Significance
Invasive species negatively impact human society and natural ecosystems and are notoriously difficult to eradicate once established. Thus, identifying potentially invasive species and preventing their introduction is the most efficient management method. However, since the time of Darwin, there has been no consensus on whether relatedness to native species positively or negatively influences the outcome of biological invasions. Here, we examine patterns of evolutionary relatedness between native and nonnative plants across hundreds of thousands of communities. We demonstrate that the presence of native species closely related to potentially invasive species is more likely to predict invasion success at larger spatial extents and in harsher climates. Invasive species prevention must account for spatial scale and climate as well as evolutionary relationships.
Keywords: biological invasions, competition, Darwin’s naturalization hypothesis, environmental filtering, spatial resolution
Abstract
Darwin proposed two seemingly contradictory hypotheses regarding factors influencing the outcome of biological invasions. He initially posited that nonnative species closely related to native species would be more likely to successfully establish, because they might share adaptations to the local environment (preadaptation hypothesis). However, based on observations that the majority of naturalized plant species in the United States belonged to nonnative genera, he concluded that the lack of competitive exclusion would facilitate the establishment of alien invaders phylogenetically distinct from the native flora (competition-relatedness hypothesis). To date, no consensus has been reached regarding these opposing hypotheses. Here, following Darwin, we use the flora of the United States to examine patterns of taxonomic and phylogenetic relatedness between native and nonnative taxa across thousands of nested locations ranging in size and extent, from local to regional scales. We find that the probability of observing the signature of environmental filtering over that of competition increases with spatial scale. Further, native and nonnative species tended to be less related in warm, humid environments. Our work provides an empirical assessment of the role of observation scale and climate in biological invasions and demonstrates that Darwin’s two opposing hypotheses need not be mutually exclusive.
Increasing anthropogenic influences across the globe have facilitated the displacement of numerous species, causing the invasion of alien species into novel, nonnative habitats (1–4). Invasions have brought about widespread ecological, evolutionary, economic, and social consequences (5–13). However, managing biological invasions has proven difficult, as has making generalizations about what traits, characteristics, and circumstances contribute to invasion success (14–18).
Two of the longest standing, and seemingly contradictory, hypotheses that seek to explain invasion success were initially proposed by Darwin in On the Origin of Species (19). Initially, Darwin posited that nonnative introduced species closely related to native species would be more likely to successfully establish, because they might share similar adaptations to the local environment with their native relatives. This “preadaptation hypothesis” emphasizes the role of environmental filtering and niche conservatism in biological invasions, which facilitate the establishment of invaders phylogenetically similar to the native community (20). However, based on the observations of Alphonse de Candolle and Asa Gray that the majority of naturalized plant species in the United States belonged to nonnative genera (21, 22), Darwin also hypothesized that “As species of the same genus have usually… some similarity in habits and constitution, and always in structure, the struggle will generally be more severe between species of the same genus, when they come into competition with each other, than between species of distinct genera” (p. 76). This notion that the lack of competitive exclusion would facilitate the establishment of alien invaders phylogenetically distinct from the native flora has become known as “Darwin’s naturalization hypothesis” (23).
A number of studies have since tested these opposing hypotheses, or “Darwin’s Naturalization Conundrum” (24), using taxonomic ranks and, more recently, molecular phylogenetics (reviewed in refs. 25–28). There does not appear to be a clear consensus—studies have shown that the relationship between relatedness and invasion/establishment success can be positive (e.g., refs. 29–33), negative (e.g., refs. 34 and 35), or nonexistent/variable (e.g., refs. 36–38). In an effort to reconcile these findings, it has been increasingly suggested that Darwin’s two opposing hypotheses need not be mutually exclusive (39–42). Rather, each hypothesis may correctly predict the outcome of biological invasions at different environments or spatial scales. For instance, a meta-analysis of the literature found that introduced alien species tended to be more closely related to natives at the local scale but less closely related to natives at the larger, regional scales (26). It has been proposed that closely related species occur in mutually exclusive patterns at small spatial scales (e.g., plot or habitat scale) where competitive interactions are likely to be more severe (43). At larger spatial scales, closely related species may be more likely to co-occur due to shared broad environmental preferences and less frequent interspecific competition (40). However, the spatial scale of biological invasion research has been historically constrained by logistical considerations and the availability of datasets, resulting in dramatic scale gaps.
