Abstract
Background
Distant metastasis (DM) is a crucial problem in management of patients with gastric cancer. Identification of the risk factors for development of DM and the prognostic factors for patients with DM is essential in development of individualized treatment of patients at the advanced stage with specific metastasis.
Material/Methods
Records of patients with gastric cancer were extracted from the Surveillance, Epidemiology, and End Results (SEER) database. Survival duration of patients with specific DM was estimated, and the prognostic factors were investigated using the Cox proportional hazard regression model. The logistic regression model was used to reveal the inherent risk factors for development of DM.
Results
Eventually, 32.6% (11,918 out of 36,588) of gastric cancer patients were diagnosed with DM between 2010 and 2015, among whom 5,361, 1,778, 1,495, and 231 patients were diagnosed with liver, lung, bone, and brain metastasis, respectively. The median overall survival for patients with DM was 5.0 (95% CI: 4.8–5.2) months, with a 5-year survival rate of 3.9%. Primary tumor site, histology types, tumor grade, T stage, N stage, surgery, chemotherapy, and the number of metastases were associated with worse survival. Younger age and higher tumor grade were positively associated with the development of DM.
Conclusions
Initial DM was found in 32.6% of patients with gastric cancer. Homogenous and heterogenous predictive factors were identified for patients with a specific metastatic site, which can be used in targeted screening and individualized treatment.
MeSH Keywords: Neoplasm Metastasis, Prognosis, Risk Factors, SEER Program, Stomach Neoplasms
Background
As one of most common cancers worldwide, gastric cancer causes many death every year, imposing a huge burden on economic and medical resources [1]. In the latest cancer statistics (2019) in the United States, it was reported that there were 17,230 newly diagnosed cases of gastric cancer and 11,140 deaths caused by gastric cancer [2]. With the development of new treatment strategies, the long-term survival outcome of patients with gastric cancer has significantly improved, especially for pre-metastatic patients, with a 5-year survival rate of approximate 70% [3]. However, the prognosis of patients with distant metastasis has remained poor.
Distant metastasis is the main criterion for stage IV gastric cancer diagnosis, and distant metastasis is correlated with worse survival [4]. The percentage of metastasis in gastric cancer patients was reported to have increased from 24% in 1990 to 44% in 2011 in the Netherlands [5]. Due to the absence of early specific clinical symptoms, many patients are diagnosed with distant metastasis. Among all patients with gastric cancer, 40.1% were found to have synchronous distant metastasis [6]. The National Comprehensive Cancer Network (NCCN) recommends that different treatments should be administered to gastric cancer patients in different stages. For gastric cancer patients in stage IV, palliative therapy is suggested [7,8]. Due to the various symptoms associated with different metastatic sites, targeted treatment should be given in a specialized department. Thus, it is important to perform distant metastasis screening, and research on the risk factors for distant metastasis is needed.
Although it is important for guiding individualized treatment, prediction of prognosis of gastric cancer patients with distant metastasis is often difficult. Compared with other organs, the liver is more likely to develop metastasis in gastric cancer patients. A previous study found that 2.43% of gastric cancer patients who received gastrectomy subsequently developed liver metastases [9]. The 2-year survival rate in gastric cancer patients with synchronous liver-only metastases was reported to be 17.2% [10]. Favorable prognostic factors for patients with gastric cancer after radical hepatectomy were reported to be: lower T and N stage, less metastases, lesions smaller than 5 cm, and negative resection margins [11]. The pulmonary metastasis rate for gastric cancer patients was reported to be 0.96%, and the median survival was 4.0 months after diagnosis of pulmonary metastasis [12]. In a study of a cohort of patients with metastatic or recurrent gastric cancer, the initial bone metastasis rate was 6.7%, and the median survival was 4.4 months after diagnosis of bone metastasis [13]. Brain metastasis has seldom been studied [14], with a reported occurrence rate of 2.33% in gastric cancer patients [15].
However, the research cited above studied specific metastasis in gastric cancer and had limited sample sizes. To thoroughly study the relative risk factors and prognosis in stage IV gastric cancer, research exploring different patterns of distant metastasis on gastric cancer in a large population is needed.
The present study assessed a gastric cancer patient cohort extracted from the Surveillance, Epidemiology, and End Results (SEER) Program database to thoroughly investigate distant metastasis in gastric cancer patients. Our analysis of the risk factors, prognostic factors, and prognosis may help develop targeted specific metastatic screening and guide individualized treatment.
Material and Methods
Study population
Data were extracted from the National Cancer Institute SEER cohort (https://seer.cancer.gov/data/). SEER*Stat Software version 8.3.6 was used to generate the data.
Patients who were initially diagnosed with gastric cancer between 2010 to 2015 were selected because sites of metastases were available after 2010. In the patients we enrolled from the SEER database, all had been followed up until at least 2018 (i.e., minimum 3-year follow-up). The primary site label was used to identify patients with gastric cancer (C16.0–C16.9). Patients diagnosed at autopsy or via death certificate and those without detailed records on distant metastasis were excluded. To investigate the prognostic factors for gastric cancer patients with distant metastasis, patients diagnosed without distant metastasis were excluded after logistic regression analysis (Figure 1).
Figure 1.
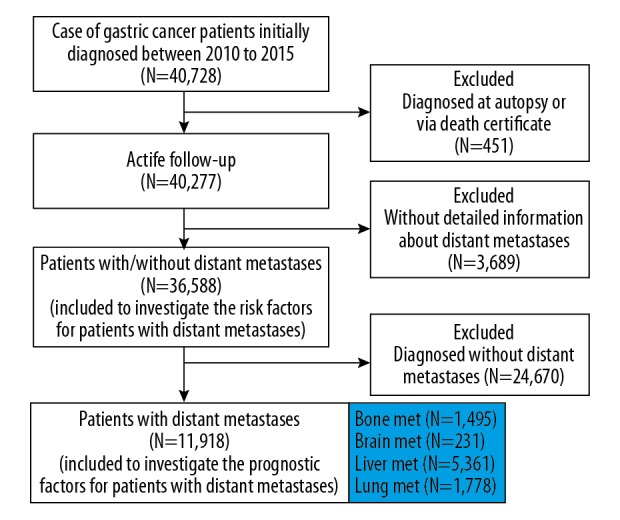
Flowchart of patient selection.
Statistical analysis
The following patient-related characteristics were included: age (<65 and ≥65 years); sex (female and male); marital status (unmarried and married); race (white, black, and others); insurance status (insured and uninsured); histological type (adenocarcinoma, mucinous adenocarcinoma, signet ring cell carcinoma, and others/unknown); primary site (proximal third, middle third, distal third, stomach, NOS, and overlapping lesion); tumor grade (I to IV: well, moderately, poorly, and undifferentiated, respectively); T stage (T0/Tis/T1, T2, T3, and T4); N stage (N0, N1, N2, and N3); the presence of lung, liver, brain, or bone metastasis; surgical treatment (no, yes); radiation treatment (no/unknown, yes); and treatment with chemotherapy (no/unknown, yes). To investigate the prognostic factors for patients with distant metastasis, the variables of ‘Number of mets’ (the sum of metastases sites) and ‘Other mets (metastasis for other sites)’ were defined. The homogenous predictive factors are variables which exert the same effect on disease development or survival prediction in subgroup analysis, while heterogeneous factors are those affecting a specific subgroup.
Categorical variables were presented as number and percentage (N,%), and Pearson chi-square (χ2) or Fisher’ exact test was used to evaluate the differences between demographic and clinicopathological variables. To identify risk factors for specific metastasis, logistic regression analysis was performed in our initial population. Variables with statistical differences in univariate logistic regression analysis were further analyzed by multivariate analysis. To identify risk factors for patients at M1 stage, patients who presented any distant metastatic sites (including liver, lung, bone, brain, and other non-specific sites) were defined as ‘M-Met’. To identify risk factors for organ-specific metastasis, patients with only liver, lung, bone, and brain metastasis were regarded as having specific metastasis. For example, to identify risk factors for liver metastasis, patients who were diagnosed with only liver metastasis were regarded as ‘Liver-Met’ and were compared with those without any metastasis and those who had other metastatic sites. Overall survival (OS) was the primary outcome in the present study, which was defined as the time from diagnosis of gastric cancer to death due to any cause. Kaplan-Meier analysis were performed to estimate the length of survival, and the differences were assessed with log-rank test. To identify the prognostic factors for patients with distant metastasis, patients at M0 stage were excluded. We tested the proportional hazards assumption. Univariate Cox proportional hazards regression analysis was performed for patients at M1 stage. Variables with statistically significant differences were further analyzed by multivariate analysis to identify the prognostic factors.
IBM SPSS Statistics (version 23.0, Armonk, NY, USA) was used for statistical analyses, and survival curves were generated using MedCalc 15.2.2 (MedCalc Software, Ostend, Belgium). All statistical tests were 2-sided, and P<0.05 was considered significant.
Ethnics statement
The SEER dataset is freely available and the data released by SEER do not require informed patient consent because cancer is a reportable disease in every state in the USA. The present study complied with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
Results
Patient characteristics
According to the pre-defined inclusion and exclusion criteria, a total of 36,588 gastric cancer patients were selected, among whom 11,918 (32.6%) cases were diagnosed at M1 stage. There were 5,361, 1,778, 1,495, and 231 patients diagnosed with liver, lung, bone, and brain metastasis, respectively. The mean age was 67.2±14.0 years, with a predominance for male patients (N=22,421, 61.3%) in the total cohort. After excluding patients without detailed information, more than half of the patients were of white race (N=25,930, 71.3%) and married (N=20,317, 58.9%), and almost of the patients were insured (N=34,269, 96.3%). The main histological subtype was adenocarcinoma (N=23,245, 63.5%), and proximal third was the most common tumor site (N=12,898, 35.3%), following by stomach, NOS (N=10,158, 27.8%) and distal third (N=7,079, 19.3%). Almost half of patients (N=17,266, 47.2%) underwent surgical treatment, and the percentage of patients receiving radiation and chemotherapy were 23.0% and 47.8%, respectively. Table 1 shows additional information on patient characteristics.
Table 1.
Description of the SEER population of patients with gastric cancer by distant metastasis at diagnosed between 2010–2015.
