Abstract
Despite it is widely accepted that intrapopulation variation is fundamental to ecological and evolutionary processes, this level of information has only recently been included into network analysis of species/population interactions. When done, it has revealed non-random patterns in the distribution of trophic resources. Nestedness in resource use among individuals is the most recurrent observed pattern, often accompanied by an absence of modularity, but no previous studies examine bipartite modularity. We use network analysis to describe the diet composition of the Balearic endemic lizard Podarcis lilfordi in 2 islets at population and individual levels, based on the occurrence of food items in fecal samples. Our objectives are to 1) compare niche structure at both levels, 2) characterize niche partition using nestedness and modularity, and 3) assess how size, sex, season, and spatial location influence niche structure. At population-level niche width was wide, but narrow at the level of the individual. Both islet networks were nested, indicating similar ranking of the food preferences among individuals, but also modular, which was partially explained by seasonality. Sex and body size did not notably affect diet composition. Large niche overlap and therefore possibly relaxed competition were observed among females in one of the islets and during spring on both islets. Likewise, higher modularity in autumn suggests that higher competition could lead to specialization in both populations, because resources are usually scarce in this season. The absence of spatial location influence on niche might respond to fine-grained spatio-temporally distribution of food resources. Behavioral traits, not included in this study, could also influence resource partitioning.
Keywords: Balearic Islands, individual diet composition, individual-level network, modularity, nestedness, population niche width
Most work on ecological networks involves 2-mode species-based networks, where nodes represent 2 interacting communities, for instance, species of plants and pollinators or hosts and parasitoids (Verhoef and Morin 2010; Pires et al. 2011; Tylianakis and Morris 2017). However, each species node is aggregated, representing a population of individuals and, whereas species, principally at least, do not interact, their individuals do. Likewise, many population models assume that individuals of a particular species are identical, but in fact individuals do differ (Bolnick et al. 2003; Dall et al. 2012). Despite the fact that individual variation within natural populations is one of the pillars of Darwinism (Bolnick et al. 2003, 2011; Svanbäck and Bolnick 2005; Dall et al. 2012; Sih et al. 2012; Wolf and Weissing 2012) and that the importance of downscaling ecological networks from species to individuals has been repeatedly stressed (Ings et al. 2009; Olesen et al. 2010; Gómez et al. 2011; Tur et al. 2014; Pettorelli et al. 2015; Moran et al. 2017; Zwolak 2018), the number of network studies at that organizational level has only recently increased significantly (e.g., Woodward and Warren 2007; Araújo et al. 2008, 2010; Tinker et al. 2012; Tur et al. 2014; Kernaléguen et al. 2016; Fernandes da Cunha et al. 2018). An individual-resource network is a bipartite network consisting of 2 types of node, one representing the individuals of a population and the other representing resources (Pires et al. 2011; Tinker et al. 2012).
This growth has improved the characterization of intrapopulation patterns of resource use, which is essential to understand how different hierarchical levels affect each other (Araújo et al. 2010; Melián et al. 2011). In particular, it has demonstrated a strong nonrandom use of trophic resources among individuals. The most recurrent pattern found is nestedness (e.g., Araújo et al. 2010; Kernaléguen et al. 2016; Fernandes da Cunha et al. 2018), which reveals that more specialized individuals use subsets of those resources that more generalized individuals use (Patterson and Atmar 1986; Bascompte et al. 2003; Araújo et al. 2008). The second pattern, less frequently found, is clustering or modularity (e.g., Araújo et al. 2008; Tinker et al. 2012; Lemos-Costa et al. 2016). Clusters or modules are network subsets more densely connected than expected if interaction among nodes was random, which translated to individual-resource networks means a presence of groups tending to use the same subset of resources (Olesen et al. 2007; Araújo et al. 2008). Some modularity indices allow considering the bipartite nature of interactions (Dormann and Strauss 2014). As far as we know, no previous studies have used bipartite modularity to analyze the niche partition of individual-resource networks.
The population resource niche width is the range of resources used by all individuals in the population; whereas the individual resource niche width has to be a subset of the population resource niche width (Roughgarden 1972). Variation in individual niche width can be driven by the sex, age, shape, size, social status, and behavior of the individuals (Schoener 1971; Bolnick et al. 2003, 2007, 2011). Such variation can be tested against the ideal free distribution theory (Fretwell and Lucas 1969). This theory predicts that individuals self-distribute on food resources to maximize their individual energy input when search and handling times (capture of resource, consumption, and digestion) are included (Schoener 1971; Bolnick et al. 2003; Svanbäck and Bolnick 2005, 2007; Araújo et al. 2011; Pires et al. 2011; Tinker et al. 2012). According to this theory and at a given time and space, the phenotypic traits of an individual constitute a complex factor that determines its niche width, but diversity of available resources (Tinker et al. 2012; Araújo et al. 2011; Svanbäck et al. 2011) and intra- and interspecific competition may be also important determining factors (Svanbäck and Bolnick 2005, 2007; Bolnick et al. 2011; Tinker et al. 2012).
This study focused on the diet of the Balearic lizard Podarcis lilfordi (Lacertidae) on 2 islets off the southern coast of Mallorca Island (Balearic archipelago, Spain), namely Na Moltona (NM) and Na Guardis (NG). Diet data are produced from analysis of fecal samples collected in different seasons and years (spring, summer, and autumn, 2011–2013). According to literature, P. lilfordi is expected to feed on invertebrates, plants, and conspecific eggs (Pérez-Mellado and Corti 1993; Traveset and Sáez 1996; Pérez-Mellado and Traveset 1999; Pérez-Cembranos et al. 2016). Niche structure was described at individual level. In addition, data on size and gender were collected. The resource niche of a lizard individual was defined qualitatively as number of different food items in its diet (degree). Since the resource niche of the entire population is equivalent to the pooled number of different links of all its individuals, together, 2 extremes of a continuum are possible 1) individual and population niche are equal or 2) the niches of the individuals has minimum overlap. Thus, the shape of the actual population niche becomes the frequency distribution of the individual niche widths. Specifically, our objectives are to 1) compare the niche structure at population and individual levels, 2) characterize niche partition by means of the network indices modularity and nestedness, and 3) assess the influence of body size, gender, season, and spatial location on niche structure.
Material and Methods
Species and study area
Podarcis lilfordi is an endemic lizard from the Balearic Islands (Western Mediterranean Sea), with more than 24 described subspecies, that is categorized as endangered at the national level and vulnerable at the global and regional levels (Viada 2006, p. 281). It is locally extinct on the 2 largest islands, Mallorca and Menorca, due to human introduction of vertebrate predators ∼2,000 years ago (Pérez-Mellado 2002). Currently, it is found on islets around Mallorca and Menorca and on the Cabrera Archipelago, southern Mallorca. During the last century, it seems to have disappeared from 4 of these islets (Mayol 2004).
