Abstract
Endoplasmic reticulum (ER) stress-induced Pancreatic β-cell dysfunction and death plays important roles in the development of diabetes. The 1,2,3-triazole derivative 1 is one of only a few structures that have thus far been identified that protect β cells against ER stress. However, this compound has narrow activity range and limited aqueous solubility. To overcome these, we designed and synthesized a new scaffold in which the triazole pharmacophore was substituted with a glycine-like amino acid. Structure-activity relationship studies on this scaffold identified a N-(2-(Benzylamino)-2-oxoethyl)benzamide analog WO5m that possesses β-cell protective activity against ER stress with much improved potency (maximal activity at 100% with EC50 at 0.1 ± 0.01 μM) and water solubility. Identification of this novel β-cell-protective scaffold thus provides a new promising modality for the treatment of diabetes.
Keywords: ER stress, Pancreatic β cell survival, Diabetes, UPR, Aqueous solubility
Keywords: ER stress, beta cell death, diabetes, beta cell survival, benzamide, anti-diabetic drug, hyperglycemia
Graphical Abstract

1. Introduction:
The endoplasmic reticulum (ER) has key roles in synthesis, folding and maturation of secreted transmembrane proteins.1–2 Upon translation, secretory, luminal and membrane proteins are translocated into the lumen of the ER where they are covalently modified and attain their proper folding.3 A balance between the ER protein loading and its folding capacity must be established to maintain the proper function of the ER. However, pathophysiological stimuli can disrupt this ER homeostasis, resulting in an accumulation of unfolded or misfolded proteins in the ER, a condition known as ER stress. ER stress triggers an evolutionarily conserved signaling cascade called the unfolded protein response (UPR). This process is mediated by three ER membrane-spanning proteins, inositol-requiring protein 1α (IRE1α), PKR-like ER kinase (PERK), and activating transcription factor 6 (ATF6), which act as unfolded protein sensors.4–7 In unstressed cells, these sensors are maintained in an inactive state through interaction with the protein chaperone binding immunoglobulin protein (BiP). Under ER stress, unfolded and misfolded proteins accumulate in the ER and bind to and sequester BiP, thereby releasing and activating the sensors8. As an initial adaptive response, IRE1α, PERK, and ATF6 each activates a series of events aimed at restoring ER homeostasis by altering the translation, folding, and post-translational modification of secreted and membrane proteins.1 Under chronic or severe ER stress, however, the UPR often fails to adequately resolve ER stress and instead activates the expression of ER stress-specific pro-apoptotic genes such as the transcription factor C/EBP-homologous protein (CHOP), eventually leading to cell death.
Type 2 Diabetes (T2D), a chronic condition of hyperglycemia, is a pervasive threat to health and a burden on the healthcare system in the United States. T2D usually develops in obese and insulin-resistant subjects with the onset of insulin-producing β cell dysfunction and death.9 Increasing evidence indicates that ER stress is a major mechanism underlying the progressive decline in β cell function and mass in T2D patients.10–11 ER stress has also been implicated in T1D and monogenic diabetes.12–13 These findings illustrate the therapeutic potential for novel drugs that block ER stress-induced β cell dysfunction and death for the treatment of diabetes.
Recent reports of chemical chaperons such as 4-phenyl butyrate (PBA)14–15 and tauroursodeoxycholic acid (TUDCA)14–17 (see Figure 1) have shown effect on ER stress modulation and antidiabetic activity in T2D animal models. However, because of their low efficacy, large dose is needed for any therapeutic treatment, which makes it unlikely that these agents can be used for the treatment for human patients due to the increased toxicity.18–19
Figure 1.

Known β-cell protecting agents against ER stress.
Recently, our group has discovered a novel small molecule, 1-(3-chloro-2-methylphenyl)-N-(2,5-dimethylphenyl)-5-methyl-1H-1,2,3-triazole-4-carboxamide (1)20 that protects β cell function and survival against ER stress with maximal activity at 97% and an EC50 value of about 6 μM.
However, the 1,2,3-triazole analog exhibited β cell protective activity at around 5~10 μM and aqueous insolubility, which becomes a hindrance for further drug development. In this study, we used 1 as a starting point to synthesize a series of novel peptide mimics and obtained several new analogs with better potency in a wider range of concentration and with improved aqueous solubility.
2. Results and discussion
Synthesis of N-(2-(Benzylamino)-2-oxoethyl)benzamide 5a-q analogs were outlined in Scheme 1 and substituents are listed in Table 1. Commercially available substituted benzoic acids 2 were coupled with corresponding substituted ethyl glycinates 3 in the presence of EDC/HOBt in dimethylformamide to yield benzoylglycine ester, which was hydrolyzed with an aqueous solution of LiOH in ethanol at 5−10 °C afforded key intermediate benzoylglycine 4. For preparation of 5a-q analogs, benzoylglycines 4 were reacted with various substituted benzyl amine in dichloromethane using HATU at 0-rt yielded N-(2-(Benzylamino)-2-oxoethyl)benzamide 5 analogs in excellent yields. All these synthesized compounds were listed in Table 1 and characterized by physical and spectral analysis data that confirmed their assigned structures.
Scheme 1.

Synthesis of compounds 5a-q. Reagents & Conditions: a) HOBt, EDC, DIPEA, DMF at 0-rt; b) LiOH/EtOH, 0-rt; c) HATU, DIPEA/DCM
Table 1.
