Abstract
Loss of sensory hair cells is the leading cause of deafness in humans. The mammalian cochlea cannot regenerate its complement of sensory hair cells. Thus at present, the only treatment for deafness due to sensory hair cell loss is the use of prosthetics, such as hearing aids and cochlear implants. In contrast, in nonmammalian vertebrates, such as birds, hair cell regeneration occurs following the death of hair cells and leads to the restoration of hearing. Regeneration in birds is successful because supporting cells that surround the hair cells can divide and are able to subsequently differentiate into new hair cells. However, supporting cells in mammals do not normally divide or trans-differentiate when hair cells are lost, and so regeneration does not occur. To understand the failure of mammalian cochlear hair cell regeneration, we need to understand the molecular mechanisms that underlie cell division control and hair cell differentiation, both during embryogenesis and in the postnatal mouse. In this review, we present a discussion of the regulation of cell proliferation in embryogenesis and during postnatal maturation. We also discuss the role of the Cip/Kip cell cycle inhibitors and Notch signaling in the control of stability of the differentiated state of early postnatal supporting cells. Finally, recent data indicate that some early postnatal mammalian supporting cells retain a latent capacity to divide and transdifferentiate into sensory hair cells. Together, these observations make supporting cells important therapeutic targets for continued efforts to induce hair cell regeneration.
Keywords: development of the inner ear, organ of Corti, hair cell regeneration, cell cycle regulation
Introduction: The Problem of Hair Cell Regeneration
Environmental factors, such as noise or ototoxic drugs, combine with a variable genetic background to predispose many individuals to hearing loss. Indeed, age-related hearing loss affects over 50% of the population by the age of 65.1,2 People become deafened primarily because the sensory hair cells in their inner ears die. As in most of the mammalian nervous system, regeneration does not occur, and so the loss of hearing due to sensory hair cell death is permanent.3,4 Aside from prosthetics, such as hearing aids and the cochlear implant, developing therapies to induce regeneration in the sensory epithelia of the inner ear offers the only hope to the millions of people suffering from hearing loss.
In contrast to the permanent nature of mammalian deafness, the avian auditory organ readily regenerates lost hair cells. The supporting cells that normally surround the sensory hair cells are capable of acting as precursors in response to hair cell loss to regenerate the sensory epithelium.5–9 This regeneration can occur in two ways: quiescent supporting cells can directly differentiate into new hair cells, or they can first divide and subsequently differentiate as new hair cells and supporting cells.4 These two regenerative processes occur sequentially in the bird, and their temporal separation suggests that they may be regulated by distinct signaling pathways.10–13 Moreover, additional mechanisms must exist to regulate the extent of regeneration, as well as to re-establish the orderly mosaic of cells that is necessary for auditory function14,15 (Fig. 1).
Figure 1.
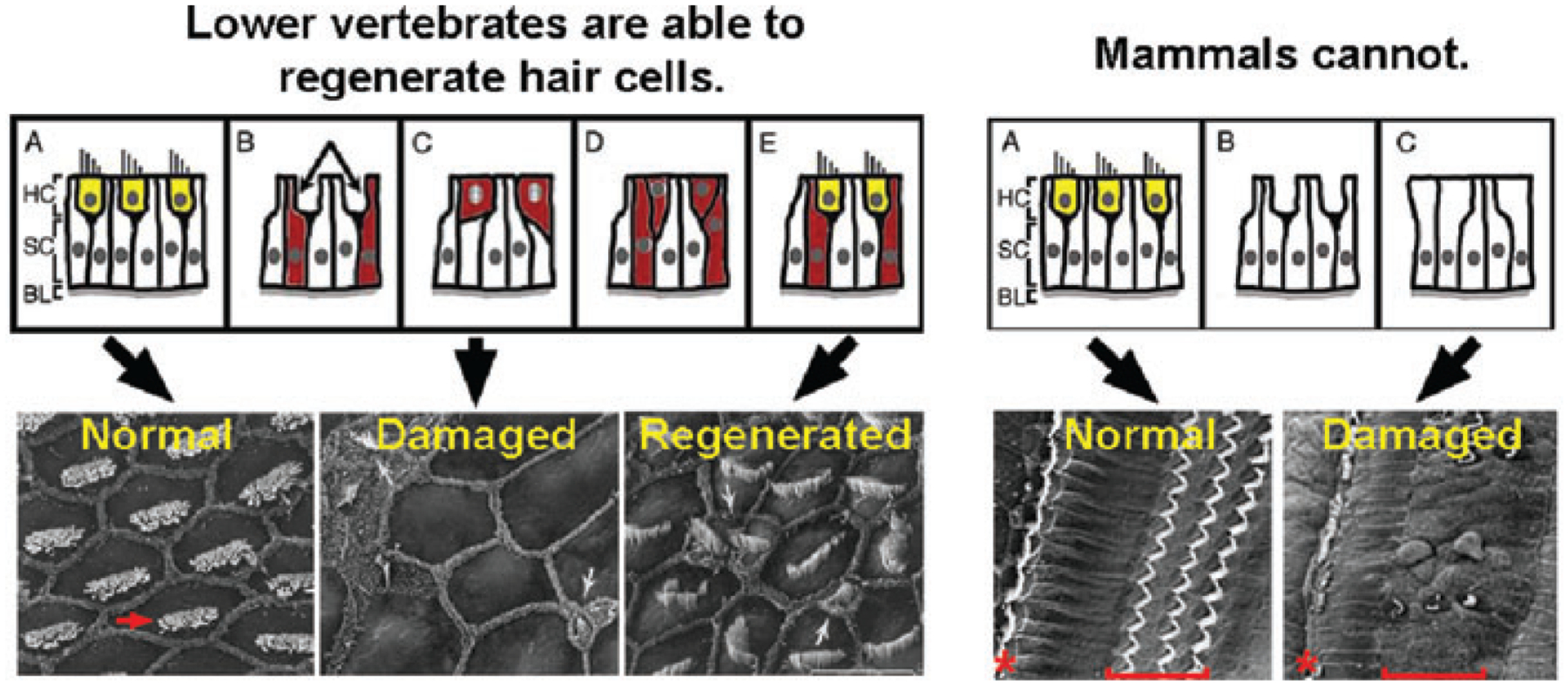