Here, following Darwin, we use the flora of the United States to empirically examine how spatial scale affects observed patterns of relatedness between native and nonnative taxa, from local forest communities to regional assemblages at an unprecedented scale. We also account for the broad effects of climate, which have been shown to influence taxonomic and phylogenetic community composition (44) and, thus, may modulate the effects of spatial scale. Although there have been efforts to incorporate scale effects in community ecology (43–47), relatively few have done so in the context of biological invasions (41, 42, 48). These studies have revealed important and perhaps representative signatures of biological invasions but have been limited in their broad generalizability due to their variable methodologies, relatively small spatial scales examined, and general confinement to certain habitat types and/or taxa. Synthesizing big data from tens of thousands of locations ranging from 1 m2 in size to thousands of square kilometers, we address these limitations and demonstrate that spatial scale does indeed affect observed signals of taxonomic and phylogenetic relatedness between native and nonnative introduced taxa.
Methods
Species Composition Data.
Local-scale plot-level data detailing the presence of species were extracted from the US Forest Service’s Forest Inventory and Analysis (FIA; https://www.fia.fs.fed.us) and National Ecological Observatory Network (NEON; https://www.neonscience.org) databases. The FIA database consists of multiscale plot‐level species inventory data from across the conterminous United States, where one field observation plot has been established for approximately every 25 km2 of forested land, thus representing the most comprehensive source of presence/absence data on forest plant species available in the United States (49). FIA plot inventory scales comprise 168.62 m2 (1/24 acres) subplots and 4,046.86 m2 (1 acre) plots. NEON plot inventories span a representative range of US climate variability across 20 ecoclimate domains. Plant taxonomic composition is recorded in 20 × 20 m square plots (400 m2) comprised of four 10 × 10 m subplots (100 m2) containing nested 10‐ and 1‐m2 subplots (50, 51). We combined species inventory data across existing surveys to attain the most comprehensive sampling possible for each plot. Plots without sufficient location information (i.e., lack of coordinates) or taxa keyed to species were not considered. County level (regional) plant species lists and species native status were compiled from the US Department of Agriculture (USDA) PLANTS Database (https://plants.sc.egov.usda.gov). As a result, our sampling focuses largely on temperate climate regions and forest plots at the local scale. In total, our dataset comprised 228,659 plots and 3,063 counties across the contiguous United States (Fig. 1).
Fig. 1.
Sampling across the United States. Blue circles represent FIA plots, and orange diamonds represent NEON plots. Red triangles at Right indicate county mean climatic values.
For each locale, we also gathered data on mean annual temperature and annual precipitation at 30 arc-second resolution from WorldClim version 2 (52) and elevation from WorldClim version 1.4 (53). In the case of counties, we calculated the mean and SD of these metrics. All spatial analyses were conducted using the raster package (54) in R v3.5.1 (55). Replication codes for these and other analyses are available in a Zenodo repository (56).
Taxonomic Analyses.
The USDA PLANTS Database taxonomic classification was used in all our survey data and, thus, applied throughout the study. The native status of species in the United States was also based on USDA PLANTS classifications. For each plot, the number of nonnative introduced species within the same genus (congeners) or family (confamilials) as native species were calculated. These measures quantify Darwin’s original observations of relatedness between nonnative taxa and the native community. However, as the number of congeneric and confamilial taxa can be affected by the total number of taxa present, which is correlated with spatial scale, we estimated the standard effect sizes (SES) of these metrics as: (observed value − expected value)/SD of the expected value (57). Expected values of the number of native congeneric and confamilial taxa were calculated from a null distribution of 1,000 random assemblages of species drawn without replacement from the species pool of the combined plot data. We thus account for effects of species richness by representing how much the observed values deviate from random expectations. Only nonnative taxa were randomized, keeping species number constant in each community.
Phylogenetic Analyses.