Effects of compounds 5a-qand 1on CHOP-Luc reporter and β cell viability against (tunicamycin) Tm
 | |||||
|---|---|---|---|---|---|
| CompoundID | R1 | R2 | R3 | Max Activity (%)a | EC50 (μM)b |
| 1c | 3-Cl,2-Me | - | 2,5-di Me | 97 | 6 ± 1 |
| 5a | 3-Cl,2-Me | H | 2,5-di Me | 45 | 18 ± 4 |
| 5b | 3-Cl,2-Me | H | 2-Pyridine | 16 | 38 ± 9 |
| 5c | 3-Cl,2-Me | H | Indazole | Cytotoxic | - |
| 5d | 3-Cl,2-Me | H | 4-OMe | 55 | 32 ± 7 |
| 5e | 3-Cl,2-Me | H | 4-SO2NH2 | 52 | 40 ± 12 |
| 5f | 3-Cl,2-Me | H | Tyramine | 41 | 34 ± 8 |
| 5g | 3-Cl,2-Me | H | 4-CF3 | 88 | 13 ± 1 |
| 5h | 3-Cl,2-Me | H | 3-OMe,4-OH | 100 | 10 ± 2 |
| 5i | 3-Cl,2-Me | H | 3-CF3 | 46 | 32 ± 7 |
| 5j | H | H | 4-CF3 | 35 | 43 ± 11 |
| 5k | 4-Et | H | 4-CF3 | 42 | 45 ± 8 |
| 5l | 4-F | H | 4-CF3 | 12 | 41 ± 6 |
| 5m (WO5m) | 3-OH | H | 4-CF3 | 100 | 0.1 ± 0.01 |
| 5n | 3-OH | H | 3-OMe,4-OH | 30 | 65 ± 8 |
| 5o | 3-OMe,4-OH | H | 4-CF3 | 43 | 29 ± 6 |
| 5p | 3-Cl,2-Me | Me | 4-CF3 | 34 | 63 ± 4 |
| 5q | 3-Cl,2-Me | Tyrosine | 4-CF3 | Cytotoxic | - |
Maximum activity value is reported as % rescue from Tm (0.15 μg/mL) -induced reduction of cell viability, as measured by intracellular ATP levels; the values for Tm treatment alone and control (DMSO, without Tm) treatment are designated as 0% and 100%, respectively.
EC50 values (the concentrations that reach half-maximal activity) for INS-1 cell viability are calculated with GraphPad Prism from the data of ten 2-fold serial titration points in all tables. All experiments were performed in triplicate.
Compound 1 contains the 1,2,3-triazole group, as shown in Figure 1.
We have previously described that compound 1 possesses the structural motif of triazole amide (Figure 1) for effective β-cell protective activity against ER stress (see Table 1).20 However, 1 showed less-desired potency within a narrow range of doses,20 and poor aqueous solubility (see Figure 2). Since 1,2,3-triazole is a scaffold frequently present in various structures with multiple bioactivities, such as antimicrobial, antiviral, and antitumor effects, we reasoned that the triazole ring could be the reason for these less-desirable properties. We therefore designed and synthesized novel analogs (5a–q, Table 1) of 1 by replacing 1,2,3-triazole with amide bond isosteres and studied the effect of resultant analogs on β-cell protection against ER stress. The newly synthesized compounds were tested for the viability of rat INS-1 β-cells in the presence of tunicamycin (Tm), a potent ER stress inducer that inhibits N-linked glycosylation of proteins. The maximum activities and the concentrations that reach half-maximal activity (EC50) of the compounds were evaluated by the degree of increase in viability of INS-1 cells co-treated with the compounds in the presence of Tm compared with Tm treatment alone.
Figure 2. Compound WO5m protects INS-1 β cells against Tm-induced apoptosis.
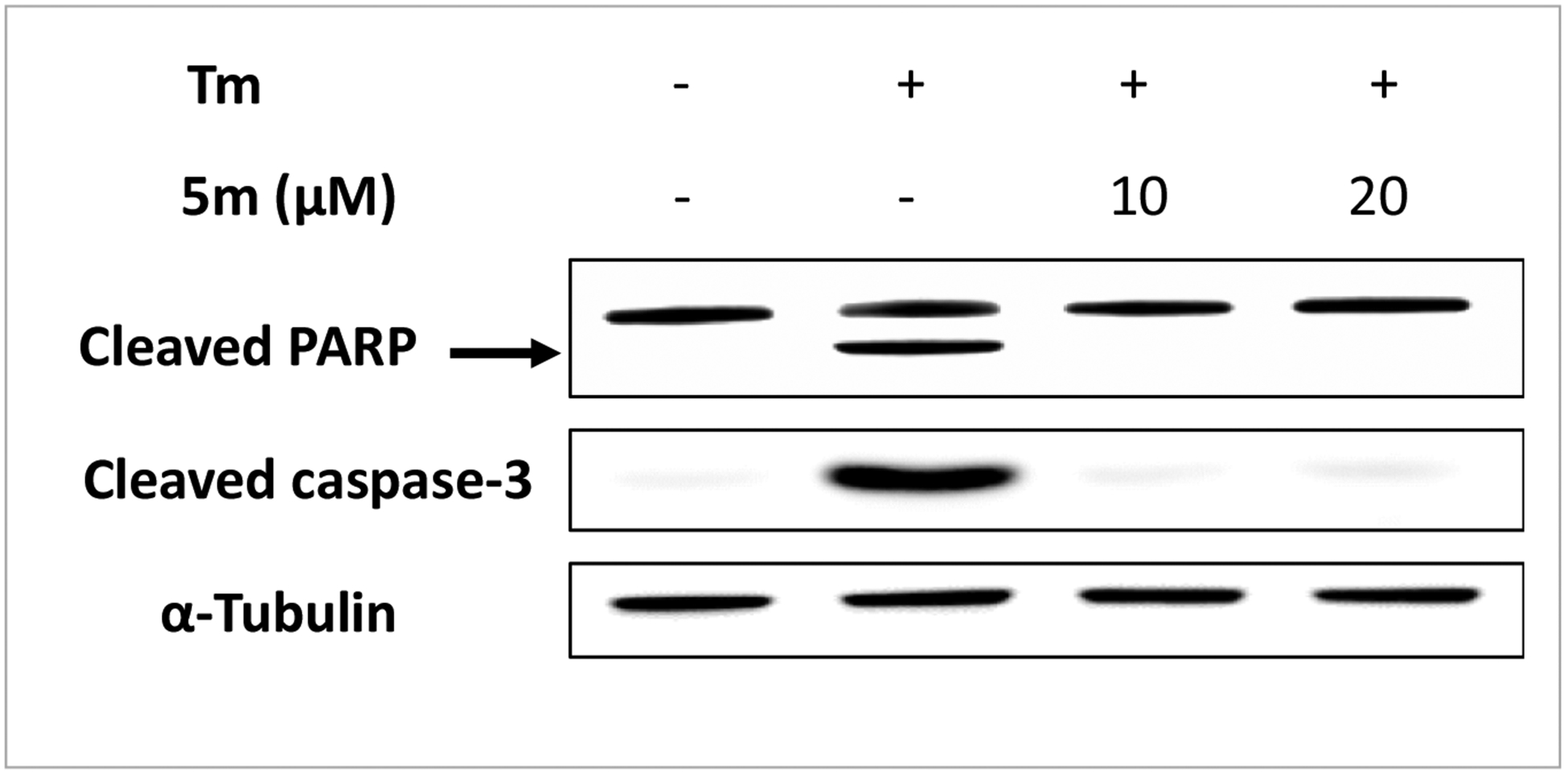
INS-1 cells were treated with or without Tm (0.3 μg/mL) in the presence of WO5m at indicated concentrations or DMSO for 24 h. Cleaved caspase-3 and PARP were determined by Western blotting. α-Tubulin was used as a loading control. The data shown are representative of 3 independent experiments.