The death of hair cells (yellow) in the cochlea of chicks (left panels) stimulates the surrounding supporting cells (red) to re-enter the cell cycle and differentiate as new hair cells. In mammals, the death of cochlear hair cells is permanent. Supporting cells do not normally re-enter the cell cycle and no regeneration occurs. The red arrow indicates the stereocilia bundle of a single chick hair cell. The red asterisk and brackets indicate the position of the single row of inner hair cells and three rows of outer hair cells typically found in mice and humans. (Scanning electron micrographs are courtesy of Dr. Ed Rubel and House Ear Institute.)
Understanding the underlying causes of the failure of hair cell regeneration in mammals is a major focus of much recent research and has important implications for the treatment of hearing loss in humans. As in birds, regulation of two basic processes, proliferation of sensory precursors and their subsequent differentiation into hair cells, is likely to underlie any regenerative therapies. In this brief discussion, we will focus primarily on recent efforts to understand how proliferation is regulated during development in the mammalian cochlea, how it might be regulated during regeneration, and how precursors differentiate into hair cells.
Control of Cell Proliferation in the Inner Ear during Development
During the development of the mouse organ of Corti, the progenitors that give rise to hair cells and supporting cells make their final cell division prior to the apparent onset of differentiation, and then do not normally divide again for the life of the animal.16 Our hope is that by studying the regulation of cell cycle exit during embryogenesis, we will get a better understanding of the failure of supporting cell proliferation when hair cells are lost.
The cyclin-dependent kinases (CDK) and their regulatory machinery are prime targets of developmental signaling to co-ordinate proliferation with differentiation.17 The mouse organ of Corti arises from a prosensory region of the elongating cochlea defined by the expression of the CDK inhibitor p27Kip1.18 Developmentally, p27Kip1 is under transcriptional control and appears in a wave of expression that begins in the apical tip of the cochlear duct and progresses to the base.19 This expression establishes a postmitotic prosensory domain within the cochlear duct prior to apparent hair cell or supporting cell differentiation20 and parallels the pattern of cell cycle exit in the organ of Corti.16
Subsequently, differentiation and patterning within this postmitotic domain initiates in the base of the cochlea and progresses in a wave toward the apex, finishing just prior to birth. Thus, cells that first become postmitotic in the apical regions of the organ of Corti are maintained in a nondividing, but undifferentiated state for several days. Loss of p27Kip1 disrupts the normal pattern of cell cycle exit, and although the onset of differentiation occurs normally, the coordination between cell cycle exit and differentiation is lost. This suggests that during development the pattern of cell cycle exit and the onset and progression of differentiation are independently regulated. In p27Kip1 knockout mice, supernumerary hair cells and supporting cells are produced. Furthermore, these mice are deaf,18,21 most likely because the biomechanical integrity of the organ of Corti is compromised. Thus, fine control of p27Kip1 expression is crucial, not only for the correct morphological development of the organ of Corti, but also for its function.
