Abstract
Animals living around people may modify their antipredator behavior as a function of proximity to humans, and this response has profound implications for whether or not a population can coexist with humans. We asked whether inland blue-tailed skinks Emoia impar modified their individual antipredator behavior as a function of differential exposure to humans. We conducted multiple consecutive flushes and recorded 2 measures of antipredator response: flight initiation distance (FID), the distance from a threatening stimulus at which an individual flees, and distance fled, the distance an individual fled after a flush. We used a multiple model comparison approach to quantify variation in individual escape behavior across multiple approaches and to test for differences in between-individual variation among populations. We found that individuals tolerated closer approach and fled shorter distances at locations with relatively less human disturbance than at locations with medium and high human disturbance, respectively. In addition, skinks living at high human disturbance sites had less variable FIDs than at low human disturbance sites. Two theories may explain these results. Selection against less favorable phenotypes has reduced behavioral variation in urban habitats and behavioral plasticity allows individuals to flexibly adjust their behavioral patterns in response to human disturbance. These results highlight the importance of studying variation within populations, at the individual level, which may better elucidate the impact that human disturbance has on the behavioral composition of populations.
Keywords: distance fled, Emoia impar, flight initiation distance, habitat sorting, habituation, urbanization
Environments are becoming increasingly urbanized, bringing wild animals into more frequent contact with humans. Urban predators often avoid humans by shifting to a more nocturnal schedule (Ditchkoff et al. 2006), whereas other species such as birds, lizards, and mammals become habituated, more tolerant of humans through repeated exposure (Samia et al. 2015). Urbanization can additionally influence individuals differently based on distinct life history factors, as seen in the combined effect of urbanization and sex on personality across metamorphic stages in butterflies (Kaiser et al. 2018). Given that habituation occurs at the level of the individual, it is important to focus on how individuals respond to humans and whether there is variance in degree of tolerance to humans. Individual differences within urban populations can influence population dynamics, especially if animals sort themselves according to degree of tolerance or if individuals in a population are similarly plastic in their response to humans.
Most vertebrates exhibit some degree of individual behavioral plasticity by adapting to stressors through behavioral changes (Øverli et al. 2007). Individual variation in response to urbanization can have profound impacts on fitness, especially through reduced escape behavior or through phenotypic diversity from microevolution (Sol et al. 2013; Miranda 2017). For instance, Iberian wall lizards Podarcis hispanica differ in their propensity to explore and be social, with bolder phenotypes more likely to colonize urban habitats. More exploratory lizards habituate to new environments more quickly, including urban ones, likely due to an enhanced ability to assess risk (Rodríguez-Prieto et al. 2010). Burrowing owls Athene cunicularia vary at the individual level in their tolerance of human disturbance and as a result, choose different places to breed based on these preferences (Carrete and Tella 2010). Thus, individual variation can have an important effect on how populations respond to humans in different environments or situations.
Habituation to humans is a likely consequence of increased exposure to humans and is likely to influence risk assessments and hence antipredator behavior. Habituation is defined as the reduction of an individual’s response to a stimulus after multiple exposures to that stimulus, whereas sensitization is an increased response to a repeated stimulus (Rankin et al. 2009). Habituation may, in some cases, transfer across contexts, such that an animal that habituates to humans may also have a reduced antipredator response to actual predators (Geffroy et al. 2015). This is seen in fox squirrels Sciurus niger where urban individuals showed reduced antipredator responses to coyote Canis latrans and red-tailed hawk Buteo jamaicensis vocalizations (McCleery 2009). This carries with it obvious negative fitness consequences. To study habituation to predatory stimuli, a subject must be exposed to multiple nonthreatening encounters with a potential predator, which requires repeated measures of the same individual across time (Rodriguez-Prieto et al. 2008; Bejder et al. 2009). For repeated approaches, the distance at which the individual flees could increase, decrease, or not change, indicating sensitization, habituation, or no effect, respectively (Blumstein 2016).
Quantifying antipredator behavior, by studying how animals escape from a potential predator, is a useful way to understand how animals respond to anthropogenic activities (Blumstein 2006; Geffroy et al. 2015). Flight initiation distance (FID), the distance between an individual and a threatening stimulus when the subject flees (Ydenberg and Dill 1986), is a widely used measure to quantify both antipredator behavior and response to humans (Ydenberg and Dill 1986; Frid and Dill 2002; Cooper and Blumstein 2015). Although FID can tell us a great amount about how human presence influences behavior, many studies of FID focus on measures of central tendency in populations (Blumstein et al. 2003; Blumstein 2006; Martínez-Abraín et al. 2008; Rodriguez-Prieto et al. 2008) instead of the variation in FID at the individual level (Carrete and Tella 2010; Møller and Garamszegi 2012). This follows a larger trend in the quantification of antipredator behavior and behavioral plasticity, which typically are studied at a population level, where population means (Møller 2010; Møller and Garamszegi 2012; Møller et al. 2013; McGowan et al. 2014; Samia et al. 2015; Garamszegi and Møller 2017) and population variances (Møller 2010; Møller and Garamszegi 2012; Møller et al. 2013; Garamszegi and Møller 2017) are compared across sites with different levels of human disturbance or along some other environmental gradient. Perhaps due to this population-level scale of study, most studies are conducted on unidentified individuals, and consist of only a single FID measurement per individual. However, Guay et al. (2013) found that swans differ in antipredator response by sex, suggesting that studies on marked individuals or that study individual-level response would provide a more nuanced view of antipredator behavior.