First, we wanted to determine whether replacing the 1,2,3-triazole group with the amino acid glycine-like group still maintains the β-cell protective activity. We synthesized 5a by replacing the 1,2,3-triazole group with the glycine-like group while maintaining other moieties intact and observed that 5a exhibited moderate activity (maximum activity = 45%, EC50 = 18.6 ± 4 μM). With this encouraging result, we explored the effect of substituents on the moieties on β-cell protection. We started investigating the effect of modifying the right ring (R3) on the 5a scaffold. We found that the glycine derivatives with modifications as 2-pyridine (5b) or indazole (5c) are not or minimally active (maximal activity at 16 and 0%, respectively), while derivatives with modifications as 4-OMe (5d), 4-SO2NH2 (5e), or Tyramine (5f) are moderately active with maximal activity from 41 to 55%, suggesting that the introduction of new hydrogen bonding groups and the extension of length in-between amide and B-ring may are not beneficial for the activity. However, excitingly, substitutions with 4-CF3 (5g) or 4-OH, 3-OMe (5h) yielded very active compounds with maximal activity at 88 and 100%, although the EC50s of both derivatives are still less desired (13 ± 1 and 10 ± 2 μM, respectively). Next, as 5g showed significant β-cell protective activity (see Table 1), to establish the suitable position of the -CF3 group in the phenyl ring of 5g, we synthesized 5h with the -CF3 group at position 3 and observed that 5h (with 3-CF3) was less potent than 5g (with 4-CF3) (5h with maximum activity = 46%, EC50 = 32 ±7 μM vs. 5g with maximum activity = 88%, EC50 = 13 ± 1 μM). These results indicate that the CF3 substitution at position 4 on phenyl ring (5g) is more favorable than at position 3 (5h) for β-cell protective activity. We therefore kept the 4-CF3 substitution on the right ring for further structure-activity relationship (SAR) studies.
We next sought to determine the suitable substituents on the left ring (R1) on β-cell protection against ER stress. The compounds synthesized with various substituents at the left ring (5j–n) are listed in Table 1. While substituting the 3-Cl,2-Me (5g) on the phenyl ring R1 with hydrogen (5j), 4-ethyl (5k), or 4-F (5l) substantially reduced their respective activities (maximal activity: 88% (5g) vs. 35% (5j), 42% (5k), or 12% (5l), and EC50 (μM): 13 ± 1 (5a) vs. 43 ± 11 (5j), 45 ± 8 (5k), or 41 ± 6 (5l)), substitution with 3-hydroxy group (5m (WO5m)) remarkably enhanced the activity with the maximum activity at 100% and EC50 = 0.1 ± 0.01 μM. These results suggest that the hydrogen bond donating group at meta position of R1 is critical for the β-cell protective activity. As we have shown that compound 5h with the 3-OMe,4-OH substituent at R3 in a scaffold containing a 3-Cl,2-Me moieties at R1 exhibited the excellent β-cell protective activity, and that the 3-OH substituent (WO5m) at R1 yielded an activity superior to that of 3-Cl,2-Me moieties (5g) in a scaffold with 4-CF3 at R3, we wondered whether an analog containing 3-OH at R1 and 3-OMe,4-OH at R3 (5n) and a second analog containing 3-OMe,4-OH at R1 with 4-CF3 at R3 (5o) are equally active. However, as shown in Table 1, these analogs exhibited a significantly compromised activity. To further explore the SAR, we studied the effect of substituent at R2 position, compounds 5p (R2 = Me) and 5q (R2 = Tyrosine) are significantly less or not active. These results indicated that the bulkier substitution at R2 position is not well tolerated.
Based on above SAR results we selected the most potent compound WO5m for further investigation. We next investigated whether WO5m treatment inhibited ER stress-induced β-cell death. We used cleaved caspase-3 and cleaved PARP for this purpose. Under chronic ER stress, caspase 3 and PARP are activated by undergoing proteolytic cleavage to trigger apoptotic cell death. Tm treatment for 24 h significantly induced both cleaved caspase-3 and cleaved PARP protein levels in INS-1 cells (Figure 2). However, WO5m co-treatment eliminated the Tm-induced cleavage of both caspase-3 and PARP (Figure 2). These results demonstrated that WO5m protects β-cells against ER stressor Tm-induced cell death.
We further investigated whether the protective effect of WO5m on β cells is specific to Tm-induced stress. We used two other ER stressors thapsigargin (TG), which disrupts intraluminal Ca2+ homeostasis in the ER and causes accumulation of unfolded proteins by inhibiting sarcoplasmic/endoplasmic reticulum Ca2+-ATPase (SERCA), and brefeldin A (BFA), which inhibits a key guanine nucleotide exchange factor essential for the transport of proteins from the ER to the Golgi, to treat INS-1 cells.21 As expected, both stressors induced significant reduction in cell viability; however, compound WO5m significantly reversed the reduction in viability in INS-1 cells treated with TG or BFA (Figure 3). All these results indicate that compound WO5m protects β cell survival against ER stress induced by all stressors tested.
Figure 3. Compound 5m protects INS-1 β cells against other ER stress inducers.