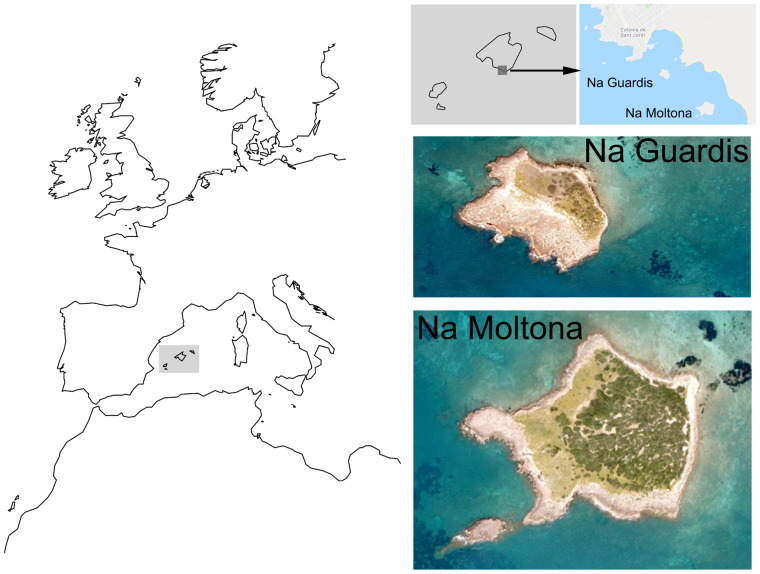
This study was conducted on 2 islets near southern Mallorca (Figure 1): NM (∼39°18′N, 3°00′E) and NG (∼39°18′N, 3°00′E). Half of NM islet (5.09 ha) is covered by shrubs, mostly Pistacia lentiscus and Phillyrea spp., about 10% by halophytic species, such as Salicornia ramosissima and the rest is bare rock and small pools (Ruiz de Infante Anton et al. 2013). NG is a 1.98 ha rocky coastline islet with a lower plant abundance and diversity than NM. More than half of the islet is covered by shrubs, about 30% by halophytic species in low densities and about 20% by herbaceous plants such as Crithmum maritimum (Ruiz de Infante Anton et al. 2013).
Figure 1.
Study areas: NG and NM islets, located on the southern coast of the Mallorca island (Balearic archipelago, Spain).
Data collection
Lizard faces were collected during 2 spring seasons (5–15 April 2011 and 9–17 April 2013), 2 autumn seasons (7–10 October 2011 and 11–22 October 2012), and during 1-day visits in June 2011. Sampling was always carried out during optimal conditions for lizard activity, that is, mainly sun mild or no wind, and temperatures between 18°C and 29°C. Lizards were caught in pitfall traps placed within or next to the vegetation (47 traps in NM and 25 traps in NG). The snout–vent length (millimeters) and weight (grams) of each lizard was measured and the photograph of each individual was taken for later identification (Moya et al. 2015). The gender of all individuals was determined in the field according to the presence or absence of developed femoral pores or, in a few cases, by counting the number of ventral scales when preprocessing individual images (Rotger et al. 2016).
Dietary analysis
To have a reference collection for the identification of seeds found in lizard faces we carried out a plant inventory on both islets. The content of each fecal sample was examined under a stereoscope and identified to the lowest possible taxonomic level. Since many of the food items were only fragments, we could not quantify consumed prey species, restricting us to qualitative data, that is, occurrence of prey in a dropping.
Data analysis
For each islet, we used these occurrence data to build adjacency qualitative matrices of occurrences of trophic interactions between lizard individuals and food items. We used R 3.4.3. (R Development Core Team 2017) to quantify nestedness (nestednodf function in “vegan 2.4-4”; Oksanen et al. 2015) at population level and modularity (computeModules function of “bipartite 2.08”, Dormann et al. 2008). This was done at both population level and for each season within each population. We used a metric of nestedness based on overlap and decreasing fill (hereafter NODF; Almeida-Neto et al. 2008) because it is less sensitive to species richness than other nestedness metrics (Almeida-Neto et al. 2008). NODF ranges from 0 (minimum nestedness) to 100 (maximum). To estimate modularity Q, we used the Beckett’s algorithm (Beckett 2016), which assumes the interactions to be bipartite; Q ranges from 0 (minimum) to 1 (maximum). To test if observed NODF and Q-values deviated significantly from our null models, we compared them against a distribution of 10,000 null model NODF values and 100 null model Q-values, respectively. We used 3 null models varying in their level of structure: Null models 1 and 2 of Bascompte et al. (2003) and Null model 3 termed quasiswap (Miklós and Podani 2004). In Null model 1, every column and row are equiprobable and, therefore, only the total number of interactions is conserved. In Null model 2, the probability of occupancy of each cell in the adjacency matrix is the average of the probabilities of occupancy of its row and column (i.e., the total number of interactions of the involved pair of nodes; Bascompte et al. 2003). Null model 3 is a so called nonsequential algorithm for binary matrices that preserves the exact row and column sums, and thus also connectance. Null models 2 and 3 are conservative for nestedness and modularity (Fortuna et al. 2010). Null model 2 might exhibit high Type II error when detecting significant modularity (Fortuna et al. 2010), and Null model 3 exhibits high Type II error when detecting significant nestedness (Ulrich and Gotelli 2007; Blüthgen et al. 2008; Fortuna et al. 2010). In both populations, we used z-scores based on Null model 3 to compare the modularity between autumn and spring networks.
We assessed the relationships between individual specialization (degree) and 3 variables related to lizard size: length, weight, and body condition index (BCI, the residuals of the regression of lizard length against weight), accounting for the factor islet (NM or NG), and the factors: 1) sex (females, males, and juveniles) or 2) season (autumn, spring, and summer) by means of 2-way generalized linear models (GLMs in R 3.4.3.; R Development Core Team 2017). We used a Poisson error distribution and log link function. To test for differences in diet composition among lizard individuals with different traits (body size and sex) and season, we performed a redundancy analysis (RDA; Rao 1964) in R 3.4.3. with the rda function of vegan 2.4-4 package (Oksanen et al. 2015). This function allows summarizing linear relationships between 1) components of the distribution of presence–absence adjacency matrix of individual-resource interactions and 2) 4 explanatory variables: sex, season, length, and weight. We tested the ordination significance by means of 999 permutations based on a reduced model.
For each islet, possible relations between spatial and 1) topological or 2) niche distances were tested by means of 2 Mantel tests based on Pearson’s correlation and 9,999 permutations. To do that, we used geographic coordinates of traps to makes a spatial distance matrix, the shortest path lengths among lizard individuals in the network to make a topological distance matrix, and Jaccard similarity indices among lizard individuals to make a niche distance matrix.
Finally for each islet, we compared intra- versus intergroup Jaccard Similarity values with respect to sex and season by means of Mann–Whitney–Wilcoxon tests using the wilcox.test function of the “stats 3.4.3.” package of R 3.4.3.