Inferring phylogenies with only taxa within the area of interest can lead to spurious calculations of evolutionary distance and diversity (58, 59). Therefore, we utilized the most widely sampled time‐calibrated seed plant phylogeny to date for our calculations of phylogenetic distance (60). In order to maximize phylogenetic coverage, we attached unrepresented species using SUNPLIN (61). Species were added within a given clade using the “branch-based method,” which chooses a location for insertion of missing species within a clade by randomly sampling the total branch length within that clade. The addition of these missing species was constrained by taxonomy; species with one or more congeners present were added to the clade defined by the most recent common ancestor (MRCA) of that genus, while species lacking congeners were added to the clade defined by the MRCA of their family. We ensured the correspondence between the taxa present in the phylogeny and our survey data using the Taxonomic Name Resolution Service, which incorporates the USDA taxonomy (62). One species, Pilostyles thurberi, a native endoparasitic plant, did not have any close relatives represented and, thus, was omitted from our analyses. This process was repeated 1,000 times to account for phylogenetic uncertainty, and all downstream analyses were conducted across the resulting set of 1,000 trees each comprising 17,468 taxa (SI Appendix, Fig. S1). The mean phylogenetic distance (MPD) and mean nearest taxon distance (MNTD) between native and nonnative introduced species were quantified to represent phylogenetic relatedness. These metrics and their standardized effect sizes were calculated using the package PhyloMeasures v2.1 (63) in R v3.5.1 (55). Expected values of MPD and MNTD were calculated from a null distribution of 1,000 random assemblages of species drawn without replacement from the species pool of the combined plot data. The SE of each metric derived from the phylogenetic replicates is presented on SI Appendix, Fig. S2. Given that not all taxa on our phylogenies are supported by molecular data, and that calculations of MNTD are much more sensitive to the topological uncertainty toward the tips of the phylogeny (demonstrated by significantly larger degrees of SE), we focused our analyses on MPD.
Statistical Analyses.
We examined the effect of plot area on the observed relatedness between native and nonnative introduced species using linear mixed models. Response variables included the standardized number of introduced taxa with native congeners/confamilials and MPD between native and introduced species. Explanatory variables included plot area (m2), which was log transformed, and mean annual temperature and annual precipitation to account for environmental variation across plots. Data source (FIA or NEON) was also included as a fixed effect, and state and higher-level plot groupings entered the models as random effects. Similar models were used to examine regional-scale county data, where county area (m2) and the SDs of mean annual temperature and annual precipitation across the area were included as fixed effects instead. We also used generalized linear models to assess whether the probability of observing greater than random relatedness between native and nonnative introduced species increased with plot/county area with the same fixed effects. Analyses were conducted using the lme4 package (64) in R v3.5.1 (55).
Results
The expected number of introduced species with native congeners was significantly greater at the regional level, accounting for differences in species richness (Fig. 2A and SI Appendix, Table S1). Both the number of introduced species with native congeners present and the probability of observing greater than random numbers of such congeneric introduced species increased with spatial scale at both local and regional levels (Fig. 2 and SI Appendix, Tables S2 and S3). Similar trends were observed for introduced species with native confamilial species (SI Appendix, Tables S4–S6).
Fig. 2.
Effect plots of spatial scale on patterns of standardized taxonomic relatedness. Depicted are the expected standardized effect sizes (SES) of the number of introduced species with native congeners in plot (local-scale) vs. county (regional-scale) (A), in response to plot area (B), county area (C), and the probability of observing greater than random standardized numbers of introduced species with native congeners in response to plot area (D) and county area (E). Shaded areas represent 95% CIs.
