Abstract
Purpose
Preoperative therapy in borderline resectable pancreatic cancer (BRPC) is intended to increase R0 resection rates. An optimal approach in BRPC is yet to be defined.
Methods and Materials
Patients with BRPC, confirmed adenocarcinoma, performance status ≤1, and adequate organ function enrolled in a single-institution, phase 2 trial. Patients received FOLFIRINOX × 6 cycles, then radiation therapy (50 Gy in 25 fractions) concurrent with fixed-dose rate gemcitabine (1 g/m2 over 100 minutes) followed by 2 additional gemcitabine infusions. Computed tomography scans were performed at 2-month intervals during treatment. Patients without distant disease were offered surgical exploration. The primary objective was R0 resection rate with an alternate hypothesis of 55%. Secondary objectives included median progression-free survival (PFS), median overall survival (OS), response rate, and safety. The trial registration number is NCT01661088.
Results
Twenty-five patients with median age of 60 years (range, 47–77 years) enrolled from November 2011 through January 2017. Twenty-one (84%) completed FOLFIRINOX and 19 (76%) completed all protocol therapy. Treatment-related grade 3 to 4 toxicities included neutropenia (40%), nausea and vomiting (28%), diarrhea (16%), and fatigue (12%). Eighteen patients (72%) underwent laparotomy, 13 (52%) were resected (all R0). The median PFS and OS in 25 patients were 13.1 months (95% confidence interval [CI], 7.3–24.7) and 24.4 months (95% CI, 12.6–40.0), respectively. For resected patients, median PFS was 21.6 months (95% CI, 8.2–37.1) and OS was 37.1 months (95% CI, 15.4–not reached).
Conclusions
Neoadjuvant therapy with FOLFIRINOX, followed by intensity modulated radiation therapy concurrent with fixed-dose-rate gemcitabine in BRPC is feasible and tolerated. Although the alternate hypothesis was not met, the OS of the resected cohort was favorable.
Introduction
Pancreatic ductal adenocarcinoma (PDAC) is an aggressive cancer with an estimated 55,440 new cases and 44,330 deaths in the United States in 2018.1 It is currently the third leading cause of cancer-related deaths in the United States1 and projected to be the second most common by 2030.2 Prognosis in PDAC remains poor, with a 5-year overall survival (OS) of 8.2% for all patients.3 Surgical resection is necessary for curative treatment; however, only a minority of patients present with resectable disease.4–6
Borderline resectable pancreatic cancer (BRPC) was initially defined radiographically in the early 2000s as localized disease with vascular involvement limiting efficacy of initial surgical therapy.7–9 More recently, the National Comprehensive Cancer Network has defined this stage by quantifying the extent of tumor involvement with the surrounding veins and arteries on imaging. When patients with BRPC undergo resection as initial therapy, there is a high likelihood of microscopic or macroscopic residual tumor,7,10,11 an outcome which leads to inferior survival compared with margin negative resection (R0).5,12 Current treatment guidelines recommend neoadjuvant therapy to increase R0 resection rates in BRPC, although an optimal approach is yet to be defined.
We previously performed a prospective, multi-institutional trial in 68 patients, predominantly with BRPC.13 Protocol therapy consisted of 2 months of neoadjuvant gemcitabine and oxaliplatin, the first cycle concurrent with radiation therapy (30 Gy in 15 fractions). Two cycles of adjuvant chemotherapy were intended. Resection rate in all treated patients was 63%, with R0 resections in 53%. Only 60% of resected patients received postoperative chemotherapy. Median survival was 18.2 months in the study population, and 27.1 months in resected patients. In consideration of these results, an increased duration and exposure to systemic treatment and intensification of local therapy was suggested.
More recently, multidrug chemotherapy regimens which improve survival in patients with metastatic PDAC, such as FOLFIRINOX (5-fluorouracil, leucovorin, oxaliplatin and irinotecan),14 have been used as neoadjuvant chemotherapy in localized pancreas cancer. Petrelli et al conducted a meta-analysis across 6 studies to evaluate the impact of FOLFIRINOX in patients with BRPC and reported a pooled resection rate of all treated patients of 68.5%, with a R0 resection rate of 63.5%.15 These studies, however, were retrospective evaluations, shared no uniform definition of BRPC, and had varied use of radiation.
We previously published our experience with radiation concurrent with gemcitabine in patients with unresectable PDAC. In this phase 1/2 trial, a 5-week course of intensity modulated radiation therapy (IMRT) (50–57.5 Gy) was given concurrently with weekly fixed-dose-rate (FDR) gemcitabine (1,000 mg/m2 over 100 minutes). FDR gemcitabine infusion likely increases intratumoral concentration of its active metabolite and enhances effects of radiation.16,17 This treatment resulted in a subsequent resection in 12 of 50 patients (24%), with resultant median OS of 32 months in that small cohort.18
With the experience detailed earlier, we designed and herein report a phase 2 clinical trial of neoadjuvant treatment in BRPC. We hypothesized that a 3-month course of FOLFIRINOX would provide systemic disease control and that a 5-week course of IMRT concurrent with FDR-gemcitabine would continue systemic therapy and maximize local disease response. Additionally, this protocol would provide 6 months of treatment or total neoadjuvant therapy, thereby insuring a conventional duration of systemic treatment in all patients. The objectives of this sequential multimodality treatment were to improve upon reported R0 resection rate and overall outcome in this patient population.
