Google Scholar]
- Hegemann A, Alcalde Abril P, Muheim R, Sjöberg S, Alerstam T. et al. 2018. Immune function and blood parasite infections impact stopover ecology in passerine birds. Oecologia 188:1011–1024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horrocks NPC, Hegemann A, Ostrowski S, Ndithia Shobrak M. et al. 2015. Environmental proxies of antigen exposure explain variation in immune investment better than indices of pace of life. Oecologia 177:281–290. [DOI] [PubMed] [Google Scholar]
- Isaksson C, Sheldon BC, Uller T, 2011. The challenges of integrating oxidative stress into life-history biology. Bioscience 61:194–202. [Google Scholar]
- Jenni-Eiermann S, Jenni L, Smith S, Costantini D, 2014. Oxidative stress in endurance flight: and unconsidered factor in bird migration. PLoS ONE 9:e97650.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenni L, Jenni-Eiermann S, 1998. Fuel supply and metabolic constraints in migrating birds. J Avian Biol 29:521–528. [Google Scholar]
- Kaiser A, 1993. A new multi-category classification of subcutaneous fat deposits of song birds. J Field Ornithol 64:246–255. [Google Scholar]
- Klasing KC, 2004. The cost of immunity. Acta Zool Sin 50:961–969. [Google Scholar]
- Lochmiller RL, Deerenberg C, 2000. Trade-offs in evolutionary immunology: just what is the cost of immunity?. Oikos 88:87–98. [Google Scholar]
- Matson KD, Horrocks NPC, Versteegh MA, Tieleman BI, 2012. Baseline haptoglobin concentrations are repeatable and predictive of certain aspects of a subsequent experimentally-induced inflammatory response. Comp Biochem Physiol A 162:7–15. [DOI] [PubMed] [Google Scholar]
- Monaghan P, Metcalfe NB, Torres R, 2009. Oxidative stress as a mediator of life-history trade-offs: mechanisms, measurements, and interpretation. Ecol Lett 12:75–92. [DOI] [PubMed] [Google Scholar]
- Müller F, Eikenaar C, Crysler ZJ, Taylor PD, Schmaljohann H, 2018. Nocturnal departure timing in songbirds facing distinct migratory challenges. J Anim Ecol 87:1102–1115. [DOI] [PubMed] [Google Scholar]
- Nebel S, Bauchinger U, Buehler DM, Langlois LA, Boyles M. et al. 2012. Constitutive immune function in European starlings Sturnus vulgaris is decreased immediately after an endurance flight in a wind tunnel. J Exp Biol 215:272–278. [DOI] [PubMed] [Google Scholar]
- Nebel S, Buehler DM, Macmillan A, Guglielmo CG, 2013. Flight performance of western sandpipers Calidirs mauri remains uncompromised when mounting an acute phase immune response. J Exp Biol 216:2752–2759. [DOI] [PubMed] [Google Scholar]
- Owen JC, Moore FR, 2008. Relationship between energetic condition and indicators of immune function in thrushes during spring migration. Can J Zool 86:638–647. [Google Scholar]
- Pap PL, Czirják GA, Vágási CI, Barta Z, Hasselquist D, 2010. Sexual dimorphism in immune function changes during the annual cycle in house sparrows. Naturwissenschaften 97:891–901. [DOI] [PubMed] [Google Scholar]
- Powers SK, Jackson MJ, 2008. Exercise-induced oxidative stress: cellular mechanisms and impact on muscle force production. Physiol Rev 88:1243–1276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Råberg L, Grahn M, Hasselquist D, Svensson E, 1998. On the adaptive significance of stress–induced immunosuppression. Proc R Soc Lond B 265:1637–1641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roitt IM, Brostoff J, Male DK, 1998. Immunology. London: Mosby. [Google Scholar]
- Safriel UN, Lavee D, 1988. Weight changes of cross-desert migrants at an oasis: do energetic considerations alone determine the length of stopover?. Oecologia 76:611–619. [DOI] [PubMed] [Google Scholar]