Maintenance of the Postmitotic State
As differentiation progresses along the length of the nascent organ of Corti, p27Kip1 is rapidly downregulated in differentiating hair cells, but is maintained at high levels in differentiating supporting cells.18 This suggests the hypothesis that changing levels of CDK inhibitors may be involved in the maintenance of the postmitotic state of hair cells and supporting cells. Moreover, the high p27Kip1 levels in supporting cells might be responsible for their lack of regenerative response when hair cells are dead.
Given that p27Kip1 is rapidly and permanently downregulated as hair cells differentiate, other mechanisms must be responsible for maintaining the postmitotic state of these cells. P19Ink4d, a member of the Ink4 family of CDK inhibitors, is expressed at relatively high levels in hair cells, and its deletion leads to a progressive hair cell loss in postnatal mice.22 This occurs because in the absence of p19Ink4d, hair cells, unable to maintain the postmitotic state, sporadically re-enter the cell cycle and ultimately undergo apoptosis. Moreover, mutation of p21Cip1, another CDK inhibitor of the Cip/Kip family, when combined with mutation of p19Ink4d causes hair cells to re-enter the cell cycle en masse after birth, and then die.23 Similarly, the loss of retinoblastoma1 (Rb1) also causes a loss of control of the postmitotic state and leads to hair cell apoptosis,24–26 suggesting that p19Ink4d and p21Cip1 might act upstream to maintain CDKs in an inactive state and protect hair cells from inappropriate phosphorylation and inactivation of Rb1. Taken together, these results indicate that, like many neurons,27 the terminally differentiated state of postnatal hair cells is incompatible with cell cycle reentry. Thus, inducing conditional proliferation in surviving hair cells does not seem to be an attractive strategy for therapeutic design.
Supporting cells are the source of new hair cells in regenerating bird auditory organs14; thus, they are an attractive target for therapeutic manipulation. To regenerate a functional cochlea, the correct ratio of hair cells to supporting cells must be reproduced in the mosaic: therefore, lost cells must be replaced through mitosis. Although p27Kip1 levels are downregulated in hair cells during differentiation, p27Kip1 levels increase in differentiating supporting cells.18,24 This high level of CDK inhibitor expression is partly responsible for maintaining the postmitotic state of supporting cells, as supporting cell proliferation occurs at a low level in the p27Kip1 knockout.21
Additional evidence for a role of p27Kip1 in regulating supporting cell quiescence comes from recent studies on purified, dissociated supporting cells grown in culture from perinatal and 2-week-old organ of Corti.28 Interestingly, wild-type perinatal cochlear supporting cells maintain a latent capacity for proliferation. When these cells are purified and placed in culture, they rapidly downregulate p27Kip1 and expand in number.28 However, they undergo an age-dependent change in their ability to divide under these conditions. Supporting cells purified from 2-week-old organ of Corti do not downregulate p27Kip1, and very few cells are able to re-enter the cell cycle.
The correlation between p27Kip1 downregulation and cell cycle reentry in perinatal supporting cells suggested a role in the failure of regeneration. However, only partial rescue of proliferation is observed in supporting cells obtained from p27Kip1-null mice,28 indicating that p27Kip1 is only one element in the machinery responsible for maintaining the postmitotic state of supporting cells. Forced expression of the F-box protein Skp2, an ubiquitin ligase that targets p27Kip129 and other cell cycle regulatory molecules, results in supporting cell proliferation in the mature guinea pig cochlea.30 Thus, both transcriptional and posttranslational mechanisms of p27Kip1 present important targets for future research and potential therapeutic manipulation.