Methods and Materials
Study design
This phase 2 trial of total neoadjuvant therapy for patients with BRPC was conducted at a single institution with a target enrollment of 31 patients. The primary objective of this trial was to increase the frequency of R0 resections in this patient population. Secondary objectives included measures of efficacy, including overall response rate, progression-free survival (PFS) and OS in addition to toxicity and tolerability of this multimodality treatment.
The study was conducted in accordance with the Declaration of Helsinki and Good Clinical Practice Guidelines of the International Conference on Harmonization (trial registration number NCT01661088). The protocol was approved by the institutional review board at the institution. All patients provided written informed consent before treatment.
Patient eligibility
Key eligibility criteria included age 18 years or older, pathologic confirmation of adenocarcinoma and meeting criteria for borderline resectable disease as defined by National Comprehensive Cancer Network Practice Guidelines in Oncology (v.2.2010) on pancreatic protocol computed tomography (CT) or magnetic resonance imaging. Borderline resectable status included superior mesenteric or portal vein involvement by tumor greater than 180° without deformity, venous involvement less than 180° with deformity, or a short segment venous occlusion; superior mesenteric artery or celiac artery contact of less than 180°; any involvement of common hepatic artery amenable to reconstruction; or direct abutment of the hepatic artery without extension to the celiac axis.
Additional inclusion criteria included an Eastern Cooperative Oncology Group performance status of 0 or 1, patient’s ability and willingness to undergo surgical resection, and adequate organ function (defined as absolute neutrophil count ≥ 1500/mm3, platelet count ≥ 100,000/mm3, serum creatinine ≤ 1.5 mg/dL, and total bilirubin < 2.0 mg/dL). Patients with a history of prior chemotherapy for pancreatic cancer or any abdominal radiation, other active systemic malignancy, ongoing infection, uncontrolled concomitant systemic disorders, or peripheral neuropathy grade 2 or higher at baseline were excluded.
Study treatment
Patients initiated treatment with FOLFIRINOX (5-fluorouracil 400 mg/m2, oxaliplatin 85 mg/m2, and irinotecan 180 mg/m2 concurrent with leucovorin 400 mg/m2 intravenously on day 1 followed by 2400 mg/m2 of 5-fluorouracil via continuous infusion over 46 hours) intended every 14 days for 6 cycles (Table E1; available online at https://doi.org/10.1016/j.ijrobp.2019.08.057). This regimen was modified with omission of 5-FU bolus and leucovorin starting with the thirteenth patient on study owing to widely accepted dose modifications to improve tolerance in the United States.19 White blood cell growth factor was used at the discretion of the treating physician. After 4 cycles of FOLFIRINOX, patients underwent imaging evaluation with pancreatic-protocol CT20 and measurement of carbohydrate antigen (CA) 19–9. Patients without evidence of disease progression continued with 2 more cycles of FOLFIRINOX and were referred for radiation therapy.
After 6 cycles of FOLFIRINOX, patients received image guided IMRT (50.0 Gy in once daily 2.0 Gy fraction) concurrent with FDR gemcitabine (1000 mg/m2 infused over 100 minutes) on days 1, 8, 22, and 29 of the 5-week course of radiation. The gross tumor volume for IMRT included the primary tumor and grossly involved regional lymph nodes identified on the pretreatment CT scan. The clinical target volume included the gross tumor volume plus a 0.5 cm expansion. Patients were treated using a breath hold technique if possible. If the patient could not tolerate a breath hold technique, then a 4-dimensional CT scan was performed and an internal target volume was constructed to account for respiratory motion. The planning target volume included the internal target volume or clinical target volume plus 0.5 cm. The mean planning target volume dose was aimed to be as close to 50 Gy as possible.18 Three to 4 weeks after completion of combined modality therapy, patients underwent repeat CT imaging evaluation. Patients without evidence of disease progression received 2 additional FDR-gemcitabine infusions to complete neoadjuvant treatment.
With completion of study therapy, patients underwent a final CT imaging for evaluation of resectability and measurement of CA 19–9. Patients were evaluated at the multidisciplinary pancreatic cancer tumor board and those without evidence of distant disease were offered surgical exploration (approximately 4–6 weeks after last chemotherapy).
Assessments
Toxicities were evaluated according to the National Cancer Institute Common Terminology Criteria for Adverse Events (version 4.0).21 Any treatment-related adverse events ≥grade 3 required holding chemotherapy until toxicity resolved to ≤grade 1. FOLFIRINOX and FDR-gemcitabine were dose adjusted according to hematologic and nonhematologic toxicities experienced before and on the day of planned therapy. The University Cancer Center Data Safety Monitoring Board monitored the study for safety.
Borderline resectable status and radiologic response were assessed by a single, board-certified abdominal radiologist using response evaluation criteria in solid tumors guidelines v1.1.22 Pathologic tumor response was evaluated by a board-certified pathologist in resected pancreatic specimens, and tumor regression was scored according to a modified Ryan scheme recommended by the College of American Pathologists.23 After protocol therapy or surgery, patients were followed every 3 months for a total of 2 years from date of registration.
Statistical methodology
The primary endpoint was to assess the frequency of R0 resection as defined by absence of both gross and microscopic involvement of resection margins by tumor. The sample size was calculated based on a null hypothesis of R0 resection rate of 30% based on neoadjuvant treatment data available at the time of study design24–26 and an alternative hypothesis of 55%. The trial was designed to have a type I error less than 0.05 and type II error less than 0.20.