Results
Population structure
A total of 129 fecal samples were collected in NM (81 in autumn, 42 in spring, and 6 in summer, Table 1 in Online Appendix I) and 62 in NG (23 in autumn, 27 in spring, and 12 in summer, Table 1 in Online Appendix I). Overall, 97 lizard individuals (41 females, 49 males, and 7 juveniles, Table 2 in Online Appendix I) could be sex identified in NM (corresponding to 104 fecal samples) and 53 lizard individuals (21 females, 28 males, and 4 juveniles, Table 2 in Online Appendix I) in NG (61 fecal samples). Both populations were strongly male-biased and had the same proportion of juveniles. Six lizards in NM and 5 in NG were captured between 2 and 4 times. Seven of the recaptured lizards were found in more than 1 season and 6 were found in more than 1 year. Each sample from recaptured lizards contained between 1 and 2 food items. Only the millipede Polydesmus sp. 1 was found in more than 1 fecal sample from the same individual. A total of 24 of the 27 seeds found in fecal samples in NM and 14 of the 16 seeds found in NG could be identified.
Table 1.
Chi-squared (Chi2) and significance (P) of 2-way GLM analyzing the relationship between BCI and specialization (degree) of lizards considering the factors islet (NM and NG) and sex (females, juveniles, and males) (N = 131)
| Effect | Chi2 | P |
|---|---|---|
| BCI | 0.932 | 0.334 |
| Sex | 1.632 | 0.442 |
| Islet | 1.758 | 0.185 |
| BCI × Sex | 0.025 | 0.987 |
| BCI × Islet | 0.240 | 0.624 |
| Sex × Islet | 4.771 | 0.092 |
| BCI × Sex × Islet | 0.686 | 0.710 |
Table 2.
Chi-squared (Chi2) and significance (P) of 2-way GLM analyzing the relationship between BCI and specialization (degree) of lizards considering the factors islet (NM and NG) and season (individuals captured in autumn, spring, and summer) (N = 124)
| Effect | Chi2 | P |
|---|---|---|
| BCI | 0.360 | 0.549 |
| Season | 5.169 | 0.075 |
| Islet | 0.079 | 0.779 |
| BCI × Season | 1.084 | 0.582 |
| BCI × Islet | 0.093 | 0.760 |
| Season × Islet | 1.504 | 0.471 |
| BCI × Season × Islet | 0.511 | 0.774 |
Individual specialization and diet composition
Most fecal samples contained few items (1–3) whereas a few contained more than 5 (Figure 1 in Online Appendix I). In our analyses of the fecal samples, we identified 43 invertebrate morphospecies (3 Arachnida, 2 millipede [Diplopoda], 35 Insecta, and 3 Mollusca; Table 3 in Online Appendix I) and 3 seed morphospecies (Rubia peregrina and 2 unidentified; Table 3 in Online Appendix I). Diet composition (Figure 2 in Online Appendix I) and frequency of occurrence (Table 4 in Online Appendix I) of food items were similar in the 2 islets. In both populations, the most frequent prey order was Coleoptera (28.7% on NM and 31.3% on NG), which was found in 37.6% and 61.5% of lizard individuals in NM and NG, respectively. The most frequent family of Coleoptera was the weevils (Curculionidae; 62.5% and 56.1%, respectively; Table 4 in Online Appendix I), but on both islets the number of items found varied seasonally. Feces of NM individuals contained most weevils in autumn, whereas those from NG did so in spring (Table 4 in Online Appendix I). In both islets, the second most frequent order was Hymenoptera (22.3% of the diet in NM and 22.1% in NG and 48.4% of the NM individuals and 46.2% of the NG individuals). The family most frequently found in this order was ants (Formicidae; 89.3% and 86.7%, respectively). The ranking of the remaining orders varied slightly between the 2 populations. The content of seeds was similar in both islets (4.4% of the diet in NM and 5.3% in NG and 12.9% of NM individuals and 13.5% of NG individuals). At NM, seeds were found in faces only in autumn and spring, being scarcer in spring than in autumn, and at NG, only in spring and summer, being very scarce in both seasons.
Table 3.
Niche overlap among lizard individuals calculated as mean Jaccard’s interaction dissimilarity (mean) and standard deviation (SD) of pairs of lizard individuals belonging to the same or different groups of sex and season in each islet
| Lizard group | Intragroup |
Intergroup |
Mann–Whitney–Wilcoxon test |
|||
|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | U | P | |
| NM | ||||||
| Total | 0.926 | 0.171 | – | – | – | – |
| Females | 0.937 | 0.151 | 0.924 | 0.174 | 1,335,900 | 0.026 |
| Males | 0.924 | 0.175 | 0.924 | 0.174 | 2,535,300 | 0.996 |
| Juveniles | 0.958 | 0.092 | 0.926 | 0.177 | 12,171 | 0.571 |
| Autumn | 0.860 | 0.242 | 0.960 | 0.117 | 3,137,700 | <0.001 |
| Spring | 0.936 | 0.137 | 0.960 | 0.117 | 1,476,100 | <0.001 |
| Summer | 0.882 | 0.155 | 0.964 | 0.089 | 5,798 | <0.001 |
| NG | ||||||
| Total | 0.941 | 0.147 | – | – | ||
| Females | 0.936 | 0.174 | 0.948 | 0.141 | 134,570 | 0.503 |
| Males | 0.939 | 0.136 | 0.944 | 0.144 | 264,450 | 0.062 |
| Juveniles | 0.917 | 0.129 | 0.922 | 0.151 | 466 | 0.766 |
| Autumn | 0.855 | 0.274 | 0.962 | 0.127 | 86,201 | <0.001 |
| Spring | 0.952 | 0.111 | 0.967 | 0.109 | 119,040 | <0.001 |
| Summer | 0.861 | 0.160 | 0.955 | 0.117 | 39,303 | <0.001 |
Statistics U and significance (P) of U-Mann–Whitney–Wilcoxon tests comparing intra- and intergroup niche overlap are shown.
Figure 2.