Urban environments also alter an individuals’ perception of risk (Cavalli et al. 2016). Perception of risk can be continually altered, such as the continuous evaluation of a predator’s behavior after an individual has begun to flee (Cooper and Blumstein 2014). Distance fled (DF) is a measure of the distance an individual flees after an approach and can be a measure of risk. In lizards, DF increases as the lizard’s perceived risk increased (Cooper and Blumstein 2014). In urban habitats, anole lizards Anolis sagrei have shorter escape distances than their forest-dwelling counterparts (Lapiedra et al. 2017). Ungulates flee longer distances in response to more threatening stimuli and as a function of habitat openness (Stankowich 2008). Risk is often perceived to be greater in areas with sparse cover, distant refuges, fast predators, and/or when approached directly (Cooper 2010; Cooper et al. 2015). Different measures of antipredator behavior are differentially influenced by human activity (Price et al. 2014). By using multiple measures, we will better be able to recognize nuances within different escape processes, as well as the differences at the individual level.
We studied how inland blue-tailed skinks Emoia impar antipredator behavior, measured by FID and DF, varied in response to human development. However, unlike the majority of previous studies, we used multiple simulated predator approaches to focus on the variability of both individual habituation and between-individual variation within a population across a human disturbance gradient. Skinks are an ideal species in which to ask these questions because they are locally abundant, and prior work established that our Mo’orean population of skinks tolerated closer approaches by humans in areas where people were more common (McGowan et al. 2014).
Materials and Methods
Study site and procedures
We studied blue-tailed skinks in Mo’orea, French Polynesia (17°32′S, 149°50′W) from 19 January to 1 February 2018 during periods of peak skink activity (07:00 AM–04:00 PM). We observed skinks in 3 locations studied by McGowan et al. (2014) that were ranked for human disturbance based on domestic animal presence (dogs, cats, and chickens) as well as human foot and vehicular traffic in the area. Our high human disturbance site (17°29.23.3′S, 149°49′44.2′W) was the Richard B. Gump South Pacific Research Station and the contiguous Manutea Tahiti – Rotui Juice Factory and Distillery, a developed area with substantial pedestrian and vehicular traffic from researchers, residents, tourists, dogs C. lupus familiaris, cats Felis catus, and chickens Gallus gallus domesticus. Our medium human disturbance site (17°31.36′S, 149°49.50′W) was an unpaved cross bay road connecting Cook’s Bay and Opunohu Bay and was characterized by minimal foot traffic but frequent vehicular traffic. Our low human disturbance site (17°52.2051′S, 149°83.1743′W) was a formerly undeveloped trail which was recently (within 2 years of our study) upgraded to service a pineapple farm (H. Teavai–Murphy, personal communication). This site was characterized by some vehicular traffic but, during our study, had the least amount of pedestrian traffic of the 3 sites. Despite this land use change, we believe that the ranking of the sites in terms of relative human presence and activities was preserved.
Three observers walked independently through these 3 locations searching for skinks. Observers did not visit the same location within a site in the same or adjacent days. This ensured that observers did not oversample a particular site. In the South Pacific, skinks are reported to live in very high densities (Rodda et al. 2001), whereas we did not formally quantify densities, our impression was that they were quite abundant at our Mo’orean study sites. Thus, it was unlikely that when an observer returned to a general location, they flushed the same skink. To avoid resampling the same individual and to avoid carryover effects from a previous flush, observers only conducted flushes on skinks that were ≥10 m apart.
When a skink was spotted, observers identified it as E. impar and ensured that it had an intact tail (not autotomized). The loss of a tail would indicate a recent predator attack and this might have systematically altered perceptions of risk. Once an individual was detected and identified, an experimental approach began. Observers were trained on a standardized protocol to collect repeated FIDs (2–4 FIDs) on a single individual by approaching at a constant rate of 0.5 m/s (Blumstein et al. 2004). Observers carried flags to mark the starting position of the skink, the FIDs and the observer’s initial location (hereafter, starting distance, SD) separately. At the beginning of the approach, a flag was dropped marking the observer’s initial location. The observer waited 30 s after dropping the flag to allow for the skink to settle, and approached the skink directly. When the skink began to flee, a flag was dropped at the observers’ position to mark the first FID. The observer then stopped, and dropped another flag to mark their SD for the second approach and waited 30 s before beginning. Although conducting the second approach, a flag was dropped at the initial position of the skink for the first flush. When the skink fled a second time, another flag was dropped at the point marking the observers’ position when the skink began to flee. If the skink was still in sight, the observer again stopped, dropped a flag and waited 30 s to repeat the process. Overall, this procedure was repeated for 2–4 total trials on the same individual. After all possible trials had been conducted on a skink, the observer measured distances between flags for all SD, FID, and DFs. SD was the distance between the flag marking SD for a separate approach and the flag marking the skink’s initial location for that trial. FID for a separate trial was found by measuring the distance between the flag marking FID and the flag marking the skink’s initial location for that trial. DF for a separate trial was found by measuring the distance between the flag marking the skink’s initial location for that trial and the flag marking the skink’s initial location for the following trial. Experimental approaches were only conducted when it was not raining and when the wind speed was ≤2 on the Beaufort scale. At the start of the first flush, we also counted the number of conspecifics ≤1 m radius of the focal subject.
Statistical analyses
All analyses were conducted in R (version 3.4.3; R Core Team 2017). We log10-transformed our FID, DF, and SD to normalize distributions. We then tested whether there was variation in FID between individual skinks. To do this, we created a null linear mixed-effects model with a dependent variable of log10FID and the random effect of individual. From this, we calculated the intraclass correlation coefficient to quantify how much of the variation in FID was explained by individual skinks. Then, we used a chi-square to test whether skinks received approximately equal numbers of 1, 2, 3, or 4 flushes across the 3 sites.