Secondary endpoints included tumor response rate, estimates of PFS and OS, as well as tolerability and toxicity. PFS was defined from date of registration to the date of documented radiologic progression or recurrence, or death, whichever occurs first. Patients without events were censored on their date of nonprotocol disease related therapy or last known clinical event. OS was defined from date of registration to date of death or last follow-up, whichever occurs first. Distributions of PFS and OS were estimated using the Kaplan-Meier product-limit method (with 95% pointwise confidence intervals [CI] for time-to-event outcomes). Associations of PFS and OS with CA 19–9 baseline levels were explored using Cox proportional hazards regression analysis.
Results
Patients
A total of 25 patients were enrolled between November 2011 and January 2017. Trial enrollment was discontinued short of the goal of 31 patients owing to slow accrual and a competing intergroup trial (A021501); no formal analysis of power was performed before closure, but it was accepted that power to evaluate our primary endpoint, R0 resection rate, would be diminished. The median age of patients was 60 years (range, 47–77 years), of which 16 (64%) were men. Patient baseline demographics and disease characteristics are summarized in Table 1.
Table 1.
Patient baseline demographics and disease characteristics (safety population)
| Characteristic | Result |
|---|---|
| Total eligible patients (n) | 25 |
| Age, y | |
| Median (range) | 60 (47–77) |
| Female, n (%) | 9(36) |
| Race, n (%) | |
| Caucasian | 23 (92) |
| American Indian/Alaska Native | 2(8) |
| Ethnicity, n (%) | |
| Hispanic | 2 (8) |
| Not Hispanic | 23 (92) |
| ECOG PS, n (%) | |
| 0 | 12 (48) |
| 1 | 13 (52) |
| Tumor location, n (%) | |
| Head/uncinate | 23 (92) |
| Body | 1 (4) |
| Tail | 1 (4) |
| CA 19–9, U/mL, median (range) | |
| At baseline | 561 (2–16,586) |
| Degree of vascular involvement, n (%) | |
| ≤ 180 SMV and/or PV only | 7 (28) |
| > 180 SMV and/or PV only | 8 (32) |
| ≤180 SMA only | 2 (8) |
| Arterial and venous combined | 8 (32) |
Abbreviations: CA = carbohydrate antigen; ECOG PS = Eastern Cooperative Oncology Group performance status; PV = portal vein; SD = standard deviation; SMA = superior mesenteric artery; SMV = superior mesenteric vein.
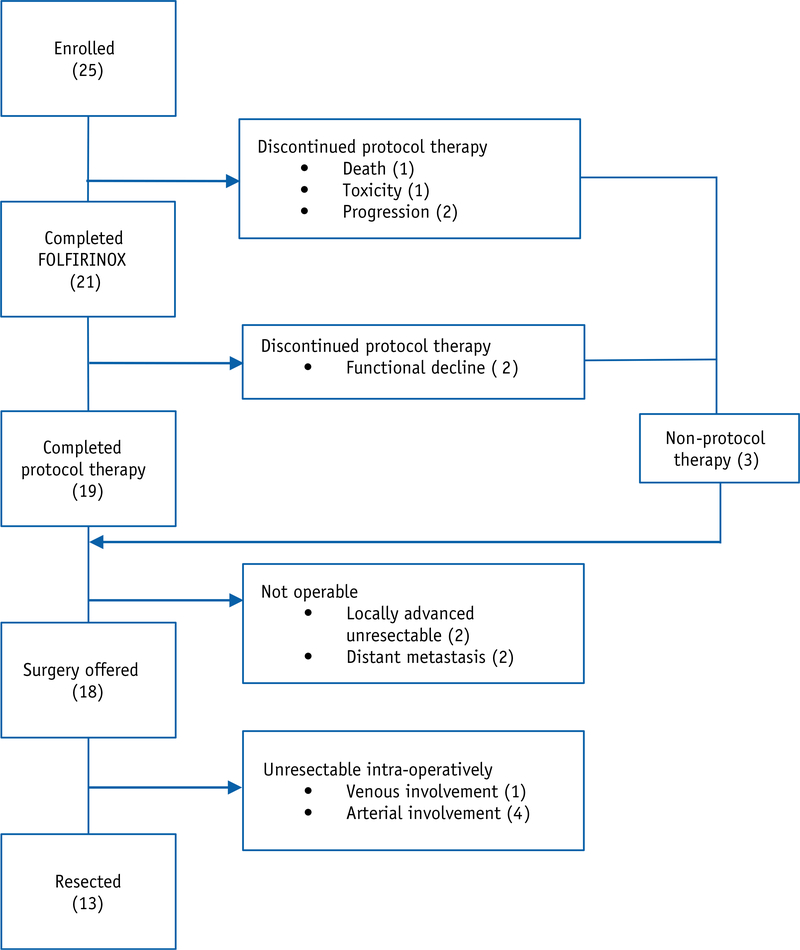
Patient disposition is summarized in Figure 1. Of the 25 patients treated, 21 (84%) patients completed FOLFIRINOX and 19 (76%) completed all protocol therapy. Four patients did not complete FOLFIRINOX because of toxicity (n = 2) and disease progression (n = 2). Two additional patients discontinued protocol therapy after completion of FOLFIRINOX at least in part due to toxicity and opted for early surgery (1) and supportive care only (1). Nineteen (76%) patients completed IMRT/FDR-gemcitabine, 16 of whom were treated using a breath hold technique during radiation.
Fig. 1.