| Subject characteristics | M-Met | Liver-Met | Lung-Met | Bone-Met | Brain-Met | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| No N (%) |
Yes N (%) |
No N (%) |
Yes N (%) |
No N (%) |
Yes N (%) |
No N (%) |
Yes N (%) |
No N (%) |
Yes N (%) |
|
| Age (χ2, P) | 378.42 | <0.001 | 7.34 | 0.007 | 7.96 | 0.005 | 76.11 | <0.001 | 18.35 | <0.001 |
| <65 | 9,094 (61.6) | 5,662 (38.4) | 12,504 (84.7) | 2,252 (15.3) | 13,928 (94.8) | 774 (5.2) | 13,991 (94.8) | 765 (5.2) | 14,631 (99.2) | 125 (0.8) |
| ≥65 | 15,576 (71.3) | 6,256 (28.7) | 18,723 (85.8) | 3,109 (14.2) | 20,828 (95.4) | 1,004 (4.6) | 21,102 (96.7) | 730 (3.3) | 21,726 (99.5) | 106 (0.5) |
| Gender (χ2, P) | 64.13 | <0.001 | 236.58 | <0.001 | 39.84 | <0.001 | 17.82 | <0.001 | 12.84 | <0.001 |
| Male | 14,768 (65.9) | 7,653 (34.1) | 18,629 (83.1) | 3,792 (16.9) | 21,205 (94.6) | 1,216 (5.4) | 21,427 (95.6) | 994 (4.4) | 22,253 (99.3) | 168 (0.7) |
| Female | 9,902 (69.9) | 4,265 (30.1) | 12,598 (88.9) | 1,569 (11.1) | 13,605 (96.0) | 562 (4.0) | 13,666 (96.5) | 501 (3.5) | 14,104 (99.6) | 63 (0.4) |
| Race (χ2, P) | 33.20 | <0.001 | 73.48 | <0.001 | 24.34 | <0.001 | 20.56 | <0.001 | 14.62 | 0.002 |
| White | 17,308 (66.7) | 8,622 (33.3) | 22,070 (85.1) | 3,860 (14.9) | 24,583 (94.8) | 1,347 (5.2) | 24,802 (95.6) | 1,128 (4.4) | 25,740 (99.3) | 190 (0.7) |
| Black | 3,450 (67.7) | 1,647 (32.3) | 4,228 (83.0) | 869 (17.0) | 4,882 (95.8) | 215 (4.2) | 4,938 (96.9) | 159 (3.1) | 5,078 (99.6) | 19 (0.4) |
| Others | 3,742 (70.0) | 1,601 (30.0) | 4,731 (88.5) | 612 (11.5) | 5,131 (96.0) | 212 (4.0) | 5,139 (96.2) | 204 (3.8) | 5,322 (99.6) | 21 (0.4) |
| Unknown | 170 (78.0) | 48 (22.0) | 198 (90.8) | 20(9.2) | 214 (98.2) | 4 (1.8) | 214 (98.2) | 4 (1.8) | 217 (99.5) | 1 (0.5) |
| Marital status (χ2, P) | 40.00 | <0.001 | 16.02 | <0.001 | 9.34 | 0.009 | 7.48 | 0.024 | 0.79 | 0.674 |
| Married | 13,557 (66.7) | 6,760 (33.3) | 17,276 (85.0) | 3,041 (15.0) | 19,335 (95.2) | 982 (4.8) | 19,469 (95.8) | 848 (4.2) | 20,183 (99.3) | 134 (0.7) |
| Unmarried | 9,565 (67.5) | 4,600 (32.5) | 12,092 (85.4) | 2,073 (14.6) | 13,444 (94.9) | 721 (5.1) | 13,580 (95.9) | 585 (4.1) | 14,079 (99.4) | 86 (0.6) |
| Unknown | 1,548 (73.5) | 558 (26.5) | 1,859 (88.3) | 247 (11.7) | 2,031 (96.4) | 75(3.6) | 2,044 (97.1) | 62 (2.9) | 2,095 (99.5) | 11 (0.5) |
| Insurance status (χ2, P) | 191.62 | <0.001 | 29.22 | <0.001 | 12.06 | 0.002 | 17.82 | <0.001 | 2.86 | 0.239 |
| Insured | 23,203 (67.7) | 11,066 (32.3) | 29,265 (85.4) | 5,004 (14.6) | 32,622 (95.2) | 1,647 (4.8) | 32,878 (95.9) | 1,391 (4.1) | 34,057 (99.4) | 212 (0.6) |
| Uninsured | 680 (52.0) | 627 (48.0) | 1,061 (81.2) | 246 (18.8) | 1,218 (93.2) | 89 (6.8) | 1,229 (94.0) | 78 (6.0) | 1,294 (99.0) | 13 (1.0) |
| Unknown | 787 (77.8) | 225 (22.2) | 901 (89.0) | 111 (11.0) | 970 (95.8) | 42 (4.2) | 986 (97.4) | 26 (2.6) | 1,006 (99.4) | 6 (0.6) |
| Year of diagnosis (χ2, P) | 11.67 | 0.040 | 11.81 | 0.038 | 4.34 | 0.501 | 13.33 | 0.020 | 1.84 | 0.871 |
| 2010 | 3,935 (67.8) | 1,871 (32.2) | 4,983 (85.8) | 823 (14.2) | 5,524 (95.1) | 282 (4.9) | 5,599 (96.4) | 207 (3.6) | 5,769 (99.4) | 37 (0.6) |
| 2011 | 3,994 (68.6) | 1,830 (31.4) | 4,956 (85.1) | 868 (14.9) | 5,566 (95.6) | 258 (4.4) | 5,601 (96.2) | 223 (3.8) | 5,787 (99.4) | 37 (0.6) |
| 2012 | 4,197 (68.2) | 1,958 (31.8) | 5316 (86.4) | 839 (13.6) | 5,866 (95.3) | 289 (4.7) | 5,913 (96.1) | 242 (3.9) | 6,121 (99.4) | 34 (0.6) |
| 2013 | 4,134 (67.2) | 2,020 (32.8) | 5,214 (84.7) | 940 (15.3) | 5,850 (95.1) | 304 (4.9) | 5903 (95.9) | 251 (4.1) | 6,119 (99.4) | 35 (0.6) |
| 2014 | 4,231 (66.9) | 2,094 (33.1) | 5411 (85.5) | 914 (14.5) | 6,003 (94.9) | 322 (5.1) | 6,054 (95.7) | 271 (4.3) | 6,281 (99.3) | 44 (0.7) |
| 2015 | 4,179 (66.1) | 2,145 (33.9) | 5,347 (84.6) | 977 (15.4) | 6,001 (94.9) | 323 (5.1) | 6,023 (95.2) | 301 (4.8) | 6,280 (99.3) | 44 (0.7) |
| Primary site (χ2, P) | 221.36 | <0.001 | 310.33 | <0.001 | 176.00 | <0.001 | 82.92 | <0.001 | 70.87 | <0.001 |
| Proximal third | 8,619 (66.8) | 4,279 (33.2) | 10,469 (81.2) | 2,429 (18.8) | 12,045 (93.4) | 853 (6.6) | 12,265 (95.1) | 633 (4.9) | 12,763 (99.0) | 135 (1.0) |
| Mid | 2,715 (69.7) | 1,178 (30.3) | 3,439 (88.3) | 454 (11.7) | 3,768 (96.8) | 125 (3.2) | 3,746 (96.2) | 147 (3.8) | 3,882 (99.7) | 11 (0.3) |
| Distal third | 5,184 (73.2) | 1,895 (26.8) | 6,323 (89.3) | 756 (10.7) | 6,883 (97.2) | 196 (2.8) | 6,915 (97.7) | 164 (2.3) | 7,066 (99.8) | 13 (0.2) |
| Stomach, NOS | 6,640 (65.4) | 3,518 (34.6) | 8,792 (86.6) | 1,366 (13.4) | 9,674 (95.2) | 484 (4.8) | 9,708 (95.6) | 450 (4.4) | 10,093 (99.4) | 65 (0.6) |
| Overlapping | 1,512 (59.1) | 1,048 (40.9) | 2,204 (86.1) | 356 (13.9) | 2,440 (95.3) | 120 (4.7) | 2,459 (96.1) | 101 (3.9) | 2,553 (99.7) | 7 (0.3) |
| Histology (χ2, P) | 758.44 | <0.001 | 570.14 | <0.001 | 88.15 | <0.001 | 173.99 | <0.001 | 13.26 | 0.004 |
| Adenoca-rcinoma | 15,256 (65.6) | 7,989 (34.4) | 19,139 (82.3) | 4,106 (17.7) | 21,951 (94.4) | 1,294 (5.6) | 22,288 (95.9) | 957 (4.1) | 23,078 (99.3) | 167 (0.7) |
| Mucous carcinoma | 381 (65.7) | 199 (34.3) | 517 (89.1) | 63 (10.9) | 546 (94.1) | 34 (5.9) | 557 (96.0) | 23 (4.0) | 578 (99.7) | 2 (0.3) |
| Signet-ring cell carcinoma | 3,497 (59.3) | 2,401 (40.7) | 5,553 (94.2) | 345 (5.8) | 5,647 (95.7) | 251 (4.3) | 5,513 (93.5) | 385 (6.5) | 5,859 (99.3) | 39 (0.7) |
| Unknown | 5,536 (80.6) | 1,329 (19.4) | 6,018 (87.7) | 847 (12.3) | 6,666 (97.1) | 199 (2.9) | 6,735 (98.1) | 130 (1.9) | 6,842 (99.7) | 23 (0.3) |
| Grade (χ2, P) | 1231.98 | <0.001 | 305.40 | <0.001 | 93.89 | <0.001 | 227.91 | <0.001 | 27.33 | <0.001 |
| I | 2,766 (92.1) | 236 (7.9) | 2,868 (95.5) | 134 (4.5) | 2,958 (98.5) | 44 (1.5) | 2,986 (99.5) | 16(0.5) | 3,001 (100.0) | 1 (0.0) |
| II | 5,655 (73.5) | 2,038 (26.5) | 6,400 (83.2) | 1,293 (16.8) | 7,310 (95.0) | 383 (5.0) | 7,515 (97.7) | 178 (2.3) | 7,641 (99.3) | 5 (0.7) |
| III | 10,775 (62.5) | 6,475 (37.5) | 14,753 (85.5) | 2,497 (14.5) | 16,374 (94.9) | 876 (5.1) | 16,346 (94.8) | 904 (5.2) | 17,149 (99.4) | 101 (0.6) |
| IV | 483 (68.2) | 225 (31.8) | 595 (84.0) | 113 (16.0) | 686 (96.9) | 22 (3.1) | 687 (97.0) | 21 (3.0) | 701 (99.0) | 7 (1.0) |
| Unknown | 4,991 (62.9) | 2,944 (37.1) | 6,611 (83.3) | 1,324 (16.7) | 7,482 (94.3) | 453 (5.7) | 7,559 (95.3) | 376 (4.7) | 7,865 (99.1) | 70 (0.9) |
| T stage (χ2, P) | 4691.69 | <0.001 | 2339.40 | <0.001 | 803.09 | <0.001 | 840.77 | <0.001 | 155.81 | <0.001 |
| T1 | 7,401 (77.8) | 2,112 (22.2) | 8,438 (88.7) | 1,075 (11.3) | 9,146 (96.1) | 367 (3.9) | 9,245 (97.2) | 268 (2.8) | 9,473 (99.6) | 40 (0.4) |
| T2 | 3,471 (84.8) | 622 (15.2) | 3,891 (95.1) | 202 (4.9) | 4,031 (98.5) | 62 (1.5) | 4,039 (98.7) | 54 (1.3) | 4,084 (99.8) | 9 (0.2) |
| T3 | 6,845 (80.0) | 1,711 (20.0) | 7,906 (92.4) | 650 (7.6) | 8,365 (97.8) | 191 (2.2) | 8,369 (97.8) | 187 (2.2) | 8,529 (99.7) | 27 (0.3) |
| T4 | 3,697 (59.3) | 2,535 (40.7) | 5,280 (84.7) | 952 (15.3) | 5,931 (95.2) | 301 (4.8) | 6,031 (96.8) | 201 (3.2) | 6,207 (99.6) | 25 (0.4) |
| Unknown | 3,256 (39.7) | 4,938 (60.3) | 5,712 (69.7) | 2,482 (30.3) | 7,337 (89.5) | 857 (10.5) | 7,409 (90.4) | 785 (9.6) | 8,064 (98.4) | 130 (1.6) |
| N stage (χ2, P) | 2899.94 | <0.001 | 1388.41 | <0.001 | 590.22 | <0.001 | 471.15 | <0.001 | 80.68 | <0.001 |
| N0 | 14,807 (76.0) | 4,677 (24.0) | 17,422 (89.4) | 2,062 (10.6) | 18,844 (96.7) | 640 (3.3) | 18,959 (97.3) | 525 (2.7) | 19,407 (99.6) | 77 (0.4) |
| N1 | 4,557 (53.1) | 4,020 (46.9) | 6,607 (77.0) | 1,970 (23.0) | 7,908 (92.2) | 669 (7.8) | 7,993 (93.2) | 584 (6.8) | 8,493 (99.0) | 84 (1.0) |
| N2 | 2,171 (76.8) | 654 (23.2) | 2,572 (91.0) | 253 (9.0) | 2,756 (97.60) | 69 (2.4) | 2,762 (97.8) | 63 (2.2) | 2,815 (99.6) | 10 (0.4) |
| N3 | 2,034 (74.9) | 683 (25.1) | 2,503 (92.1) | 214 (7.9) | 2,650 (97.5) | 67 (2.5) | 2,655 (97.7) | 62 (2.3) | 2,704 (99.5) | 13 (0.5) |
| Unknown | 1,101 (36.9) | 1,884 (63.1) | 2,123 (71.1) | 862 (28.9) | 2,652 (88.8) | 333 (11.2) | 2,724 (91.3) | 261 (8.7) | 2,938 (98.4) | 47 (1.6) |
| Surgery (χ2, P) | 8952.99 | <0.001 | 3808.23 | <0.001 | 1391.97 | <0.001 | 1199.06 | <0.001 | 163.73 | <0.001 |
| None | 8,707 (45.4) | 10,488 (54.6) | 14,298 (74.5) | 4,897 (25.5) | 17,496 (91.1) | 1,699 (8.9) | 17,756 (92.5) | 1,439 (7.5) | 18,977 (98.9) | 218 (1.1) |
| Yes | 15,853 (91.8) | 1,413 (8.2) | 16,808 (97.3) | 458 (2.7) | 17,189 (99.6) | 77 (0.4) | 17,212 (99.7) | 54 (0.3) | 17,253 (99.9) | 13 (0.1) |
| Unknown | 110 (86.6) | 17 (13.4) | 121 (95.3) | 6(4.7) | 125 (98.4) | 2 (1.6) | 125 (98.4) | 2 (1.6) | 127 (100.0) | 0 (0.0) |
| Radiation therapy (χ2, P) | 510.46 | <0.001 | 244.63 | <0.001 | 20.13 | <0.001 | 44.61 | <0.001 | 190.20 | <0.001 |
| No/unknown | 18,148 (64.4) | 10,031 (35.6) | 23,605 (83.8) | 4,574 (16.2) | 26,732 (94.9) | 1,447 (5.1) | 27,134 (96.3) | 1,045 (3.7) | 28,089 (99.7) | 90 (0.3) |
| Yes | 6,522 (77.6) | 1,887 (22.4) | 7,622 (90.6) | 787 (9.4) | 8,078 (96.1) | 331 (3.9) | 7,959 (94.6) | 450 (5.4) | 8,268 (98.3) | 141 (1.7) |
| Chemical therapy (χ2, P) | 782.93 | <0.001 | 171.88 | <0.001 | 39.20 | <0.001 | 62.04 | <0.001 | 8.09 | 0.005 |
| No/unknown | 14,124 (74.0) | 4,965 (26.0) | 16,735 (87.7) | 2,354 (12.3) | 18,290 (95.8) | 799 (4.2) | 18,458 (96.7) | 631 (3.3) | 18,990 (99.5) | 99 (0.5) |
| Yes | 10,546 (60.3) | 6,953 (39.7) | 14,492 (82.8) | 3,007 (17.2) | 16,520 (94.4) | 979 (5.6) | 16,635 (95.1) | 864 (4.9) | 17,367 (99.2) | 132 (0.8) |
| Vital status (χ2, P) | 5077.26 | <0.001 | 1866.53 | <0.001 | 725.16 | <0.001 | 643.67 | <0.001 | 75.14 | <0.001 |
| Alive | 11,792 (91.0) | 1,166 (9.0) | 12,457 (96.1) | 501 (3.9) | 12,858 (99.2) | 100 (0.8) | 12,888 (99.5) | 70 (0.5) | 12,939 (99.9) | 19 (0.1) |
| Dead | 12,878 (54.5) | 10,752 (45.5) | 18,770 (79.4) | 4,860 (20.6) | 21,952 (92.9) | 1,678 (7.1) | 22,205 (94.0) | 1,425 (6.0) | 23,418 (99.1) | 212 (0.9) |
| Number of mets (χ2, P) | 3248.96 | <0.001 | 6493.04 | <0.001 | 16646.25 | <0.001 | 7459.10 | <0.001 | 1968.49 | <0.001 |
| ≤1 | 24,670 (70.3) | 10,413 (29.7) | 31,025 (88.4) | 4,058 (11.6) | 34,432 (98.1) | 651 (1.9) | 34,299 (97.8) | 784 (2.2) | 34,995 (99.7) | 88 (0.3) |
| >1 | 0 (0.0) | 1,505 (100.0) | 202 (13.4) | 1,303 (86.6) | 378 (25.1) | 1,127 (74.9) | 794 (52.8) | 711 (47.2) | 1,362 (90.5) | 143 (9.5) |
SEER – Surveillance, Epidemiology, and End Results; Met – metastases; NOS – not otherwise specified.
