Abstract
In a number of species, males and females have different ecological roles and therefore might be required to solve different problems. Studies on humans have suggested that the 2 sexes often show different efficiencies in problem-solving tasks; similarly, evidence of sex differences has been found in 2 other mammalian species. Here, we assessed whether a teleost fish species, the guppy, Poecilia reticulata, displays sex differences in the ability to solve problems. In Experiment 1, guppies had to learn to dislodge a disc that occluded a feeder from which they had been previously accustomed to feed. In Experiment 2, guppies had to solve a version of the detour task that required them to learn to enter a transparent cylinder from the open sides to reach a food reward previously freely available. We found evidence of sex differences in both problem-solving tasks. In Experiment 1, females clearly outperformed males, and in Experiment 2, guppies showed a reversed but smaller sex difference. This study indicates that sex differences may play an important role in fish’s problem-solving similar to what has previously been observed in some mammalian species.
Keywords: fish cognition, individual differences, learning abilities, problem solving, sex differences
In their natural habitat, animals are often required to solve problems, and the solution to these problems may provide access to resources important for fitness, such as food, refuge, and mates, thereby providing direct selective pressures on the underlying cognitive processes (Cole and Quinn 2012; Cole et al. 2012; Cauchard et al. 2013). The fundamental cognitive process that searches for solutions to a given problem is often referred to as problem solving, but it is recognized that solving problems may require the interaction of several other processes, such as learning, memory, and abstraction (Wang and Chiew 2010).
Considering that males and females in many species differ considerably in their ecology, it seems possible that the 2 sexes face different problems. In the last 2 decades, increasing evidence has shown that ecological sex differences can be associated with cognitive sex differences that may have evolved via natural and sexual selection (Jones et al. 2003; Jonasson 2005; Carazo et al. 2014; Lucon-Xiccato and Bisazza 2017a). For example, in species in which the male has a larger home range than the female, males often exhibit greater spatial abilities (Gaulin and FitzGerald 1986; Jones et al. 2003); reverse sex differences have been reported in species in which the female has a more complex spatial behaviour than the male (Astié et al. 1998). Accordingly, we expect the presence of sex differences in problem solving in species in which males and females face different ecological challenges and we can predict that the direction of these sex differences would vary according to species and the specific task investigated.
Several cases of sex differences have been reported in relation to problem solving in humans (Carey 1958; Milton 1959; Graf and Riddell 1972; Allen 1974; Johnson 1984; Gallagher et al. 2000). For example, Johnson (1984) administered a battery of 20 problem-solving tests to more than 1000 undergraduates, finding males averaged 35% more correct responses than females. According to some authors, modern sex differences might reflect selective pressures in our evolutionary past (Geary 1996). However, results of these types of studies are controversial because they are affected by a number of non-cognitive factors, such as gender stereotypes (Inzlicht and Ben-Zeev 2000) or different access to education (Guiso et al. 2008).
Studies on problem solving are still scarce in other species. One sex was observed to be more effective in problem solving than the other in 2 mammalian species: Suricata suricatta males were more likely than females to obtain a food reward in a set of problem solving apparatuses that required them to rotate a lid, pull a tab or a wire, and rip open a lid (Thornton and Samson 2012); and Canis familiaris males showed higher success rates than females in a task that required them to open a box to obtain a food reward (Duranton et al. 2015). Conversely, the 2 sexes’ performances were comparable in 2 other instances, releasing pins and sliding panels to access a food reward in Pan troglodytes and pulling a string to open a door and access the nest box in Parus major (Cole et al. 2011; Hopper et al. 2014). Testing the idea of evolutionary origins of sex differences in problem solving obviously requires data from many more animal species.
Recent studies have reported cognitive sex differences in teleost fish (reviewed in Lucon-Xiccato and Bisazza 2017a). Teleost fish may be an important resource for understanding the evolution of cognitive abilities because they may provide information about vertebrates’ ancestral state (Bshary and Brown 2014). The ∼30,000 species of teleosts display virtually all the levels of sexual differentiation in ecology, allowing us to explore hypotheses about selective pressures that cause cognitive sex differences. Research on fish has reported sex differences in tasks requiring discrimination between 2 visual stimuli, such as a colour discrimination learning task (Lucon-Xiccato et al. 2019), reversal learning task (Lucon-Xiccato and Bisazza 2014), and quantity discrimination tasks (Lucon-Xiccato et al. 2016; Etheredge et al. 2018). Sex differences have been also reported in some spatial learning tasks requiring the fish to learn the path that allows them to exit a maze (Fabre et al. 2014; Lucon-Xiccato and Bisazza 2017b, 2017c). Problem solving, that is, finding the solution to a situation to obtain a goal, has not been investigated in fish in this respect.
In the present research, we attempted to investigate whether fish display sex differences in problem solving, using the guppy, Poecilia reticulata, as the study species. The guppy is a teleost fish characterized by a large sexual differentiation in morphology, behaviour, foraging strategy, and spatial ecology (Magurran and Garcia 2000; Magurran 2005). Guppies show sex differences favouring females in several cognitive capacities that may affect problem solving performance, such as the ability to flexibly modify their behaviour (Lucon-Xiccato and Bisazza 2014). We therefore expected females to display greater problem-solving performance than males in this species. We tested our prediction using 2 distinct paradigms. We observed guppies for their ability to learn to remove an obstacle (Experiment 1) and to detour around a transparent obstacle (Experiment 2) to reach a food resource.