Patient disposition. A general schema of patient disposition including treatments received and reasons for discontinuation of treatment.
Toxicity
Treatment-related hematologic and nonhematologic ≥grade 3 adverse events are summarized in Table 2. A majority of patients (72%) experienced at least 1 ≥grade 3 toxicity related to treatment. The most frequently reported nonhematologic adverse events related to treatment were nausea and vomiting (40%), diarrhea (16%), and fatigue (12%), mainly during FOLFIRINOX treatment. One patient died of cardiac arrest, assessed as probably related to FOLFIRINOX therapy. A second patient discontinued protocol treatment owing to coronary spasm, and a third patient discontinued owing to decline in functional status. Growth factor was administered in 14 (56%) patients during FOLFIRINOX. All 19 patients who initiated IMRT/gemcitabine completed that therapy.
Table 2.
Grade 3 or higher treatment-related adverse events
| Event | During FOLFIRINOX | During IMRT/Gemcitabine | Total (N = 25) |
|---|---|---|---|
| All hematologic AEs | n | n | n (%) |
| Neutropenia | 3 | 7 | 10 (40) |
| Leukopenia | 0 | 8 | 8 (32) |
| Lymphopenia | 1 | 7 | 8 (32) |
| Febrile neutropenia | 2 | 0 | 2(8) |
| Anemia | 0 | 2 | 2 (8) |
| Nonhematologic AEs | |||
| Nausea | 4 | 1 | 5 (20) |
| Vomiting | 3 | 2 | 5 (20) |
| Diarrhea | 3 | 1 | 4(16) |
| Fatigue | 2 | 1 | 3 (12) |
| Abdominal pain | 1 | 1 | 2 (8) |
| Oral mucositis / esophagitis | 2 | 0 | 2 (8) |
| AST / ALT increased | 0 | 2 | 2 (8) |
| Anorexia | 0 | 1 | 1 (4) |
| Chest pain / coronary spasm | 1 | 0 | 1 (4) |
| Cardiac arrest / death | 1 | 0 | 1 (4) |
| Cholangitis | 1 | 0 | 1 (4) |
| Gastric perforation | 0 | 1 | 1 (4) |
Abbreviations: AE = adverse event; ALT = alanine transaminase; AST = aspartate transaminase.
Efficacy
Tumor response and change in CA 19–9 during therapy
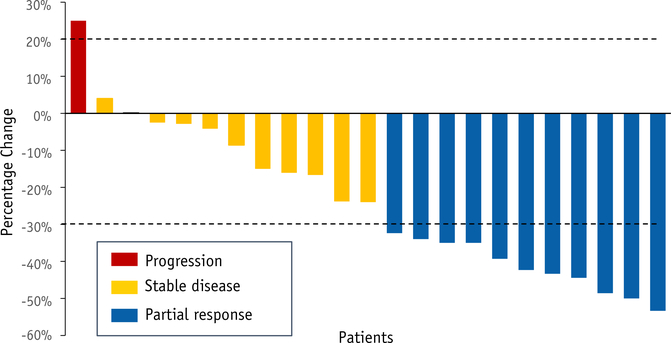
Twenty-three patients (92%) underwent at least 1 restaging scan, of which 11 patients experienced a partial response and an additional 11 had stable disease per response evaluation criteria in solid tumors v1.1 for a best overall response rate of 44% and disease control rate of 88% (Fig. 2). Partial responses were noted at 2 months (n = 3), after IMRT/gemcitabine (n = 6) and at treatment completion (n = 2). A partial response to therapy was not statistically significant for resection (P = .99) nor for OS (P= .35).
Fig. 2.
Tumor response based on response evaluation criteria in solid tumors criteria. Best overall response rate is 44% and disease control rate is 88%. Two patients with no follow-up scans were considered nonevaluable. Dotted line above x-axis represents 20% increase in sum of target lesions from nadir, and below x-axis represents 30% decrease in sum of target lesions from baseline.
Excluding nonsecretors (n = 4), the median baseline CA 19–9 level was 561 U/mL (range, 85–16,586 U/mL). CA 19–9 at baseline was not significantly associated with resection (P = .53), PFS (P = .36), or OS (P = .08). The median CA 19–9 after completion of 2 months of FOLFIRINOX (n = 19) was 362 U/mL (range, 15–3055 U/mL), and after IMRT and gemcitabine (n = 15) was 88 U/mL (range, 10–1649 U/mL). Nineteen patients had at least 1 follow-up CA 19–9 of which 16 (84.2%) had >50% decline in CA 19–9 on therapy.
Twelve patients (48%) did not undergo resection. Three patients discontinued protocol therapy owing to progression or deterioration on FOLFIRINOX. Two patients developed liver metastasis after completion of all therapy, 2 patients were judged unresectable radiologically, and 5 were deemed unresectable intraoperatively owing to extensive vascular involvement.