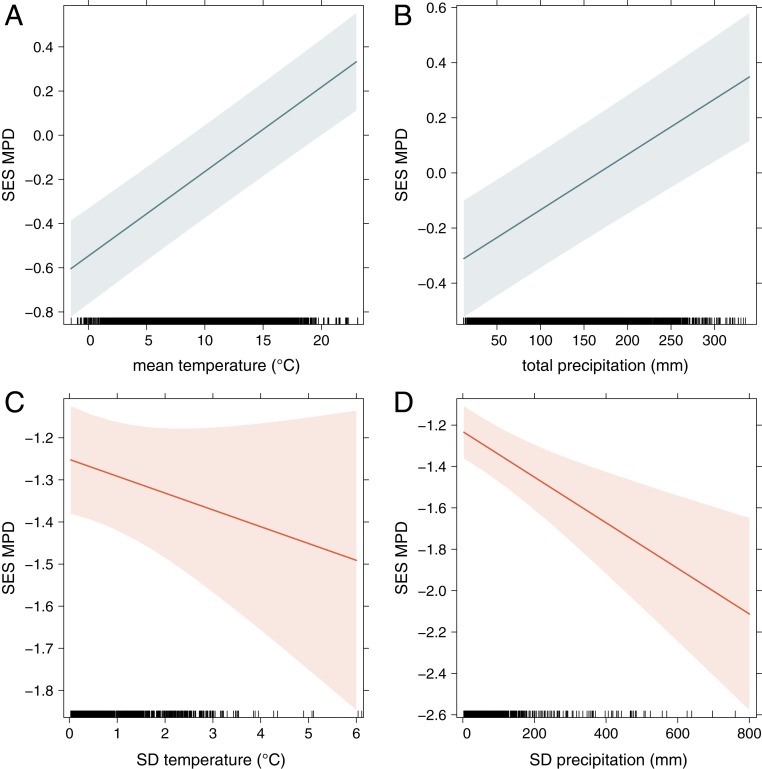
The standardized effect sizes of MPD between native and introduced species was significantly lower at the regional level, indicating greater phylogenetic clustering compared to plot level surveys (Fig. 3A and SI Appendix, Table S7). MPD between native and introduced species decreased with spatial scale (Fig. 3 B and C and SI Appendix, Table S8), while the probability of observing phylogenetic clustering (SES.MPD < 0) increased at both local and regional levels (Fig. 3 D and E and SI Appendix, Table S9). Further, MPD tended to increase with temperature and precipitation at local scales (Fig. 4 A and B and SI Appendix, Table S8). At the regional scales, MPD decreased in response to increasing climatic variation (Fig. 4 C and D and SI Appendix, Table S8).
Fig. 3.
Effect plots of spatial scale on patterns of standardized phylogenetic relatedness. Depicted are the expected SES of MPD (SES MPD) between native and introduced species in plot (local-scale) vs. county (regional-scale) (A), in response to plot area (B), county area (C), and the probability of observing greater than random standardized numbers of introduced species with native congeners in response to plot area (D) and county area (E). Shaded areas represent 95% CIs.
Fig. 4.
Effect plots of climate on patterns of standardized phylogenetic relatedness. Depicted are the expected standardized MPD (SES MPD) between native and introduced species in response to plot mean annual temperature (A), plot annual precipitation (B), the SD of mean annual temperature across each county (C), and SD of annual precipitation across each county (D). Shaded areas represent 95% CIs.
Discussion
Explaining why certain nonnative introduced species successfully establish is a major challenge (25, 65). Although it has been long thought that the success of an invasive species can be explained by its relatedness to resident native taxa since Darwin proposed the idea, empirical tests have yielded conflicting results. Our study highlights some of the conceptual reasons why this might be the case through a unique, large-scale empirical test. Following Darwin, we examined the taxonomic and phylogenetic relatedness between native and nonnative species in the United States across an unprecedented number of locations and demonstrate that spatial scale and climate can influence these patterns. As it has been shown that the presence and number of invaders (66) as well as metrics of (phylogenetic) relatedness can be influenced by species richness (46, 67), we standardized all measures of relatedness to represent departures from random expectations accounting for species richness in each locale.
As Darwin observed, many of the nonnative species introduced to the United States do not have native congeners (∼40%). However, we find that introduced species were more likely to have native congeners (and confamilials) present at larger spatial scales. Further, the phylogenetic distance between native and introduced species decreased with spatial scale at both local and regional levels. Although variation exists at the level of individual communities regardless of size, it is more likely to find introduced species to be closely related to the native community at larger spatial extents. This suggests that at broad regional scales (e.g., county-scale or larger), invasion success can be predicted by preadapted traits and niche preferences. Coexisting species are often more closely related than expected due to the effect of environmental filtering on the composition of regional communities (29–31, 45, 68). At smaller, local spatial scales, introduced species tended to appear less related to native taxa, potentially signaling the presence of neutral dynamics and/or density-dependent mechanisms associated with limiting (ecological) similarity such as competition and enemy escape/resistance (41, 57, 70, 71). As the environment is generally homogeneous at local scales, individuals are more likely to interact directly and compete over the same pool of limited resources and, thus, there may be a limit to the similarity of competing species that can coexist (71). These spatial patterns are supported by meta-analyses of previous studies of biological invasions (25, 26) and the general observation that plant communities tend to be phylogenetically and functionally overdispersed at finer spatial scales, whereas clustering is found across coarser spatial scales, where environmental gradients tend to be stronger (20, 72). Thus, we may predict that nonnative species with close native relatives will have a higher chance of successfully establishing within the general area but are less likely to do so in close proximity of said relatives.