To determine how individuals varied in their response to subsequent flushes, we fitted a series of increasingly complex linear mixed-effects models in lme4 (Bates et al. 2015) and lmerTest (Kuznetsova et al. 2017). All models included the following fixed effects: site, observer, and SD, which we included to, respectively, investigate differences between site, evaluate observer effects, and account for the association between SDs and FID (see Table 1 for FID models, Table 4 for DF models). Models varied by their inclusion of a random intercept or a random intercept and random slope and are specified below. By including different types of random effects in the models we accounted for variance in behavior within individuals across sites. This allowed us to explore the relationships between individual behavior and site in different ways and, ultimately, to find which relationship held the greatest explanatory power.
Table 1.
Models fitted in lme4 to study variation in FIDs of skinks
| Model | Fixed effects | Random effects | AIC |
|---|---|---|---|
| M0FID—Regression | Observer, site, logSD | N/A | N/A |
| M1FID—Random intercept | Observer, site, logSD, trial | Intercept: skink | −191.7 |
| M2FID—Random intercept/fixed interaction | Observer, site, logSD, trial, site*trial | Intercept: skink | −191.7 |
| M3FID—Random intercept/random slope | Observer, site, logSD, trial | Intercept: skinkSlope: trial | −196.7 |
| M4FID—Random intercept/random slope with site–trial interactions | Observer, site, logSD, trial, site*trial | Intercept: skinkSlope: trial | −195.23 |
Dependent variable is logFID for all models. Bold text indicates model with lowest AIC.
Indicates an effect interaction.
Table 4.
Models fitted in lme4 to study variation in the distance skinks fled
| Model | Fixed effects | Random effects | AIC |
|---|---|---|---|
| M0DF—basic regression | Observer, site, logSD | N/A | N/A |
| M1DF—random intercept trial | Observer, site, logSD, trial | Intercept: skink | 38.75 |
| M2DF—random intercept with site–trial interaction | Observer, site, logSD, trial, site*trial | Intercept: skink | 42.26 |
| M3DF—random intercept/random slope with site–trial interactions | Observer, site, logSD, trial | Intercept: skinkSlope: trial | 41.94 |
| M4DF—random intercept/random slope with site–trial interactions | Observer, site, logSD, trial, site*trial | Intercept: skinkSlope: trial | 45.41 |
Dependent variable is logDF for all models. Bold text indicates model with lowest AIC.
Indicates an effect interaction.
Wefirst created a basic Ordinary Least Squares (OLS) regression (M0) with the fixed effects mentioned above. This allowed us to examine variation in escape behavior explained by these fixed factors. We created a second, random intercept model (M1) by adding trial as a fixed effect to M0. Adding the fixed effect of trial allowed us to account for individual habituation in escape behavior as well as determine whether trial significantly explained the variation in the data. We then created a random intercept, random slope model (M2) by adding a random slope of trial to M1. By adding random slope of trial, we allowed each individual to exhibit different slopes in their antipredator responses across multiple trials. This allowed us to determine the overall direction and degree of each individual’s response to multiple approaches. Lastly, we created 2 additional models, (M3 and M4) by adding a site–trial interaction as a fixed effect to both M1 and M2, respectively. M3 thus examined the between-individual variation in escape behavior averaged across trials at each site with varied individual intercepts but a common mean slope. M4 examined between-individual variation in escape behavior across trials at each site with varied individual intercepts and slopes reflective of each individual’s habituation response across trials.
To determine the best-fit model, we used data-driven model comparison to identify the best supported model based on Akaike Information Criterion (AIC) comparisons (Zuur et al. 2009). Then, we evaluated the significance of the fixed effects in the best model and conducted a pair-wise means test on site, if significant, in the best model.
To test for variation of FID for individuals among sites (Bolker 2013), we fitted 2 linear mixed-effects models in nlme (Pinheiro et al. 2017). Both models had trial, observer, SD, site, and the interaction between site and trial as fixed effects. Our models again differed in their inclusion of random intercept or random slope and random intercept, examining 2 different levels of within individual variation. Our first model included the random intercept of individuals (S0), and our second model had both random intercept of individuals and the random effect of site (S1). S0 thus measured whether there was a difference between individuals in their mean response, whereas S1 measured whether individuals differed across sites. We compared these 2 models using the AIC comparisons to determine whether site explained significant variation in FID. If the model comparison was significant, we inferred that there were differences in individual plasticity among sites.
We visualized our results with spaghetti plots made with ggplot2 (Wickham 2009). Assumptions of our models were evaluated by plotting residuals, testing for normality with the Shapiro–Wilks tests, and examining Q–Q plots. We used an identical workflow to study factors affecting variation in DF.
Results
We observed a total of 87 skinks: 39 at the human-dominated site (16 with 4 FID trials, 16 with 3 FID trials, and 7 with 2 FID trials); 32 along the dirt road (13 with 4 FID trials, 8 with 3 FID trials, and 11 with 2 FID trials); and 16 along the more pristine trail (7 with 4 FID trials, 5 with 3 FID trials, and 4 with 2 FID trials). There was no significant difference across site in the number of successive flushes (χ2 = 0.37; P = 0.999). Bad weather (rain and high wind) during our research trip prevented further data collection.