Materials and Methods
Subjects
We tested 24 guppies (12 males and 12 females) in Experiment 1 and another 24 guppies (12 males and 12 females) in Experiment 2. We used 6-month-old guppies of an ornamental strain commonly called “snakeskin cobra green.” This strain has been bred in the laboratory since 2012 and was derived from ∼200 individuals bought at a local pet shop. We routinely use this strain of guppies in experiments with complex training procedures because they habituate more quickly than wild guppies to interaction with humans. Moreover, unlike wild guppies, the domestic strain does not show marked size differences between the 2 sexes. This similarity is particularly useful in the study of sex differences because it prevents collinearity between size and sex, although previous studies on this strain showed that variation in size does not explain variation between sexes in cognitive performance (Lucon-Xiccato and Bisazza 2014, 2016; Lucon-Xiccato et al. 2015). After completing the experiments, we checked fish size by measuring the average standard length of each individual from 5 frames retrieved from the video recordings. We did not find sex differences in size in the samples of males and females used in Experiment 1 (males: median 1.92 cm, IQR 1.84 cm–1.96 cm; females: median 1.94 cm, IQR 1.86 cm–1.97 cm; Wilcoxon test: N = 24, W = 88, P = 0.378); in Experiment 2, females were ∼0.1 cm larger than males (males: median 1.74 cm, IQR 1.68 cm–1.81 cm; females: median 1.89 cm, IQR 1.77 cm–1.99 cm; Wilcoxon test: N = 24, W = 112, P = 0.021). We therefore controlled for a size effect in the statistical analysis. The experiments took place in 2017. Before the experiments, the guppies were maintained in 400-L plastic tanks with water at 26 ± 1°C, natural gravel bottom, plants, water filters, and 30-W fluorescent lamps set to provide a 12h: 12 h light/dark photoperiod. We provided the guppies commercial food flakes (Aqua Tropical, Isola Vicentina, Italy) and Artemia salina nauplii as daily food.
Experiment 1
We used a new task developed based on previous studies on guppies’ learning abilities (Lucon-Xiccato and Bisazza 2014, 2016; Miletto Petrazzini et al. 2017). In a series of training trials, we trained the guppies to feed on feeders provided with a small hole. Once the guppies met a learning criterion for the training phase, we placed an opaque disc over the feeders’ hole, blocking direct access to the food. The guppies had to learn to dislodge the disc to access the food. They could accomplish this task by pecking at and pushing the disc with the snout.
Apparatus
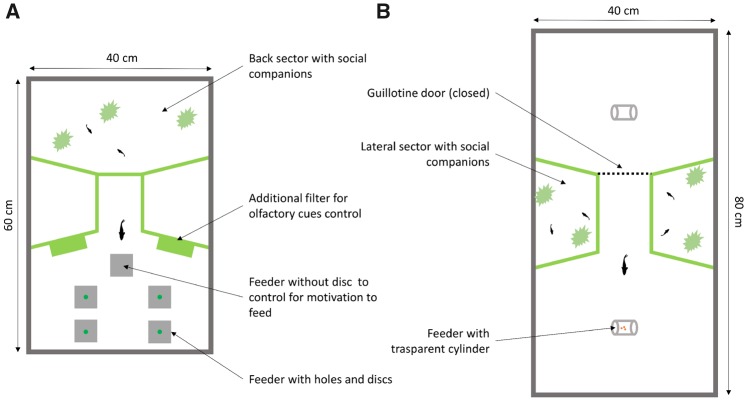
We built 6 identical apparatuses using 60 cm × 40 cm × 35-cm glass tanks filled with 30 cm of water (Figure 1a). Each apparatus was divided into 3 sectors: back sector (17 cm × 40 cm), front sector (23 cm × 40 cm), and a start box (8 cm × 12 cm) in the centre. The back sector contained plants, filters, and 4 immature conspecifics to prevent social isolation of the subjects, which could affect cognitive performance (Brandão et al. 2015). The subject was housed in the front sector for the entire experiment and could not enter the back sector due to the presence of a grid net at the end of the start box. In the front sector, the bottom consisted of a plasticized paper sheet with a gravel pattern printed on it. This bottom was used because in preliminary experiments using a natural gravel bottom, we often observed the guppies searching for food among the gravel instead of participating in the test. In the front sector, we also placed 2 additional filters (3 cm × 8 cm × 10 cm), which were activated only during the test trials (see below). The front sector was also inhabited by 1 adult conspecific of the opposite sex of the subject and 2 immature conspecifics as social companions. These fish were moved to another tank before the start of each experimental trial. The start box was equipped with a sliding Plexiglas door. The apparatus was lit by a 36-W fluorescence lamp. We also placed a camera 50 cm above the front sector to record the trials.
Figure 1.
Top view of the experimental apparatuses used in (A) Experiment 1 and in (B) Experiment 2.