Survival estimation and prognostic factors for patients with metastasis
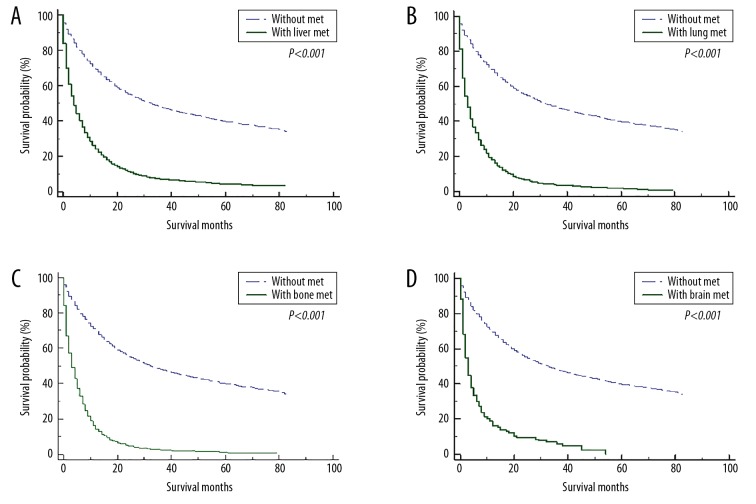
For patients without distant metastasis, the median overall survival was 32.0 (95% CI: 30.7–33.3) months, and the 1-, 2-, 3-, and 5-year survival rates were 69.3%, 55.4%, 47.9%, and 39.8%, respectively. On the contrary, the median overall survival for metastatic patients was 5.0 (95% CI: 4.8–5.2) months, and the 1-, 2-, 3-, and 5-year survival rates were 25.9%, 11.6%, 6.7%, and 3.9%, respectively. The 1-year survival rates for patients with liver, lung, bone, and brain metastasis were 24.0%, 18.0%, 14.0%, and 16.2%, respectively. The corresponding 5-year survival rates were 4.4%, 1.6%, 1.2%, and 0%, respectively. The survival curves for gastric cancer patients with or without metastasis to liver (Figure 2A), lung (Figure 2B), bone (Figure 2C), and brain (Figure 2D) are illustrated in Figure 2.
Figure 2.
The overall survival for gastric cancer patients with or without metastasis to liver (A), lung (B), bone (C), and brain (D).
Supplementary Table 1 shows P values for results of testing the proportion hazards assumption. Most of the factors did not violate the proportional hazards assumption. As shown in Supplementary Table 2, primary site, T stage, and treatment by surgery and chemotherapy were associated with survival in univariate Cox regression analysis. Other variables, such as age, marital status, histology types, tumor grade, N stage, radiation treatment, and presence or absence of other metastasis, were associated with specific metastasis patients. After adjusting all these characteristics in multivariate analysis, factors significantly associated with survival outcome for patients with liver metastasis were: age ≥65 years (HR=1.19, 95% CI 1.12–1.26); tumor grade (II HR=1.64, 95% CI 1.21–2.22; III HR=2.23, 95% CI 1.66–3.00; IV HR=1.91, 95% CI 1.21–2.82); T stage (T2 HR=0.80, 95% CI 0.64–0.99; T3 HR=0.88, 95% CI 0.77–1.00); N3 stage (HR=1.23, 95% CI 1.01–1.50); surgery (HR=0.45, 95% CI 0.38–0.52); chemotherapy (HR=0.30, 95% CI 0.27–0.34); and more other metastases (HR=1.38, 95% CI 1.24–1.55). In patients with lung metastasis, the following factors were associated with overall survival: tumor grade III (HR=1.53, 95% CI 1.02–2.29); T4 stage (HR=1.27, 95% CI 1.06–1.53); surgery (HR=0.74, 95% CI 0.55–0.99); chemotherapy (HR=0.31, 95% CI 0.26–0.37); and more other metastases (HR=1.51, 95% CI 1.28–1.77). Table 2 provides additional information on the results of multivariate Cox regression analysis.
Table 2.
Multivariable Cox regression for analyzing the prognostic factors for gastric cancer patients with distance metastases.
| Subject characteristics | M-Met | Liver-Met | Lung-Met | Bone-Met | Brain-Met | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| HR (95% CI) | P-value | HR (95% CI) | P-value | HR (95% CI) | P-value | HR (95% CI) | P-value | HR (95% CI) | P-value | |
| Age | ||||||||||
| <65 | 1.00 (Reference) | 1.00 | 1.00 (Reference) | 1.00 | 1.00 (Reference) | 1.00 | 1.00 (Reference) | 1.00 | 1.00 (Reference) | 1.00 |
| ≥65 | 1.19 (1.12–1.26) | <0.001 | 1.16 (1.06–1.28) | 0.002 | 0.94 (0.81–1.11) | 0.479 | 0.96 (0.82–1.13) | 0.652 | NA | NA |
| Marital status | ||||||||||
| Married | 1.00 (Reference) | 1.00 | 1.00 (Reference) | 1.00 | 1.00 (Reference) | 1.00 | 1.00 (Reference) | 1.00 | 1.00 (Reference) | 1.00 |
| Unmarried | 1.02 (0.96–1.08) | 0.631 | 0.95 (0.86–1.04) | 0.244 | 1.10 (0.94–1.29) | 0.233 | 0.95 (0.81–1.12) | 0.544 | NA | NA |
| Primary site | ||||||||||
| Proximal third | 1.00 (Reference) | 1.00 | 1.00 (Reference) | 1.00 | 1.00 (Reference) | 1.00 | 1.00 (Reference) | 1.00 | 1.00 (Reference) | 1.00 |
| Mid | 1.09 (0.98–1.22) | 0.107 | 0.99 (0.83–1.18) | 0.880 | 1.12 (0.81–1.56) | 0.490 | 1.55 (1.17–2.05) | 0.002 | 2.90 (0.86–9.85) | 0.088 |
| Distal third | 0.93 (0.85–1.02) | 0.135 | 0.91 (0.79–1.04) | 0.167 | 1.02 (0.79–1.31) | 0.892 | 1.30 (0.98–1.74) | 0.071 | 2.32 (1.00–5.41) | 0.051 |
| Stomach, NOS | 0.96 (0.89–1.04) | 0.349 | 0.93 (0.82–1.05) | 0.241 | 1.11 (0.91–1.37) | 0.306 | 0.98 (0.81–1.19) | 0.837 | 1.13 (0.69–1.85) | 0.625 |
| Overlapping | 1.17 (1.05–1.30) | 0.004 | 1.29 (1.08–1.55) | 0.005 | 1.25 (0.93–1.67) | 0.137 | 1.09 (0.81–1.46) | 0..575 | 0.42 (0.09–1.96) | 0.272 |
| Histology | ||||||||||
| Adenocarcinoma | 1.00 (Reference) | 1.00 | 1.00 (Reference) | 1.00 | 1.00 (Reference) | 1.00 | 1.00 (Reference) | 1.00 | 1.00 (Reference) | 1.00 |
| Mucous carcinoma | 1.05 (0.82–1.34) | 0.696 | 1.42 (0.89–2.28) | 0.142 | NA | NA | NA | NA | NA | NA |
| Signet-ring cell carcinoma | 1.03 (0.95–1.11) | 0.514 | 1.02 (0.85–1.23) | 0.843 | NA | NA | NA | NA | NA | NA |
| Unknown | 0.74 (0.65–0.84) | <0.001 | 0.80 (0.68–0.95) | 0.012 | NA | NA | NA | NA | NA | NA |
| Grade | ||||||||||
| I | 1.00 (Reference) | 1.00 | 1.00 (Reference) | 1.00 | 1.00 (Reference) | 1.00 | 1.00 (Reference) | 1.00 | 1.00 (Reference) | 1.00 |
| II | 1.55 (1.24–1.92) | <0.001 | 1.64 (1.21–2.22) | 0.001 | 1.08 (0.71–1.64) | 0.726 | NA | NA | NA | NA |
| III | 2.00 (1.62–2.48) | <0.001 | 2.23 (1.66–3.00) | <0.001 | 1.53 (1.02–2.29) | 0.039 | NA | NA | NA | NA |
| IV | 1.84 (1.39–2.43) | <0.001 | 1.91 (1.29–2.82) | 0.001 | 1.04 (0.51–2.13) | 0.920 | NA | NA | NA | NA |
| T stage | ||||||||||
| T1 | 1.00 (Reference) | 1.00 | 1.00 (Reference) | 1.00 | 1.00 (Reference) | 1.00 | 1.00 (Reference) | 1.00 | 1.00 (Reference) | 1.00 |
| T2 | 0.83 (0.73–0.93) | 0.002 | 0.80 (0.64–0.99) | 0.039 | 0.98 (0.69–1.39) | 0.904 | 0.54 (0.39–0.74) | <0.001 | 1.67 (0.79–3.53) | 0.184 |
| T3 | 0.92 (0.84–1.00) | 0.042 | 0.88 (0.77–1.00) | 0.048 | 1.16 (0.93–1.44) | 0.183 | 0.89 (0.73–1.09) | 0.262 | 1.34 (0.74–2.40) | 0.333 |
| T4 | 1.15 (1.06–1.24) | 0.001 | 1.07 (0.95–1.21) | 0.241 | 1.27 (1.06–1.53) | 0.011 | 1.11 (0.91–1.36) | 0.309 | 2.28 (1.28–4.07) | 0.005 |
| N stage | ||||||||||
| N0 | 1.00 (Reference) | 1.00 | 1.00 (Reference) | 1.00 | 1.00 (Reference) | 1.00 | 1.00 (Reference) | 1.00 | 1.00 (Reference) | 1.00 |
| N1 | 1.06 (0.99–1.31) | 0.122 | 1.05 (0.95–1.17) | 0.350 | NA | NA | NA | NA | NA | NA |
| N2 | 1.11 (0.99–1.24) | 0.074 | 1.15 (0.95–1.38) | 0.146 | NA | NA | NA | NA | NA | NA |
| N3 | 1.19 (1.06–1.34) | 0.003 | 1.23 (1.01–1.50) | 0.040 | NA | NA | NA | NA | NA | NA |
| Surgery | ||||||||||
| No | 1.00 (Reference) | 1.00 | 1.00 (Reference) | 1.00 | 1.00 (Reference) | 1.00 | 1.00 (Reference) | 1.00 | 1.00 (Reference) | 1.00 |
| Yes | 0.43 (0.39–0.47) | <0.001 | 0.45 (0.38–0.52) | <0.001 | 0.74 (0.55–0.99) | 0.045 | 0.54 (0.38–0.75) | <0.001 | 0.32 (0.13–0.79) | 0.014 |
| Radiation | ||||||||||
| No/unknown | 1.00 (Reference) | 1.00 | 1.00 (Reference) | 1.00 | 1.00 (Reference) | 1.00 | 1.00 (Reference) | 1.00 | 1.00 (Reference) | 1.00 |
| Yes | 0.93 (0.86–1.00) | 0.053 | NA | NA | 0.99 (0.81–1.20) | 0.903 | 0.94 (0.80–1.12) | 0.499 | 0.91 (0.56–1.46) | 0.683 |
| Chemotherapy | ||||||||||
| No/unknown | 1.00 (Reference) | 1.00 | 1.00 (Reference) | 1.00 | 1.00 (Reference) | 1.00 | 1.00 (Reference) | 1.00 | 1.00 (Reference) | 1.00 |
| Yes | 0.32 (0.30–0.35) | <0.001 | 0.30 (0.27–0.34) | <0.001 | 0.31 (0.26–0.37) | <0.001 | 0.28 (0.24–0.34) | <0.001 | 0.24 (0.14–0.42) | <0.001 |
| Number of mets | ||||||||||
| ≤1 | 1.00 (Reference) | 1.00 | – | – | – | – | – | – | – | – |
| >1 | 1.48 (1.35–1.63) | <0.001 | – | – | – | – | – | – | – | – |
| Other mets | ||||||||||
| No | – | – | 1.00 (Reference) | 1.00 | 1.00 (Reference) | 1.00 | 1.00 (Reference) | 1.00 | 1.00 (Reference) | 1.00 |
| Yes | – | – | 1.38 (1.24–1.55) | <0.001 | 1.51 (1.28–1.77) | <0.001 | NA | NA | 1.47 (0.90–2.38) | 0.123 |
Met – metastases; HR – hazard ratio; CI – confidence interval; NOS – not otherwise specified; NA – not available.