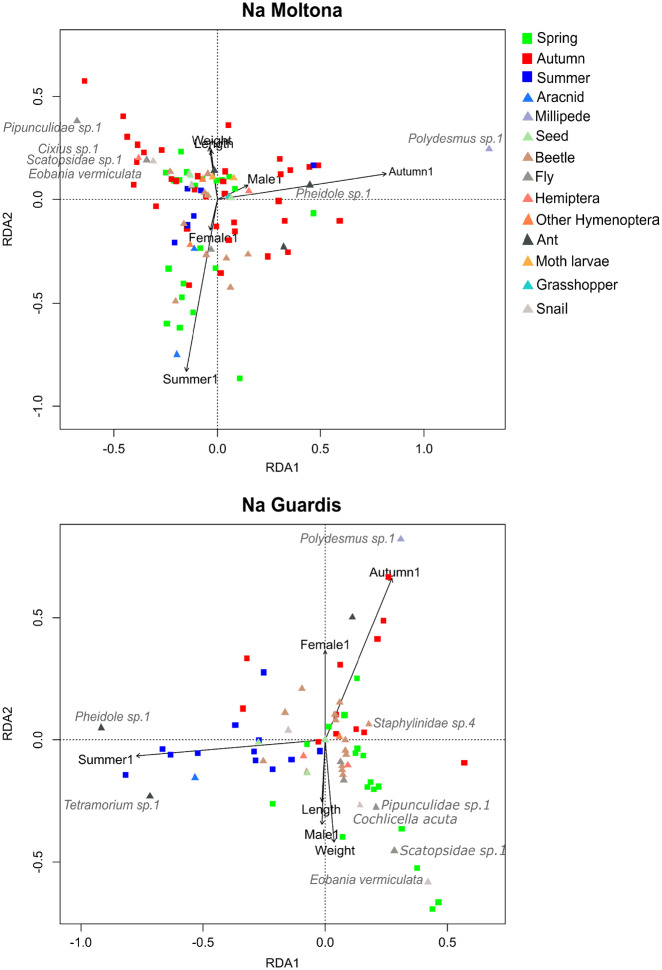
Ordination of the first and second constrained axes of RDA of the relationships between diet composition of lizard individuals and 4 explanatory variables (arrows): sex (female, juvenile, and male), season (autumn, spring, and summer), body length and body weight in the islets NM (left), and NG (right). Symbols indicate lizard individuals (squares) and food items (triangles). Colors of squares indicate the season in which the lizard was captured and colors of the triangles indicate the type of food item (see legend). Only Axis 1 and the explanatory variable season significantly influenced the ordination.
Network structure
At both sites, networks were moderately nested, but significantly higher than predicted from Null models 1 and 2 (Table 5 in Online Appendix I; NODFNM = 10.77; NODFNG = 10.22), although significance disappeared when compared with Null model 3. Modularity values (NM: Q = 0.55; NG: Q = 0.58) were only significantly higher than predicted from Null model 3 (Table 5 in Online Appendix I). Modularity z-scores were higher in autumn (NM: 1.92; NG: 3.65) than in spring (NM: 1.16; NG: −0.72).
Lizard traits versus specialization and diet composition
We did not detect any significant relationship between individual specialization (degree) and length, weight, and BCI (only BCI results are shown, Tables 1 and 2). At NM, the RDA was significant (F6, 79 = 1.8, P = 0.001) and extracted 1 significant axis that explained 54.8% of the constrained variance and 7.1% of the total variance (F1, 79 = 5.8, P = 0.001). Only the predictor variable “season” significantly influenced the ordination, separating lizard individuals in autumn from those in spring. The autumn food items that mostly influenced the ordination were the millipede “Polydesmus sp. 1” and the ant “Pheidole sp. 1”, whereas 2 Diptera “Pipunculidae sp. 1” and “Scatopsidae sp. 1”, and the Hemiptera “Cixius sp. 1” were mainly found in lizards from spring (Figure 2). At NG, the RDA was also significant (F6,45 = 1.5, P = 0.002) and extracted 1 significant axis that explained 34.3% of the constrained variance and 6.6% of the total variance (F1,45 = 3.1; P = 0.016). In this islet, season was also the only predictor that significantly influenced the ordination, separating lizard individuals from autumn, spring, and summer. The food items that mostly influenced the ordination in this case were the millipede “Polydesmus sp. 1” and the Coleoptera “Staphylinidae sp. 4” in autumn; the 2 snails Eobania vermiculata and Cochlicella acuta and the Diptera “Scatopsidae sp. 1” and “Pipunculidae sp. 1” in spring; and the 2 ants “Tetramorium sp. 1”; and “Pheidole sp. 1” in summer (Figure 2).
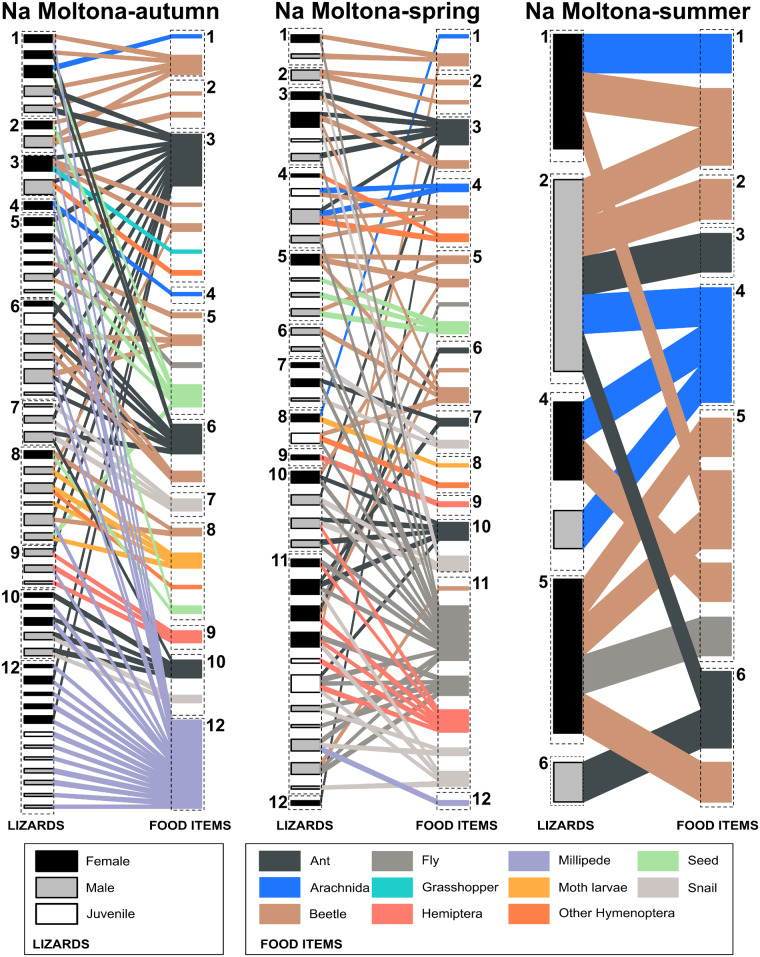
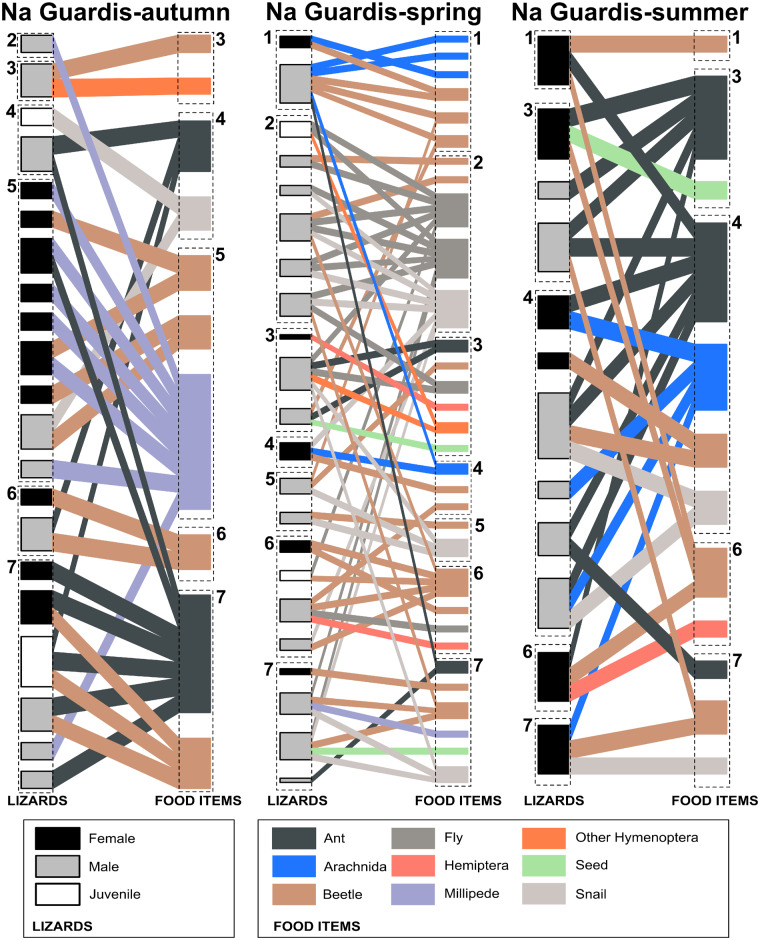
The composition of modules in both networks was consistent with the RDA (Figures 3 and 4). In both networks, Diptera species mainly occur in modules dominated by lizards from the spring (Module 11 in NM, Module 3 in NG, Figures 3 and 4) whereas millipede species mainly occurred in modules dominated by lizards from autumn (Module 12 in NM and Module 5 in NG, Figures 3 and 4). As shown by both module composition and RDA ordination, the consumption of more than 1 food item (flies, ants, and seeds), which may require more handling experience by an individual, was rare. This was especially true for NM males and NG females. Geographic distance was not significantly related to either topological (NM: R = −0.002, P = 0.51; NG: R = 0.036, P = 0.24) or niche distance (NM: R = 0.009, P = 0.36; NG: R = −0.006, P = 0.56).
Figure 3.
Network module composition of the interactions between lizard individuals (left boxes) and food items (right boxes) of NM separated in 3 seasonal subnetworks. The width of the boxes is proportional to the percentage of interactions in the seasonal subnetwork. Colors of left boxes indicate lizard sex (see legend). Colors of right boxes indicate the food item group (see legend). Numbers and dashed line boxes refer to modules in the 3-season-compiled network. Note that not all modules have species in all seasons.
Figure 4.
Network modules composition of the interactions between lizard individuals (left boxes) and food items (right boxes) of NG separated in 3 seasonal subnetworks. See Figure 3 for details.
Niche overlap among individual lizards