FID
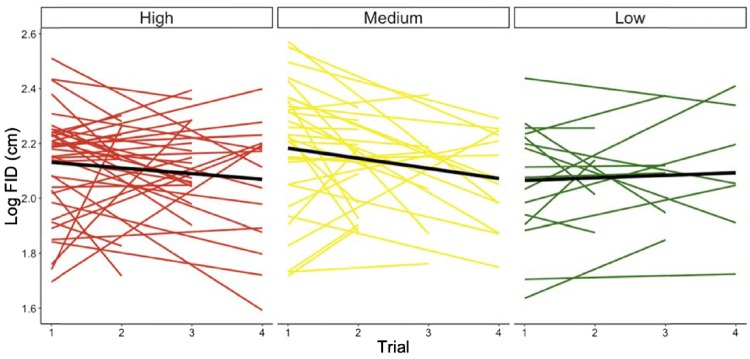
Skink identity alone explained 47% of the variation in FID. For individual response to multiple flushes, 2 models had approximately the same AIC (M3 AIC= −196.7, M4 AIC = −195.23, Table 1, δAIC = 1.5). We discuss M3, the random intercept, random slope model without a significant interaction between site and trial (Table 1) because it had the smallest AIC, but conclusions for the 2 models are similar. After accounting for significant observer and SD effects, we found that neither site nor trial explained significant variation in FID (Table 2). However, a pair-wise comparison of site means revealed that skinks tolerated significantly closer approaches at the low human disturbance site versus the medium human disturbance site (estimate = −0.1, t = −2.40, P = 0.02, reference level low). There were no significant differences in site means between high- and medium-disturbance sites (estimate = 0.0, t = −1.06, P = 0.29, reference level high) or between high- and low-disturbance sites (estimate = 0.1, t = 1.63, P = 0.11, reference level high). Overall, FID did not change significantly across trial and did not exhibit a pattern across the human disturbance gradient (Figure 1).
Table 2.
Fixed effects from M3, the random intercept, random slope model without the interaction of trial and site explaining variation in individual FID response to multiple flushes
| Variable | Estimate (SE) | t | P |
|---|---|---|---|
| (Intercept) | 0.697 | 1.914 | 0.057 |
| Observer | |||
| A | −0.155 | −4.178 | <0.001 |
| B | −0.008 | −0.220 | 0.826 |
| Site | |||
| Medium | 0.035 | 1.055 | 0.294 |
| Low | −0.068 | −1.632 | 0.106 |
| logSD | 0.590 | 4.210 | <0.001 |
| Trial | −0.006 | −0.525 | 0.601 |
Significant effects are in bold.
Figure 1.
Skink FID responses across multiple approaches. Shown are the means and slopes of log10 FID from individuals after multiple flushes at the 3 sites which vary in their degree of human exposure. Thick black line indicates the average slope per site. There is no significant difference in average slopes across sites.
The best model that explained variation in FID for individuals among sites was the random intercept, random-effect model (S1) rather than the random intercept model (S0, AICrandom intercept, random effect = −147.4, AICrandom intercept = −144.1). Based on this significant model comparison, we infer that site accounted for significant variation in individual plasticity (Figure 1). Skinks from the highest disturbance site had the least variation in their FIDs across multiple flushes (standard deviation = 0.078), whereas the medium-disturbance site had an intermediate level of variation (standard deviation = 0.099). The most variation in individual FIDs occurred at the low-disturbance site (standard deviation = 0.167). Finally, SD and observer effects were also significant in our model (Table 3).
Table 3.
Fixed effects from the random intercept, random slope model (S1) including interaction of site and trial explaining variation in FID for individuals among sites
| Variable | Estimate (SE) | t | P |
|---|---|---|---|
| (Intercept) | 0.663 | 1.884 | 0.061 |
| Observer | |||
| A | −0.196 | −5.357 | <0.001 |
| B | −0.043 | −1.166 | 0.247 |
| Site | |||
| Medium | 0.081 | 1.570 | 0.120 |
| Low | −0.131 | −1.789 | 0.077 |
| LogSD | 0.613 | 4.50 | <0.001 |
| Trial | −0.003 | −0.260 | 0.796 |
| Trial: low | 0.027 | 1.13 | 0.258 |
| Trial: medium | −0.238 | −1.245 | 0.215 |
Significant effects are in bold.
DF
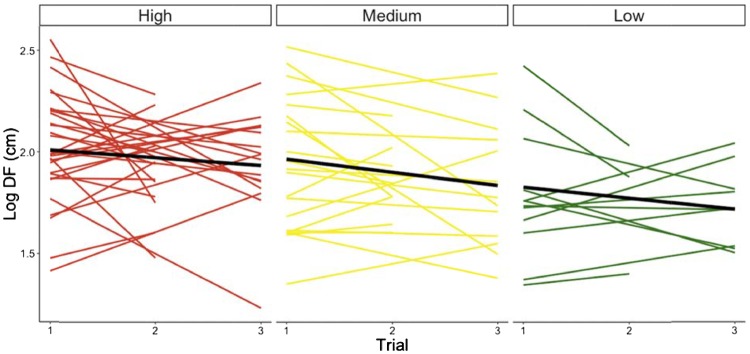
Skink identity alone explained 39% of the variation in DF. For variation in individual response to multiple flushes, the random intercept with trial model (M1, Table 4) was the best mixed model (δAIC = 2.22). After accounting for observer effects, we found that significant variation in DF was explained by trial, but not by SD or site. However, a pair-wise comparison of DF across sites revealed that skinks fled significantly shorter distances at the low-disturbance site when compared with high-disturbance site (estimate = 0.2, t = 2.62, P = 0.010, reference level high), but not when compared with the medium-disturbance site (estimate = −0.1, t = −1.50, P = 0.14, reference level low). The distance high-disturbance site skinks fled was not statistically different from their DF at the medium-disturbance site (estimate = 0.1, t = 1.33, P = 0.19, reference level high) (Table 5). Overall, DF decreased across trial and decreased across sites from high human disturbance to low human disturbance (Figure 2).