Risk factors for distant metastases
In our cohort, there were 11,918 patients presenting distant metastasis. The number of cases with bone, brain, liver, and lung metastasis was 1495, 231, 5361, and 1778, respectively. As shown in Supplementary Table 3, the following factors were significantly associated with developing distant metastasis and bone, liver, or lung metastasis: age, sex, race, insurance status, primary tumor site, histological type, grade, T stage, and N stage, and all of these variables except insurance status and T stage were also associated with developing brain metastasis.
The multivariate regression analysis suggested several independent risk factors. Younger age and higher tumor grade were positively associated with developing distant metastasis, including all four organs. Proximal third of stomach was the most common primary site for tumor metastasis. Patients without insurance were more likely to have distant metastasis. T stage and N stage were independent risk factors. More details on the results of the multivariate analysis are provided in Table 3.
Table 3.
Multivariable logistic regression for analyzing the risk factors for developing distant metastases in patients with gastric cancer.
| Subject characteristics | M-Met | Liver-Met | Lung-Met | Bone-Met | Brain-Met | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| OR (95% CI) | P-value | OR (95% CI) | P-value | OR (95% CI) | P-value | OR (95% CI) | P-value | OR (95% CI) | P-value | |
| Age | ||||||||||
| <65 | 1.00 (Reference) | 1.00 | 1.00 (Reference) | 1.00 | 1.00 (Reference) | 1.00 | 1.00 (Reference) | 1.00 | 1.00 (Reference) | 1.00 |
| ≥65 | 0.68 (0.63–0.72) | <0.001 | 0.87 (0.79–0.96) | 0.005 | 0.80 (0.68–0.94) | 0.008 | 0.71 (0.59–0.85) | <0.001 | 0.50 (0.35–0.71) | <0.001 |
| Gender | ||||||||||
| Male | 1.00 (Reference) | 1.00 | 1.00 (Reference) | 1.00 | 1.00 (Reference) | 1.00 | 1.00 (Reference) | 1.00 | 1.00 (Reference) | 1.00 |
| Female | 0.94 (0.87–1.01) | 0.076 | 0.73 (0.66–0.82) | <0.001 | 0.90 (0.75–1.07) | 0.215 | 0.82 (0.67–1.00) | 0.056 | 0.80 (0.52–1.21) | 0.287 |
| Race | ||||||||||
| White | 1.00 (Reference) | 1.00 | 1.00 (Reference) | 1.00 | 1.00 (Reference) | 1.00 | 1.00 (Reference) | 1.00 | 1.00 (Reference) | 1.00 |
| Black | 1.01 (0.92–1.12) | 0.817 | 1.37 (1.20–1.57) | <0.001 | 0.99 (0.77–1.27) | 0.947 | 0.69 (0.50–0.95) | 0.022 | 0.49 (0.23–1.07) | 0.073 |
| Others | 0.82 (0.74–0.90) | <0.001 | 0.82 (0.71–0.95) | 0.008 | 0.89 (0.70–1.13) | 0.330 | 0.96 (0.74–1.25) | 0.760 | 0.62 (0.32–1.20) | 0.154 |
| Insurance status | ||||||||||
| Insured | 1.00 (Reference) | 1.00 | 1.00 (Reference) | 1.00 | 1.00 (Reference) | 1.00 | 1.00 (Reference) | 1.00 | 1.00 (Reference) | 1.00 |
| Uninsured | 1.48 (1.25–1.75) | <0.001 | 1.48 (1.18–1.85) | 0.001 | 1.52 (1.06–2.16) | 0.021 | 1.39 (0.93–2.08) | 0.107 | NA | NA |
| Primary site | ||||||||||
| Proximal third | 1.00 (Reference) | 1.00 | 1.00 (Reference) | 1.00 | 1.00 (Reference) | 1.00 | 1.00 (Reference) | 1.00 | 1.00 (Reference) | 1.00 |
| Mid | 1.05 (0.93–1.18) | 0.446 | 0.79 (0.66–0.94) | 0.009 | 0.53 (0.39–0.73) | <0.001 | 0.64 (0.46–0.91) | 0.012 | 0.25 (0.10–0.63) | 0.003 |
| Distal third | 0.70 (0.63–0.77) | <0.001 | 0.62 (0.54–0.72) | <0.001 | 0.37 (0.28–0.48) | <0.001 | 0.29 (0.21–0.41) | <0.001 | 0.17 (0.08–0.37) | <0.001 |
| Stomach, NOS | 1.04 (0.94–1.14) | 0.465 | 0.71 (0.63–0.82) | <0.001 | 0.65 (0.52–0.80) | <0.001 | 0.88 (0.70–1.11) | 0.267 | 0.39 (0.23–0.65) | <0.001 |
| Overlapping | 1.32 (1.16–1.50) | <0.001 | 0.86 (0.72–1.04) | 0.125 | 0.77 (0.57–1.04) | 0.085 | 0.81 (0.58–1.14) | 0.229 | 0.30 (0.12–0.74) | 0.009 |
| Histology | ||||||||||
| Adenoca-rcinoma | 1.00 (Reference) | 1.00 | 1.00 (Reference) | 1.00 | 1.00 (Reference) | 1.00 | 1.00 (Reference) | 1.00 | 1.00 (Reference) | 1.00 |
| Mucous carcinoma | 0.78 (0.59–1.02) | 0.720 | 0.43 (0.26–0.69) | 0.001 | 0.80 (0.41–1.56) | 0.508 | 0.97 (0.45–2.08) | 0.938 | NA | NA |
| Signet-ring cell carcinoma | 0.95 (0.86–1.03) | 0.218 | 0.31 (0.26–0.38) | <0.001 | 0.78 (0.61–0.98) | 0.035 | 1.34 (1.07–1.67) | 0.011 | 1.03 (0.62–1.72) | 0.918 |
| Unknown | 0.82 (0.71–0.94) | 0.003 | 1.22 (1.04–1.45) | 0.018 | 0.66 (0.47–0.93) | 0.018 | 1.05 (0.72–1.53) | 0.797 | 0.61 (0.27–1.37) | 0.233 |
| Grade | ||||||||||
| I | 1.00 (Reference) | 1.00 | 1.00 (Reference) | 1.00 | 1.00 (Reference) | 1.00 | 1.00 (Reference) | 1.00 | 1.00 (Reference) | 1.00 |
| II | 3.53 (2.89–4.32) | <0.001 | 4.14 (3.16–5.43) | <0.001 | 2.26 (1.47–3.48) | <0.001 | 4.72(2.14–10.40) | <0.001 | 11.32 (1.53–83.77) | 0.017 |
| III | 5.46 (4.49–6.66) | <0.001 | 4.41 (3.37–5.78) | <0.001 | 2.64 (1.72–4.04) | <0.001 | 9.49 (4.37–20.62) | <0.001 | 9.20 (1.25–67.85) | 0.029 |
| IV | 4.93 (3.77–6.44) | <0.001 | 4.90 (3.41–7.05) | <0.001 | 1.53 (0.73–3.22) | 0.263 | 5.15 (1.90–14.02) | 0.001 | 20.76 (2.47–174.32) | 0.005 |
| T stage | ||||||||||
| T1 | 1.00 (Reference) | 1.00 | 1.00 (Reference) | 1.00 | 1.00 (Reference) | 1.00 | 1.00 (Reference) | 1.00 | 1.00 (Reference) | 1.00 |
| T2 | 0.48 (0.43–0.54) | <0.001 | 0.29 (0.23–0.35) | <0.001 | 0.33 (0.23–0.45) | <0.001 | 0.40 (0.28–0.56) | <0.001 | NA | NA |
| T3 | 0.56 (0.51–0.62) | <0.001 | 0.41 (0.36–0.47) | <0.001 | 0.40 (0.32–0.49) | <0.001 | 0.45 (0.36–0.58) | <0.001 | NA | NA |
| T4 | 1.59 (1.44–1.75) | <0.001 | 1.14 (1.00–1.30) | 0.048 | 1.11 (0.90–1.37) | 0.329 | 0.78 (0.61–1.01) | 0.054 | NA | NA |
| N stage | ||||||||||
| N0 | 1.00 (Reference) | 1.00 | 1.00 (Reference) | 1.00 | 1.00 (Reference) | 1.00 | 1.00 (Reference) | 1.00 | 1.00 (Reference) | 1.00 |
| N1 | 2.17 (2.00–2.35) | <0.001 | 2.24 (2.00–2.50) | <0.001 | 2.05 (1.71–2.46) | <0.001 | 2.35 (1.91–2.90) | <0.001 | 1.81 (1.23–2.66) | 0.003 |
| N2 | 0.91 (0.80–1.02) | 0.116 | 0.95 (0.79–1.15) | 0.595 | 0.77 (0.55–1.07) | 0.119 | 1.01 (0.70–1.45) | 0.967 | 0.70 (0.33–1.49) | 0.354 |
| N3 | 0.85 (0.75–0.96) | 0.008 | 0.88 (0.73–1.06) | 0.182 | 0.64 (0.45–0.91) | 0.013 | 0.91 (0.63–1.31) | 0.608 | 1.40 (0.73–2.67) | 0.315 |
Met – metastases; OR – odds ratio; CI – confidence interval; NOS – not otherwise specified; NA – not available.
Discussion
Distant metastasis is a serious problem during cancer management. The median overall survival time for gastric cancer patients with distant metastasis was approximately 4.0 months in our analysis. Synchronous metastasis was present in 32.6% of patients with primary gastric cancer. The poor survival and high metastasis rate in gastric cancer suggested that further research should be performed to thoroughly investigate the related predictive factors for prognosis and prevalence of distant metastasis.
To promote the survival of patients at stage IV, developing a comprehensive treatment strategy has been the global focus. Surgery of the primary cancer and chemotherapy can improve survival for patients with metastasis to liver, lung, bone, and brain. In the present study, chemotherapy was the main treatment for patients with distant metastasis and it was offered to 56.1% of patients with liver metastasis, while surgery of primary gastric cancer was performed in only 8.5% of patients. The proportion of gastric resection and chemotherapy was consistent with a previous study in Europe [16]. As previously reported, chemotherapy was the main treatment for patients with liver metastasis, and conversion surgery can be considered in some selected patients [17]. Similarly, according to a survey from two European and Japanese gastric cancer study groups, preoperative chemotherapy followed by resection of both primary and liver lesions was the recommended option for patients without extrahepatic metastasis [18], an similar percentages of chemotherapy and surgery were performed in patients with metastasis to lung and bone. Based on the records from the Metastatic Lung Tumor Study Group of Japan, the 5-year survival rate was 28% after pulmonary metastatic tumor resection [19]. For bone metastasis in gastric cancer, a metastasis rate of 3.8% was reported, and palliative chemotherapy was a significant factor for improved survival [20]. Brain metastasis was rare and no prediction was made in large cohort. In our study, based on the analysis of 231 patients with brain metastasis, we found similar benefits from surgery and chemotherapy. Currently, chemotherapy is the standard first-line treatment for advanced gastric cancer patients and has shown good performance [21]. More than 50 years since chemotherapy was first introduced, infusional 5-FU has been accepted as the main component of most combination regimens in stage IV gastric cancer [22], while paclitaxel is a widely used second-line chemotherapy drug [23]. Adverse effects and resistance to chemotherapy in clinical practice have recently focused attention on developing combination therapy [23,24]. Further study is needed to reveal the underlying causes of adverse effects and chemotherapy resistance.
Except for the homogenous prognostic factors for all metastatic sites in our analysis, age older than 65 years, higher grade (II, III, and IV), and N4 were associated with worse survival in patients with liver metastasis. Tumor grade III and T4 stage were independent factors associated with pulmonary metastasis. T4 stage was also associated with worse survival for patients with brain metastasis. All these negative factors should be considered in the prediction of survival in patients with specific metastasis.
To improve long-term survival and quality of life, the negative influence of distant metastasis on survival must be determined. Thus, timely screening and early diagnosis of the possible metastasis is important before treatment. PET/CT has been the main strategy for distant metastasis screening in gastric cancer [25]. However, due to limited medical resources, the screening should be offered to gastric cancer patients with higher risk of distant metastasis. Thus, the prediction of possible distant metastasis is crucial in clinical practice.
Although many studies have evaluated the survival and related factors for gastric cancer patients with metastasis, few studies have investigated risk factors for distant metastasis. The risk factors for the development of bone metastasis were evaluated in a study including 1,342 patients with metastatic gastric cancer, in which 141 (10.5%) patients presented bone metastasis and predictive factors included age younger than 65 years, signet ring cell histology and location than 2/3 of stomach [13]. In our study, homogenous risk factors for all the metastatic sites were age less than 65 years, tumor in the proximal third of the stomach, higher grade, and N1 stage. Male sex, black race, and uninsured status were also associated with higher risk of liver metastasis. Histological type showed different effects on metastasis. The clinicopathological factors revealed in our study can guide the identification of patients with distant metastasis.
In addition to predictive clinicopathological characteristics, some blood tests can also be used for prediction; for example, the serum level of the bone alkaline phosphatase was reported to be correlated with bone metastasis [26]. More advanced techniques have been developed to predict distant metastasis, including high-quality image-based artificial intelligence technologies [27]. Based on radiomics analysis and selected clinical characteristics, constructed nomograms can be used to predict metastasis to the liver [28], lymph nodes [29], and peritoneum [30]. Gene expression [31] and metastasis-associated protein [32] have been studied for their value as potential predictive biomarkers for distant metastasis in gastric cancer. All these promising tools at different levels can be further applied and validated to assist prediction of metastasis.