In addition to spatial scale, we show that environmental variables can influence the relatedness between native and introduced species. Our results indicate that at smaller, local spatial scales (i.e., plot-level), introduced species tend to be less phylogenetically related to natives in warmer and wetter climates. At larger, regional scales (i.e., county-level), introduced species tended to be more closely related to the native flora in areas with higher variation in precipitation and temperature, i.e., where environmental gradients are more pronounced. This suggests that the effects of competition may be more observable in comparatively homogeneous, benign environments, which can support a large diversity of taxa. Along these lines, phylogenetic clustering has frequently been associated with harsher environmental conditions such as colder temperatures and lower precipitation, as competition can be greatly reduced (44, 72–75), and adaptations to harsh environments can be phylogenetically conserved (76). Although we examined hundreds of thousands of locales across a wide range of environments, our plot-level sampling is constrained to largely temperate climates and terrestrial forest plots of the western and northeastern United States, and further examinations of more diverse assemblages and environmental factors are needed to test this hypothesis on a global scale. In particular, edaphic variables, such as soil nitrogen content (77), and anthropogenic disturbance (78) can influence invasive plant establishment in addition to climatic variables. It is also possible that other measures of relatedness such as MNTD may show different patterns, but it has been suggested that mean distances that relate to the entire community more effectively capture the signal of different assembly processes (79, 80).
Although we examined the most common climatic variables associated with plant distributions and diversity (81–84), other environmental factors may also affect patterns of clustering and overdispersion between introduced species and natives, as can the taxonomic scope of analyses (25, 46). Focusing on more specific clades can help elucidate how invasion success manifests among close relatives; however, as most community assemblages comprise a wide diversity of evolutionary lineages, we chose to analyze all seed plants to better understand the general effect of spatial scale on patterns of relatedness in biological invasions. Molecular data for most plant species are not yet available (60, 85), and it is possible that additional data on the phylogenetic relationships of the taxa examined could change our findings. Nonetheless, our phylogenies represent one of the most comprehensive reconstructions of seed plant evolutionary history to date, and the effect of phylogenetic uncertainty on the calculation of phylogenetic distance—assessed across 1,000 randomizations—was minimal. Further, our analyses of taxonomic relatedness yielded largely similar results. Importantly, interpretations of phylogenetic and taxonomic relatedness patterns rely upon the assumption that key traits associated with environmental adaptation and biotic interactions are evolutionarily conserved; i.e., closely related taxa share similar traits and preferences (28, 31, 86, 87). Although this is likely the case in general, relatedness is not always a good predictor of the ecological mechanisms that contribute to the establishment of nonnative introduced taxa (27, 88, 89).
Along these lines, our results do not strictly imply that the effects of environmental filtering outweigh those of competition at larger spatial scales (and/or harsher environments) and vice versa. Both mechanisms function across all scales and situations (90, 91)—barring the colonization of a completely empty habitat, a successful invader must both be minimally adapted to the introduced environment and able to compete with established native taxa in the vicinity. What we demonstrate is that the probability of observing the signature of environmental filtering over that of competition increases with spatial scale, and that this can at least partially explain Darwin’s naturalization conundrum (Fig. 5). As is such, the results of studies at different spatial resolutions are not directly comparable, and spatial scale and its implications should always be considered in the analysis and interpretation of biological invasions and community assembly patterns in general.
Fig. 5.
Expected changes in the probability of observing patterns of clustering versus overdispersion between native (black) and introduced (orange shrub) taxa across spatial scale.
Data Availability Statement.
Data discussed in the paper are publicly available through the USDA Forest Inventory and Analysis website (https://www.fia.fs.fed.us), the National Ecological Observatory Network data portal (https://data.neonscience.org), and the WorldClim database (https://www.worldclim.org). Replication code is available in a Zenodo repository (doi: 10.5281/zenodo.3710499).