Stability of the Differentiated State of Supporting Cells in the Postnatal Organ of Corti
In uninjured birds and mammals, auditory hair cells and supporting cells maintain their differentiated state through the life of the animal. When hair cells are lost in birds, supporting cells can undergo direct transdifferentiation into hair cells in response to the loss of hair cells.12,13,31 This is marked by the upregulation of the basic helix-loop-helix (bHLH) transcription factor Atoh1 in the differentiating cell,13 followed by the expression of other hair cell specific genes.12 In mammals, it is unknown what molecular changes the surrounding supporting cells undergo in response to hair cell loss. Although supporting cells do not undergo transdifferentiation to replace lost hair cells, they are able to maintain and apparently expand the tight junctions at their apical surface in order to maintain epithelial integrity. In some cases, the supporting cell phenotype is lost as well, and a flat, cuboidal epithelium appears.32
In spite of the lack of supporting cell transdifferentiation in response to hair cell loss in mature mammals, a number of experiments have indicated a latent capacity for embryonic and perinatal mammalian supporting cells to differentiate into hair cells. In embryonic organ of Corti, laser ablation of nascent hair cells led to the recruitment of surrounding supporting cells.33 More recently, White28 and colleagues showed that purified perinatal mouse supporting cells were also capable of direct transdifferentiation into hair cells in specific culture conditions. This latent capacity was also observed in young, hearing mice.28
Given that Atoh1 is the earliest known marker for hair cell differentiation, both in embryogenesis and avian regeneration, understanding its regulation may be key to stimulating regeneration in mammals. Indeed, forced expression of Atoh1 in prenatal rodent supporting cells causes the differentiation of these cells into sensory hair cells, both in vitro34 and in vivo.35,36 Several lines of evidence suggest that Atoh1 in supporting cells may be negatively regulated through lateral inhibition via Notch signaling. First, Notch receptors and ligands, as well as downstream effectors, such as the Hes/Hey family of bHLH transcription factors, are all expressed in supporting cells.37–39 Second, genetic disruption of Notch signaling during development leads to the production of supernumerary hair cells.38,40–42 In addition, disruption of Notch signaling by pharmacological inhibition of gamma-secretase leads to the direct transdifferentiation of embryonic and perinatal supporting cells into hair cells, and is accompanied by the downregulation of some members of the Hes/Hey family and by the upregulation of Atoh1.39,43–46 Taken together, these results suggest that most supporting cells are held in the differentiated state through a process of Notch-dependent lateral inhibition, signaled either through neighboring hair cells, supporting cells, or both (but see Doetzlhofer and co-workers46 for one exception).
Conclusion: Mammalian Cochlear Regeneration
To regenerate a functional organ, lost cells must be replaced in kind, number, and position. The maturational changes in the organ of Corti that limit this capacity remain unknown. Given that, in damaged avian auditory sensory epithelia, supporting cells are able to replenish lost hair cells, it is important to evaluate the capacities of mammalian supporting cells for proliferation and hair cell generation. Current research, briefly summarized above, suggests that embryonic and perinatal mammalian supporting cells retain a latent capacity for regeneration. These studies give hope and direction to investigations aimed at stimulating regeneration in the inner ear. Further research on molecular pathways that control proliferation, differentiation, and patterning the mammalian cochlea will be vital for the design of therapies to regenerate human hearing.
Footnotes
Conflicts of Interest
The authors declare no conflicts of interest.