Our work has some limitations. First, the SEER database only recorded synchronous metastatic patients; therefore, patients developing distant metastasis later in their course were not analyzed. Although our analysis revealed some important factors predicting distant metastasis in gastric cancer, only the liver, lung, bone, and brain metastatic sites were available, and the lack of data on other metastatic sites may impair the accuracy of our findings. The significant predictive factors need to be externally validated in different databases or multiple centers. Furthermore, other useful information such as genetic or clinical tests were not available in the SEER database, and these should be analyzed and incorporated into the predictive model to establish a more accurate and robust tool for patient stratification.
Conclusions
Initial distant metastasis was recorded in 32.6% of patients with gastric cancer in the SEER database. Patients with distant metastasis had significantly shorter survival than those without metastasis. Homogeneity and heterogeneity were identified in the risk factors for specific distant metastasis and the prognostic factors of gastric cancer patients. A series of factors were found to be correlated with distant metastasis, including: age, sex, race, insurance status, primary tumor site, histological type, grade, T stage, and N stage. These factors might be used in auxiliary individualized evaluation and prediction in the future. Our findings may improve individualized evaluation and prediction of gastric cancer patients.
Supplementary Data
Supplementary Table 1.
P values for the results of proportion hazards assumption test.
| Subject characteristics | M-Met | Liver-Met | Lung-Met | Bone-Met | Brain-Met |
|---|---|---|---|---|---|
| P-value | P-value | P-value | P-value | P-value | |
| Age | 0.039 | 0.103 | 0.187 | 0.576 | 0.654 |
| Gender | 0.482 | 0.001 | 0.070 | 0.647 | 0.971 |
| Race | 0.802 | 0.956 | 0.813 | 0.147 | 0.562 |
| Marital Status | 0.716 | 0.091 | 0.516 | 0.226 | 0.845 |
| Insurance Status | 0.300 | 0.328 | 0.659 | 0.999 | 0.592 |
| Primary site | 0.381 | 0.475 | 0.695 | 0.329 | 0.568 |
| Histology | 0.263 | 0.587 | 0.218 | 0.718 | 0.993 |
| Grade | 0.097 | 0.160 | 0.006 | 0.904 | 0.340 |
| T Stage | 0.022 | 0.656 | 0.900 | 0.720 | 0.930 |
| N Stage | 0.170 | 0.430 | 0.548 | 0.432 | 0.674 |
| Surgery | 0.002 | 0.338 | 0.509 | 0.269 | 0.507 |
| Radiation | 0.178 | 0.243 | 0.043 | 0.804 | 0.462 |
| Chemotherapy | <0.001 | <0.001 | 0.004 | 0.066 | 0.600 |
| Number of mets | 0.244 | – | – | – | – |
| Other mets | – | 0.506 | 0.768 | 0.181 | 0.602 |
Supplementary Table 2.
Univariable Cox regression for analyzing the prognostic factors for gastric cancer patients with distant metastases.
| Subject characteristics | M-Met | Liver-Met | Lung-Met | Bone-Met | Brain-Met | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| HR (95% CI) | P-value | HR (95% CI) | P-value | HR (95% CI) | P-value | HR (95% CI) | P-value | HR (95% CI) | P-value | |
| Age | ||||||||||
| <65 | 1.00 (Reference) | 1.00 | 1.00 (Reference) | 1.00 | 1.00 (Reference) | 1.00 | 1.00 (Reference) | 1.00 | 1.00 (Reference) | 1.00 |
| ≥65 | 1.31 (1.26–1.36) | <0.001 | 1.34 (1.26–1.42) | <0.001 | 1.17 (1.06–1.29) | 0.001 | 1.12 (1.01–1.24) | 0.036 | 1.14 (0.87–1.49) | 0.355 |
| Gender | ||||||||||
| Male | 1.00 (Reference) | 1.00 | 1.00 (Reference) | 1.00 | 1.00 (Reference) | 1.00 | 1.00 (Reference) | 1.00 | 1.00 (Reference) | 1.00 |
| Female | 0.98 (0.94–1.02) | 0.333 | 0.95 (0.89–1.01) | 0.116 | 1.02 (0.92–1.13) | 0.702 | 1.04 (0.94–1.17) | 0.442 | 1.02 (0.75–1.38) | 0.894 |
| Race | ||||||||||
| White | 1.00 (Reference) | 1.00 | 1.00 (Reference) | 1.00 | 1.00 (Reference) | 1.00 | 1.00 (Reference) | 1.00 | 1.00 (Reference) | 1.00 |
| Black | 0.99 (0.93–1.04) | 0.597 | 0.95 (0.88–1.02) | 0.166 | 1.12 (0.97–1.30) | 0.135 | 1.16 (0.98–1.37) | 0.084 | 1.16 (0.72–1.87) | 0.540 |
| Others | 0.99 (0.94–1.05) | 0.746 | 0.99 (0.91–1.09) | 0.850 | 1.14 (0.98–1.32) | 0.950 | 1.06 (0.91–1.24) | 0.460 | 1.29 (0.81–2.05) | 0.290 |
| Marital status | ||||||||||
| Married | 1.00 (Reference) | 1.00 | 1.00 (Reference) | 1.00 | 1.00 (Reference) | 1.00 | 1.00 (Reference) | 1.00 | 1.00 (Reference) | 1.00 |
| Unmarried | 1.18 (1.13–1.23) | <0.001 | 1.15 (1.09–1.22) | <0.001 | 1.21 (1.10–1.34) | <0.001 | 1.13 (1.02–1.26) | 0.025 | 1.03 (0.78–1.38) | 0.825 |
| Insurance status | ||||||||||
| Insured | 1.00 (Reference) | 1.00 | 1.00 (Reference) | 1.00 | 1.00 (Reference) | 1.00 | 1.00 (Reference) | 1.00 | 1.00 (Reference) | 1.00 |
| Uninsured | 1.05 (0.96–1.14) | 0.303 | 1.14 (0.99–1.30) | 0.067 | 1.07 (0.85–1.35) | 0.562 | 1.08 (0.84–1.38) | 0.548 | 1.54 (0.86–2.78) | 0.148 |
| Primary site | ||||||||||
| Proximal third | 1.00 (Reference) | 1.00 | 1.00 (Reference) | 1.00 | 1.00 (Reference) | 1.00 | 1.00 (Reference) | 1.00 | 1.00 (Reference) | 1.00 |
| Mid | 1.06 (0.99–1.14) | 0.084 | 1.04 (0.93–1.15) | 0.495 | 1.18 (0.97–1.43) | 0.100 | 1.29 (1.08–1.55) | 0.006 | 1.69 (0.89–3.23) | 0.112 |
| Distal third | 1.02 (0.97–1.08) | 0.488 | 1.04 (0.96–1.14) | 0.328 | 1.18 (1.01–1.38) | 0.043 | 1.32 (1.10–1.57) | 0.002 | 1.43 (0.81–2.54) | 0.222 |
| Stomach, NOS | 1.06 (1.01–1.11) | 0.017 | 1.00 (0.93–1.07) | 0.983 | 1.19 (1.06–1.34) | 0.003 | 1.18 (1.04–1.33) | 0.010 | 1.44 (1.06–1.96) | 0.021 |
| Overlapping | 1.17 (1.09–1.26) | <0.001 | 1.29 (1.15–1.44) | <0.001 | 1.32 (1.08–1.60) | 0.006 | 1.17 (0.95–1.46) | 0.147 | 0.97 (0.40–2.37) | 0.944 |
| Histology | ||||||||||
| Adenoca-rcinoma | 1.00 (Reference) | 1.00 | 1.00 (Reference) | 1.00 | 1.00 (Reference) | 1.00 | 1.00 (Reference) | 1.00 | 1.00 (Reference) | 1.00 |
| Mucous carcinoma | 1.02 (0.88–1.17) | 0.845 | 1.43 (1.11–1.85) | 0.005 | 1.00 (0.70–1.42) | 0.995 | 0.73 (0.47–1.14) | 0.163 | 0.95 (0.24–3.85) | 0.944 |
| Signet-ring cell carcinoma | 1.06 (1.01–1.11) | 0.018 | 1.17 (1.04–1.31) | 0.007 | 1.13 (0.99–1.30) | 0.080 | 1.01 (0.90–1.14) | 0.831 | 1.35 (0.95–1.92) | 0.099 |
| Unknown | 0.67 (0.63–0.71) | <0.001 | 0.63 (0.58–0.69) | <0.001 | 1.07 (0.92–1.26) | 0.372 | 1.07 (0.88–1.29) | 0.502 | 1.11 (0.70–1.77) | 0.665 |
| Grade | ||||||||||
| I | 1.00 (Reference) | 1.00 | 1.00 (Reference) | 1.00 | 1.00 (Reference) | 1.00 | 1.00 (Reference) | 1.00 | 1.00 (Reference) | 1.00 |
| II | 1.51 (1.30–1.77) | <0.001 | 1.57 (1.28–1.92) | <0.001 | 1.24 (0.89–1.73) | 0.204 | 0.74 (0.44–1.24) | 0.251 | NA | NA |
| III | 1.83 (1.57–2.12) | <0.001 | 2.02 (1.66–2.47) | <0.001 | 1.65 (1.20–2.28) | 0.002 | 0.95 (0.58–1.55) | 0.825 | NA | NA |
| IV | 1.34 (1.09–1.65) | 0.005 | 1.46 (1.11–1.92) | 0.008 | 1.12 (0.65–0.94) | 0.691 | 0.88 (0.46–1.70) | 0.700 | NA | NA |
| T stage | ||||||||||
| T1 | 1.00 (Reference) | 1.00 | 1.00 (Reference) | 1.00 | 1.00 (Reference) | 1.00 | 1.00 (Reference) | 1.00 | 1.00 (Reference) | 1.00 |
| T2 | 0.71 (0.64–0.78) | <0.001 | 0.59 (0.50–0.70) | <0.001 | 0.90 (0.68–1.20) | 0.456 | 0.58 (0.43–0.80) | 0.001 | 1.30 (0.62–2.72) | 0.486 |
| T3 | 0.71 (0.66–0.76) | <0.001 | 0.68 (0.61–0.75) | <0.001 | 0.90 (0.75–1.07) | 0.225 | 0.83 (0.69–1.01) | 0.059 | 0.85 (0.50–1.46) | 0.561 |
| T4 | 0.92 (0.86–0.97) | 0.004 | 0.91 (0.83–1.00) | 0.043 | 1.18 (1.01–1.38) | 0.042 | 1.01 (0.84–1.22) | 0.932 | 1.72 (1.02–2.91) | 0.043 |
| N stage | ||||||||||
| N0 | 1.00 (Reference) | 1.00 | 1.00 (Reference) | 1.00 | 1.00 (Reference) | 1.00 | 1.00 (Reference) | 1.00 | 1.00 (Reference) | 1.00 |
| N1 | 1.01 (0.97–1.06) | 0.572 | 1.09 (1.02–1.16) | 0.012 | 0.98 (0.88–1.10) | 0.758 | 1.09 (0.97–1.23) | 0.155 | 1.20 (0.87–1.67) | 0.271 |
| N2 | 0.84 (0.77–0.92) | <0.001 | 0.99 (0.86–1.13) | 0.867 | 0.96 (0.75–1.24) | 0.745 | 0.93 (0.71–1.21) | 0.571 | 0.86 (0.43–1.73) | 0.676 |
| N3 | 0.86 (0.79–0.93) | <0.001 | 1.01 (0.87–1.17) | 0.944 | 1.11 (0.86–1.44) | 0.410 | 0.95 (0.72–1.24) | 0.686 | 1.32 (0.71–2.44) | 0.383 |
| Surgery | ||||||||||
| No | 1.00 (Reference) | 1.00 | 1.00 (Reference) | 1.00 | 1.00 (Reference) | 1.00 | 1.00 (Reference) | 1.00 | 1.00 (Reference) | 1.00 |
| Yes | 0.51 (0.48–0.55) | <0.001 | 0.51 (0.46–0.57) | <0.001 | 0.73 (0.57–0.92) | 0.009 | 0.66 (0.50–0.87) | 0.003 | 0.48 (0.25–0.91) | 0.025 |
| Radiation | ||||||||||
| No/unknown | 1.00 (Reference) | 1.00 | 1.00 (Reference) | 1.00 | 1.00 (Reference) | 1.00 | 1.00 (Reference) | 1.00 | 1.00 (Reference) | 1.00 |
| Yes | 0.91 (0.87–0.96) | 0.001 | 1.01 (0.93–1.09) | 0.845 | 0.80 (0.71–0.91) | <0.001 | 0.89 (0.79–0.99) | 0.036 | 0.67 (0.51–0.89) | 0.005 |
| Chemotherapy | ||||||||||
| No/unknown | 1.00 (Reference) | 1.00 | 1.00 (Reference) | 1.00 | 1.00 (Reference) | 1.00 | 1.00 (Reference) | 1.00 | 1.00 (Reference) | 1.00 |
| Yes | 0.37 (0.36–0.39) | <0.001 | 0.37 (0.34–0.39) | <0.001 | 0.36 (0.32–0.40) | <0.001 | 0.38 (0.34–0.42) | <0.001 | 0.41 (0.31–0.55) | <0.001 |
| Number of mets | ||||||||||
| ≤1 | 1.00 (Reference) | 1.00 | – | – | – | – | – | – | – | – |
| >1 | 1.44 (1.37–1.53) | <0.001 | – | – | – | – | – | – | – | – |
| Other mets | ||||||||||
| No | – | – | 1.00 (Reference) | 1.00 | 1.00 (Reference) | 1.00 | 1.00 (Reference) | 1.00 | 1.00 (Reference) | 1.00 |
| Yes | – | – | 1.41 (1.32–1.50) | <0.001 | 1.27 (1.15–1.40) | <0.001 | 1.01 (0.91–1.12) | 0.813 | 1.34 (1.01–1.78) | 0.040 |
Met – metastases; HR – hazard ratio; CI – confidence interval; NOS – not otherwise specified; NA – not available.