Supplementary Material
Acknowledgments
This research was supported by the University of Arizona Office of Research, Discovery, and Innovation and the Bridging Biodiversity and Conservation Science initiative. B.J.E. and B.S.M. were supported by NSF ABI-1565118 and NSF HDR-1934790. We thank J. D. Shaw for informative discussions regarding FIA plot data.
Footnotes
The authors declare no competing interest.
This article is a PNAS Direct Submission.
Data deposition: Data discussed in the paper are publicly available through the USDA Forest Inventory and Analysis website (https://www.fia.fs.fed.us), the National Ecological Observatory Network data portal (https://data.neonscience.org), and the WorldClim database (https://www.worldclim.org). Replication code is available in a Zenodo repository (doi: 10.5281/zenodo.3710499).
See online for related content such as Commentaries.
This article contains supporting information online at https://www.pnas.org/lookup/suppl/doi:10.1073/pnas.1918100117/-/DCSupplemental.
References
- 1.Vitousek P. M., Mooney H. A., Lubchenco J., Melillo J. M., Human domination of Earth’s ecosystems. Science 277, 494–499 (1997). [Google Scholar]
- 2.D’Antonio C. M., Vitousek P. M., Biological invasions by exotic grasses, the grass/fire cycle, and global change. Annu. Rev. Ecol. Syst. 23, 63–87 (1992). [Google Scholar]
- 3.Dukes J. S., Mooney H. A., Does global change increase the success of biological invaders? Trends Ecol. Evol. 14, 135–139 (1999). [DOI] [PubMed] [Google Scholar]
- 4.Mooney H. A., Hobbs R. J., Invasive Species in a Changing World (Island Press, Washington, DC, 2000). [Google Scholar]
- 5.Crowl T. A., Crist T. O., Parmenter R. R., Belovsky G., Lugo A. E., The spread of invasive species and infectious disease as drivers of ecosystem change. Front. Ecol. Environ. 6, 238–246 (2008). [Google Scholar]
- 6.Pimentel D., Zuniga R., Morrison D., Update on the environmental and economic costs associated with alien-invasive species in the United States. Ecol. Econ. 52, 273–288 (2005). [Google Scholar]
- 7.Pimentel D., “Invasive plants: Their role in species extinctions and economic losses to agriculture in the USA” in Management of Invasive Weeds, Inderjit X., Ed. (Spinger, Dordrecht, 2009), pp. 1–7. [Google Scholar]
- 8.Mooney H. A., Cleland E. E., The evolutionary impact of invasive species. Proc. Natl. Acad. Sci. U.S.A. 98, 5446–5451 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Crowley S. L., Hinchliffe S., McDonald R. A., Conflict in invasive species management. Front. Ecol. Environ. 15, 133–141 (2017). [Google Scholar]
- 10.Pyšek P., Richardson D. M., Invasive species, environmental change and management, and health. Annu. Rev. Environ. Resour. 35, 25–55 (2010). [Google Scholar]
- 11.Sala O. E., et al. , Global biodiversity scenarios for the year 2100. Science 287, 1770–1774 (2000). [DOI] [PubMed] [Google Scholar]
- 12.Sax D. F., Gaines S. D., Colloquium paper: Species invasions and extinction: The future of native biodiversity on islands. Proc. Natl. Acad. Sci. U.S.A. 105 (suppl. 1), 11490–11497 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mack R. N., Lonsdale W. M., Humans as global plant dispersers: Getting more than we bargained for: Current introductions of species for aesthetic purposes present the largest single challenge for predicting which plant immigrants will become future pests. Bioscience 51, 95–102 (2001). [Google Scholar]
- 14.Rejmánek M., Richardson D. M., What attributes make some plant species more invasive? Ecology 77, 1655–1661 (1996). [Google Scholar]
- 15.Kolar C. S., Lodge D. M., Progress in invasion biology: Predicting invaders. Trends Ecol. Evol. 16, 199–204 (2001). [DOI] [PubMed] [Google Scholar]
- 16.Moles A. T., Gruber M. A. M., Bonser S. P., A new framework for predicting invasive plant species. J. Ecol. 96, 13–17 (2008). [Google Scholar]