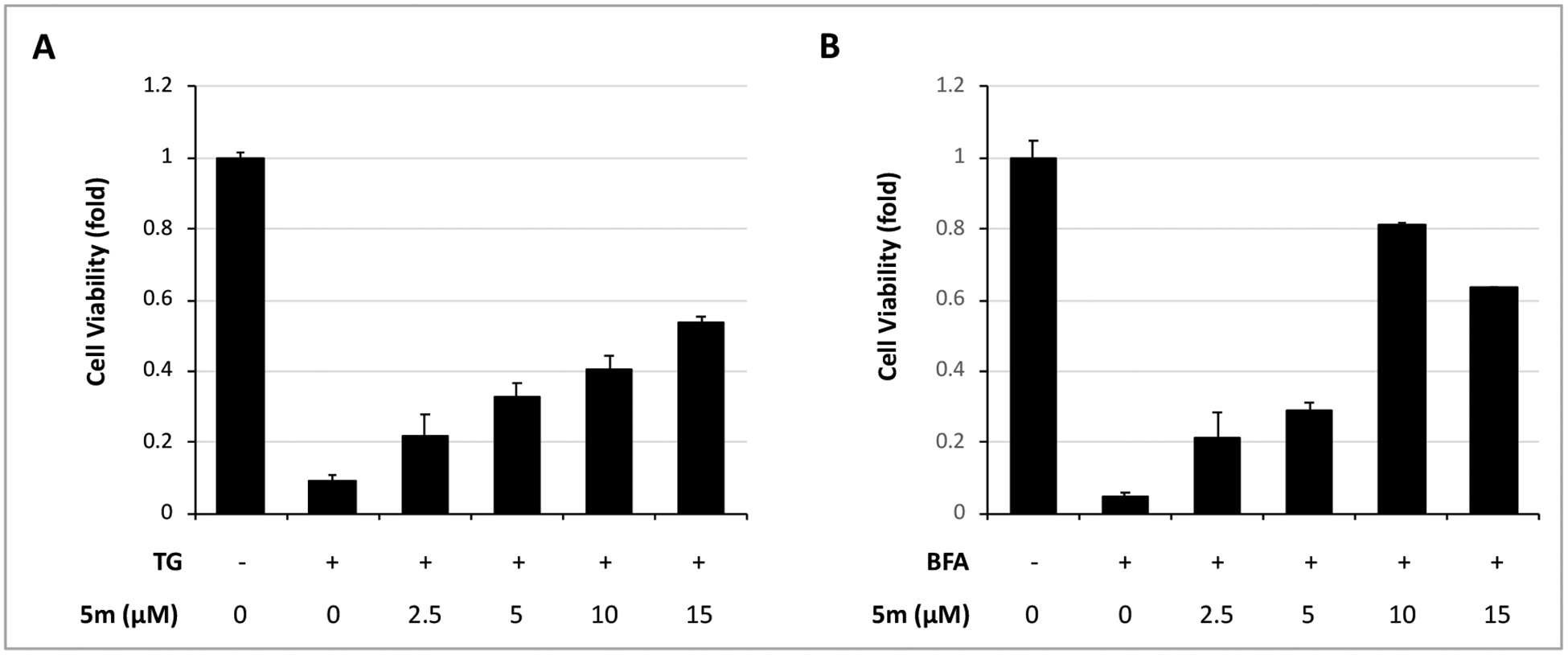
INS-1 cells were treated with or without TG (18 nM) (A) or with or without BFA (0.2 μg/mL) (B) in the presence of WO5m at indicated concentrations or DMSO for 72 h. The cell viability was determined by CellTiter-Glo. The values for control (DMSO, without Tm) treatment were normalized as 1. The results in all panels are the means of 3 replicate wells and are representative of 3 independent experiments.
Finally, as a major drawback of compound 1 is its poor water solubility, we investigated whether the newly synthesized compounds obtain the improvement in aqueous solubility. In the SAR studies, we hypothesized that substituting the 1,2,3-triazole moiety with the amino acid glycinelike group likely improves the water solubility of the newly synthesized compounds. Using a thermodynamic turbidimetric assay we evaluated the aqueous solubility of the newly synthesized compounds. As shown in Figure 4, as expected, several compounds including 5g, 5h, and WO5m exhibited improved water solubility compare to 1. In contrast to compound 1 that did not show any water solubility (i.e., near 0 μm concentration), WO5m exhibited complete solubility at the concentration of approximately 50 μm in H2O (Figure 4). 5a and 5f exhibited ever higher water solubility than WO5m, with ~75 and ~200 50 μm in H2O, respectively.
Figure 4. Aqueous solubility of newly synthesized compounds.

Aqueous solubility was assessed as the degree of turbidity of compounds at indicated concentrations in aqueous solutions, detected by absorbance at 620 nm. The experiments were performed in triplicate, with the results as the means ± SD of triplicate wells.
3. Conclusion
In this study, we have discovered a series of N-(2-(Benzylamino)-2-oxoethyl)benzamide derivatives capable of protecting pancreatic β cells from ER stress-induced death. Although we initiated the SAR studies using compound 1, a 1,2,3-triazole-4-carboxamide derivative, as the starting scaffold, we identified a novel scaffold that possesses potent β-cell protective activity. In particular, compound WO5m exhibited a maximal β-cell protective activity at 100% with EC50 at 0.1 ± 0.01 μM. Furthermore, this class of compounds also possesses far better water solubility than the original 1,2,3-triazole derivatives, a property critical for further drug development. In summary, we have discovered a novel scaffold of β cell-protective compounds against ER stress with excellent potency and aqueous solubility.
Supporting information:
Methods and Materials
1. Chemistry:
All commercial chemicals were used as obtained and all solvents were purified by the standard procedures prior to use. Flash column chromatography was performed with E Merck silica gel (230–400 mesh). NMR spectra were measured against the peak of tetramethylsilane by Varain Unity Inova 400 NMR (400 MHz) spectrometers. All tested compounds were evaluated on the Agilent HPLC systems usingACE-C18 column (250 × 4.6 mm) was used as the stationary phase. HPLC conditions include a flow rate of 1.0 mL/min using water and acetonitrile as solvents and a detection wavelength of 254 nm and determined to be ≥95% pure.
General procedure for the synthesis of (4)
To a stirred solution of substituted benzoic acid (1 equivalents) in dimethylformamide was added EDC (1 equivalents), HOBt (1 equivalents) cooled to 0–5 ˚C and added DIPEA (2 equivalents) stirred for 10min at same temperature. After 10 min, appropriate amine (1 equivalent) was dropwise added and continued stirring for overnight. After TLC confirmation, reaction mixture was evaporated residue was extracted with ethyl acetate and washed with water. Organic layer was dried over anhydrous sodium sulfate. Solvent was removed under vacuum to provide crude residue which was purified by flash column chromatography.
Hydrolysis: Ester derivative dissolved in 10% Aq. Ethanol and 2 equivalents of LiOH dissolved in water was added to the reaction mixture and vigorously stirred for 5h. after completion of reaction, evaporated the reaction solvent and acidified with 1N HCl and filtered to collect the desired compound as a white solid (66%).