Supplementary Table 3.
Univariable logistic regression for analyzing the risk factors for developing distant metastases in patients with gastric cancer.
| Subject characteristics | M-Met | Liver-Met | Lung-Met | Bone-Met | Brain-Met | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| OR (95% CI) | P-value | OR (95% CI) | P-value | OR (95% CI) | P-value | OR (95% CI) | P-value | OR (95% CI) | P-value | |
| Age | ||||||||||
| <65 | 1.00 (Reference) | 1.00 | 1.00 (Reference) | 1.00 | 1.00 (Reference) | 1.00 | 1.00 (Reference) | 1.00 | 1.00 (Reference) | 1.00 |
| ≥65 | 0.65 (0.62–0.67) | <0.001 | 0.92 (0.87–0.98) | 0.007 | 0.87 (0.79–0.96) | 0.005 | 0.63 (0.57–0.70) | <0.001 | 0.57 (0.44–0.74) | <0.001 |
| Gender | ||||||||||
| Male | 1.00 (Reference) | 1.00 | 1.00 (Reference) | 1.00 | 1.00 (Reference) | 1.00 | 1.00 (Reference) | 1.00 | 1.00 (Reference) | 1.00 |
| Female | 0.83 (0.79–0.87) | <0.001 | 0.61 (0.57–0.65) | <0.001 | 0.72 (0.65–0.80) | <0.001 | 0.79 (0.71–0.88) | <0.001 | 0.59 (0.44–0.79) | <0.001 |
| Race | ||||||||||
| White | 1.00 (Reference) | 1.00 | 1.00 (Reference) | 1.00 | 1.00 (Reference) | 1.00 | 1.00 (Reference) | 1.00 | 1.00 (Reference) | 1.00 |
| Black | 0.96 (0.90–1.02) | 0.193 | 1.18 (1.08–1.27) | <0.001 | 0.80 (0.69–0.93) | 0.004 | 0.71 (0.60–0.84) | <0.001 | 0.51 (0.32–0.81) | 0.005 |
| Others | 0.86 (0.81–0.92) | <0.001 | 0.74 (0.68–0.81) | <0.001 | 0.75 (0.65–0.87) | <0.001 | 0.87 (0.75–1.02) | 0.080 | 0.54 (0.34–0.84) | 0.007 |
| Marital status | ||||||||||
| Married | 1.00 (Reference) | 1.00 | 1.00 (Reference) | 1.00 | 1.00 (Reference) | 1.00 | 1.00 (Reference) | 1.00 | 1.00 (Reference) | 1.00 |
| Unmarried | 0.96 (0.92–1.01) | 0.121 | 0.97 (0.92–1.04) | 0.392 | 1.06 (0.96–1.17) | 0.279 | 0.99 (0.89–1.10) | 0.841 | 0.92 (0.70–1.21) | 0.548 |
| Insurance status | ||||||||||
| Insured | 1.00 (Reference) | 1.00 | 1.00 (Reference) | 1.00 | 1.00 (Reference) | 1.00 | 1.00 (Reference) | 1.00 | 1.00 (Reference) | 1.00 |
| Uninsured | 1.93 (1.73–2.16) | <0.001 | 1.36 (1.18–1.56) | <0.001 | 1.45 (1.16–1.81) | 0.001 | 1.50 (1.19–1.90) | 0.001 | 1.61 (0.92–2.83) | 0.096 |
| Primary site | ||||||||||
| Proximal third | 1.00 (Reference) | 1.00 | 1.00 (Reference) | 1.00 | 1.00 (Reference) | 1.00 | 1.00 (Reference) | 1.00 | 1.00 (Reference) | 1.00 |
| Mid | 0.87 (0.81–0.94) | 0.001 | 0.57 (0.51–0.63) | <0.001 | 0.47 (0.39–0.57) | <0.001 | 0.76 (0.63–0.91) | 0.003 | 0.27 (0.15–0.50) | <0.001 |
| Distal third | 0.74 (0.69–0.79) | <0.001 | 0.52 (0.47–0.56) | <0.001 | 0.40 (0.34–0.47) | <0.001 | 0.46 (0.39–0.55) | <0.001 | 0.17 (0.10–0.31) | <0.001 |
| Stomach, NOS | 1.07 (1.01–1.13) | 0.020 | 0.67 (0.62–0.72) | <0.001 | 0.71 (0.63–0.79) | <0.001 | 0.90 (0.79–1.02) | 0.089 | 0.61 (0.45–0.82) | 0.001 |
| Overlapping | 1.40 (1.28–1.52) | <0.001 | 0.70 (0.62–0.79) | <0.001 | 0.69 (0.57–0.85) | <0.001 | 0.80 (0.64–0.99) | 0.037 | 0.26 (0.12–0.56) | 0.001 |
| Histology | ||||||||||
| Adenocar-cinoma | 1.00 (Reference) | 1.00 | 1.00 (Reference) | 1.00 | 1.00 (Reference) | 1.00 | 1.00 (Reference) | 1.00 | 1.00 (Reference) | 1.00 |
| Mucous carcinoma | 1.00 (0.84–1.19) | 0.977 | 0.57 (0.44–0.74) | <0.001 | 1.06 (0.74–1.50) | 0.760 | 0.96 (0.63–1.47) | 0.856 | 0.48 (0.12–1.93) | 0.300 |
| Signet-ring cell carcinoma | 1.31 (1.24–1.39) | <0.001 | 0.29 (0.26–0.33) | <0.001 | 0.75 (0.66–0.87) | <0.001 | 1.63 (1.44–1.84) | <0.001 | 0.92 (0.65–1.31) | 0.640 |
| Unknown | 0.46 (0.43–0.49) | <0.001 | 0.66 (0.61–0.71) | <0.001 | 0.51 (0.44–0.59) | <0.001 | 0.45 (0.37–0.54) | <0.001 | 0.47 (0.30–0.72) | 0.001 |
| Grade | ||||||||||
| I | 1.00 (Reference) | 1.00 | 1.00 (Reference) | 1.00 | 1.00 (Reference) | 1.00 | 1.00 (Reference) | 1.00 | 1.00 (Reference) | 1.00 |
| II | 4.22 (3.66–4.87) | <0.001 | 4.32 (3.60–5.19) | <0.001 | 3.52 (2.57–4.83) | <0.001 | 4.42 (2.65–7.39) | <0.001 | 20.42 (2.82–147.80) | 0.003 |
| III | 7.04 (6.15–8.07) | <0.001 | 3.62 (3.03–4.33) | <0.001 | 3.60 (2.65–4.88) | <0.001 | 10.32 (6.29–16.95) | <0.001 | 17.68 (2.47–126.74) | 0.004 |
| IV | 5.46 (4.44–6.71) | <0.001 | 4.07 (3.12–5.30) | <0.001 | 2.16 (1.28–3.62) | 0.004 | 5.71 (2.96–10.99) | <0.001 | 29.97 (3.68–243.96) | 0.001 |
| T stage | ||||||||||
| T1 | 1.00 (Reference) | 1.00 | 1.00 (Reference) | 1.00 | 1.00 (Reference) | 1.00 | 1.00 (Reference) | 1.00 | 1.00 (Reference) | 1.00 |
| T2 | 0.63 (0.57–0.69) | <0.001 | 0.41 (0.35–0.48) | <0.001 | 0.38 (0.29–0.50) | <0.001 | 0.46 (0.34–0.62) | <0.001 | 0.52 (0.25–1.08) | 0.078 |
| T3 | 0.88 (0.82–0.94) | <0.001 | 0.65 (0.58–0.72) | <0.001 | 0.57 (0.48–0.68) | <0.001 | 0.77 (0.64–0.93) | 0.007 | 0.75 (0.46–1.22) | 0.248 |
| T4 | 2.40 (2.24–2.58) | <0.001 | 1.42 (1.29–1.55) | <0.001 | 1.27 (1.08–1.48) | 0.003 | 1.15 (0.96–1.38) | 0.141 | 0.95 (0.58–1.57) | 0.853 |
| N stage | ||||||||||
| N0 | 1.00 (Reference) | 1.00 | 1.00 (Reference) | 1.00 | 1.00 (Reference) | 1.00 | 1.00 (Reference) | 1.00 | 1.00 (Reference) | 1.00 |
| N1 | 2.79 (2.65–2.95) | <0.001 | 2.52 (2.35–2.70) | <0.001 | 2.49 (2.23–2.79) | <0.001 | 2.64 (2.34–2.98) | <0.001 | 2.49 (1.83–3.40) | <0.001 |
| N2 | 0.95 (0.87–1.05) | 0.320 | 0.83 (0.73–0.95) | 0.008 | 0.74 (0.57–0.95) | 0.017 | 0.82 (0.63–1.07) | 0.150 | 0.90 (0.46–1.73) | 0.743 |
| N3 | 1.06 (0.97–1.17) | 0.196 | 0.72 (0.62–0.84) | <0.001 | 0.74 (0.58–0.96) | 0.023 | 0.84 (0.65–1.10) | 0.210 | 1.21 (0.67–2.18) | 0.523 |
Met – metastases; OR – odds ratio; CI – confidence interval; NOS – not otherwise specified.
Footnotes
Conflicts of interest
None.