- 17.Pyšek P., Richardson D. M., “Traits associated with invasiveness in alien plants: Where do we stand?” in Biological Invasions, Nentwig W., Ed. (Springer, 2008), pp. 97–125. [Google Scholar]
- 18.Rejmánek M., A theory of seed plant invasiveness: The first sketch. Biol. Conserv. 78, 171–181 (1996). [Google Scholar]
- 19.Darwin C., On the Origin of Species by Means of Natural Selection, (Murray, 1859). [Google Scholar]
- 20.Ricotta C., et al. , Phyloecology of urban alien floras. J. Ecol. 97, 1243–1251 (2009). [Google Scholar]
- 21.Torrey J., Gray A., A Flora of North America: Containing Abridged Descriptions of All the Known Indigenous and Naturalized Plants Growing North of Mexico; Arranged According to the Natural System (Wiley and Putnam, 1841). [Google Scholar]
- 22.De Candolle A., Géographie Botanique Raisonnée ou Exposition des Faits Principaux et des Lois Concernant la Distribution Géographique des Plantes de l’Époque Actuelle (Masson, 1855), vol. V. [Google Scholar]
- 23.Daehler C. C., Darwin’s naturalization hypothesis revisited. Am. Nat. 158, 324–330 (2001). [DOI] [PubMed] [Google Scholar]
- 24.Diez J. M., Sullivan J. J., Hulme P. E., Edwards G., Duncan R. P., Darwin’s naturalization conundrum: Dissecting taxonomic patterns of species invasions. Ecol. Lett. 11, 674–681 (2008). [DOI] [PubMed] [Google Scholar]
- 25.Gallien L., Carboni M., The community ecology of invasive species: Where are we and what’s next? Ecography 40, 335–352 (2017). [Google Scholar]
- 26.Ma C., et al. , Different effects of invader-native phylogenetic relatedness on invasion success and impact: A meta-analysis of Darwin’s naturalization hypothesis. Proc. Biol. Sci. 283, 20160663 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Thuiller W., et al. , Resolving Darwin’s naturalization conundrum: A quest for evidence. Divers. Distrib. 16, 461–475 (2010). [Google Scholar]
- 28.Cadotte M. W., Campbell S. E., Li S. P., Sodhi D. S., Mandrak N. E., Preadaptation and naturalization of nonnative species: Darwin’s two fundamental insights into species invasion. Annu. Rev. Plant Biol. 69, 661–684 (2018). [DOI] [PubMed] [Google Scholar]
- 29.Park D. S., Potter D., A reciprocal test of Darwin’s naturalization hypothesis in two mediterranean-climate regions. Glob. Ecol. Biogeogr. 24, 1049–1058 (2015). [Google Scholar]
- 30.Park D. S., Potter D., A test of Darwin’s naturalization hypothesis in the thistle tribe shows that close relatives make bad neighbors. Proc. Natl. Acad. Sci. U.S.A. 110, 17915–17920 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Park D. S., Potter D., Why close relatives make bad neighbours: Phylogenetic conservatism in niche preferences and dispersal disproves Darwin’s naturalization hypothesis in the thistle tribe. Mol. Ecol. 24, 3181–3193 (2015). [DOI] [PubMed] [Google Scholar]
- 32.Cadotte M. W., Dinnage R., Tilman D., Phylogenetic diversity promotes ecosystem stability. Ecology 93, 223–233 (2012). [Google Scholar]
- 33.Cadotte M. W., et al. , Phylogenetic patterns differ for native and exotic plant communities across a richness gradient in Northern California. Divers. Distrib. 16, 892–901 (2010). [Google Scholar]
- 34.Schaefer H., Hardy O. J., Silva L., Barraclough T. G., Savolainen V., Testing Darwin’s naturalization hypothesis in the Azores. Ecol. Lett. 14, 389–396 (2011). [DOI] [PubMed] [Google Scholar]
- 35.Strauss S. Y., Webb C. O., Salamin N., Exotic taxa less related to native species are more invasive. Proc. Natl. Acad. Sci. U.S.A. 103, 5841–5845 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Alexandrou M. A., et al. , Evolutionary relatedness does not predict competition and co-occurrence in natural or experimental communities of green algae. Proc. Biol. Sci. 282, 20141745 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Castro S. A., Escobedo V. M., Aranda J., Carvallo G. O., Evaluating Darwin’s naturalization hypothesis in experimental plant assemblages: Phylogenetic relationships do not determine colonization success. PLoS One 9, e105535 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ng J., Weaver W. N., Laport R. G., Testing Darwin’s Naturalization Conundrum using phylogenetic relationships: Generalizable patterns across disparate communities? Divers. Distrib. 25, 361–373 (2019). [Google Scholar]