Niche overlap of lizards at NM was significantly higher among females than between females and males and juveniles (Table 3). Such differences were not detected at NG. At both sites, niche overlap among individuals was significantly lower within the same season than among seasons, and, niche overlap was significantly higher among spring lizards than summer and autumn lizards (Table 3).
Discussion
The broad food niche of P. lilfordi at population level contrasted with the low individual food niche (specialization, degree) in both populations considered. Such a high intrapopulation variation suggests that the lizards respond plastically to variation in food availability. The network analysis suggested the same. The significant nestedness pattern, albeit low, gives a deeper insight into the nature of the individual diets. A few lizards had a relatively wide diet whereas most lizards had a diet that was a subset of that of the generalists. The absent or low modularity supports this conclusion, that is, the few generalists destroy any modularity pattern. The diet seems not be driven by variation in body size or sex but partially reflects the seasonality of food. This effect of season was removed by analyzing seasonal networks. Now a stronger modularity was observed, especially at NG in the autumn. At this level, diet differences were also observed between the 2 sexes. The variation between populations and seasons reflects the observed variation in food availability. For example, higher modularity in autumn than in spring indicates lower specialization in spring, in which availability of resources is usually higher. The absence of any relationship between geographic and network topological and niche distances of individuals in any of the populations might be driven by fine-grained spatio-temporally patchy distribution of food resources.
The diet was highly diverse including 40 morphotaxa of arthropods, 3 species of molluscs and seeds from 3 plant species. The dominance of weevils (Cuculionidae) and ants, and the low seed consumption in spring at NM is in accordance with Pérez-Mellado (1989), Pérez-Mellado and Corti (1993) and Pérez-Cembranos et al. (2016). Pérez-Mellado and Corti (1993) have suggested that omnivorous (i.e., both plant and animal diet) lizards like P. lilfordi eat more plants and ants when other arthropods are scarce because immobile and clumped prey/plants increase net energy intake. We found a lower frequency and fewer types of plant structures in feces of P. lilfordi than previous studies and certainly lower than the expected for island populations (Pérez-Mellado and Corti 1993; Brown and Pérez-Mellado 1994; Pérez-Cembranos et al. 2016). In addition, the expected summer increase in plant consumption (Pérez-Mellado and Corti 1993) was not observed. Nevertheless, we cannot discard the possibility that some plant material could be underestimated, for example, food like nectar and pollen (Valido and Olesen 2019; see Study caveats). We only observed an increase in ant consumption in the summer at NG (Figure 3). This complex pattern is consistent with the high spatio-temporal (both intra- and interannual) variation in the diet of P. lilfordi recently reported (Pérez-Cembranos et al. 2016).
To achieve this high food generalization at population level, P. lilfordi and other arthropod-consuming lacertids forage on a suboptimal diet of low profitable but clumped preys (ants, Pérez-Mellado and Corti 1993; Carretero 2004), flying preys (flies, Pérez-Mellado and Corti 1993; Brown and Pérez-Mellado 1994), and plants (Pérez-Mellado and Corti 1993, and our study). The contrast between the high generalization found at population level and the low level among individuals shows that the former encompasses a niche differentiation at an individual level, although some nestedness was observed in our study. Such contrast has been frequently documented for vertebrate and invertebrate diets (Van Valen 1965; Werner and Sherry 1987; Schatz et al. 1995; Bolnick et al. 2003, 2007; Araújo et al. 2010, 2011; Ballesteros et al. 2014), including 20 reptile species (Araújo et al. 2011). The study site setting and our methodology may in part have affected our results. For example, more recapture and resource availability data would be needed to better characterize the species adaptability at the individual level, that is, to know if high trophic plasticity leads to either a divergent ecological specialization of individuals or opportunistic behavior; opportunism being defined as a frequent diet switch driven by food availability variation.