References
- 1.Davis RR, Kozel P & Erway LC. 2003. Genetic influences in individual susceptibility to noise: a review. Noise Health 5: 19–28. [PubMed] [Google Scholar]
- 2.Seidman MD, Ahmad N & Bai U. 2002. Molecular mechanisms of age-related hearing loss. Ageing Res. Rev 1: 331–343. [DOI] [PubMed] [Google Scholar]
- 3.Chardin S & Romand R. 1995. Regeneration and mammalian auditory hair cells. Science 267: 707–711. [DOI] [PubMed] [Google Scholar]
- 4.Roberson DW & Rubel EW. 1994. Cell division in the gerbil cochlea after acoustic trauma. Am. J. Otol 15: 28–34. [PubMed] [Google Scholar]
- 5.Baird RA, Steyger PS & Schuff NR. 1996. Mitotic and nonmitotic hair cell regeneration in the bullfrog vestibular otolith organs. Ann. N. Y. Acad. Sci 781: 59–70. [DOI] [PubMed] [Google Scholar]
- 6.Balak KJ, Corwin JT & Jones JE. 1990. Regenerated hair cells can originate from supporting cell progeny: evidence from phototoxicity and laser ablation experiments in the lateral line system. J. Neurosci 10: 2502–2512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Corwin JT & Cotanche DA. 1988. Regeneration of sensory hair cells after acoustic trauma. Science 240: 1772–1774. [DOI] [PubMed] [Google Scholar]
- 8.Ryals BM & Rubel EW. 1988. Hair cell regeneration after acoustic trauma in adult Coturnix quail. Science 240: 1774–1776. [DOI] [PubMed] [Google Scholar]
- 9.Weisleder P & Rubel EW. 1992. Hair cell regeneration in the avian vestibular epithelium. Exp. Neurol 115: 2–6. [DOI] [PubMed] [Google Scholar]
- 10.Bhave SA et al. 1995. Cell cycle progression in gentamicin-damaged avian cochleas. J. Neurosci 15: 4618–4628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Janas JD, Cotanche DA & Rubel EW. 1995. Avian cochlear hair cell regeneration: stereological analyses of damage and recovery from a single high dose of gentamicin. Hear Res. 92: 17–29. [DOI] [PubMed] [Google Scholar]
- 12.Duncan LJ et al. 2006. Differential expression of unconventional myosins in apoptotic and regenerating chick hair cells confirms two regeneration mechanisms. J. Comp. Neurol 499: 691–701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cafaro J, Lee GS & Stone JS. 2007. Atoh1 expression defines activated progenitors and differentiating hair cells during avian hair cell regeneration. Dev. Dyn 236: 156–170. [DOI] [PubMed] [Google Scholar]
- 14.Stone JS & Cotanche DA. 2007. Hair cell regeneration in the avian auditory epithelium. Int. J. Dev. Biol 51: 633–647. [DOI] [PubMed] [Google Scholar]
- 15.Stone JS & Rubel EW. 1999. Delta1 expression during avian hair cell regeneration. Development 126: 961–973. [DOI] [PubMed] [Google Scholar]
- 16.Ruben RJ 1967. Development of the inner ear of the mouse: a radioautographic study of terminal mitoses. Acta. Otolaryngol. Suppl 220:221–244. [PubMed] [Google Scholar]
- 17.Borriello A et al. 2007. p27Kip1 metabolism: a fascinating labyrinth. Cell Cycle 6: 1053–1061. [DOI] [PubMed] [Google Scholar]
- 18.Chen P & Segil N. 1999. p27(Kip1) links cell proliferation to morphogenesis in the developing organ of Corti. Development 126: 1581–1590. [DOI] [PubMed] [Google Scholar]
- 19.Lee YS, Liu F & Segil N. 2006. A morphogenetic wave of p27Kip1 transcription directs cell cycle exit during organ of Corti development. Development 133: 2817–2826. [DOI] [PubMed] [Google Scholar]
- 20.Chen P et al. 2002. The role of Math1 in inner ear development: uncoupling the establishment of the sensory primordium from hair cell fate determination. Development 129: 2495–2505. [DOI] [PubMed] [Google Scholar]
- 21.Lowenheim H et al. 1999. Gene disruption of p27(Kip1) allows cell proliferation in the postnatal and adult organ of corti. Proc. Natl. Acad. Sci. USA 96: 4084–4088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chen P et al. 2003. Progressive hearing loss in mice lacking the cyclin-dependent kinase inhibitor Ink4d. Nat. Cell Biol 5: 422–426. [DOI] [PubMed] [Google Scholar]
- 23.Laine H et al. 2007. p19(Ink4d) and p21(Cip1) collaborate to maintain the postmitotic state of auditory hair cells, their codeletion leading to DNA damage and p53-mediated apoptosis. J. Neurosci 27: 1434–1444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mantela J et al. 2005. The retinoblastoma gene pathway regulates the postmitotic state of hair cells of the mouse inner ear. Development 132: 2377–2388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sage C et al. 2005. Proliferation of functional hair cells in vivo in the absence of the retinoblastoma protein. Science 307: 1114–1118. [DOI] [PubMed] [Google Scholar]