Procedure
The experiment consisted of 3 phases: habituation to the apparatus and procedure, training to feed on the feeder, and problem-solving test. Guppies randomly chosen from the stock tanks were initially housed in the experimental apparatus for a 3-day habituation. During the habituation, fish could swim in the entire apparatus (front sector and start box) and underwent a feeding schedule that varied each day. On Day 1, we fed the fish according to the schedule used in the maintenance. On Day 2, we fed the guppies 3 times on the bottom of the tank by delivering crumbled fish flakes mixed with water using a Pasteur plastic pipette. On Day 3, the experimenter gently guided the subject into the start box by means of a transparent panel and then closed the guillotine door and added an opaque plastic panel in front of the door. The subject was thus prevented from seeing the front sector. Meanwhile, the experimenter delivered the food as previously described. After 2 min, the experimenter removed the plastic door and lifted the guillotine door, allowing the subject to enter the front sector and consume the food. This trial was repeated 3 times.
In the following phase of the experiment, the guppies underwent training to feed in the feeders, which would be used for problem solving. The feeders were gray plastic plates (5 cm × 5 cm, 0.8 cm high) with a hole (Ø 10 mm, 4 mm deep) in the centre (Figure 1a). In each trial of the training phase, after segregating the subject in the start box as previously described, the experimenter placed 5 feeders on the bottom of the experimental compartment. The food was delivered inside the hole of each feeder. Once the experimenter opened the guillotine door, the fish could enter the front sector and consume the food. This trial was performed 3 times per day. Guppies that did not feed on any feeder within 10 min after entering the front sector repeated the trial later. The guppies had to reach a learning criterion of 2 out of 3 trials in which they consumed the food in at least 4 out of 5 feeders presented in the trial before being admitted to the following problem-solving test phase.
In the problem-solving test phase, we tested guppies as described for the previous phase. However, the experimenter placed 4 small plastic green discs (Ø 1.5 mm, 1 mm high) above the hole of 4 feeders. To reach the food concealed in these covered feeders, the fish had to dislodge the discs. If a subject did not dislodge the discs during the trial, it could be either because it did not solve the problem or because it was not motivated to feed. To control for the latter factor, we placed an additional feeder with food but with no disc in front of the entrance of the front sector. We excluded from the analysis the trials in which subjects did not eat at least the uncovered feeder because in those trials, the guppies were likely not motivated to feed. In the trials of this phase, the experimenter also added food into the apparatus’ additional filters to permeate the apparatus with food olfactory cues, motivate the subject to feed, and prevent the subject from smelling the food smell in the feeders. The test trials lasted 30 min. Each subject underwent 18 test trials, divided between 6 days.
We scored the guppies’ performances using the video recordings of the trials. We used 3 variables to measure the performance: 1) the number of days necessary to reach the learning criterion in the training to use the feeder; 2) the number of discs dislodged in each trial of the problem-solving test phase (repeated-measures binomial variable bounded with 4 possible discs, for example, 1 out of 4 or 3 out of 4); 3) the time (s) to dislodge the first disc in each trial of the problem-solving test phase, with the maximum trial-length value (1800 s) assigned to guppies that did not dislodge any disc (right skewed variable, log transformed before the analysis).
Experiment 2
In Experiment 2, we used a modified version of the detour task, which is often used to study spatial problem solving (e.g., Smith and Litchfield 2010; Marshall-Pescini et al. 2016; Nawroth et al. 2016). After training guppies to feed on a feeder, we placed the feeder in a transparent cylinder. Therefore, the food was visible in the feeder as during the training trials, but the fish could reach it by swimming directly toward it. They could solve the problem by detouring around the cylinder and entering it from the open, lateral sides.
Apparatus
We used an apparatus and procedure recently developed in our laboratory (Lucon-Xiccato et al. 2017). We built 6 identical apparatuses using 80 cm × 40 cm × 35-cm glass tanks filled with 30 cm of water (Figure 1b). Each apparatus has an hourglass shape, with 2 identical experimental sectors (30 cm × 40 cm) connected by a central corridor (12 cm × 9 cm). A semi-transparent guillotine door (10 cm × 8 cm) was situated between each sector and the corridor to control the passage of fish between the 2 experimental sectors. The experimental sectors were as described for the front sector of Experiment 1 and housed the subjects during the experiments. With this apparatus, the test could be performed alternately in the 2 experimental sectors. Therefore, after the fish solved the trial in 1 experimental sector, the experimenter could set the new trial in the opposite sector. We chose this apparatus for Experiment 2 because it was expected to reduce disturbance to the fish and allow for more trials per day than those conducted in Experiment 1. Two additional sectors, placed beside the corridor, housed immatures guppies, abundant vegetation, and a filter, similar to the back compartment in Experiment 1. A green net separated these additional sectors from the other sectors. An 18-W fluorescent lamp uniformly illuminated each experimental sector. A video camera was positioned 50 cm above each sector.
Procedure
Experiment 2 consisted of 3 sequential phases: habituation to the apparatus and procedure, training to feed on the feeder, and problem solving. We initially placed the subjects, randomly selected from maintenance tanks, in the apparatus for a 3-day habituation. During the habituation, we kept a group of 4 immature social companions in the apparatus to facilitate familiarization with the new environment. We subsequently removed these companions during the trials as described for Experiment 1, leaving only the fish in the lateral sectors as social companions. On Day 1, we fed the subject as in the maintenance tanks. On Day 2, we fed the subject 5 times in the 2 experimental sectors alternately, using a Pasteur pipette. On Day 3, we fed the fish as on Day 2, but we started to habituate the subjects to the guillotine door’s movements by closing the door of the experimental sector opposite the one with the subject before inserting the pipette into the water. When the subject tried to reach the pipette, we lifted the guillotine door, and we let the subject enter the experimental sector and feed.