- Salewski V, Schaub M, 2007. Stopover duration of Paleartic passerine migrants in the western Sahara: independent of fat stores?. Ibis 149:223–236. [Google Scholar]
- Salewski V, Schmaljohann H, Liechti F, 2010. Spring passerine migrants stopping over in the Sahara are not fall-outs. J Ornithol 151:371–378. [Google Scholar]
- Sandberg R, Moore FR, 1996. Fat stores and arrival on the breeding grounds: reproductive consequences for passerine migrants. Oikos 77:577–581. [Google Scholar]
- Schaub M, Jenni L, 2001. Variation in fuelling rates among sites, days and individuals in migrating passerine birds. Funct Ecol 15:584–594. [Google Scholar]
- Schmaljohann H, Naef-Daenzer B, 2011. Body condition and wind support initiate the shift of migratory direction and timing of nocturnal departure in a songbird. J Anim Ecol 80:1115–1122. [DOI] [PubMed] [Google Scholar]
- Schmaljohann H, Buchmann M, Fox JW, Bairlein F, 2012a. Tracking migration routes and the annual cycle of a trans: Sahara songbird migrant. Behav Ecol Sociobiol 66:915–922. [Google Scholar]
- Schmaljohann H, Fox JW, Bairlein F, 2012b. Phenotypic response to environmental cues, orientation and migration costs in songbirds flying halfway around the world. Anim Behav 84:623–640. [Google Scholar]
- Schmaljohann H, Korner-Nievergelt F, Naef-Daenzer B, Nagel R, Maggini I. et al. 2013. Stopover optimization in a long-distance migrant: the role of fuel load and nocturnal take-off time in Alaskan northern wheatears Oenanthe oenanthe. Front Zool 10:26.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmaljohann H, Meier C, Arlt D, Bairlein F, van Oosten H. et al. 2015. Proximate causes of avian protandry differ between subspecies with contrasting migration challenges. Behav Ecol 27:321–331. [Google Scholar]
- Schmaljohann H, Eikenaar C, 2017. How do energy stores and changes in these affect departure decisions in migratory birds? A critical view on stopover departure studies and some future perspectives. J Comp Physiol A 203:411–429. [DOI] [PubMed] [Google Scholar]
- Schwenke RA, Lazzaro BP, Wolfner MF, 2016. Reproduction—immunity trade—offs in insects. Annu Rev Entomol 61:239–256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skrip M, Bauchinger U, Goymann W, Fusani L, Cardinale M. et al. 2015. Migrating songbirds at stopover prepare for, and recover from, oxidative challenges posed by long-distance flight. Ecol Evol 5:3198–3209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skrip MM, Seeram NP, Yuan T, Ma H, McWilliams SR, 2016. Dietary antioxidants and flight exercise in female birds affect allocation of nutrients to eggs: how carry-over effects work. J Exp Biol 219:2716–2725. [DOI] [PubMed] [Google Scholar]
- Sköld-Chiriac S, Nord A, Nilsson J, Hasselquist D, 2014. Physiological and behavioral responses to an acute-phase response in zebra finches: immediate and short-term effects. Physiol Biochem Zool 87:288–298. [DOI] [PubMed] [Google Scholar]
- Thomas JS, 2000. Overview of plasma proteins In: Feldman BF, Zinkl JG, Jain NC, Schalm OW, editors. Schlam’s Veterinary Hematology. Philadelphia: Lippincott Williams and Wilkins; 891–898. [Google Scholar]
- Wone B, Ojha J, Contreras H, Davidowitz D, 2014. More is not always better: a hidden cost of the flight-fecundity trade-off in the hawk moth Manduca sexta. Faseb J 28:S1100.2. [Google Scholar]
- Wilcoxen TE, Boughton RK, Schoech SJ, 2010. Selection on innate immunity and body condition in Florida scrub-jays throughout an epidemic. Biol Lett 6:552–554. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.