Table 5.
Fixed effects from the best fit model, random intercept with trial model (M1), explaining individual variation in DF response to multiple flushes
| Variable | Estimate (SE) | t | P |
|---|---|---|---|
| (Intercept) | 1.588 | 2.423 | 0.016 |
| Observer | |||
| A | 0.212 | 3.752 | <0.001 |
| B | 0.027 | 0.467 | 0.642 |
| Site | |||
| Medium | −0.068 | −1.328 | 0.188 |
| Low | −0.166 | −2.621 | 0.010 |
| SD | 0.152 | 0.602 | 0.548 |
| Trial | −0.049 | −2.068 | 0.040 |
Significant effects are in bold.
Figure 2.
Skink DF responses across multiple approaches. Shown are the means and slopes of log10 DF from individuals after multiple flushes at the 3 sites which vary in their degree of human exposure. Thick black line indicates the average slope per site. Low site average slope is significantly lower than high site average slope.
For variation of DF for individuals among sites, we found that our best model remained the random intercept model (S0, AICrandom intercept = 78.78; AICrandom intercept, random effect = 81.44). This suggested that site did not significantly explain variance in DF. For the fixed effects, only observer effects were significant (Figure 2; Table 6).
Table 6.
Fixed effects from the random intercept model with site–trial interaction (S0) explaining variation in individual DF response for individuals among sites
| Variable | Estimate | t | P |
|---|---|---|---|
| (Intercept) | 1.580 | 2.346 | 0.021 |
| Observer | |||
| A | 0.213 | 3.632 | 0.005 |
| B | 0.027 | 0.452 | 0.652 |
| Site | |||
| Medium | −0.027 | −0.266 | 0.791 |
| Low | −0.196 | −1.621 | 0.109 |
| logSD | −0.0128 | −0.161 | 0.873 |
| Trial | −0.044 | −1.263 | 0.210 |
| Trial: low | 0.017 | 0.286 | 0.776 |
| Trial: medium | −0.024 | −0.476 | 0.635 |
Significant effects are in bold.
Discussion
Different antipredator behaviors may be differentially influenced by human activity. We found that skinks tolerated the closest approach distance (lowest FID) at the low-disturbance site, but that that they did not differ in their approach distances between the medium- and high-disturbance sites or between the high- and low-disturbance sites. To summarize, skinks living in areas with limited human and domestic animal exposure allowed the observer to get closer to them, whereas those living in areas with humans, dogs, cats, and chickens fled at greater distances. These results are consistent with an underlying process of sensitization and this result was unexpected given prior results (McGowan et al. 2014). This pattern was repeated for DF. Skinks had the lowest DF at the low-disturbance site and the highest DF at the high-disturbance site with nonsignificant differences between low- and medium-disturbance sites and medium- and high-disturbance sites.
This pattern for DF is likely a consequence of each skink’s proximity to vegetation at the more natural sites, which they use as refuge. Although not formally quantified, there appeared to be more natural vegetation at the low-disturbance site than at either the high-disturbance site or medium-disturbance site. Thus, skinks fleeing to cover did not have to move as far to reach a refuge at the low-disturbance site. This pattern is consistent with prior findings, which found that the distance ungulates fled (Stankowich 2008) and the distance lizards fled (Cooper and Wilson 2007) increased with increasing distance from refuges. Animals also have greater FIDs when they are farther from a refuge (Cooper and Wilson 2007). Thus, the more “natural” habitat, rather than variation human disturbance, may explain the pattern of sensitization that our results showed for FID.
Sensitization within urban habitats may also increase with increased predation risk because antipredator behavior is costly and should only increase with predator pressure (Brock et al. 2015). Human presence triggers a stress response in many species, indicating that humans are perceived as a threat (Villanueva et al. 2011). In addition, humans frequently introduce novel predators to urban habitats through their domesticated pets, such as dogs and chickens. Lizards, mammals, and birds in urban areas experience high predation rates due to cats and other domesticated animals (Koenig et al. 2002; Loyd et al. 2013). Domesticated animals also have higher population densities around areas of high human occupation. Our skinks are known to be eaten by chickens, which roam freely near our high-disturbance site. The presence of these predators at high density may increase skink predator wariness and antipredator responses.
Individual-level variation in antipredator behavior also covaried with the human disturbance gradient and we found the lowest variance at the highest disturbance site, intermediate variance at the medium site, and the highest variance at the lowest disturbance site. We do not believe that this was because of substantial differences in habitat at the different sites; skinks were found in leaf litter near trees and shrubs at all sites. To summarize, skinks living closest to humans were less variable, whereas those living in more natural sites had the greatest variability in their response to experimental approaches. Individual variance across sites could not be evaluated for DF because the best model did not include site as a random effect; a finding that suggested that site explained no significant variation in DF.