In the following phase, we trained the subjects to use the feeder, a 4 cm × 4-cm green panel placed on the bottom of the experimental sector. After closing the subject in 1 experimental sector, the experimenter placed the feeder 15 cm from the guillotine door and delivered a small quantity of food (a ∼3 mm × 3-mm crumbled flake) over it. The experimenter then lifted the guillotine door and let the subject consume the food on the feeder. This procedure was repeated 5 times per day and continued until the fish reached a learning criterion of 4 out of 5 successful trials in a day (trials in which the subject found the food on the feeder within 10 min).
After reaching the training phase’s learning criterion, the subject started the test phase with the problem to be solved. The procedure was identical to that described for the training phase, but a transparent cylinder (Ø 3.5 cm, 4 cm long) enclosed the feeder. Therefore, the subject had to detour around the transparent cylinder and reach the food reward by entering the cylinder from the side. We performed 30 test trials divided between 6 days.
We scored the guppies’ performances using the video recordings of the trials like in Experiment 1. We measured 3 variables: 1) the number of days necessary to reach the learning criterion in the training to use the feeder, 2) whether the subject succeeded in each trial of the problem-solving phase, that is, it entered the transparent cylinder sideways and did not touch the cylinder (repeated-measures binomial variable with 2 possible values: “success” and “failure”), and 3) the time (s) to reach the food in each trial of the problem-solving phase (repeated measures, right skewed variable, log transformed before the analysis).
Statistical analysis
We analysed the data using R version 3.4.0 (The R Foundation for Statistical Computing, Vienna, Austria, http://www.r-project.org). We used 2-tailed tests and significance threshold of P = 0.05.
We analysed the number of days the fish took to reach the training phase’s learning criterion (for Experiments 1 and 2) with a Wilcoxon test to compare males and females and with a Spearman’s rank correlation to determine whether days to criterion correlates with the performance in the problem solving phase.
We analysed the data from the problem-solving phase (for both experiments) using 5 generalized linear mixed-effects models (GLMM) fitted with the “glmmadmb” function of the “glmmADMB” R package. In Experiment 1, we used 1 model for success in the trial and 2 models for time to solve the task and in Experiment 2, we used 1 model for success in the trial and 1 model for time to solve the task. In these models, we always included trial number and sex as fixed effects and subject ID as a random effect to account for repeated trials. We fitted the data on success in the trials using binomial response distribution. In the model for Experiment 1, success in the trials was a matrix with number of discs dislodged and the number of discs not dislodged in each trial, whereas in the model for Experiment 2, it was a binary variable indicating whether the subjects touched the cylinder (“failure” and “success”). We similarly fitted the models for time to solve the task, but we used Gaussian error distribution (data were log transformed to deal with right-skewed distribution).
Lastly, we checked the role of fish size (standard length) in problem-solving performance because in Experiment 2, we detected a small size difference between males and females. We used Spearman’s correlations to determine whether the guppies’ size (standard length) correlated with problem-solving performance (percentage correct trials and average time to solve the task). We performed these correlations on the entire sample and on males and females separately.
In the problem-solving phase of Experiment 1, 1 female did not perform the last day of the problem solving test phase, so we analysed its performance only up to Day 5. We also dropped 7 trials in which the fish did not eat the control food in the uncovered feeder from the analysis (final data set: 422 trials), assuming that in these trials, the fish were not motivated to feed. The trials in which subjects did not eat the food were equally distributed between the 2 sexes (N males = 3; N females = 4). This finding suggested an absence of sex differences in motivation in participate to the trials.
In the problem-solving phase of Experiment 2, we dropped 40 trials (N females = 11; N males = 29) in which the fish did not eat the reward. Experiment 2’s final data set included 680 trials.
Ethical note
Experiments were conducted in accordance with the law of the country in which they were performed (Italy, D.L. 4 March 2014, n. 26). The Ethical Committee of University of Padova reviewed and approved all the experimental procedures (protocol n. 33/2015). No physical invasive manipulations were performed on the fish during the experiments and no fish showed signs of distress, such as freezing. At the end of the experiments, all subjects were released into stock tanks.
Results
Experiment 1
Training to feed on the feeder
During the initial training, guppies learned to feed on the feeder in 2 days (median), with a range 2–5 days. All 12 females achieved this criterion in 2 days of training, whereas among males, 7 subjects took 2 days, 3 subjects took 3 days, 1 subject took 4 days, and 1 subject took 5 days (median 2 days, range 2–5 days). The comparison between the sexes demonstrated that females achieved the learning criterion significantly faster than males (Wilcoxon test: N = 24, W = 42, P = 0.016). The number of days necessary to learn to feed in the feeder did not predict the number of discs dislodged in the problem solving of males (Spearman’s correlation: N = 12, ρ = −0.219, P = 0.493); in females, it was not possible to perform this test because their performance in the training phase had variance equal to zero (i.e., all females took 2 days to complete the phase).