- 26.Sage C et al. 2006. Essential role of retinoblastoma protein in mammalian hair cell development and hearing. Proc. Natl. Acad. Sci. USA 103: 7345–7350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Liu DX & Greene LA. 2001. Neuronal apoptosis at the G1/S cell cycle checkpoint. Cell Tissue Res 305: 217–228. [DOI] [PubMed] [Google Scholar]
- 28.White PM et al. 2006. Mammalian cochlear supporting cells can divide and trans-differentiate into hair cells. Nature 441: 984–987. [DOI] [PubMed] [Google Scholar]
- 29.Sabile A et al. 2006. Regulation of p27 degradation and S-phase progression by Ro52 RING finger protein. Mol. Cell Biol 26: 5994–6004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Minoda R et al. 2007. Manipulating cell cycle regulation in the mature cochlea. Hear Res. 232: 44–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Roberson DW, Alosi JA & Cotanche DA. 2004. Direct transdifferentiation gives rise to the earliest new hair cells in regenerating avian auditory epithelium. J. Neurosci. Res 78: 461–471. [DOI] [PubMed] [Google Scholar]
- 32.Izumikawa M et al. 2008. Response of the flat cochlear epithelium to forced expression of Atoh1. Hear Res. 240: 52–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kelley MW, Talreja DR & Corwin JT. 1995. Replacement of hair cells after laser microbeam irradiation in cultured organs of corti from embryonic and neonatal mice. J. Neurosci 15: 3013–3026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zheng JL et al. 2000. Hes1 is a negative regulator of inner ear hair cell differentiation. Development 127: 4551–4560. [DOI] [PubMed] [Google Scholar]
- 35.Shou J, Zheng JL & Gao WQ. 2003. Robust generation of new hair cells in the mature mammalian inner ear by adenoviral expression of Hath1. Mol. Cell Neurosci 23: 169–179. [DOI] [PubMed] [Google Scholar]
- 36.Izumikawa M et al. 2005. Auditory hair cell replacement and hearing improvement by Atoh1 gene therapy in deaf mammals. Nat. Med 11: 271–276. [DOI] [PubMed] [Google Scholar]
- 37.Lewis AK et al. 1998. Distinct expression patterns of notch family receptors and ligands during development of the mammalian inner ear. Mech. Dev 78: 159–163. [DOI] [PubMed] [Google Scholar]
- 38.Zine A & de Ribaupierre F. 2002. Notch/Notch ligands and Math1 expression patterns in the organ of Corti of wild-type and Hes1 and Hes5 mutant mice. Hear Res. 170: 22–31. [DOI] [PubMed] [Google Scholar]
- 39.Hayashi T et al. 2008. Hesr1 and Hesr2 may act as early effectors of Notch signaling in the developing cochlea. Dev. Biol 316: 87–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kiernan AE, Xu J & Gridley T. 2006. The Notch ligand JAG1 is required for sensory progenitor development in the mammalian inner ear. PLoS Genet. 2: e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zine A et al. 2001. Hes1 and Hes5 activities are required for the normal development of the hair cells in the mammalian inner ear. J. Neurosci 21: 4712–4720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zine A, Van De Water TR & de Ribaupierre F. 2000. Notch signaling regulates the pattern of auditory hair cell differentiation in mammals. Development 127: 3373–3383. [DOI] [PubMed] [Google Scholar]
- 43.Tang LS, Montemayor C & Pereira FA. 2006. Sensorineural hearing loss: potential therapies and gene targets for drug development. IUBMB Life 58: 525–530. [DOI] [PubMed] [Google Scholar]
- 44.Takebayashi S et al. 2007. Multiple roles of Notch signaling in cochlear development. Dev. Biol 307: 165–178. [DOI] [PubMed] [Google Scholar]
- 45.Hori R et al. 2007. Pharmacological inhibition of Notch signaling in the mature guinea pig cochlea. Neuroreport 18: 1911–1914. [DOI] [PubMed] [Google Scholar]
- 46.Doetzlhofer A et al. 2008. Hey2 regulation by FGF provides a Notch-independent mechanism for maintaining pillar cell fate in the organ of Corti. Developmental Cell. 16: 58–69. [DOI] [PMC free article] [PubMed] [Google Scholar]