The pattern of reduced variation in FID at the individual level in the high-disturbance site is consistent with natural selection, phenotypic sorting, or behavioral plasticity (Møller et al. 2015). Phenotypic sorting is the process by which individuals settle in different habitats based on their personal tolerance to each local environment (Edelaar et al. 2008). Sorting has been suggested to explain the higher abundance of bold over shy birds in urban environments (Clergeau et al. 2006; Croci et al. 2008). However, due to their small body size, skinks likely have low dispersal capability, making phenotypic sorting unlikely. This leaves behavioral plasticity or natural selection as the most likely explanations. Changes in selection pressure often cause changes in antipredator behavior (Cooper et al. 2015), and it is possible that individuals more adapted to human presence were more likely to survive and reproduce. This selection pressure would ultimately reduce phenotypic variation as behaviors less-suited to the urban environment were lost. Behavioral plasticity is the ability of an individual to change their behavior when faced with novel environmental challenges. Although our population may be individually flexible in their behavior over a human disturbance gradient, we did not explicitly measure this in our study.
Urbanization has been known to affect escape behavior in lizards, whereas the resulting behavioral changes are highly species specific, selection appears to be the most likely cause in most cases. Common garden skinks Lampropholis guichenoti had longer FIDs and sprint speeds in urban environments (consistent with sensitization), but similar DFs between both urban and natural sites (Prosser et al. 2006). Western fence lizards Sceloporus occidentalis on 2 separate college campuses had reduced antipredator responses compared with rural lizards around each site (Sparkman et al. 2018), which is a finding consistent with habituation. Several invasive lizard species in Southern California were found to have less variation in risk-taking behavior when compared with the native Western fence lizards Sceloporus occidentalis (Putman BR et al., manuscript under review), which may contribute to their success in urban habitats. Our own study found that skinks had lowest variation in FID at high-disturbance sites, supporting the idea that urban development acts as an ecological homogenizer (McKinney 2006), which decreases phenotypic difference in behavioral responses of animals adapting to life in human-populated habitats.
Behavioral plasticity may alter individual phenotypes and it is possible that skinks exposed to humans have changed their antipredator responses to best suit the new environment. This was found for the Indian rock agama Psammophilus dorsalis where urbanized males chose lower perches that were closer to refuges than rural males. Urban males also had lower average FIDs and less variation in FID than rural lizards, with plasticity and prior habituation as the likely explanation (Batabyal et al. 2017). We are thus unable to rule out either the possible effect of an individually plastic response to humans or natural selection, as mechanisms for explaining reduced variation. Indeed, both mechanisms could be at work.
There are consequences for the loss of variation in escape behavior within urban areas. One such consequence may be a possible loss of the genetic diversity underlying behavioral diversity (Hallsson and Björklund 2012; Smith and Blumstein 2013). This loss of both behavioral and genetic diversities could reduce a population’s resilience when faced with subsequent perturbations (Laikre 2010). If individual variation in behavioral plasticity is generally decreasing in urban areas, it could reduce species’ ability to evolve and persist in the Anthropocene. Thus, understanding the effects of urbanization on individual variation in escape behavior is important in order to better predict which individual characteristics will be more likely to persist in urban populations. Here, we studied individual variation on unmarked individuals and thus we could not quantify variation on a longer time scale, although prior work has shown that plasticity may vary over different timescales (Highcock and Carter 2014; Biro and Stamps 2015; Johnson et al. 2017). Future studies would benefit from studying plasticity of marked individuals over longer time scales (e.g., Highcock and Carter 2014).
Acknowledgments
Skinks were studied under University of California Los Angeles (UCLA) Animal Use Protocol 2000-147 (11 January 2018) and under permission of the Government of French Polynesia (permit approved on 9 November 2017). By design, playbacks led to only brief responses. Animals were neither captured nor marked as part of this study. We thank the station staff at the Richard B. Gump South Pacific Research Station for providing housing and accommodation; Andy Lin from the UCLA Institute for Digital Research and Education for statistical advice; and Bree Putman for comments on a previous draft. All data are available from the authors on request.
Funding
We thank the UCLA Office of Instructional Development and the UCLA Department of Ecology and Evolutionary Biology for partial support.
References
- Batabyal A, Balakrishna S, Thaker M, 2017. A multivariate approach to understanding shifts in escape strategies of urban lizards. Behav Ecol Sociobiol 71:83. [Google Scholar]
- Bates D, Maechler M, Bolker B, 2015. Fitting linear mixed-effects models using lme4. J Stat Software 67:1–48. [Google Scholar]
- Bejder L, Samuels A, Whitehead H, Finn H, Allen S, 2009. Impact assessment research: use and misuse of habituation, sensitisation and tolerance in describing wildlife responses to anthropogenic stimuli. Mar Ecol Prog Ser 395:177–185. [Google Scholar]
- Biro PA, Stamps JA, 2015. Using repeatability to study physiological and behavioural traits: ignore time-related change at your peril. Anim Behav 105:223–230. [Google Scholar]
- Blumstein DT, Anthony LL, Harcourt R, Ross G, 2003. Testing a key assumption of wildlife buffer zones: is flight initiation distance a species-specific trait? Biol Conserv 110:97–100. [Google Scholar]
- Blumstein DT, Runyan A, Seymour M, Nicodemus A, Ozgul A. et al. , 2004. Locomotor ability and wariness in yellow‐bellied marmots. Ethology 110:615 [Google Scholar]
- Blumstein DT, 2006. Developing an evolutionary ecology of fear: how life history and natural history traits affect disturbance tolerance in birds. Anim Behav 71:389–399. [Google Scholar]
- Blumstein DT, 2016. Habituation and sensitization: new thoughts about old ideas. Anim Behav 120:255–262. [Google Scholar]
- Bolker B, 2013. Factor-Specific Variances in R http://rpubs.com/bbolker/6298 (21 May 2019, date last accessed).