General procedure for the synthesis of (5)
To a mixture of the acid (1equivalent) in DCM was added DIPEA (3 equivalents) and HATU (1 equivalents). The mixture was stirred for 5 min, and then the appropriate amine (1 equivalents) was added. The reaction mixture was stirred at rt for 30 min. The completion of the reaction was monitored by TLC. The solvent was removed in vacuo to obtain the crude which was purified by flash chromatography to provide product (78%)
3-Chloro-N-(2-((2,5-dimethylbenzyl)amino)-2-oxoethyl)-2-methylbenzamide (5a)
The title compound was prepared following general procedures B: off-white powder. 1H NMR (CDCl3, 400 MHz) δ: 7.50 (d, J = 7.8 Hz, 1H), 7.41 (d, J = 7.8 Hz, 1H), 7.19 (m, 3H), 7.14 (t, J = 7.7 Hz, 1H), 6.97 (bs, 1H), 6.76 (bs, 1H), 4.50 (d, J = 5.7 Hz, 2H), 4.17 (d, J = 5.0 Hz, 2H), 2.37 (s, 3H), 2.29 (s, 6H). 13C NMR (100MHz, CDCl3) δ: 171.0, 167.9, 156.5, 148.1, 139.6, 136.8, 132.5, 132.1, 127.2, 125.5, 128.5, 127.2, 125.4, 124.1, 120.9, 48.3, 43.3, 21.6, 18.9, 17.2. HPLC purity 98.31%
3-Chloro-2-methyl-N-(2-oxo-2-((pyridin-2-ylmethyl)amino)ethyl)benzamide (5b)
The title compound was prepared following general procedures B: a brown solid. 1H NMR (CDCl3, 400 MHz) δ: 8.67 (d, J = 7.8 Hz, 1H), 7.76 (t, J = 7.6 Hz, 1H), 7.50 (d, J = 7.8 Hz, 1H), 7.14 (m, 4H), 4.41 (d, J = 5.7 Hz, 2H), 3.92 (d, J = 5.0 Hz, 2H), 2.37 (s, 3H). 13C NMR (100MHz, CDCl3) δ: 169.5, 168.3, 155.5, 149.1, 137.9, 136.9, 135.8, 134.2, 130.8, 126.8, 125.3, 122.6, 122.0, 44.3, 43.2, 17.2. HPLC purity 96.11%
N-(2-((1H-benzo[d]imidazol-6-yl)amino)-2-oxoethyl)-3-chloro-2-methylbenzamide (5c)
The title compound was prepared following general procedures B: a white powder. 1H NMR (DMSO-d6, 400 MHz) δ: 12.89 (s, 1H), 10.19 (s, 1H), 8.90 (t, J = 5.7 Hz 1H), 8.12 (s, 1H), 7.90 (m, 3H), 7.67 (d, J = 8.7 Hz, 1H), 7.33 (t, J = 8.5 Hz 2H), 7.12 (d, J = 8.6 Hz 1H), 4.10 (d, J = 5.6 Hz, 2H), 2.35 (s, 3H). 13C NMR (100MHz, DMSO-d6) δ: 168.5, 167.1, 142.0, 138.6, 136.8, 136.7, 135.2, 134.4, 134.4, 132.7, 127.2, 123.7, 115.8, 109.5, 43.8, 17.1. HPLC purity 95.02%
3-Chloro-N-(2-((4-methoxybenzyl)amino)-2-oxoethyl)-2-methylbenzamide (5d)
The title compound was prepared following general procedures B: a white powder. 1H NMR (DMSO-d6, 400 MHz) δ: 7.39 (d, J = 8.0 Hz, 1H), 7.29 (m, 5H), 7.15 (d, J = 7.7 Hz, 1H), 7.86 (d, J = 7.6 Hz, 1H), 7.47 (t, J = 7.2 Hz, 1H), 7.22 (d, J = 7.8 Hz, 2H), 6.89 (d, J = 7.8 Hz, 1H ), 6.69 (bs, 1H), 4.23 (d, J = 5.6 Hz, 2H), 3.09 (s, 2H), 3.76 (s, 3H), 2.43 (s, 3H). 13C NMR (100MHz, DMSO-d6) δ: 169.3, 168.2, 158.8, 137.3, 136.8, 134.4, 132.7, 132.1, 130.8, 130.2, 128.8, 127.2, 125.7, 124.4, 123.6, 123.1, 55.8, 43.9, 43.6, 17.2. HPLC purity 99.00%
3-Chloro-2-methyl-N-(2-oxo-2-((4-sulfamoylbenzyl)amino)ethyl)benzamide (5e)
The title compound was prepared following general procedures B: a white powder. 1H NMR (DMSO-d6, 400 MHz) δ: 8.67 (bs, 1H), 8.58 (bs, 1H), 7.76 (d, J = 7.9 Hz, 2H), 7.49 (m, 3H), 7.31 (m, 4H), 4.38 (d, J = 5.7 Hz, 2H), 3.90 (d, J = 5.8 Hz, 2H), 2.37 (s, 3H). 13C NMR (100MHz, DMSO-d6) δ: 168.0, 167.9, 142.7, 141.7, 138.6, 133.3, 132.5, 129.1, 126.6, 126.2, 125.2, 124.7, 41.5, 40.9, 15.9. HPLC purity 95.30%
3-Chloro-N-(2-((4-hydroxyphenethyl)amino)-2-oxoethyl)-2-methylbenzamide (5f)
The title compound was prepared following general procedures B: a white powder. 1H NMR (DMSO-d6, 400 MHz) δ: 7.77 (s, 1H), 7.49 (bs, 1H), 7.45 (bs, 1H), 7.25 (m, 1H), 6.99 (d, J = 7.8 Hz, 2H), 6.67 (d, J = 7.6 Hz, 2H), 3.55 (d, J = 5.6 Hz, 2H), 2.80 (t, J = 5.8 Hz, 2H), 2.58 (t, J = 5.6 Hz, 2H), 2.35 (s, 3H). 13C NMR (100MHz, DMSO-d6) δ: 167.1, 163.0, 155.0, 136.8, 134.4, 134.4, 132.1, 130.2, 127.2, 125.4, 115.8, 43.8, 40.4, 35.1, 17.1. HPLC purity 98.10%
3-Chloro-2-methyl-N-(2-oxo-2-((4-(trifluoromethyl)benzyl)amino)ethyl)benzamide (5g)