Problem solving: success in the trials
In the problem-solving phase, guppies dislodged 423 discs overall. The success in solving the task significantly increased with trials (χ21 = 101.166, P < 0.0001; Figure 2a). Sex significantly predicted success in solving the task (discs dislodged per trial: males: median 0%, IQR 0–13.89%; females: median 31.94%, IQR 13.60–66.93%; GLMM: χ21 = 8.439, P = 0.004; Figure 2a). However, there was a significant interaction between sex and trial (χ21 = 18.428, P < 0.0001), indicating that females improved their performance in dislodging the discs across trials whereas males did not or did it less markedly (Figure 2a). The presence of the random effect individual in the model significantly increased the amount of variance explained (P < 0.0001), which suggested large between-individual variance in performance. The size of the guppies did not correlate with the percentage of discs dislodged (Spearman’s correlation: all subjects: N = 24, ρ = −0.075, P = 0.729; males: N = 12, ρ = −0.305, P = 0.334; females: N = 12, ρ = −0.055, P = 0.865).
Figure 2.
Performance of males (light gray) and females (dark gray) in Experiment 1: (A) percentage of discs dislodged across the days of the problem-solving phase; time to solve the task calculated (B) including and (C) excluding the trials in which the subjects did not dislodge any disc. Data points represent mean and bars represent standard error.
Problem solving: time to dislodge the first disc
In a first analysis, we included all trials, assigning the maximum value of 1800 s (trial duration) to trials in which fish did not dislodge any disc. Males showed a significantly higher time compared with females (time to dislodge the first disc: males: median 1800 s, IQR 1800 s–1800 s; females: median 1800 s, IQR 485 s—1800 s; GLMM: χ21 = 5.330, P = 0.021). There was a significant decrease in time to solve the task over trials (χ21 = 58.152, P < 0.0001), and a significant sex × trial interaction (χ21 = 22.809, P < 0.0001; Figure 2b).
The interaction between sex and trial in the previous analysis might indicate that females became faster in dislodging the first disc compared with males across the series of trials. However, given that males opened less discs than females (see analysis on success on the trials), male dataset included more values of 1800 s and this might have affected the analysis. Therefore, we repeated the analysis on time considering only trials in which at least 1 disc was dislodged (N = 161). In this latter analysis, only the effect of trial remained significant (trial: χ21 = 13.458, P < 0.001; sex: χ21 = 0.091, P = 0.763; sex × trial interaction: χ21 = 1.721, P = 0.190; Figure 2c). Therefore, the effects of sex and the interaction in the previous model were due to the increase in the number of trials in which females dislodged a disc rather than to the fact that females learned to dislodge a disc faster than males. The inclusion of individual as random effect significantly increased the variance explained by the model (P < 0.0001). The size of the guppies did not correlate with the time to dislodge the first disc (Spearman’s correlation: all subjects: N = 24, ρ = 0.009, P = 0.966; males: N = 12, ρ = 0.306, P = 0.334; females: N = 12, ρ = −0.125, P = 0.699).
Experiment 2
Training to feed on the feeder
During the initial training, guppies learned to find the food in the feeder in 2 days, median, with a range of 2–5 days, and with no difference between the 2 sexes (males: median 2 days, range 2 to 5 days; females: median 2 days, range 2–3 days; Wilcoxon test: N = 24, W = 59.5, P = 0.285). The number of days necessary to learn the use of the feeder did not predict the proportion of correct responses in the following problem solving phase (Spearman’s correlation: N = 24, ρ = 0.309, P = 0.142).
Problem solving: success in the trials
In the problem-solving phase, males showed a higher rate of success (i.e., reaching the food without touching the transparent cylinder) compared with females (correct trials: males: median 59.63%, IQR 56.04–66.37%; females: median 53.44%, IQR 45.83–59.48%; GLMM: χ21 = 4.873, P = 0.027; Figure 3a). The likelihood of success did not significantly vary across trials (χ21 = 0.708, P = 0.400). The interaction between sex and trial was close to the threshold for statistical significance (χ21 = 3.685, P = 0.055; Figure 3a), suggesting that the performance of the 2 sexes might have varied differently across trials. The presence of the random effect (individual fish) in the model significantly contributed to the variance explained (P = 0.013). The size of the guppies did not correlate with the percentage of correct trials (Spearman’s correlation: all subjects: N = 24, ρ = −0.096, P = 0.656; males: N = 12, ρ = 0.133, P = 0.683; females: N = 12, ρ = 0.140, P = 0.667).
Figure 3.
Performance of males (light gray) and females (dark gray) in Experiment 2: (A) % of correct responses across the days of the problem-solving phase; (B) time to solve the task. Data points represent mean and bars represent standard error.
Problem solving: time to solve the task
The time to solve the task in the problem solving phase was not significantly explained by sex (males: median 95.24 s, IQR 65.90 s–135.45 s; females: median 50.44 s, IQR 16.98 s–119.56 s; GLMM: χ21 = 1.227, P = 0.268). There was a substantial reduction of the time to solve the task across trials (χ21 = 17.028, P < 0.0001; Figure 3b); this effect was qualified by an interaction with sex (χ21 = 16.894, P < 0.0001; Figure 3b), indicating that the 2 sexes initially took similar time to solve the task, but then females diminished more than males did. Individual random effect significantly increased the variance explained by the model (P < 0.0001). The size of the guppies did not correlate with the time to solve the problem (Spearman’s correlation: all subjects: N = 24, ρ = 0.286, P = 0.175; males: N = 12, ρ = 0.406, P = 0.193; females: N = 12, ρ = 0.490, P = 0.110).
Discussion
Sex differences in problem solving, the process that leads animals to seek and evaluate solutions to a given situation to reach a certain goal (Wang and Chiew 2010), have been often reported in humans (e.g., Milton 1959; Allen 1974; Johnson 1984; Gallagher et al. 2000), and in few cases in other mammals (e.g., Thornton and Samson 2012). This study showed that fish may also exhibit sex differences in the ability to solve problems and that the direction of the sex difference varies with the task.