- Brock KM, Bednekoff PA, Pafilis P, Foufopoulos J, 2015. Evolution of antipredator behavior in an island lizard species, Podarcis erhardii (Reptilia: lacertidae): the sum of all fears? Evolution 69:216–231. [DOI] [PubMed] [Google Scholar]
- Cavalli M, Baladrón AV, Isacch JP, Biondi LM, Bó MS, 2016. Differential risk perception of rural and urban Burrowing Owls exposed to humans and dogs. Behav Processes 124:60–65. [DOI] [PubMed] [Google Scholar]
- Carrete M, Tella JL, 2010. Individual consistency in flight initiation distances in burrowing owls: a new hypothesis on disturbance-induced habitat selection. Biol Lett 6:167–170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clergeau P, Croci S, Jokimäki J, Kaisanlahti-Jokimäki M-L, Dinetti M, 2006. Avifauna homogenisation by urbanisation: analysis at different European latitudes. Biol Conserv 127:336–344. [Google Scholar]
- Cooper WE, Jr, 2010. Economic escape In: Breed MD, Moore J, editors. Encyclopedia of Animal Behavior. Oxford: Academic Press; 588–595. [Google Scholar]
- Cooper WE Jr, Blumstein DT, 2014. Novel effects of monitoring predators on costs of fleeing and not fleeing explain flushing early in economic escape theory. Behav Ecol 25:44–52. [Google Scholar]
- Cooper WE Jr, Blumstein DT, 2015. Escape behavior: importance, scope, and variables In: Cooper WE Jr, Blumstein DT, editors. Escaping from Predators: An Integrative View of Escape Decisions. Cambridge: Cambridge University Press; 18–37. [Google Scholar]
- Cooper WE Jr, Blumstein DT, Samia DSM, Stankowich T, 2015. Best practice for the study of escape behavior In: Cooper WE Jr, Blumstein DT, editors. Escaping from Predators: An Integrative View of Escape Decisions. Cambridge: Cambridge University Press, 407–419. [Google Scholar]
- Cooper WE Jr, Wilson DS, 2007. Beyond optimal escape theory: microhabitats as well as predation risk affect escape and refuge use by the phrynosomatid lizard Sceloporus virgatus. Behaviour 144:1235–1254. [Google Scholar]
- Croci S, Butet A, Clergeau P, 2008. Does urbanization filter birds on the basis of their biological traits. Condor 110:223–240. [Google Scholar]
- Ditchkoff SS, Saalfeld ST, Gibson CJ, 2006. Animal behavior in urban ecosystems: modifications due to human-induced stress. Urban Ecosyst 9:5–12. [Google Scholar]
- Edelaar P, Siepielski AM, Clobert J, 2008. Matching habitat choice causes directed gene flow: a neglected dimension in evolution and ecology. Evolution 62:2462–2472. [DOI] [PubMed] [Google Scholar]
- Frid A, Dill L, 2002. Human-caused disturbance stimuli as a form of predation risk. Conserv Ecol 6:11. [Google Scholar]
- Garamszegi LZ, Møller AP, 2017. Partitioning within-species variance in behaviour to within and between-population components for understanding evolution. Ecol Lett 20:599–608. [DOI] [PubMed] [Google Scholar]
- Geffroy B, Samia DS, Bessa E, Blumstein DT, 2015. How nature-based tourism might increase prey vulnerability to predators. Trends Ecol Evol 30:755–765. [DOI] [PubMed] [Google Scholar]
- Guay PJ, Lorenz RD, Robinson RW, Symonds MR, Weston MA, 2013. Distance from water, sex and approach direction influence flight distances among habituated black swans. Ethology 119:552–558. [Google Scholar]
- Hallsson LR, Björklund M, 2012. Selection in a fluctuating environment leads to decreased genetic variation and facilitates the evolution of phenotypic plasticity. J Evol Biol 25:1275–1290. [DOI] [PubMed] [Google Scholar]
- Highcock L, Carter AJ, 2014. Intraindividual variability of boldness is repeatable across contexts in a wild lizard. PLoS ONE 9:e95179.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson GC, Karajah MT, Mayo K, Armenta TC, Blumstein DT, 2017. The bigger they are the better they taste: size predicts predation risk and anti-predator behavior in giant clams. J Zool 301:102–107. [Google Scholar]
- Kaiser A, Merckx T, Van Dyck H, 2018. Urbanisation and sex affect the consistency of butterfly personality across metamorphosis. Behav Ecol Sociobiol 72:188. [Google Scholar]
- Koenig J, Shine R, Shea G, 2002. The dangers of life in the city: patterns of activity, injury and mortality in suburban lizards Tiliqua scinoides. J Herpetol 36:62–68. [Google Scholar]
- Kuznetsova A, Brockhoff PB, Christensen RHB, 2017. LmerTest package: tests in linear mixed effects models. J Stat Software 82:1–26. [Google Scholar]
- Laikre L, 2010. Genetic diversity is overlooked in international conservation policy implementation. Conserv Genet 11:349–354. [Google Scholar]
- Lapiedra O, Chejanovski Z, Kolbe JJ, 2017. Urbanization and biological invasion shape animal personalities. Glob Change Biol 23:592–603. [DOI] [PubMed] [Google Scholar]
- Loyd KAT, Hernandez SM, Carroll JP, Abernathy KJ, Marshall GJ, 2013. Quantifying free-roaming domestic cat predation using animal-borne video cameras. Biol Conserv 160:183–189. [Google Scholar]