The title compound was prepared following general procedures B: a white powder. 1H NMR (CDCl3, 400 MHz) δ: 7.56 (d, J = 7.8 Hz, 2H), 7.43 (d, J = 7.8 Hz, 1H), 7.37 (d, J = 7.9 Hz, 2H), 7.22 (s, 1H),7.14 (t, J = 7.7 Hz, 1H), 6.97 (bs, 1H), 6.76 (bs, 1H), 4.51 (d, J = 5.7 Hz, 2H), 4.17 (d, J = 5.0 Hz, 2H), 2.37 (s, 3H). 13C NMR (100MHz, CDCl3) δ: 170.0, 168.6, 141.7, 137.3, 135.9, 134.1, 131.1, 127.8, 126.8, 125.65, 125.2, 43.7, 43.1, 17.2. HPLC purity 97.17%
3-Chloro-N-(2-((4-hydroxy-3-methoxybenzyl)amino)-2-oxoethyl)-2-methylbenzamide (5h)
The title compound was prepared following general procedures B: a white powder. 1H NMR (DMSO-d6, 400 MHz) δ: 8.82 (s, 1H), 8.59 (t, J = 5.7 Hz, 1H), 8.30 (t, J = 5.6 Hz, 1H) 7.49 (d, J = 7.7 Hz, 1H), 7.34 (d, J = 7.4 Hz, 1H) 7.26 (t, J = 7.6 Hz, 1H) 6.85 (s, 1H), 6.68 (m, 2H), 4.20 (d, J = 5.6 Hz, 2H) 3.86 (d, J = 5.8 Hz, 2H), 3.74 (s, 3H), 2.35 (s, 3H). 13C NMR (100MHz, DMSO-d6) δ: 169.1, 168.9, 147.9, 145.8, 139.8, 134.6, 133.5, 130.5, 130.2, 127.5, 126.4, 120.16, 115.6, 112.1, 56.0, 42.8, 42.4, 17.1. HPLC purity 99.31%
3-Chloro-2-methyl-N-(2-oxo-2-((3-(trifluoromethyl)benzyl)amino)ethyl)benzamide (5i)
The title compound was prepared following general procedures B: a white powder. 1H NMR (CDCl3, 400 MHz) δ: 7.54 (d, J = 7.9 Hz, 2H), 7.42 (d, J = 7.8 Hz, 1H ), 7.35 (d, J = 8.0 Hz, 2H), 7.22 (d, J = 7.6 Hz, 1H), 7.13 (m, 2H), 6.86 (bs, 1H), 4.50 (d, J = 5.8 Hz, 2H), 4.19 (d, J = 5.0 Hz, 2H), 2.35 (s, 3H). 13C NMR (100MHz, DMSO-d6) δ: 170.1, 167.6, 137.3, 136.8, 134.4, 132.7, 132.1, 130.8, 130.2, 128.8, 127.2, 125.7, 124.4, 123.6, 123.1, 43.9, 43.6, 17.2. HPLC purity 97.68%
N-(2-Oxo-2-((4-(trifluoromethyl)benzyl)amino)ethyl)benzamide (5j)
The title compound was prepared following general procedures B: a white powder. 1H NMR (DMSO-d6, 400 MHz) δ: 8.82 (bs, 1H), 8.55 (bs, 1H) 7.88 (d, J = 7.4 Hz, 2H), 7.68 (d, J = 7.8 Hz, 2H), 7.50 (m, 5H) 4.38 (d, J = 5.6 Hz, 2H) 3.93 (d, J = 5.8 Hz, 2H); 13C NMR (100MHz, DMSO-d6) δ: 169.5, 167.6, 142.9, 133.7, 131.5, 128.2, 127.6, 127.2, 125.2, 126.4, 43.5, 42.5 HPLC purity 95.81%
4-Ethyl-N-(2-oxo-2-((4-(trifluoromethyl)benzyl)amino)ethyl)benzamide (5k)
The title compound was prepared following general procedures B: a white Solid. 1H NMR (DMSO-d6, 400 MHz) δ: 8.75 (t, J = 5.7 Hz, 1H), 8.54 (t, J = 5.6 Hz, 1H), 7.83 (d, J = 7.8 Hz, 2H), 7.68 (d, J = 8.0 Hz, 2H), 7.49 (d, J = 7.8 Hz, 2H), 7.31 (d, J = 7.8 Hz, 2H), 4.38 (d, J = 5.8 Hz, 2H) 3.91 (d, J = 5.8 Hz, 2H), 2.65 (q, J = 7.5 Hz, 2H), 1.19 (t, J = 7.5 Hz, 3H). 13C NMR (100MHz, DMSO-d6) δ: 168.5, 165.6, 150.3, 146.6, 143.6, 130.6, 126.9, 126.7, 124.2, 119.9, 41.9, 40.8, 27.2, 14.5. HPLC purity 96.55%
4-fluoro-N-(2-oxo-2-((4-(trifluoromethyl)benzyl)amino)ethyl)benzamide (5l)
The title compound was prepared following general procedures A: a white powder. 1H NMR (DMSO-d6, 400 MHz) δ: 8.88 (t, J = 5.6 Hz, 1H), 8.55 (t, J = 5.7 Hz, 1H), 7.97 (m, 2H), 7.74 (d, J = 7.9 Hz 2H), 7.44 (d, J = 8.0 Hz 2H), 7.31 (m, 4H), 4.35 (d, J = 5.8 Hz, 2H), 3.91 (d, J = 5.7 Hz, 2H), 13C NMR (100MHz, DMSO-d6) δ: 170.5, 167.1, 166.3, 141.2, 129.8, 129.1, 129.0, 128.5, 124.9, 115.8, 43.8, 43.3 HPLC purity 97.72%
3-Hydroxy-N-(2-oxo-2-((4-(trifluoromethyl)benzyl)amino)ethyl)benzamide (5m)
The title compound was prepared following general procedures B: a white powder. 1H NMR (DMSO-d6, 400 MHz) δ: 9.65 (s, 1H), 8.80 (s, 1H), 8.64 (t, J = 5.4 Hz, 1H), 8.28 (t, J = 5.6 Hz, 1H) 7.27 (m, 3H), 6.91 (d, J = 7.2 Hz, 1H), 6.83 (s, 1H), 6.67 (m, 2H) 4.18 (d, J = 5.6 Hz, 2H) 3.85 (d, J = 5.8 Hz, 2H), 13C NMR (100MHz, DMSO-d6) δ: 169.3, 166.9, 157.7, 147.8, 145.7, 135.9, 130.6, 129.6, 129.0, 120.0, 118.6, 118.3, 115.5, 114.8, 112.0, 43.3, 42.0. HPLC purity 99.28%
3-Hydroxy-N-(2-((4-hydroxy-3-methoxybenzyl)amino)-2-oxoethyl)benzamide (5n)
The title compound was prepared following general procedures B: a white powder. 1H NMR (DMSO-d6, 400 MHz) δ: 9.86 (s, 1H), 8.79 (s, 1H), 8.63 (t, J = 5.4 Hz, 1H), 8.32 (t, J = 5.6 Hz, 1H) 7.27 (m, 3H), 6.91 (d, J = 7.2 Hz, 1H), 6.83 (s, 1H), 6.67 (m, 2H) 4.18 (d, J = 5.6 Hz, 2H) 3.85 (d, J = 5.8 Hz, 2H), 3.74 (s, 3H), 13C NMR (100MHz, DMSO-d6) δ: 169.3, 166.9, 157.7, 147.8, 145.7, 135.9, 130.6, 129.6, 120.0, 118.6, 118.3, 115.5, 114.8, 112.1, 55.9, 43.6, 42.2. HPLC purity 97.48%