Source of support: The present study was supported by Chongqing Natural Science Foundation Program (cstc2019jcyj-msxmX0466), the Top Talent Training Program of the First Affiliated Hospital of PLA Army Medical University (SWH2018BJKJ-12), and the Natural Science Foundation of China (No. 81802508 and No. 81903398)
References
- 1.Thrift AP, El-Serag HB. Burden of gastric cancer. Clin Gastroenterol Hepatol. 2020;18(3):534–42. doi: 10.1016/j.cgh.2019.07.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2019. Cancer J Clin. 2019;69(1):7–34. doi: 10.3322/caac.21551. [DOI] [PubMed] [Google Scholar]
- 3.Rawla P, Barsouk A. Epidemiology of gastric cancer: Global trends, risk factors and prevention. Prz Gastroenterol. 2019;14(1):26–38. doi: 10.5114/pg.2018.80001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Leiting JL, Grotz TE. Advancements and challenges in treating advanced gastric cancer in the West. World J Gastro Oncol. 2019;11(9):652–64. doi: 10.4251/wjgo.v11.i9.652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bernards N, Creemers GJ, Nieuwenhuijzen GAP, et al. No improvement in median survival for patients with metastatic gastric cancer despite increased use of chemotherapy. Ann Oncol. 2013;24(12):3056–60. doi: 10.1093/annonc/mdt401. [DOI] [PubMed] [Google Scholar]
- 6.Qiu M, Shi S, Chen Z, et al. Frequency and clinicopathological features of metastasis to liver, lung, bone, and brain from gastric cancer: A SEER-based study. Cancer Med. 2018;7(8):3662–72. doi: 10.1002/cam4.1661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ma T, Wu ZJ, Xu H, et al. Nomograms for predicting survival in patients with metastatic gastric adenocarcinoma who undergo palliative gastrectomy. BMC Cancer. 2019;19(1):852. doi: 10.1186/s12885-019-6075-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Omari J, Drewes R, Orthmer M, et al. Treatment of metastatic gastric adenocarcinoma with image-guided high-dose rate, interstitial brachytherapy as second-line or salvage therapy. Diagn Interv Radiol. 2019;25(5):360–67. doi: 10.5152/dir.2019.18390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Markar SR, Mackenzie H, Mikhail S, et al. Surgical resection of hepatic metastases from gastric cancer: Outcomes from national series in England. Gastric Cancer. 2017;20(2):379–86. doi: 10.1007/s10120-016-0604-6. [DOI] [PubMed] [Google Scholar]
- 10.Liu J, Li J, Zhai R, et al. Predictive factors improving survival after gastric and hepatic surgical treatment in gastric cancer patients with synchronous liver metastases. Chin Med J (Engl) 2012;125(2):165–71. [PubMed] [Google Scholar]
- 11.Montagnani F, Crivelli F, Aprile G, et al. Long-term survival after liver metastasectomy in gastric cancer: Systematic review and meta-analysis of prognostic factors. Cancer Treat Rev. 2018;69:11–20. doi: 10.1016/j.ctrv.2018.05.010. [DOI] [PubMed] [Google Scholar]
- 12.Kong JH, Lee J, Yi C, et al. Lung metastases in metastatic gastric cancer: Pattern of lung metastases and clinical outcome. Gastric Cancer. 2012;15(3):292–98. doi: 10.1007/s10120-011-0104-7. [DOI] [PubMed] [Google Scholar]
- 13.Kim YJ, Kim SH, Kim JW, et al. Gastric cancer with initial bone metastasis: A distinct group of diseases with poor prognosis. Eur J Cancer. 2014;50(16):2810–21. doi: 10.1016/j.ejca.2014.08.003. [DOI] [PubMed] [Google Scholar]
- 14.Tamura S, Takeno A, Miki H, et al. [Clinical outcomes in patients with brain metastasis from gastric cancer]. Gan To Kagaku Ryoho. 2011;38(12):2093–96. [PubMed] [Google Scholar]
- 15.Cavanna L, Seghini P, Di Nunzio C, et al. Gastric cancer with brain metastasis and the role of human epidermal growth factor 2 status. Oncol Lett. 2018;15(4):5787–91. doi: 10.3892/ol.2018.8054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Claassen YHM, Bastiaannet E, Hartgrink HH, et al. International comparison of treatment strategy and survival in metastatic gastric cancer. BJS Open. 2018;3(1):56–61. doi: 10.1002/bjs5.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhang F, Huang X, Song Y, et al. Conversion surgery for stage IV gastric cancer. Front Oncol. 2019;9:1158. doi: 10.3389/fonc.2019.01158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kataoka K, Kinoshita T, Moehler M, et al. Current management of liver metastases from gastric cancer: What is common practice? New challenge of EORTC and JCOG. Gastric Cancer. 2017;20(5):904–12. doi: 10.1007/s10120-017-0696-7. [DOI] [PubMed] [Google Scholar]
- 19.Shiono S, Sato T, Horio H, et al. Outcomes and prognostic factors of survival after pulmonary resection for metastatic gastric cancer. Eur J Cardiothorac. 2013;43(1):e13–16. doi: 10.1093/ejcts/ezs574. [DOI] [PubMed] [Google Scholar]
- 20.Turkoz FP, Solak M, Kilickap S, et al. Bone metastasis from gastric cancer: The incidence, clinicopathological features, and influence on survival. J Gastric Cancer. 2014;14(3):164–72. doi: 10.5230/jgc.2014.14.3.164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Liu Y, Chen L, Zhang R, et al. Efficacy and safety of elemene combined with chemotherapy in advanced gastric cancer: A Meta-analysis. Medicine (Baltimore) 2020;99(11):e19481. doi: 10.1097/MD.0000000000019481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Digklia A, Wagner AD. Advanced gastric cancer: Current treatment landscape and future perspectives. World J Gastroenterol. 2016;22(8):2403–14. doi: 10.3748/wjg.v22.i8.2403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ajani JA, D’Amico TA, Almhanna K, et al. Gastric cancer, Version 3.2016, NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw. 2016;14(10):1286–312. doi: 10.6004/jnccn.2016.0137. [DOI] [PubMed] [Google Scholar]
- 24.Biagioni A, Skalamera I, Peri S, et al. Update on gastric cancer treatments and gene therapies. Cancer Metastasis Rev. 2019;38(3):537–48. doi: 10.1007/s10555-019-09803-7. [DOI] [PubMed] [Google Scholar]
- 25.Kawanaka Y, Kitajima K, Fukushima K, et al. Added value of pretreatment (18)F-FDG PET/CT for staging of advanced gastric cancer: Comparison with contrast-enhanced MDCT. Eur J Radiol. 2016;85(5):989–95. doi: 10.1016/j.ejrad.2016.03.003. [DOI] [PubMed] [Google Scholar]
- 26.Lim SM, Kim YN, Park KH, et al. Bone alkaline phosphatase as a surrogate marker of bone metastasis in gastric cancer patients. BMC Cancer. 2016;16:385. doi: 10.1186/s12885-016-2415-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ma W, Zhao Y, Ji Y, et al. Breast cancer molecular subtype prediction by mammographic radiomic features. Acad Radiol. 2019;26(2):196–201. doi: 10.1016/j.acra.2018.01.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tsurumaru D, Nishimuta Y, Muraki T, et al. Gastric cancer with synchronous and metachronous hepatic metastasis predicted by enhancement pattern on multiphasic contrast-enhanced CT. Eur J Radiol. 2018;108:165–71. doi: 10.1016/j.ejrad.2018.09.030. [DOI] [PubMed] [Google Scholar]
- 29.Wang Y, Liu W, Yu Y, et al. CT radiomics nomogram for the preoperative prediction of lymph node metastasis in gastric cancer. Eur Radiol. 2020;30(2):976–86. doi: 10.1007/s00330-019-06398-z. [DOI] [PubMed] [Google Scholar]
- 30.Liu S, He J, Liu S, et al. Radiomics analysis using contrast-enhanced CT for preoperative prediction of occult peritoneal metastasis in advanced gastric cancer. Eur Radiol. 2020;30(1):239–46. doi: 10.1007/s00330-019-06368-5. [DOI] [PubMed] [Google Scholar]
- 31.Jin X, Zhu Z, Shi Y. Metastasis mechanism and gene/protein expression in gastric cancer with distant organs metastasis. Bull Cancer. 2014;101(1):E1–12. [PubMed] [Google Scholar]
- 32.Okugawa Y, Mohri Y, Tanaka K, et al. Metastasis-associated protein is a predictive biomarker for metastasis and recurrence in gastric cancer. Oncol Rep. 2016;36(4):1893–900. doi: 10.3892/or.2016.5054. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Table 1.
P values for the results of proportion hazards assumption test.
| Subject characteristics | M-Met | Liver-Met | Lung-Met | Bone-Met | Brain-Met |
|---|---|---|---|---|---|
| P-value | P-value | P-value | P-value | P-value | |
| Age | 0.039 | 0.103 | 0.187 | 0.576 | 0.654 |
| Gender | 0.482 | 0.001 | 0.070 | 0.647 | 0.971 |
| Race | 0.802 | 0.956 | 0.813 | 0.147 | 0.562 |
| Marital Status | 0.716 | 0.091 | 0.516 | 0.226 | 0.845 |
| Insurance Status | 0.300 | 0.328 | 0.659 | 0.999 | 0.592 |
| Primary site | 0.381 | 0.475 | 0.695 | 0.329 | 0.568 |
| Histology | 0.263 | 0.587 | 0.218 | 0.718 | 0.993 |
| Grade | 0.097 | 0.160 | 0.006 | 0.904 | 0.340 |
| T Stage | 0.022 | 0.656 | 0.900 | 0.720 | 0.930 |
| N Stage | 0.170 | 0.430 | 0.548 | 0.432 | 0.674 |
| Surgery | 0.002 | 0.338 | 0.509 | 0.269 | 0.507 |
| Radiation | 0.178 | 0.243 | 0.043 | 0.804 | 0.462 |
| Chemotherapy | <0.001 | <0.001 | 0.004 | 0.066 | 0.600 |
| Number of mets | 0.244 | – | – | – | – |
| Other mets | – | 0.506 | 0.768 | 0.181 | 0.602 |
Supplementary Table 2.
Univariable Cox regression for analyzing the prognostic factors for gastric cancer patients with distant metastases.
| Subject characteristics | M-Met | Liver-Met | Lung-Met | Bone-Met | Brain-Met | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| HR (95% CI) | P-value | HR (95% CI) | P-value | HR (95% CI) | P-value | HR (95% CI) | P-value | HR (95% CI) | P-value | |
| Age | ||||||||||
| <65 | 1.00 (Reference) | 1.00 | 1.00 (Reference) | 1.00 | 1.00 (Reference) | 1.00 | 1.00 (Reference) | 1.00 | 1.00 (Reference) | 1.00 |
| ≥65 | 1.31 (1.26–1.36) | <0.001 | 1.34 (1.26–1.42) | <0.001 | 1.17 (1.06–1.29) | 0.001 | 1.12 (1.01–1.24) | 0.036 | 1.14 (0.87–1.49) | 0.355 |
| Gender | ||||||||||
| Male | 1.00 (Reference) | 1.00 | 1.00 (Reference) | 1.00 | 1.00 (Reference) | 1.00 | 1.00 (Reference) | 1.00 | 1.00 (Reference) | 1.00 |
| Female | 0.98 (0.94–1.02) | 0.333 | 0.95 (0.89–1.01) | 0.116 | 1.02 (0.92–1.13) | 0.702 | 1.04 (0.94–1.17) | 0.442 | 1.02 (0.75–1.38) | 0.894 |
| Race | ||||||||||
| White | 1.00 (Reference) | 1.00 | 1.00 (Reference) | 1.00 | 1.00 (Reference) | 1.00 | 1.00 (Reference) | 1.00 | 1.00 (Reference) | 1.00 |
| Black | 0.99 (0.93–1.04) | 0.597 | 0.95 (0.88–1.02) | 0.166 | 1.12 (0.97–1.30) | 0.135 | 1.16 (0.98–1.37) | 0.084 | 1.16 (0.72–1.87) | 0.540 |
| Others | 0.99 (0.94–1.05) | 0.746 | 0.99 (0.91–1.09) | 0.850 | 1.14 (0.98–1.32) | 0.950 | 1.06 (0.91–1.24) | 0.460 | 1.29 (0.81–2.05) | 0.290 |
| Marital status | ||||||||||
| Married | 1.00 (Reference) | 1.00 | 1.00 (Reference) | 1.00 | 1.00 (Reference) | 1.00 | 1.00 (Reference) | 1.00 | 1.00 (Reference) | 1.00 |