Resection ate and pathologic response
Eighteen patients (72%) were offered surgery (Fig. 1). Of these, 5 were judged unresectable at laparotomy owing to unreconstructable involvement of superior mesenteric vein, superior mesenteric artery, hepatic artery, or common hepatic artery. In total, 13 (52%) patients underwent resection, all of which were R0 resections. The median time from enrollment to surgery was 7 months. Venous resection and reconstruction was performed in 9 patients (69.2%; 4 portal vein and 5 superior mesenteric vein). The R0 resection rate in the intent to treat population was observed to be 52% (95% CI, 31.3%−72.2%), with R0 defined as absence of tumor cells at the resection margin, and did not reach our a priori alternative hypothesis of 55%; however, it was significantly different (P = .026) from our historical null hypothesis estimate of 30% using an exact 2-sided binomial test. One of the 13 resections was noted to have tumor cells within 1 mm of the margin. Pathologic tumor regression grades were noted to be no response (n = 2), partial response (n = 9), and near complete response (n = 2). The median number of regional lymph nodes examined were 13 (range, 5–25), and 7 patients (53.8%) had metastatic nodal disease. After surgical resection, the median length of stay was 7 days, with 3 patients readmitted within 30 days owing to delayed gastric emptying, acute renal failure, and ascites. There was no 30-day or 60-day mortality after surgery.
Survival
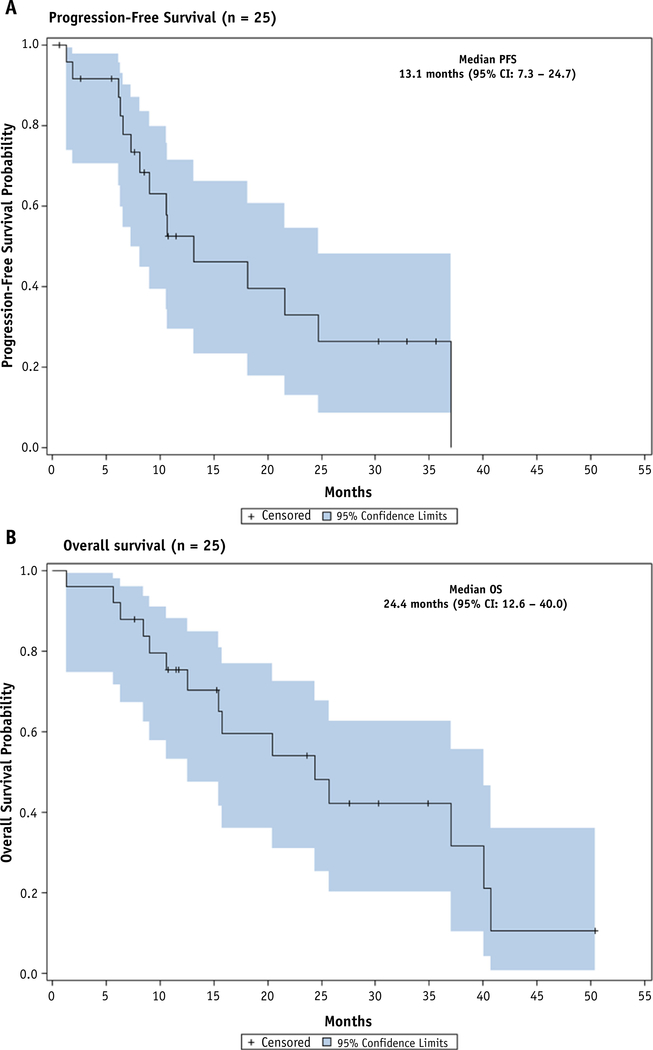
The median OS in the intent to treat population was 24.4 months (95% CI, 12.6–40.0), including 12.6 months (95% CI, 5.7–40.0) for unresected patients and 37.1 months (95% CI, 15.4-not reached) for resected patients (Fig. 3). The OS rates for all study patients at 12 and 24 months were 75.4% and 54.2%, respectively. The median PFS was 13.1 months (95% CI, 7.3–24.7) for all patients, 8.9 months (95% CI, 1.9-not reached) for unresected patients, and 21.6 months (95% CI, 8.2–37.1) for resected patients. The PFS rates in the intent to treat population at 12 and 24 months were 54.7% and 30.4%, respectively.
Fig. 3.
Progression-free survival and overall survival. Median progression-free survival for intention to treat population is 13.1 months (95% confidence interval [CI]: 7.3–24.7) and overall survival is 24.4 months (95% CI: 12.6–40.0). Shaded area represents 95% CI for time-to-event outcomes. Tick marks represent censored patients.
Of the 13 patients who underwent surgery, 5 patients have experienced loco-regional recurrence as the initial site of treatment failure and 3 patients have developed distant metastasis in lung (n = 2) and liver (n = 1).
Discussion
This phase 2 trial evaluated the efficacy of FOLFIRINOX followed by IMRT concurrent with FDR-gemcitabine in patients with borderline resectable pancreatic adenocarcinoma. The R0 resection rate in the intent to treat population was observed to be 52%, and although improved from the historical R0 resection rate of 30%,24,25 did not meet the alternate hypothesis of 55%. The exact 95% confidence interval for the estimate varies from 31.3% to 72.2%, which suggests that the treatment schema does significantly improve the R0 resection rate above our historical estimate of 30%. The combination of FOLFIRINOX followed by FDR gemcitabine with concurrent radiation therapy did have an acceptable safety profile in this patient population with no new or unexpected toxicities observed.