The nested pattern of P. lilfordi diet agrees with previous findings in other individual-resource networks (e.g., Araújo et al. 2008, 2010; Ballesteros et al. 2014; Fernandes da Cunha et al. 2018) and suggests rank preferences (Araújo et al. 2010) or at least the same top-ranked prey (Lemos-Costa et al. 2016) among individuals. Similar results of Null models 1 and 2 suggest higher nestedness than expected for estimated resources availability. Concordant with the ideal free distribution diet theory (Fretwell and Lucas 1969), most individuals should consume preferable resources whereas only the most generalists should consume less preferable resources (Araújo et al. 2010). Likewise, the modular pattern found suggests group-level individual specialization, which may have important implications for conservation. On the one hand, it means lower vulnerability to changes in resource availability. On the other hand, it leads to functional intrapopulation heterogeneity, which potentially affects prey (animals and plants) heterogeneity, for example, increasing the quantity and quality of seed dispersal (Zwolak 2018). Since P. lilfordi individuals are relatively sedentary (Terrasa et al. 2009; Calviño-Cancela et al. 2012), spatial distribution of resources might explain modularity better than individual preferences. Mantel tests found no relationship between topological or niche distances among individuals and geographic distances among traps where they were captured; however, the spatial characterization of habitat type at a small scale could allow a more accurate assessment of the influence of resource spatial distribution on modular structure. Differences in results of modularity significance when using Null model 2 or 3 might be due to the small increase of connectance produced by Null model 2. Although these 2 null models produce fairly reliable results with modularity (Fortuna et al. 2010), further studies are needed to better understand the sensitivity of modularity to these structural effects.
In both networks, the redundancy, modularity, and niche overlap analyses identified niche partitioning which partially may be explained by the variation in seasonal availability of food items. Higher niche overlap among females at NM is concordant with the greater sexual dimorphism on this island (Rotger 2016). On NG, however, male niche was significantly wider than that of the female and we explain that by the despotic behavior of males (Pérez-Mellado et al. 2015). Contrary to the expected higher specialization when resource availability increases (Schoener 1971; Werner and Hall 1974), diet overlap was higher in spring in both islets, suggesting relaxed competition in this season. Indeed, the higher modularity in autumn than in spring is consistent with a stronger competition when resources are scarcer, which can also increase specialization (Araújo et al. 2011). In addition to morphological traits and seasonality, behavioral traits and spatial heterogeneity can influence resource niche partition (Toscano et al. 2016). We conclude that niche partition in island lizard populations is driven by a complex of factors. Besides sex, seasonality and trade-offs related to the capabilities required to handle and consume each type of food item, other factors such as spatial distribution of resources, individual personality, and experience (age) contribute to individual competitive ability and thus, to niche partition.
Study caveats
Downscaling from species to individuals in the study of trophic interactions in wild populations is a challenging task because it is difficult to obtain sufficient sample size to characterize the ecological specialization of each individual within a population. This study provides information on the factors that may influence the intrapopulation distribution of P. lilfordi resources. However, to demonstrate individual specialization, future studies should include at least 2 dietary analyses per individual (Araújo et al. 2011). Likewise, independent information about resource availability would be needed to analyze specialization among individuals in an ecological context. The low number of sampled juvenile individuals, as they are more difficult to capture in pitfall traps than adults (Tenan et al. 2013), excluded an analysis of the effect of age on diet composition in this study. In general, fecal sampling has been shown to be as good as stomach/digestive tract analysis (Pérez-Mellado et al. 2011). However, it cannot be excluded that some of soft-bodied preys were destroyed during digestion and thus plant material could be underestimated, such as nectar and fruit pulp, as samples were dried before inspection rather than stored in alcohol. Finally, quantitative data, if present, rather than qualitative data may give better estimates of some network metrics (Blüthgen et al. 2006).
Concluding remarks
The analysis of trophic interactions within animal populations is revealing nonrandom patterns, which indicates heterogeneity in the distribution of resources. In an increasing number of species, generalization shows to be greater at the population level than at the individual level. Our results provide evidence in favor of this trend for 2 lacertid populations. The diets of P. lilfordi were quite similar in their composition, with a dominance of insects, especially Curculionidae and Formicidae. As in most previous studies on individual-resource networks, these interactions showed moderate but significant nestedness, but contrary to most of them they also showed moderate but significant modularity, both in annual and autumn networks. Lizard individuals, therefore, differed in their niche width but had a similar ranking of resources use, which seems to be driven, at least partially, by seasonality, differences in competition ability between sexes, and also likely by the skill acquired in handling certain types of items. On the one hand, our results suggest high plasticity of P. lilfordi to variation in resource availability. On the other hand, they also showed a niche resource partition even within the same season (autumn), suggesting that individuals from the same population of P. lilfordi are not ecologically equivalents. Thus further studies are needed to unravel the mechanisms underlying interindividual diet variation, which may affect population dynamics of this endangered endemic lizard species.
Supplementary Material
Acknowledgment
The authors are especially grateful to Xavier Canyelles for identification of diet components in the feces and 2 anonymous reviewers for valuable suggestions.
Funding
This work is framed within projects CGL2017-88122-P and BFU2009-09359 financed by the Spanish Government.