| Unmarried | 1.18 (1.13–1.23) | <0.001 | 1.15 (1.09–1.22) | <0.001 | 1.21 (1.10–1.34) | <0.001 | 1.13 (1.02–1.26) | 0.025 | 1.03 (0.78–1.38) | 0.825 |
| Insurance status | ||||||||||
| Insured | 1.00 (Reference) | 1.00 | 1.00 (Reference) | 1.00 | 1.00 (Reference) | 1.00 | 1.00 (Reference) | 1.00 | 1.00 (Reference) | 1.00 |
| Uninsured | 1.05 (0.96–1.14) | 0.303 | 1.14 (0.99–1.30) | 0.067 | 1.07 (0.85–1.35) | 0.562 | 1.08 (0.84–1.38) | 0.548 | 1.54 (0.86–2.78) | 0.148 |
| Primary site | ||||||||||
| Proximal third | 1.00 (Reference) | 1.00 | 1.00 (Reference) | 1.00 | 1.00 (Reference) | 1.00 | 1.00 (Reference) | 1.00 | 1.00 (Reference) | 1.00 |
| Mid | 1.06 (0.99–1.14) | 0.084 | 1.04 (0.93–1.15) | 0.495 | 1.18 (0.97–1.43) | 0.100 | 1.29 (1.08–1.55) | 0.006 | 1.69 (0.89–3.23) | 0.112 |
| Distal third | 1.02 (0.97–1.08) | 0.488 | 1.04 (0.96–1.14) | 0.328 | 1.18 (1.01–1.38) | 0.043 | 1.32 (1.10–1.57) | 0.002 | 1.43 (0.81–2.54) | 0.222 |
| Stomach, NOS | 1.06 (1.01–1.11) | 0.017 | 1.00 (0.93–1.07) | 0.983 | 1.19 (1.06–1.34) | 0.003 | 1.18 (1.04–1.33) | 0.010 | 1.44 (1.06–1.96) | 0.021 |
| Overlapping | 1.17 (1.09–1.26) | <0.001 | 1.29 (1.15–1.44) | <0.001 | 1.32 (1.08–1.60) | 0.006 | 1.17 (0.95–1.46) | 0.147 | 0.97 (0.40–2.37) | 0.944 |
| Histology | ||||||||||
| Adenoca-rcinoma | 1.00 (Reference) | 1.00 | 1.00 (Reference) | 1.00 | 1.00 (Reference) | 1.00 | 1.00 (Reference) | 1.00 | 1.00 (Reference) | 1.00 |
| Mucous carcinoma | 1.02 (0.88–1.17) | 0.845 | 1.43 (1.11–1.85) | 0.005 | 1.00 (0.70–1.42) | 0.995 | 0.73 (0.47–1.14) | 0.163 | 0.95 (0.24–3.85) | 0.944 |
| Signet-ring cell carcinoma | 1.06 (1.01–1.11) | 0.018 | 1.17 (1.04–1.31) | 0.007 | 1.13 (0.99–1.30) | 0.080 | 1.01 (0.90–1.14) | 0.831 | 1.35 (0.95–1.92) | 0.099 |
| Unknown | 0.67 (0.63–0.71) | <0.001 | 0.63 (0.58–0.69) | <0.001 | 1.07 (0.92–1.26) | 0.372 | 1.07 (0.88–1.29) | 0.502 | 1.11 (0.70–1.77) | 0.665 |
| Grade | ||||||||||
| I | 1.00 (Reference) | 1.00 | 1.00 (Reference) | 1.00 | 1.00 (Reference) | 1.00 | 1.00 (Reference) | 1.00 | 1.00 (Reference) | 1.00 |
| II | 1.51 (1.30–1.77) | <0.001 | 1.57 (1.28–1.92) | <0.001 | 1.24 (0.89–1.73) | 0.204 | 0.74 (0.44–1.24) | 0.251 | NA | NA |
| III | 1.83 (1.57–2.12) | <0.001 | 2.02 (1.66–2.47) | <0.001 | 1.65 (1.20–2.28) | 0.002 | 0.95 (0.58–1.55) | 0.825 | NA | NA |
| IV | 1.34 (1.09–1.65) | 0.005 | 1.46 (1.11–1.92) | 0.008 | 1.12 (0.65–0.94) | 0.691 | 0.88 (0.46–1.70) | 0.700 | NA | NA |
| T stage | ||||||||||
| T1 | 1.00 (Reference) | 1.00 | 1.00 (Reference) | 1.00 | 1.00 (Reference) | 1.00 | 1.00 (Reference) | 1.00 | 1.00 (Reference) | 1.00 |
| T2 | 0.71 (0.64–0.78) | <0.001 | 0.59 (0.50–0.70) | <0.001 | 0.90 (0.68–1.20) | 0.456 | 0.58 (0.43–0.80) | 0.001 | 1.30 (0.62–2.72) | 0.486 |
| T3 | 0.71 (0.66–0.76) | <0.001 | 0.68 (0.61–0.75) | <0.001 | 0.90 (0.75–1.07) | 0.225 | 0.83 (0.69–1.01) | 0.059 | 0.85 (0.50–1.46) | 0.561 |
| T4 | 0.92 (0.86–0.97) | 0.004 | 0.91 (0.83–1.00) | 0.043 | 1.18 (1.01–1.38) | 0.042 | 1.01 (0.84–1.22) | 0.932 | 1.72 (1.02–2.91) | 0.043 |
| N stage | ||||||||||
| N0 | 1.00 (Reference) | 1.00 | 1.00 (Reference) | 1.00 | 1.00 (Reference) | 1.00 | 1.00 (Reference) | 1.00 | 1.00 (Reference) | 1.00 |
| N1 | 1.01 (0.97–1.06) | 0.572 | 1.09 (1.02–1.16) | 0.012 | 0.98 (0.88–1.10) | 0.758 | 1.09 (0.97–1.23) | 0.155 | 1.20 (0.87–1.67) | 0.271 |
| N2 | 0.84 (0.77–0.92) | <0.001 | 0.99 (0.86–1.13) | 0.867 | 0.96 (0.75–1.24) | 0.745 | 0.93 (0.71–1.21) | 0.571 | 0.86 (0.43–1.73) | 0.676 |
| N3 | 0.86 (0.79–0.93) | <0.001 | 1.01 (0.87–1.17) | 0.944 | 1.11 (0.86–1.44) | 0.410 | 0.95 (0.72–1.24) | 0.686 | 1.32 (0.71–2.44) | 0.383 |
| Surgery | ||||||||||
| No | 1.00 (Reference) | 1.00 | 1.00 (Reference) | 1.00 | 1.00 (Reference) | 1.00 | 1.00 (Reference) | 1.00 | 1.00 (Reference) | 1.00 |
| Yes | 0.51 (0.48–0.55) | <0.001 | 0.51 (0.46–0.57) | <0.001 | 0.73 (0.57–0.92) | 0.009 | 0.66 (0.50–0.87) | 0.003 | 0.48 (0.25–0.91) | 0.025 |
| Radiation | ||||||||||
| No/unknown | 1.00 (Reference) | 1.00 | 1.00 (Reference) | 1.00 | 1.00 (Reference) | 1.00 | 1.00 (Reference) | 1.00 | 1.00 (Reference) | 1.00 |
| Yes | 0.91 (0.87–0.96) | 0.001 | 1.01 (0.93–1.09) | 0.845 | 0.80 (0.71–0.91) | <0.001 | 0.89 (0.79–0.99) | 0.036 | 0.67 (0.51–0.89) | 0.005 |
| Chemotherapy | ||||||||||
| No/unknown | 1.00 (Reference) | 1.00 | 1.00 (Reference) | 1.00 | 1.00 (Reference) | 1.00 | 1.00 (Reference) | 1.00 | 1.00 (Reference) | 1.00 |
| Yes | 0.37 (0.36–0.39) | <0.001 | 0.37 (0.34–0.39) | <0.001 | 0.36 (0.32–0.40) | <0.001 | 0.38 (0.34–0.42) | <0.001 | 0.41 (0.31–0.55) | <0.001 |
| Number of mets | ||||||||||
| ≤1 | 1.00 (Reference) | 1.00 | – | – | – | – | – | – | – | – |
| >1 | 1.44 (1.37–1.53) | <0.001 | – | – | – | – | – | – | – | – |
| Other mets | ||||||||||
| No | – | – | 1.00 (Reference) | 1.00 | 1.00 (Reference) | 1.00 | 1.00 (Reference) | 1.00 | 1.00 (Reference) | 1.00 |
| Yes | – | – | 1.41 (1.32–1.50) | <0.001 | 1.27 (1.15–1.40) | <0.001 | 1.01 (0.91–1.12) | 0.813 | 1.34 (1.01–1.78) | 0.040 |
Met – metastases; HR – hazard ratio; CI – confidence interval; NOS – not otherwise specified; NA – not available.
Supplementary Table 3.
Univariable logistic regression for analyzing the risk factors for developing distant metastases in patients with gastric cancer.
| Subject characteristics | M-Met | Liver-Met | Lung-Met | Bone-Met | Brain-Met | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| OR (95% CI) | P-value | OR (95% CI) | P-value | OR (95% CI) | P-value | OR (95% CI) | P-value | OR (95% CI) | P-value | |
| Age | ||||||||||
| <65 | 1.00 (Reference) | 1.00 | 1.00 (Reference) | 1.00 | 1.00 (Reference) | 1.00 | 1.00 (Reference) | 1.00 | 1.00 (Reference) | 1.00 |
| ≥65 | 0.65 (0.62–0.67) | <0.001 | 0.92 (0.87–0.98) | 0.007 | 0.87 (0.79–0.96) | 0.005 | 0.63 (0.57–0.70) | <0.001 | 0.57 (0.44–0.74) | <0.001 |
| Gender | ||||||||||
| Male | 1.00 (Reference) | 1.00 | 1.00 (Reference) | 1.00 | 1.00 (Reference) | 1.00 | 1.00 (Reference) | 1.00 | 1.00 (Reference) | 1.00 |
| Female | 0.83 (0.79–0.87) | <0.001 | 0.61 (0.57–0.65) | <0.001 | 0.72 (0.65–0.80) | <0.001 | 0.79 (0.71–0.88) | <0.001 | 0.59 (0.44–0.79) | <0.001 |
| Race | ||||||||||
| White | 1.00 (Reference) | 1.00 | 1.00 (Reference) | 1.00 | 1.00 (Reference) | 1.00 | 1.00 (Reference) | 1.00 | 1.00 (Reference) | 1.00 |
| Black | 0.96 (0.90–1.02) | 0.193 | 1.18 (1.08–1.27) | <0.001 | 0.80 (0.69–0.93) | 0.004 | 0.71 (0.60–0.84) | <0.001 | 0.51 (0.32–0.81) | 0.005 |
| Others | 0.86 (0.81–0.92) | <0.001 | 0.74 (0.68–0.81) | <0.001 | 0.75 (0.65–0.87) | <0.001 | 0.87 (0.75–1.02) | 0.080 | 0.54 (0.34–0.84) | 0.007 |
| Marital status | ||||||||||
| Married | 1.00 (Reference) | 1.00 | 1.00 (Reference) | 1.00 | 1.00 (Reference) | 1.00 | 1.00 (Reference) | 1.00 | 1.00 (Reference) | 1.00 |
| Unmarried | 0.96 (0.92–1.01) | 0.121 | 0.97 (0.92–1.04) | 0.392 | 1.06 (0.96–1.17) | 0.279 | 0.99 (0.89–1.10) | 0.841 | 0.92 (0.70–1.21) | 0.548 |
| Insurance status | ||||||||||
| Insured | 1.00 (Reference) | 1.00 | 1.00 (Reference) | 1.00 | 1.00 (Reference) | 1.00 | 1.00 (Reference) | 1.00 | 1.00 (Reference) | 1.00 |
| Uninsured | 1.93 (1.73–2.16) | <0.001 | 1.36 (1.18–1.56) | <0.001 | 1.45 (1.16–1.81) | 0.001 | 1.50 (1.19–1.90) | 0.001 | 1.61 (0.92–2.83) | 0.096 |
| Primary site | ||||||||||
| Proximal third | 1.00 (Reference) | 1.00 | 1.00 (Reference) | 1.00 | 1.00 (Reference) | 1.00 | 1.00 (Reference) | 1.00 | 1.00 (Reference) | 1.00 |
| Mid | 0.87 (0.81–0.94) | 0.001 | 0.57 (0.51–0.63) | <0.001 | 0.47 (0.39–0.57) | <0.001 | 0.76 (0.63–0.91) | 0.003 | 0.27 (0.15–0.50) | <0.001 |
| Distal third | 0.74 (0.69–0.79) | <0.001 | 0.52 (0.47–0.56) | <0.001 | 0.40 (0.34–0.47) | <0.001 | 0.46 (0.39–0.55) | <0.001 | 0.17 (0.10–0.31) | <0.001 |
| Stomach, NOS | 1.07 (1.01–1.13) | 0.020 | 0.67 (0.62–0.72) | <0.001 | 0.71 (0.63–0.79) | <0.001 | 0.90 (0.79–1.02) | 0.089 | 0.61 (0.45–0.82) | 0.001 |
| Overlapping | 1.40 (1.28–1.52) | <0.001 | 0.70 (0.62–0.79) | <0.001 | 0.69 (0.57–0.85) | <0.001 | 0.80 (0.64–0.99) | 0.037 | 0.26 (0.12–0.56) | 0.001 |
| Histology | ||||||||||
| Adenocar-cinoma | 1.00 (Reference) | 1.00 | 1.00 (Reference) | 1.00 | 1.00 (Reference) | 1.00 | 1.00 (Reference) | 1.00 | 1.00 (Reference) | 1.00 |
| Mucous carcinoma | 1.00 (0.84–1.19) | 0.977 | 0.57 (0.44–0.74) | <0.001 | 1.06 (0.74–1.50) | 0.760 | 0.96 (0.63–1.47) | 0.856 | 0.48 (0.12–1.93) | 0.300 |
| Signet-ring cell carcinoma | 1.31 (1.24–1.39) | <0.001 | 0.29 (0.26–0.33) | <0.001 | 0.75 (0.66–0.87) | <0.001 | 1.63 (1.44–1.84) | <0.001 | 0.92 (0.65–1.31) | 0.640 |
| Unknown | 0.46 (0.43–0.49) | <0.001 | 0.66 (0.61–0.71) | <0.001 | 0.51 (0.44–0.59) | <0.001 | 0.45 (0.37–0.54) | <0.001 | 0.47 (0.30–0.72) | 0.001 |
| Grade | ||||||||||
| I | 1.00 (Reference) | 1.00 | 1.00 (Reference) | 1.00 | 1.00 (Reference) | 1.00 | 1.00 (Reference) | 1.00 | 1.00 (Reference) | 1.00 |
| II | 4.22 (3.66–4.87) | <0.001 | 4.32 (3.60–5.19) | <0.001 | 3.52 (2.57–4.83) | <0.001 | 4.42 (2.65–7.39) | <0.001 | 20.42 (2.82–147.80) | 0.003 |
| III | 7.04 (6.15–8.07) | <0.001 | 3.62 (3.03–4.33) | <0.001 | 3.60 (2.65–4.88) | <0.001 | 10.32 (6.29–16.95) | <0.001 | 17.68 (2.47–126.74) | 0.004 |
| IV | 5.46 (4.44–6.71) | <0.001 | 4.07 (3.12–5.30) | <0.001 | 2.16 (1.28–3.62) | 0.004 | 5.71 (2.96–10.99) | <0.001 | 29.97 (3.68–243.96) | 0.001 |
| T stage | ||||||||||
| T1 | 1.00 (Reference) | 1.00 | 1.00 (Reference) | 1.00 | 1.00 (Reference) | 1.00 | 1.00 (Reference) | 1.00 | 1.00 (Reference) | 1.00 |
| T2 | 0.63 (0.57–0.69) | <0.001 | 0.41 (0.35–0.48) | <0.001 | 0.38 (0.29–0.50) | <0.001 | 0.46 (0.34–0.62) | <0.001 | 0.52 (0.25–1.08) | 0.078 |
| T3 | 0.88 (0.82–0.94) | <0.001 | 0.65 (0.58–0.72) | <0.001 | 0.57 (0.48–0.68) | <0.001 | 0.77 (0.64–0.93) | 0.007 | 0.75 (0.46–1.22) | 0.248 |
| T4 | 2.40 (2.24–2.58) | <0.001 | 1.42 (1.29–1.55) | <0.001 | 1.27 (1.08–1.48) | 0.003 | 1.15 (0.96–1.38) | 0.141 | 0.95 (0.58–1.57) | 0.853 |
| N stage | ||||||||||
| N0 | 1.00 (Reference) | 1.00 | 1.00 (Reference) | 1.00 | 1.00 (Reference) | 1.00 | 1.00 (Reference) | 1.00 | 1.00 (Reference) | 1.00 |
| N1 | 2.79 (2.65–2.95) | <0.001 | 2.52 (2.35–2.70) | <0.001 | 2.49 (2.23–2.79) | <0.001 | 2.64 (2.34–2.98) | <0.001 | 2.49 (1.83–3.40) | <0.001 |
| N2 | 0.95 (0.87–1.05) | 0.320 | 0.83 (0.73–0.95) | 0.008 | 0.74 (0.57–0.95) | 0.017 | 0.82 (0.63–1.07) | 0.150 | 0.90 (0.46–1.73) | 0.743 |
| N3 | 1.06 (0.97–1.17) | 0.196 | 0.72 (0.62–0.84) | <0.001 | 0.74 (0.58–0.96) | 0.023 | 0.84 (0.65–1.10) | 0.210 | 1.21 (0.67–2.18) | 0.523 |
Met – metastases; OR – odds ratio; CI – confidence interval; NOS – not otherwise specified.