Recently, 2 similar phase 2 trials evaluating the role of neoadjuvant therapy in borderline resectable PDAC were reported. Katz et al published the results of A021101, a multi-institutional phase 2 trial evaluating FOLFIRINOX followed by radiation concurrent with capecitabine in patients with borderline resectable PDAC.27 Of the 22 eligible patients, 15 patients (68%) underwent resection, of which 14 resections were R0 (64%). More recently, Murphy et al reported the results from their institution with use of FOLFIRINOX followed by capecitabine-radiation in a similar patient population.28 Resection rates and R0 resection in 48 eligible patients were reported as 67% and 65%, respectively. In comparison to these trials, we report an overall and R0 resection rate of 52% and 52% of all eligible patients, respectively. Differences in resection rates between trials may be due to the small number of patients, varying definitions of borderline resectable PDAC, and intraoperative surgical decision making. In our study and these 2 trials, the median OS of the intent to treat population (21.7–37.7 months) and in resected patients is (likely >36 months) comparable to adjuvant trial populations, despite patients with BRPC presenting with more advanced disease.
Components of a multimodality treatment of BRPC may include systemic therapy, radiation, and surgery. An approach of total neoadjuvant therapy, as used here, is attractive in that the intact patient is most fit for nonoperative therapies and a 6-month interval of treatment permits assessment of tumor biology and behavior of the systemic component of disease, thus reserving surgery for those most likely to benefit from it. Contingent to this approach is that neoadjuvant treatment must be efficacious and, perhaps more importantly, tolerable. FOLFIRINOX is currently the standard systemic treatment for BRPC in this situation.27,28 However, in our study, progression of disease or toxicity with FOLFIRINOX resulted in protocol discontinuation in 24% of treated patients. This result and the recognition that not all patients are appropriate for FOLFIRINOX permits investigation of other multiagent combinations including, but not limited to, gemcitabine and nab-paclitaxel.29 Although grade 3 or higher hematologic toxicities were more frequently observed during chemoradiation than with FOLFIRINOX, myelosuppression during combined treatment was related in part to prior FOLFIRINOX, was easily managed with protocol specified gemcitabine dose reductions or treatment delays, and all patients completed chemoradiation.
As systemic treatment improves, local disease control assumes greater importance. Surgically treated pancreas cancer is associated with a high risk of local recurrence, and radiation therapy is expected to increase local disease control.30 We have performed a series of trials combining gemcitabine at 1000 mg/m2 with radiation, taking advantage of the potent radiation sensitization of gemcitabine in combination with its’ systemic activity.13,18,31 Others have used capecitabine during radiation27,32 owing to improved efficacy shown in a randomized phase 2 clinical trial compared with gemcitabine at 300 mg/m2 dose.33 The persistence of local failure in our study suggests that chemoradiation is not yet optimized, and we have pursued the use of molecularly targeted agents in preclinical studies34,35 and in a clinical trial for patients with locally advanced pancreatic cancer.36 Increasingly, stereotactic body radiation therapy (SBRT) is under investigation as a component of neoadjuvant treatment.37 SBRT has the advantage of shortening the radiation course, but it does not offer an opportunity to use radiosensitizing chemotherapy which may confer selective tumor cell killing. At the time of this report, accrual to the SBRT arm to the ongoing Alliance trial (A021501) was halted when the interim data analysis met the protocol specified futility boundary for the R0 resection rate. Ultimately, the timing, technique and contributions of radiation and chemoradiation therapy in BRPC will require randomized clinical trials.
Finally, resection after neoadjuvant treatment in BRPC is dependent on local disease extent, as well as patient characteristics and surgical approach. It has been demonstrated that radiologic response is not necessary for a subsequent R0 resection, although CA19–9 response may predict for R0 resection.13,38 Importantly, continuing contact on adjacent vessels does not preclude R0 resection, rather, the likelihood of resection is related to surgeon experience and willingness to perform potentially complicated resections, including vascular resection and reconstruction.13,27,28 In this study, 69% of resected patients required venous resection or reconstruction. Vascular resections, including arterial resection or reconstruction, and hence resection rates in reported series are as dependent on the surgeon’s judgment and intraoperative decision making as the neoadjuvant treatment. The value of such resections can only be judged by careful reporting of patient outcomes using intention-to-treat analysis and descriptions of local and distant disease control and survival.
There are inherent limitations to the trial, including enrollment at a single institution, small patient population, and selection factors which influence accrual (patient and investigator). Study therapy was delivered; however, in a prospective manner and in the context of an experienced multidisciplinary group with eligibility and response assessment completed by an abdominal radiologist and pathology reassessed by a gastrointestinal pathologist.
Conclusions
In summary, trial therapy with FOLFIRINOX and FDR-gemcitabine with IMRT in patients with borderline resectable PDAC did not meet its primary endpoint. However the data from this and other similar trials suggest an improved R0 resection rate, acceptable safety profile, and possibly prolonged survival with the use of FOLFIRINOX and radiation therapy compared with historical data.15,39 Further prospective and comparative evaluation of different forms of radiation therapy with or without radiation sensitizers, in addition to systemic approaches including investigational agents, is necessary in this patient population. Finally, choice of therapy guided by molecular characterization of an individual’s cancer may further enhance outcomes after multimodality treatment.
Supplementary Material
Summary.
Preoperative therapy in borderline resectable pancreatic cancer (BRPC) is intended to increase R0 resection rates. An optimal approach in BRPRC is not yet defined. We conducted a phase 2 trial in 25 patients with BRPC treated with neoadjuvant FOLFIRINOX followed by concurrent gemcitabine-intensity modulated radiation therapy to determine R0 resection rate, median progression free survival, and overall survival. This specific neoadjuvant regimen is feasible and tolerated. R0 resection rate was 52% with favorable overall survival.