References
- Almeida-Neto M, Guimarães P, Guimarães PR Jr, Loyola RD, Ulrich W, 2008. A consistent metric for nestedness analysis in ecological systems: reconciling concept and quantification. Oikos 117:1227–1239. [Google Scholar]
- Araújo MS, Guimarães PR Jr, Svanbäck R, Pinheiro A, Guimarães P. et al. , 2008. Network analysis reveals contrasting effects of intraspecific competition on individual vs. population diets. Ecology 89:1981–1993. [DOI] [PubMed] [Google Scholar]
- Araújo MS, Martins EG, Cruz LD, Fernandes FR, Linhares AX. et al. , 2010. Nested diets: a novel pattern of individual-level resource use. Oikos 119:81–88. [Google Scholar]
- Araújo MS, Bolnick DI, Layman CA, 2011. The ecological causes of individual specialisation. Ecol Lett 14:948–958. [DOI] [PubMed] [Google Scholar]
- Ballesteros Y, Polidori C, Tormos J, Baños-Picón L, Daniel J, 2014. Complex-to-predict generational shift between nested and clustered organization of individual prey networks in digger wasps. PLoS ONE 9:e102325.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bascompte J, Jordano P, Melián CJ, Olesen JM, 2003. The nested assembly of plant-animal mutualistic networks. Proc Natl Acad Sci U S A 100:9383–9387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beckett SJ, 2016. Improved community detection in weighted bipartite networks. R Soc Open Sci 3:140536.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blüthgen N, Menzel F, Blüthgen N, 2006. Measuring specialization in species interaction networks. BMC Ecol 6:9.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blüthgen N, Fründ J, Vázquez DP, Menzel F, 2008. What do interaction network metrics tell us about specialization and biological traits? Ecology 89:3387–3399. [DOI] [PubMed] [Google Scholar]
- Bolnick DI, Svanbäck R, Fordyce JA, Yang LH, Davis JM. et al. , 2003. The ecology of individuals: incidence and implications of individual specialization. Am Nat 161:1–28. [DOI] [PubMed] [Google Scholar]
- Bolnick D, Svanbäck R, Araújo MS, Persson L, 2007. Comparative support for the niche variation hypothesis that more generalized populations also are more heterogeneous. Proc Natl Acad Sci U S A 104:10075–10079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolnick DI, Amarasekare P, Araújo MS, Bürger R, Levine JM. et al. , 2011. Why intraspecific trait variation matters in community ecology. Trends Ecol Evol 26:183–192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown RP, Pérez-Mellado V, 1994. Ecological energetics and food acquisition in dense Menorcan islet populations of the lizard Podarcis lilfordi. Funct Ecol 8:427–434. [Google Scholar]
- Calviño-Cancela M, Escudero M, Rodríguez-Pérez J, Cano E, Vargas P. et al. , 2012. The role of seed dispersal, pollination and historical effects on genetic patterns of an insular plant that has lost its only seed disperser. J Biogeogr 39:1996–2006. [Google Scholar]
- Carretero MA, 2004. From set menu to a la carte. Linking issues in trophic ecology of Mediterranean lacertids. Ital J Zool 2:121–133. [Google Scholar]
- Dall SRX, Bell AM, Bolnick DI, Ratnieks FLW, 2012. An evolutionary ecology of individual differences. Ecol Lett 15:1189–1198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dormann CF, Gruber B, Fründ J, 2008. Introducing the bipartite package: analysing ecological networks. R News 8:8–11. [Google Scholar]
- Dormann CF, Strauss R, 2014. A method for detecting modules in quantitative bipartite networks. Methods Ecolo Evol 5:90–98. [Google Scholar]
- Fernandes da Cunha A, Wolff L, Hahn SN, 2018. Seasonal changes at population and individual levels in the diet of juvenile catfish in a Neotropical floodplain. J Freshw Ecol 33:273–284. [Google Scholar]
- Fortuna MA, Stouffer DB, Olesen JM, Jordano P, Mouillot D. et al. , 2010. Nestedness versus modularity in ecological networks: two sides of the same coin? J Anim Ecol 79:811–817. [DOI] [PubMed] [Google Scholar]
- Fretwell SD, Lucas HL, 1969. On territorial behavior and other factors influencing habitat distribution in birds. Acta Biotheor 14:16–36. [Google Scholar]
- Gómez JM, Perfectti F, Jordano P, 2011. The functional consequences of mutualistic network architecture. PLoS ONE 6:e16143.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ings TC, Montoya JM, Bascompte J, Blüthgen N, Brown L. et al. , 2009. Ecological networks: beyond food webs. J Anim Ecol 78:253–269. [DOI] [PubMed] [Google Scholar]
- Kernaléguen L, Dorville N, Ierodiaconou D, Hoskins AJ, Baylis AM. et al. , 2016. From video recordings to whisker stable isotopes: a critical evaluation of timescale in assessing individual foraging specialisation in Australian fur seals. Oecologia 180:657–670. [DOI] [PubMed] [Google Scholar]
- Lemos-Costa P, Pires MM, Araújo MS, de Aguiar MAM, Guimarães PR Jr, 2016. Network analyses support the role of prey preferences in shaping resource use patterns within five animal populations. Oikos 125:492–501. [Google Scholar]
- Mayol J, 2004. A conservation proposal for most endangered insular lizards in the Balearics In: Pérez-Mellado V, Riera N, Perera A, editors. The Biology of Lacertids Lizards: Evolutionary and Ecological Perspectives. Vol. 8 Recerca: Institut Menorquí d’Estudis; 231–238. [Google Scholar]
- Melián CJ, Vilas C, Baldó F, González-Ortegón E, Drake P. et al. , 2011. Eco-evolutionary dynamics of individual-based food webs In: Belgrano A, Reiss J, editors. Advances in Ecological Research. Vol. 45 Amsterdam, The Netherlands; 225–268. [Google Scholar]
- Miklós I, Podani J, 2004. Randomization of presence-absence matrices: comments and new algorithms. Ecology 85:86–92. [Google Scholar]
- Moran NP, Wong BBM, Thompson RM, 2017. Weaving animal temperament into food webs: implications for biodiversity. Oikos 126:917–930. [Google Scholar]
- Moya O, Mansilla PL, Madrazo S, Igual JL, Rotger A. et al. , 2015. APHIS: a new software for photo-matching in ecological studies. Ecol Inform 27:64–70. [Google Scholar]
- Oksanen K, Blanchet FG, Kindt R, Legendre P, Minchin PR. et al. , 2015. Vegan: Community Ecology Package. Rpackage version 2.3-0.