In Experiment 1, females largely outperformed males in learning to dislodge a disc to reach a food item concealed underneath. At the end of the experiment, the number of discs dislodged by females was ∼3 times that of males. Once an individual learned to accomplish the task, the time taken to dislodge a disc did not vary according to its sex. The sex difference in number of discs dislodged therefore occurred because females were more likely to learn to dislodge the discs, not because of sex differences in the motorial skills (e.g., physical strength) necessary to dislodge the discs.
In Experiment 2, males outperformed females in reaching a food reward behind a transparent barrier, but the performance difference was relatively small. In Experiment 2, males were slightly smaller than females; however, we did not find statistical evidence that the size of the fish predicted their problem-solving performance. Interestingly, females were significantly faster than males at the end of Experiment 2, in which males achieved higher accuracy, suggesting that a trade-off between speed and accuracy occurred in this task (Chittka et al. 2009). Two previous studies, 1 on guppies (Lucon-Xiccato and Bisazza 2016) and 1 on 3-spined sticklebacks, Gasterosteus aculeatus (Mamuneas et al. 2014), revealed sex differences in time to solve the task that occurred in the absence of trade-offs. Our result might be due to females being more motivated to reach the visible food than males, causing females to try to reach the food passing through the transparent cylinder rather than detouring (van Horik et al. 2018).
The sex differences in our 2 experiments add to the growing evidence of sex differences in specific cognitive tasks in guppies and other fish species (reviewed in Lucon-Xiccato and Bisazza 2017a). For example, female guppies outperformed males in shoal-size discrimination (Lucon-Xiccato et al. 2016), and male Salaria fluviatilis outperformed females in learning to solve a spatial task using landmarks (Fabre et al. 2014). The problem-solving tasks administered in the present study were more complex and arguably required sets of cognitive abilities to be solved. For instance, in Experiment 1’s problem, guppies needed to remember that the food was previously present in the feeder’s hole, to flexibly try multiple strategies to reach the food, to persist in the attempts to remove the discs, and to learn and memorize an efficient motorial sequence to dislodge the discs. In Experiment 1 and marginally in Experiment 2, the sex difference was also modulated by the trial, indicating that learning might be involved, as previously reported in this species for a spatial task and a visual-discrimination task (Lucon-Xiccato and Bisazza 2017b; Lucon-Xiccato et al. 2019). The present study therefore indicates that guppies also show sex differences in complex tasks requiring a number of processes and abilities to be successfully performed. More important, these complex problems might be similar to the tasks that guppies face in their natural environment, such as when learning to exploit a new food source.
The sex difference in guppies’ problem solving varied with the task, possibly because multiple cognitive abilities were involved in the solution of the 2 tasks. One can speculate that the problem’s solution in Experiment 1 likely entailed a certain degree of persistence, because dislodging the discs required a series of pushes with the snout. Solving the problem of Experiment 2 likely required, along with achieving the spatial detour problem, an inhibition of the behaviour of swimming directly toward the food (Kabadayi et al. 2018). The use of these contrasting abilities could have caused the opposite sex differences in the 2 experiments. High variation in the direction of sex difference has already been observed in guppies (Lucon-Xiccato and Bisazza 2017a), and in the other species deeply investigated for sex differences, that is humans (Halpern 2013), rats and mice (Jonasson 2005). Therefore, available data indicates that selection has favoured, for each sex, specialization in solving certain tasks rather than higher general cognitive abilities in a sex.
The conventional explanation for sex differences in cognitive performance is that they are the consequence of past selection on male and female cognitive abilities. However, an alternative explanation for this variation is that during ontogeny, males and females are exposed to different stimuli and experience, for example because of sex differences in ecology. For instance, in guppies, males and females have often been reported to live in different parts of the river (Croft et al. 2006; Darden and Croft 2008). Our study was not designed to address the effect of ontogeny on problem solving. However, it is worth noting that the fish used in our experiments were domestic guppies, bred and maintained in our laboratory under controlled conditions. Males and females were unlikely to be exposed to different stimuli and experiences during development and could not separate in different habitats. Accordingly, our study seems to suggest that the sex differences in problem solving that guppies showed occurred to the diverse selective pressures acting on males and females in their evolutionary past.
A side finding of our study was our detection of significant between-individual variance in performance, which might indicate individual differences in problem solving. Size differences between the subjects did not account for this individual variation in performance. Individual differences in the ability to solve problems have been observed in humans (Carroll and Maxwell 1979), other mammals (Hopper et al. 2014), and birds (Cole et al. 2011). Evidence already shows individual differences in some cognitive abilities of guppies (Lucon-Xiccato and Dadda 2017a, 2017b). However, our study does not allow us to confirm that individual guppies show different problem solving abilities. For each individual, we observed performance in a single task because we tested different sets of subjects in the 2 experiments. With this design, high between-individual variance might be explained by a single cognitive ability involved in the specific task, such as learning or memory, rather than by a general effectiveness in solving problems. Future studies should therefore observe the same set of individuals in multiple problem-solving tasks, an approach that permits measurements of whether some individuals consistently outperform others in problem solving (Griffin et al. 2015).