4-hydroxy-3-methoxy-N-(2-oxo-2-((4-(trifluoromethyl)benzyl)amino)ethyl)benzamide (5o)
The title compound was prepared following general procedures B: White Solid. 1H NMR (DMSO-d6, 400 MHz) δ: 9.89 (s, 1H), 8.87 (s, 1H), 8.24 (s, 1H), 7.54 (d, J = 7.7 Hz, 2H), 7.34 (d, J = 7.4 Hz, 1H) 7.32 (s, 1H), 7.26 (m, 3H) 4.22 (d, J = 5.6 Hz, 2H), 4.09 (d, J = 5.5 Hz, 2H), 3.74 (s, 3H). 13C NMR (100MHz, DMSO-d6) δ: 170.1, 167.9, 152.2, 151.0, 139.8, 129.0, 128.5, 126.4, 120.1, 117.6, 115.1, 56.1, 43.9, 43.3. HPLC purity 94.69%
3-Chloro-2-methyl-N-(1-oxo-1-((4-(trifluoromethyl)benzyl)amino)propan-2-yl)benzamide (5p)
The title compound was prepared following general procedures B: off-white powder. 1H NMR (DMSO-d6, 400 MHz) δ: 8.51 (m, 2H), 7.68 (d, J = 7.9 Hz, 2H), 7.49 (d, J = 8.2 Hz, 3H) 7.28 (m, 2H), 4.41 (m, 3H), 2.31 (s, 3H), 1.32 (d, J = 7.0 Hz, 3H). 13C NMR (100MHz, DMSO-d6) δ: 171.0, 167.9, 141.2, 136.8, 134.4, 132.9, 132.1, 129.0, 128.5, 127.2, 124.9, 124.1, 54.9, 43.6, 17.9, 17.2. HPLC purity 98.33%
3-Chloro-N-(3-(4-hydroxyphenyl)-1-oxo-1-((4-(trifluoromethyl)benzyl)amino)propan-2-yl)-2-methylbenzamide (5q)
The title compound was prepared following general procedures B: white solid. 1H NMR (CD3OD, 400 MHz) δ: 7.57 (d, J = 8.0 Hz, 3H), 7.42 (d, J = 6.7 Hz, 1H), 7.29 (d, J = 7.9 Hz, 2H), 7.15 (m, 2H), 7.10 (d, J = 8.2 Hz, 2H), 6.72 (d, J = 8.2 Hz, 2H) 4.77 (t, J = 7.9 Hz, 1H), 4.43 (q, J = 8.2 Hz, 2H), 2.99 (m, 2H), 2.21 (s, 3H). 13C NMR (100MHz, DMSO-d6) δ: 172.1, 167.5, 155.2, 141.2, 136.8, 134.4, 132.9, 132.1, 129.2, 129.0, 128.5, 127.2, 125.5, 124.9, 124.1, 115.8, 57.8, 43.6, 37.5, 17.2. HPLC purity 97.69%
2. Biology
2.2.1. Cell culture
Tunicamycin (Tm), thapsigargin (TG), and brefeldin A (BFA) were obtained from Sigma–Aldrich (St Louis, MO) and these chemicals were dissolved in DMSO for experiments. INS-1 cells were cultured in RPMI 1640 medium (Corning) supplemented with 10% FBS (Atlanta Biologicals), 10 mM HEPES (Gibco-Life Technologies), 1 mM sodium pyruvate (Corning), 50 μM 2-mercaptoethanol (Sigma–Aldrich), and antibiotics (100 UI/mL penicillin and 100 μg/mL streptomycin; Corning) and maintained in a humidified 5% CO2 atmosphere at 37 ˚C.
2.2.2. Cell Viability Assay
INS-1 cells were resuspended in RPMI 1640 medium containing 10% FBS and plated at 5 × 103 cells/ (40 μL.well) into white clear bottom 384-well plates (Greiner) using an automated liquid handler (Biotek). After 24 h incubation at 37 °C, library compounds were added to the wells at the indicated concentration using a pin-transfer robot (PerkinElmer). Tunicamycin (Tm) in RPMI 1640 medium containing 10% FBS was then added at a final concentration of 0.15 μg/mL. TG and BFA were treated in the same fashion for the designated concentrations. After 72 h, the medium was removed and 20 μL of CellTiter-Glo reagent which measures intracellular ATP levels as an indicator of viability (Promega) was added. Luminescence was measured 10 min later using an Envision plate reader (PerkinElmer).
2.2.3. Western blotting
INS-1 cells were seeded at 8 × 105 cells/dish in 60-mm dishes and treated for the indicated times. Cells were then washed with phosphate-buffered saline (PBS) and lysed with lysis buffer (Cell Signaling Technology, Danvers, MA) containing EDTA and phosphatase inhibitors. Aliquots of 20 μg total protein were separated on 12% SDS–PAGE gels (Life Technologies) and transferred to PVDF membranes. The membranes were probed with primary antibodies followed by the appropriate HRP-conjugated secondary antibodies (goat anti rabbit IgG and goat anti-mouse IgG, 1:5000; Santa Cruz Biotechnology, Santa Cruz, CA). Blots were then developed using Pierce ECL Western Blotting Substrate (cat no 32106). The primary antibodies and dilutions used were Caspase 3 (1:1000; Cell Signaling Technology, cat no 9664S), PARP (1:1000; Cell Signaling Technology, cat no 9542S), and α-Tubulin (1:1000; Santa Cruz Biothechnology, cat no sc8035).