- Martínez-Abraín A, Oro D, Conesa D, Jiménez J, 2008. Compromise between seabird enjoyment and disturbance: the role of observed and observers. Environ Conserv 35:104–108. [Google Scholar]
- McCleery RA, 2009. Changes in fox squirrel anti-predator behaviors across the urban-rural gradient. Landsc Ecol 24:483. [Google Scholar]
- McGowan MM, Patel PD, Stroh JD, Blumstein DT, 2014. The effect of human presence and human activity on risk assessment and flight initiation distance in skinks. Ethology 120:1081–1089. [Google Scholar]
- McKinney ML, 2006. Urbanization as a major cause of biotic homogenization. Biol Conserv 127:247–260. [Google Scholar]
- Miranda AC, 2017. Mechanisms of behavioural change in urban animals: the role of microevolution and phenotypic plasticity In: Murgui E, Hedblom M, editors. Ecology and Conservation of Birds in Urban Environments. Cham: Springer; 113–132. [Google Scholar]
- Møller AP, 2010. Interspecific variation in fear responses predicts urbanization in birds. Behav Ecol 21:365–371. [Google Scholar]
- Møller AP, Garamszegi LZ, 2012. Between individual variation in risk-taking behavior and its life history consequences. Behav Ecol 23:843–853. [Google Scholar]
- Møller AP, Grim T, Ibáñez-Álamo JD, Markó G, Tryjanowski P, 2013. Change in flight initiation distance between urban and rural habitats following a cold winter. Behav Ecol 24:1211–1217. [Google Scholar]
- Møller AP, Tryjanowski P, Díaz M, Kwieciński Z, Indykiewicz P. et al. , 2015. Urban habitats and feeders both contribute to flight initiation distance reduction in birds. Behav Ecol 26:861–865. [Google Scholar]
- Øverli Ø, Sørensen C, Pulman KGT, Pottinger TG, Korzan W. et al. , 2007. Evolutionary background for stress-coping styles: relationships between physiological, behavioral, and cognitive traits in non-mammalian vertebrates. Neurosci Biobehav Rev 31:396–412. [DOI] [PubMed] [Google Scholar]
- Pinheiro J, Bates D, DebRoy S, Sarkar D, R Core Team, 2019. nlme: Linear and Nonlinear Mixed Effects Models. R package version 3.1–140, https://CRAN.R-project.org/package=nlme (21 May 2019, date last accessed).
- Price MV, Strombom EH, Blumstein DT, 2014. Human activity affects the perception of risk by mule deer. Curr Zool 60:693–699. [Google Scholar]
- Prosser C, Hudson S, Thompson MB, 2006. Effects of urbanization on behavior, performance, and morphology of the garden skink Lampropholis guichenoti. J Herpetol 40:151–159. [Google Scholar]
- Rankin CH, Abrams T, Barry RJ, Bhatnagar S, Clayton DF. et al. , 2009. Habituation revisited: an updated and revised description of the behavioral characteristics of habituation. Neurobiol Learn Mem 92:135–138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- R Core Team, 2017. R: A Language and Environment for Statistical Computing https://www.R-project.org/ (21 May 2019, date last accessed).
- Rodda GH, Perry GAD, Rondeau RJ, Lazell J, 2001. The densest terrestrial vertebrate. J Trop Ecol 17:331–338. [Google Scholar]
- Rodriguez-Prieto I, Fernández-Juricic E, Martín J, Regis Y, 2008. Antipredator behavior in blackbirds: habituation complements risk allocation. Behav Ecol 20:371–377. [Google Scholar]
- Rodríguez-Prieto I, Martín J, Fernández-Juricic E, 2010. Individual variation in behavioral plasticity: direct and indirect effects of boldness, exploration and sociability on habituation to predators in lizards. Proc R Soc B 278:266–273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samia DSM, Nakagawa S, Nomura F, Rangel TF, Blumstein DT, 2015. Increased tolerance to humans among disturbed wildlife. Nat Commun 6:8877.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith BT, Blumstein DT, 2013. Animal personalities and conservation biology: the importance of behavioral diversity In: Carere C, Maestripieri D, editors. Animal Personalities: Behavior, Physiology, and Evolution. Chicago (IL: ): University of Chicago Press; 379–411. [Google Scholar]
- Sol D, Lapiedra O, González-Lagos C, 2013. Behavioural adjustments for a life in the city. Anim Behav 85:1101–1112. [Google Scholar]
- Sparkman A, Howe S, Hynes S, Hobbs B, Handal K, 2018. Parallel behavioral and morphological divergence in fence lizards on two college campuses. PLoS ONE 13:e0191800.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stankowich T, 2008. Ungulate flight responses to human disturbance: a review and meta-analysis. Biol Conserv 141:2159–2173. [Google Scholar]
- Villanueva C, Walker BG, Bertellotti M, 2011. A matter of history: effects of tourism on physiology, behavior and breeding parameters in Magellanic penguins Spheniscus magellanicus at two colonies in Argentina. J Onithol 153:219–228. [Google Scholar]
- Wickham H, 2009. Ggplot2: Elegant Graphics for Data Analysis. Cham: Springer. [Google Scholar]
- Ydenberg RC, Dill LM, 1986. The economics of fleeing from predators. Adv Study Behav 16:229–249. [Google Scholar]
- Zuur A, Ieno EN, Walker N, Saveliev AA, Smith GM, 2009. Mixed Effects Models and Extensions in Ecology with R. Cham: Springer. [Google Scholar]