In conclusion, this study suggests that sex differences in problem solving can also be found in guppies. Selective pressures acting on males and females might differ in a large number of vertebrates and might lead to the evolution of cognitive sex differences in the ability to solve problems. More comparative research on this topic is needed, in particular including species in which males and females show different levels of ecological difference to determine which selective pressures may cause the evolution of sex differences.
Acknowledgments
We would like to thank Alice Andreose, Alberto Mair, Lorenzo Tausani, and Martina Zanutto for their help in testing the animals. We have no competing interests.
Funding
Funding was provided by PRIN 2015 Grant (prot.: 2015FFATB7) to A.B. from Ministero dell’Istruzione, Università e Ricerca (MIUR, Italy) and FIR2018 and FAR2018 grants to T.L.X. from University of Ferrara.
Authors’ Contributions
All the authors conceived the study and contributed to the final version of the manuscript. T.L.X. and E.G. collected the data; T.L.X. analysed the data and drafted the manuscript.
References
- Allen MJ, 1974. Sex differences in spatial problem-solving styles. Percept Motor Skill 39:843–846. [Google Scholar]
- Astié AA, Kacelnik A, Reboreda JC, 1998. Sexual differences in memory in shiny cowbirds. Anim Cogn 1:77–82. [DOI] [PubMed] [Google Scholar]
- Brandão ML, Braithwaite VA, Gonçalves-de-Freitas E, 2015. Isolation impairs cognition in a social fish. Appl Anim Behav Sci 171:204–210. [Google Scholar]
- Bshary R, Brown C, 2014. Fish cognition. Curr Biol 24:R947–R950. [DOI] [PubMed] [Google Scholar]
- Carazo P, Noble DW, Chandrasoma D, Whiting MJ, 2014. Sex and boldness explain individual differences in spatial learning in a lizard. Proc Roy Soc Lond B Bio 281:20133275.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carey GL, 1958. Sex differences in problem-solving performance as a function of attitude differences. J Abnorm Soc Psychol 56:256–260. [DOI] [PubMed] [Google Scholar]
- Carroll JB, Maxwell SE, 1979. Individual differences in cognitive abilities. Ann Rev Psychol 30:603–640. [DOI] [PubMed] [Google Scholar]
- Cauchard L, Boogert NJ, Lefebvre L, Dubois F, Doligez B, 2013. Problem-solving performance is correlated with reproductive success in a wild bird population. Anim Behav 85:19–26. [Google Scholar]
- Chittka L, Skorupski P, Raine NE, 2009. Speed-accuracy tradeoffs in animal decision making. Trend Ecol Evol 24:400–407. [DOI] [PubMed] [Google Scholar]
- Cole EF, Cram DL, Quinn JL, 2011. Individual variation in spontaneous problem-solving performance among wild great tits. Anim Behav 81:491–498. [Google Scholar]
- Cole EF, Morand-Ferron J, Hinks AE, Quinn JL, 2012. Cognitive ability influences reproductive life history variation in the wild. Curr Biol 22:1808–1812. [DOI] [PubMed] [Google Scholar]
- Cole EF, Quinn JL, 2012. Personality and problem-solving performance explain competitive ability in the wild. Proc Roy Soc Lond B Bio 279:1168–1175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Croft DP, Morrell LJ, Wade AS, Piyapong C, Ioannou CC. et al. , 2006. Predation risk as a driving force for sexual segregation: a cross–population comparison. Am Nat 167:867–878. [DOI] [PubMed] [Google Scholar]
- Darden SK, Croft DP, 2008. Male harassment drives females to alter habitat use and leads to segregation of the sexes. Biol Lett 4:449–451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duranton C, Rödel HG, Bedossa T, Belkhir S, 2015. Inverse sex effects on performance of domestic dogs Canis familiaris in a repeated problem-solving task. J Comp Psychol 129:84–87. [DOI] [PubMed] [Google Scholar]
- Etheredge RI, Avenas C, Armstrong MJ, Cummings ME, 2018. Sex-specific cognitive-behavioural profiles emerging from individual variation in numerosity discrimination in Gambusia affinis. Anim Cogn 21:37–53. [DOI] [PubMed] [Google Scholar]
- Fabre N, García-Galea E, Vinyoles D, 2014. Spatial learning based on visual landmarks in the freshwater blenny Salaria fluviatilis (Asso, 1801). Learn Mot 48:47–54. [Google Scholar]
- Gallagher AM, De Lisi R, Holst PC, McGillicuddy-De Lisi AV. et al. , 2000. Gender differences in advanced mathematical problem solving. J Exp Child Psychol 75:165–190. [DOI] [PubMed] [Google Scholar]
- Gaulin SJ, FitzGerald RW, 1986. Sex differences in spatial ability: an evolutionary hypothesis and test. Am Nat 127:74–88. [Google Scholar]
- Geary DC, 1996. Sexual selection and sex differences in mathematical abilities. Behav Brain Sci 19:229–247. [Google Scholar]
- Graf RO, Riddell JC, 1972. Sex differences in problem-solving as a function of problem context. J Educ Res 65:451–452. [Google Scholar]
- Griffin AS, Guillette LM, Healy SD, 2015. Cognition and personality: an analysis of an emerging field. Trend Ecol Evol 30:207–214. [DOI] [PubMed] [Google Scholar]