- Olesen MJ, Bascompte J, Dupont YL, Jordano P, 2007. The modularity of pollination networks. Proc Natl Acad Sci U S A 104:19891–19896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olesen JM, Dupont YL, O’Gorman EJ, Ings TC, Layer K. et al. , 2010. From Broadstone to Zackenberg: space, time and hierarchies in ecological networks In: Woodward G, editor Advances in Ecological Research: Ecological Networks. San Diego (CA: ): Elsevier Academic Press Inc; 1–69. [Google Scholar]
- Patterson BD, Atmar W, 1986. Nested subset and structure of insular mammalian faunas and archipelagos In: Heaney LR, Patterson BD, editors. Island Biogeography of Mammals. London: Academic Press; 65–82. [Google Scholar]
- Pérez-Cembranos A, León A, Pérez-Mellado V, 2016. Omnivory of an insular lizard: sources of variation in the diet of Podarcis lilfordi (Squamata, Lacertidae). PLoS ONE 11:e0148947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pérez-Mellado V, 1989. Estudio ecológico de la lagartija balear Podarcis lilfordi (Gunther, 1874) en Menorca. Revista de Menorca 53:455–511. [Google Scholar]
- Pérez-Mellado V, 2002. Análisis regional de la herpetofauna española - Baleares In: Pleguezuelos JM, Márquez R, Lizana M, editors. Atlas y Libro Rojo de Los Anfibios y de España. Madrid: Dirección General de Conservación de la Naturaleza; 468. [Google Scholar]
- Pérez-Mellado V, Pérez-Cembranos A, Garrido M, Luiselli M, Corti C, 2011. Using faecal samples in lizard dietary studies. Amphibia-Reptilia 32:1–7. [Google Scholar]
- Pérez-Mellado V, García-Díez T, Hernández-Estévez JA, Tavecchia G, 2015. Behavioural processes, ephemeral resources and spring population dynamics of an insular lizard, Podarcis lilfordi (Squamata: Lacertidae). Ital J Zool 82:556–564. [Google Scholar]
- Pérez-Mellado V, Corti C, 1993. Dietary adaptations and herbivory in lacertid lizards of genus Podarcis from western Mediterranean islands (Reptilia: Sauria). Bonn Zool Beitr 44:193–220. [Google Scholar]
- Pérez-Mellado V, Traveset A, 1999. Relationships between plants and Mediterranean lizards. Nat Croat 8:275–285. [Google Scholar]
- Pettorelli N, Hilborn A, Duncan C, Durant SM, 2015. Individual variability: the missing component to our understanding of predator-prey interactions. Adv Ecol Res 52:19–44. [Google Scholar]
- Pires MM, Guimarães PR Jr, Araújo MS, Giaretta AA, Costa JCL. et al. , 2011. The nested assembly of individual-resource networks. J Anim Ecol 80:896–903. [DOI] [PubMed] [Google Scholar]
- R Development Core Team, 2017. R: A Language and Environment for Statistical Computing. Vienna: R Foundation for Statistical Computing. [Google Scholar]
- Rao CR, 1964. The use and interpretation of principal component analysis in applied research. Sankhya B SANA 26:329–358. [Google Scholar]
- Rotger A, 2016. Evolutionary Demography of the Balearic Wall Lizard (Podarcis Lilfordi). Barcelona: Universitat de Barcelona; https://dialnet.unirioja.es/servlet/tesis? codigo=177967 (26 May 2019, date last accessed). [Google Scholar]
- Rotger A, Igual JM, Smith JJ, Tavecchia G, 2016. Relative role of population density and climatic factors in shaping the body growth rate of Lilford’s wall lizard Podarcis lilfordi. Can J Zool 94:207–215. [Google Scholar]
- Roughgarden J, 1972. Evolution of niche width. Am Nat 106:683–718. [Google Scholar]
- Ruiz de Infante Anton J, Rotger A, Igual JM, Tavecchia G, 2013. Estimating lizard population density: an empirical comparison between line-transect and capture-recapture methods. Wildlife Res 40:552–560. [Google Scholar]
- Schatz B, Lachaud J-P, Beugnon G, 1995. Spatial fidelity and individual foraging specializations in the Neotropical ant Ectatomma ruidum Roger (Hymenoptera, Formicidae). Sociobiology 26:269–282. [Google Scholar]
- Schoener TW, 1971. Theory of feeding strategies. Annu Rev Ecol Syst 2:369–404. [Google Scholar]
- Sih A, Cote J, Evans M, Fogarty S, Pruitt J, 2012. Ecological implications of behavioural syndromes. Ecol Lett 15:278–289. [DOI] [PubMed] [Google Scholar]
- Svanbäck R, Rydberg C, Leonardsson K, Englund G, 2011. Diet specialization in a fluctuating population of Saduria entomon: a consequence of resource or forager densities? Oikos 120:848–854. [Google Scholar]
- Svanbäck R, Bolnick DI, 2005. Intraspecific competition affects the strength of individual specialization: an optimal diet theory model. Evol Ecol Res 7:993–1012. [Google Scholar]
- Svanbäck R, Bolnick DI, 2007. Intraspecific competition drives increased resource use diversity within a natural population. Proc R Soc Lond [Biol] 274:839–844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tenan S, Rotger A, Igual JM, Moya O, Royle JA. et al. , 2013. Population abundance, size structure and sex-ratio in an insular lizard. Ecol Modell 267:39–47. [Google Scholar]
- Terrasa B, Pérez-Mellado V, Brown RP, Picornell A, Castro JA. et al. , 2009. Foundations for conservation of intraspecific genetic diversity revealed by analysis of phylogeographical structure in the endangered endemic lizard Podarcis lilfordi. Divers Distrib 15:207–221. [Google Scholar]
- Tinker TM, Guimarães PR, Novak M, Marquitti FM, Bodkin JL. et al. , 2012. Structure and mechanism of diet specialisation: testing models of individual variation in resource use with sea otters. Ecol Lett 15:475–483. [DOI] [PubMed] [Google Scholar]
- Toscano BJ, Gownaris NJ, Heerhartz SM, Monaco CJ, 2016. Personality, foraging behavior and specialization: integrating behavioral and food web ecology at the individual level. Oecologia 182:55–69. [DOI] [PubMed] [Google Scholar]
- Traveset A, Sáez E, 1996. Pollination of Euphorbia dendroides by lizards and insects: spatio-temporal variation in patterns of flower visitation. Oecologia 111:241–248. [DOI] [PubMed] [Google Scholar]
- Tur C, Vigalondo B, Trøjelsgaard K, Olesen JM, Traveset A, 2014. Downscaling pollen-transport networks to the level of individuals. J Anim Ecol 83:306–317. [DOI] [PubMed] [Google Scholar]
- Tylianakis JM, Morris RJ, 2017. Ecological networks across environmental gradients. Annu Rev Ecol Evol Syst 48:25–48. [Google Scholar]
- Ulrich W, Gotelli NJ, 2007. Null model analysis of species nestedness patterns. Ecology 88:1824–1831. [DOI] [PubMed] [Google Scholar]
- Valido A, Olesen JM, 2019. Frugivory and seed dispersal by lizards: a global review. Front Ecol Evol 7 10.3389/fevo.2019.00049. [DOI] [Google Scholar]
- Van Valen L, 1965. Morphological variation and width of ecological niche. Am Nat 99:377–390. [Google Scholar]
- Verhoef HA, Morin PJ, 2010. Community Ecology: Processes, Models, and Applications. Oxford: Oxford University Press. [Google Scholar]
- Viada C, 2006. Libro Rojo de Los Vertebrados de Las Baleares. 3rd edn.Palma: Conselleria de Medi Ambient, Govern de les Illes Balears. [Google Scholar]
- Werner EE, Hall DJ, 1974. Optimal foraging and the size selection of prey by the bluegill sunfish Lepomis macrochirus. Ecology 55:1042–1052. [Google Scholar]
- Werner TK, Sherry TW, 1987. Behavioral feeding specialisation in Pinaroloxias inornata, the “Darwin’s Finch” of Cocos Island, Costa Rica. Proc Natl Acad Sci USA 84:5506–5510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf M, Weissing FJ, 2012. Animal personalities: consequences for ecology and evolution. Trends Ecol Evol 8:452–461. [DOI] [PubMed] [Google Scholar]
- Woodward G, Warren PH, 2007. Body size and predatory interactions in freshwaters: scaling from individuals to communities In: Hildrew AG, Raffaelli D, Edmonds-Brown R, editors. Body Size: The Structure and Function of Aquatic Ecosystems. Cambridge: Cambridge University Press; 98–117. [Google Scholar]
- Zwolak R, 2018. How intraspecific variation in seed-dispersing animals matters for plants. Biol Rev 93:897–913. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.