Acknowledgments
We thank the patients and their families for their participation in the study. We would also like to acknowledge all the investigators and research staff for enrolling their patients on this trial. This trial was supported by the University of Michigan Rogel Cancer Center and the National Cancer Institute of the National Institutes of Health under award number P30CA046592 and K08CA234222 (JS).
Funding: Research reported in this publication was supported by the National Cancer Institute of the National Institutes of Health under award number P30CA046592 and K08CA234222 (JS).
Disclosures: E.K. reports personal fees from Armo, personal fees from Vicus, personal fees from Guardant Health, grants from BMS, grants from Astellas, grants from Samumed, grants from Boston Biomedical, grants from Halozyme, grants from EpicentRx, grants from Merck, grants from Oncomed, and grants from Celgene, outside the submitted work.
V.S. reports institutional grants from Celgene, Incyte, Bristol-Myers Squibb, Agios, Clovis, Halozyme, Merck, and MedImmune. He has also served in the role of a consultant for NewLink Genetics, Incyte, Celgene, and Halozyme.
M.M.Z. reports grants from Halozyme, grants from Oncomed, grants from Merck, and grants from Medimmune, outside the submitted work.
Footnotes
Supplementary material for this article can be found at https://doi.org/10.1016/j.ijrobp.2019.08.057.
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2018. CA Cancer J Clin 2018;68:7–30. [DOI] [PubMed] [Google Scholar]
- 2.Rahib L, Smith BD, Aizenberg R, et al. Projecting cancer incidence and deaths to 2030: The unexpected burden of thyroid, liver, and pancreas cancers in the United States. Cancer Res 2014;74:2913–2921. [DOI] [PubMed] [Google Scholar]
- 3.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2017. CA Cancer J Clin 2017;67:7–30. [DOI] [PubMed] [Google Scholar]
- 4.Hidalgo M, Cascinu S, Kleeff J, et al. Addressing the challenges of pancreatic cancer: Future directions for improving outcomes. Pancreatology 2015;15:8–18. [DOI] [PubMed] [Google Scholar]
- 5.Kumar R, Herman JM, Wolfgang CL, et al. Multidisciplinary management of pancreatic cancer. Surg Oncol Clin N Am 2013;22: 265–287. [DOI] [PubMed] [Google Scholar]
- 6.National Comprehensive Cancer Network. Pancreatic Adenocarcinoma (Version 2.2018). Available at: https://www.nccn.org/professionals/physician_gls/pdfpancreatic.pdf Accessed July 22, 2018.
- 7.Varadhachary GR, Tamm EP, Abbruzzese JL, et al. Borderline resectable pancreatic cancer: Definitions, management, and role of preoperative therapy. Ann Surg Oncol 2006;13:1035–1046. [DOI] [PubMed] [Google Scholar]
- 8.Callery MP, Chang KJ, Fishman EK, et al. Pretreatment assessment of resectable and borderline resectable pancreatic cancer: Expert consensus statement. Ann Surg Oncol 2009;16:1727–1733. [DOI] [PubMed] [Google Scholar]
- 9.Bockhorn M, Uzunoglu FG, Adham M, et al. Borderline resectable pancreatic cancer: A consensus statement by the International Study Group of Pancreatic Surgery (ISGPS). Surgery 2014;155:977–988. [DOI] [PubMed] [Google Scholar]
- 10.Kang CM, Chung YE, Park JY, et al. Potential contribution of preoperative neoadjuvant concurrent chemoradiation therapy on margin-negative resection in borderline resectable pancreatic cancer. J Gastrointest Surg 2012;16:509–517. [DOI] [PubMed] [Google Scholar]
- 11.Evans DB, Erickson BA, Ritch P. Borderline resectable pancreatic cancer: Definitions and the importance of multimodality therapy. Ann Surg Oncol 2010;17:2803–2805. [DOI] [PubMed] [Google Scholar]
- 12.Sohn TA, Yeo CJ, Cameron JL, et al. Resected adenocarcinoma of the pancreasd616 patients: Results, outcomes, and prognostic indicators. J Gastrointest Surg 2000;4:567–579. [DOI] [PubMed] [Google Scholar]
- 13.Kim EJ, Ben-Josef E, Herman JM, et al. A multi-institutional phase 2 study of neoadjuvant gemcitabine and oxaliplatin with radiation therapy in patients with pancreatic cancer. Cancer 2013;119(15):2692–2700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Conroy T, Desseigne F, Ychou M, et al. FOLFIRINOX versus gemcitabine for metastatic pancreatic cancer. N Engl J Med 2011;364: 1817–1825. [DOI] [PubMed] [Google Scholar]
- 15.Petrelli F, Coinu A, Borgonovo K, et al. FOLFIRINOX-based neoadjuvant therapy in borderline resectable or unresectable pancreatic cancer: A meta-analytical review of published studies. Pancreas 2015; 44:515–521. [DOI] [PubMed] [Google Scholar]
- 16.Lawrence TS, Chang EY, Hahn TM, Hertel LW, Shewach DS. Radiosensitization of pancreatic cancer cells by 2’,2’-difluoro-2’-deoxycytidine. Int J Radiat Oncol Biol Phys 1996;34(4):867–872. [DOI] [PubMed] [Google Scholar]
- 17.Grunewald R, Abbruzzese JL, Tarassoff P, Plunkett W. Saturation of 2’,2’-difluorodeoxycytidine 5’-triphosphate accumulation by mononuclear cells during a phase I trial of gemcitabine. Cancer Chemother Pharmacol 1991;27(4):258–262. [DOI] [PubMed] [Google Scholar]
- 18.Ben-Josef E, Schipper M, Francis IR, et al. A phase I/II trial of intensity-modulated radiation (IMRT) dose escalation with concurrent fixed- dose rate gemcitabine (FDR-G) in patients with unresectable pancreatic cancer. Int J Radiat Oncol Biol Phys 2012;84(5):1166–1171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mahaseth H, Brutcher E, Kauh J, et al. Modified FOLFIRINOX regimen with improved safety and maintained efficacy in pancreatic adenocarcinoma. Pancreas 2013;42:1311–1315. [DOI] [PubMed] [Google Scholar]
- 20.Al-Hawary M Role of imaging in diagnosing and staging pancreatic cancer. J Natl Compr Cancer Netw 2016;14:678–680. [DOI] [PubMed] [Google Scholar]
- 21.Common Terminology Criteria for Adverse Events (CTCAE). USDept of Health and Human Services/National Institutes of Health/-National Cancer Institute website. Available at: http://ctep.cancer.gov/protocolDevelopment/electronic_applications/ctc.htm Accessed October 17, 2017.