- Guiso L, Monte F, Sapienza P, Zingales L, 2008. Culture, gender, and math. Science 320:1164–1165. [DOI] [PubMed] [Google Scholar]
- Halpern DF, 2013. Sex Differences in Cognitive Abilities. Mahwah (NJ: ): Erlbaum. [Google Scholar]
- Hopper LM, Price SA, Freeman HD, Lambeth SP, Schapiro SJ. et al. , 2014. Influence of personality, age, sex, and estrous state on chimpanzee problem-solving success. Anim Cogn 17:835–847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inzlicht M, Ben-Zeev T, 2000. A threatening intellectual environment: why females are susceptible to experiencing problem-solving deficits in the presence of males. Psychol Sci 11:365–371. [DOI] [PubMed] [Google Scholar]
- Johnson ES, 1984. Sex differences in problem solving. J Educ Psychol 76:1359–1371. [Google Scholar]
- Jonasson Z, 2005. Meta-analysis of sex differences in rodent models of learning and memory: a review of behavioral and biological data. Neurosci Biobehav Rev 28:811–825. [DOI] [PubMed] [Google Scholar]
- Jones CM, Braithwaite VA, Healy SD, 2003. The evolution of sex differences in spatial ability. Behav Neurosci 117:403–411. [DOI] [PubMed] [Google Scholar]
- Kabadayi C, Bobrowicz K, Osvath M, 2018. The detour paradigm in animal cognition. Anim Cogn 21:21–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucon-Xiccato T, Bisazza A, 2014. Discrimination reversal learning reveals greater female behavioural flexibility in guppies. Biol Lett 10:20140206. [Google Scholar]
- Lucon-Xiccato T, Bisazza A, 2016. Male and female guppies differ in speed but not in accuracy in visual discrimination learning. Anim Cogn 19:733–744. [DOI] [PubMed] [Google Scholar]
- Lucon-Xiccato T, Bisazza A, 2017a. Individual differences in cognition among teleost fishes. Behav Process 141:184–195. [DOI] [PubMed] [Google Scholar]
- Lucon-Xiccato T, Bisazza A, 2017b. Sex differences in spatial abilities and cognitive flexibility in the guppy. Anim Behav 123:53–60. [Google Scholar]
- Lucon-Xiccato T, Bisazza A, 2017c. Complex maze learning by fish. Anim Behav 125:69–75. [Google Scholar]
- Lucon-Xiccato T, Dadda M, 2017a. Individual guppies differ in quantity discrimination performance across antipredator and foraging contexts. Behav Ecol Sociobiol 71:13. [Google Scholar]
- Lucon-Xiccato T, Dadda M, 2017b. Personality and cognition: sociability negatively predicts shoal size discrimination performance in guppies. Front Psychol 8:1118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucon-Xiccato T, Dadda M, Bisazza A, 2016. Sex differences in discrimination of shoal size in the guppy Poecilia reticulata. Ethology 122:481–491. [Google Scholar]
- Lucon-Xiccato T, Gatto E, Bisazza A, 2017. Fish perform like mammals and birds in inhibitory motor control tasks. Sci Rep 7:13144.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucon-Xiccato T, Manabe K, Bisazza A, 2019. Guppies learn faster to discriminate between red and yellow than between two shapes. Ethology 125:82–91. [Google Scholar]
- Lucon-Xiccato T, Miletto Petrazzini ME, Agrillo C, Bisazza A, 2015. Guppies discriminate between two quantities of food items but prioritize item size over total amount. Anim Behav 107:183–191. [Google Scholar]
- Magurran AE, 2005. Evolutionary Ecology: The Trinidadian Guppy. Oxford: Oxford University Press. [Google Scholar]
- Magurran AE, Garcia CM, 2000. Sex differences in behaviour as an indirect consequence of mating system. J Fish Biol 57:839–857. [Google Scholar]
- Mamuneas D, Spence AJ, Manica A, King AJ, 2014. Bolder stickleback fish make faster decisions, but they are not less accurate. Behav Ecol 26:91–96. [Google Scholar]
- Marshall-Pescini S, Frazzi C, Valsecchi P, 2016. The effect of training and breed group on problem-solving behaviours in dogs. Anim Cogn 19:571–579. [DOI] [PubMed] [Google Scholar]
- Miletto Petrazzini ME, Bisazza A, Agrillo C, Lucon-Xiccato T, 2017. Sex differences in discrimination reversal learning in the guppy. Anim Cogn 20:1081–1091. [DOI] [PubMed] [Google Scholar]
- Milton GA, 1959. Sex differences in problem solving as a function of role appropriateness of the problem content. Psychol Rep 5:705–708. [Google Scholar]
- Nawroth C, Baciadonna L, McElligott AG, 2016. Goats learn socially from humans in a spatial problem-solving task. Anim Behav 121:123–129. [Google Scholar]
- Smith BP, Litchfield CA, 2010. How well do dingoes, Canis dingo, perform on the detour task? Anim Behav 80:155–162. [Google Scholar]
- Thornton A, Samson J, 2012. Innovative problem solving in wild meerkats. Anim Behav 83:1459–1468. [Google Scholar]
- van Horik JO, Langley EJ, Whiteside MA, Laker PR, Beardsworth CE. et al. , 2018. Do detour tasks provide accurate assays of inhibitory control? Proc Roy Soc Lond B Bio 285:20180150.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Chiew V, 2010. On the cognitive process of human problem solving. Cogn Syst Res 11:81–92. [Google Scholar]