2.2.4. Solubility Assay
Each compound was prepared at concentrations from 40 mM to 0.1 mM in DMSO in a serial-dilution manner. 2 μL of each of the above compound solutions was then dispensed directly to ~198 μL of pure H2O in a 96-well white clear bottom microplate. Resulting solutions were shaken at 37 °C temperature 1–2 h on a rotary shaker at varying shaking rates to reach equilibrium solubility. The turbidity of the solution was measured using UV-visible spectrophotometry at 620 nm.
Acknowledgments:
This work was supported by Oklahoma Center for the Advancement of Science and Technology and National Institutes of Health (DK108887, DK116017) to W.W.
Footnotes
The authors claim no conflict of interest.
References:
- 1.Ron D; Walter P, Signal integration in the endoplasmic reticulum unfolded protein response. Nat Rev Mol Cell Biol 2007, 8 (7), 519–29. [DOI] [PubMed] [Google Scholar]
- 2.van Meer G; Voelker DR; Feigenson GW, Membrane lipids: where they are and how they behave. Nat Rev Mol Cell Biol 2008, 9 (2), 112–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kaufman RJ, Orchestrating the unfolded protein response in health and disease. J Clin Invest 2002, 110 (10), 1389–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fonseca SG; Gromada J; Urano F, Endoplasmic reticulum stress and pancreatic beta-cell death. Trends in endocrinology and metabolism: TEM 2011, 22 (7), 266–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Back SH; Kaufman RJ, Endoplasmic reticulum stress and type 2 diabetes. Annual review of biochemistry 2012, 81, 767–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Papa FR, Endoplasmic reticulum stress, pancreatic beta-cell degeneration, and diabetes. Cold Spring Harbor perspectives in medicine 2012, 2 (9), a007666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hetz C; Chevet E; Harding HP, Targeting the unfolded protein response in disease. Nat. Rev. Drug Discov 2013, 12 (9), 703–19. [DOI] [PubMed] [Google Scholar]
- 8.Bertolotti A; Zhang Y; Hendershot LM; Harding HP; Ron D, Dynamic interaction of BiP and ER stress transducers in the unfolded-protein response. Nat Cell Biol 2000, 2 (6), 326–32. [DOI] [PubMed] [Google Scholar]
- 9.Prentki M; Nolan CJ, Islet beta cell failure in type 2 diabetes. The Journal of clinical investigation 2006, 116 (7), 1802–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ozcan U; Cao Q; Yilmaz E; Lee AH; Iwakoshi NN; Ozdelen E; Tuncman G; Gorgun C; Glimcher LH; Hotamisligil GS, Endoplasmic reticulum stress links obesity, insulin action, and type 2 diabetes. Science 2004, 306 (5695), 457–61. [DOI] [PubMed] [Google Scholar]
- 11.Ozcan U; Yilmaz E; Ozcan L; Furuhashi M; Vaillancourt E; Smith RO; Gorgun CZ; Hotamisligil GS, Chemical chaperones reduce ER stress and restore glucose homeostasis in a mouse model of type 2 diabetes. Science 2006, 313 (5790), 1137–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tersey SA; Nishiki Y; Templin AT; Cabrera SM; Stull ND; Colvin SC; Evans-Molina C; Rickus JL; Maier B; Mirmira RG, Islet beta-cell endoplasmic reticulum stress precedes the onset of type 1 diabetes in the nonobese diabetic mouse model. Diabetes 2012, 61 (4), 818–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Eizirik DL; Cardozo AK; Cnop M, The role for endoplasmic reticulum stress in diabetes mellitus. Endocrine reviews 2008, 29 (1), 42–61. [DOI] [PubMed] [Google Scholar]
- 14.Engin F; Hotamisligil GS, Restoring endoplasmic reticulum function by chemical chaperones: an emerging therapeutic approach for metabolic diseases. Diabetes Obes Metab 2010, 12 Suppl 2, 108–15. [DOI] [PubMed] [Google Scholar]
- 15.Montane J; de Pablo S; Castano C; Rodriguez-Comas J; Cadavez L; Obach M; Visa M; Alcarraz-Vizan G; Sanchez-Martinez M; Nonell-Canals A; Parrizas M; Servitja JM; Novials A, Amyloid-induced beta-cell dysfunction and islet inflammation are ameliorated by 4-phenylbutyrate (PBA) treatment. FASEB J 2017, 31 (12), 5296–5306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Engin F; Yermalovich A; Nguyen T; Hummasti S; Fu W; Eizirik DL; Mathis D; Hotamisligil GS, Restoration of the unfolded protein response in pancreatic beta cells protects mice against type 1 diabetes. Sci Transl Med 2013, 5 (211), 211ra156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ramalho RM; Viana RJ; Low WC; Steer CJ; Rodrigues CM, Bile acids and apoptosis modulation: an emerging role in experimental Alzheimer’s disease. Trends Mol Med 2008, 14 (2), 54–62. [DOI] [PubMed] [Google Scholar]
- 18.Lee J; Liu J; Feng X; Salazar Hernandez MA; Mucka P; Ibi D; Choi JW; Ozcan U, Withaferin A is a leptin sensitizer with strong antidiabetic properties in mice. Nat Med 2016, 22 (9), 1023–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Liu J; Lee J; Salazar Hernandez MA; Mazitschek R; Ozcan U, Treatment of obesity with celastrol. Cell 2015, 161 (5), 999–1011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Duan H; Arora D; Li Y; Setiadi H; Xu D; Lim HY; Wang W, Identification of 1,2,3-triazole derivatives that protect pancreatic beta cells against endoplasmic reticulum stress-mediated dysfunction and death through the inhibition of C/EBP-homologous protein expression. Bioorg Med Chem 2016, 24 (12), 2621–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Oslowski CM; Urano F, Measuring ER stress and the unfolded protein response using mammalian tissue culture system. Methods Enzymol 2011, 490, 71–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Oslowski CM; Urano F, The binary switch that controls the life and death decisions of ER stressed beta cells. Curr Opin Cell Biol 2011, 23 (2), 207–15. [DOI] [PMC free article] [PubMed] [Google Scholar]