- 22.Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: Revised RECIST guideline (version 1.1). Eur J Cancer 2009;45:228–247. [DOI] [PubMed] [Google Scholar]
- 23.Washington MK, Berlin J, Branton P, et al. Protocol for the examination of specimens from patients with carcinoma of the pancreas. Coll Am Pathol 2016. [Google Scholar]
- 24.Small W Jr., Berlin J, Freedman GM, et al. Full-dose gemcitabine with concurrent radiation therapy in patients with nonmetastatic pancreatic cancer: A multicenter phase II trial. J Clin Oncol 2008;26:942–947. [DOI] [PubMed] [Google Scholar]
- 25.Katz MH, Pisters PW, Evans DB, et al. Borderline resectable pancreatic cancer: The importance of this emerging stage of disease. J Am Coll Surg 2008;206:833–846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gillen S, Schuster T, Meyer Zum Büschenfelde C, et al. Preoperative/neoadjuvant therapy in pancreatic cancer: A systematic review and meta-analysis of response and resection percentages. PLoS Med 2010;7:e1000267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Katz MH, Shi Q, Ahmad SA, et al. Preoperative modified FOLFIRINOX treatment followed by capecitabine-based chemoradiation for borderline resectable pancreatic cancer: Alliance for clinical trials in oncology trial A021101. JAMA Surg 2016;151:e161137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Murphy JE, Wo JY, Ryan DP, et al. Total neoadjuvant therapy with FOLFIRINOX followed by individualized chemoradiotherapy for borderline resectable pancreatic adenocarcinoma. JAMA Oncol 2018;4:963–969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Von Hoff DD, Ervin T, Arena FP, et al. Increased survival in pancreatic cancer with nab-paclitaxel plus gemcitabine. N Engl J Med 2013;369: 1691–1703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Van Laethem JL, Hammel P, Mornex F, et al. Adjuvant gemcitabine alone versus gemcitabine-based chemoradiotherapy after curative resection for pancreatic cancer : A randomized EORTC-40013–22012 / FFCD-9203 / GERCOR phase II study. J Clin Oncol 2010;28:4450–4456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Talamonti MS, Small W Jr., Mulcahy MF, et al. A multi-institutional phase II trial of preoperative full-dose gemcitabine and concurrent radiation for patients with potentially resectable pancreatic carcinoma. Ann Surg Oncol 2006;13:150–158. [DOI] [PubMed] [Google Scholar]
- 32.Katz MH, Marsh R, Herman JM, et al. Borderline resectable pancreatic cancer: Need for standardization and methods for optimal clinical trial design. Ann Surg Oncol 2013;20:2787–2795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mukherjee S, Hurt CN, Bridgewater J, et al. Gemcitabine-based or capecitabine-based chemoradiotherapy for locally advanced pancreatic cancer ( SCALOP ): A multicentre, randomised, phase 2 trial. Lancet Oncol 2013;14:317–326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wei D, Li H, Yu J, et al. Radiosensitization of human pancreatic cancer cells by MLN4924, an investigational NEDD8-activating enzyme inhibitor. Cancer Res 2013;72(1):282–293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Engelke CG, Parsels LA, Qian Y, et al. Sensitization of pancreatic cancer to chemoradiation by the Chk1 inhibitor, MK8776. Clin Cancer Res 2014;19(16):4412–4421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cuneo KC, Morgan M, Schipper MJ, et al. Phase I study of definitive chemoradiation with gemcitabine and the WEE1 inhibitor AZD1775 in unresectable pancreatic cancer. J Clin Oncol 2017;35 (4_suppl,TPS512–TPS512). [Google Scholar]
- 37.Herman JM, Chang DT, Goodman KA, et al. Phase 2 multi-institutional trial evaluating gemcitabine and stereotactic body radiotherapy for patients with locally advanced unresectable pancreatic adenocarcinoma. Cancer 2015;121:1128–1137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Boone BA, Steve J, Zenati MS, et al. Serum CA 19–9 response to neoadjuvant therapy is associated with outcome in pancreatic adenocarcinoma. Ann Surg Oncol 2014;21:4351–4358. [DOI] [PubMed] [Google Scholar]
- 39.Tang K, Lu W, Qin W, et al. Neoadjuvant therapy for patients with borderline resectable pancreatic cancer: A systematic review and meta-analysis of response and resection percentages. Pancreatology 2016;16:28–37. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.