- 39.Procheş S., Wilson J. R. U., Cowling R. M., How much evolutionary history in a 10 x 10 m plot? Proc. Biol. Sci. 273, 1143–1148 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Procheş Ş., Wilson J. R. U., Richardson D. M., Rejmánek M., Searching for phylogenetic pattern in biological invasions. Glob. Ecol. Biogeogr. 17, 5–10 (2008). [Google Scholar]
- 41.Cavender-Bares J., Keen A., Miles B., Phylogenetic structure of Floridian plant communities depends on taxonomic and spatial scale. Ecology 87 (suppl. 7), S109–S122 (2006). [DOI] [PubMed] [Google Scholar]
- 42.Carboni M., et al. , Darwin’s naturalization hypothesis: Scale matters in coastal plant communities. Ecography 36, 560–568 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Swenson N. G., Enquist B. J., Thompson J., Zimmerman J. K., The influence of spatial and size scale on phylogenetic relatedness in tropical forest communities. Ecology 88, 1770–1780 (2007). [DOI] [PubMed] [Google Scholar]
- 44.Park D. S., Razafindratsima O. H., Anthropogenic threats can have cascading homogenizing effects on the phylogenetic and functional diversity of tropical ecosystems. Ecography 42, 148–161 (2019). [Google Scholar]
- 45.Swenson N. G., Phylogenetic resolution and quantifying the phylogenetic diversity and dispersion of communities. PLoS One 4, e4390 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Swenson N. G., Enquist B. J., Pither J., Thompson J., Zimmerman J. K., The problem and promise of scale dependency in community phylogenetics. Ecology 87, 2418–2424 (2006). [DOI] [PubMed] [Google Scholar]
- 47.Hardy O. J., Testing the spatial phylogenetic structure of local communities: Statistical performances of different null models and test statistics on a locally neutral community. J. Ecol. 96, 914–926 (2008). [Google Scholar]
- 48.Davies K. F., Cavender-Bares J., Deacon N., Native communities determine the identity of exotic invaders even at scales at which communities are unsaturated. Divers. Distrib. 17, 35–42 (2011). [Google Scholar]
- 49.Smith W. B., Forest inventory and analysis: A national inventory and monitoring program. Environ. Pollut. 116 (suppl. 1), S233–S242 (2002). [DOI] [PubMed] [Google Scholar]
- 50.Barnett D. T., et al. , The plant diversity sampling design for the national ecological observatory network. Ecosphere 10, e02603 (2019). [Google Scholar]
- 51.Thorpe A. S., et al. , Introduction to the sampling designs of the national ecological observatory network terrestrial observation system. Ecosphere 7, e01627 (2016). [Google Scholar]
- 52.Fick S. E., Hijmans R. J., WorldClim 2: New 1‐km spatial resolution climate surfaces for global land areas. Int. J. Climatol. 37, 4302–4315 (2017). [Google Scholar]
- 53.Hijmans R. J., Cameron S. E., Parra J. L., Jones P. G., Jarvis A., Very high resolution interpolated climate surfaces for global land areas. Int. J. Climatol. 25, 1965–1978 (2005). [Google Scholar]
- 54.Hijmans R. J., et al. , raster: Geographic data analysis and modeling –In: R. (R Packag. version 2.9.5, 2016).
- 55.R Core Team (2018) R: A Language and Environment for Statistical Computing (R Foundation for Statistical Computing, Vienna). Available at https://www.R-project.org/.
- 56.Park D. S., Feng X., Maitner B. S., Darwin’s naturalization conundrum can be explained by spatial scale: R replication code. (2020). https://zenodo.org/record/3710499. Deposited 13 March 2020. [DOI] [PMC free article] [PubMed]
- 57.Webb C. O., Ackerly D. D., McPeek M. a., Donoghue M. J., Phylogenies and community ecology. Annu. Rev. Ecol. Syst. 33, 475–505 (2002). [Google Scholar]
- 58.Park D. S., Worthington S., Xi Z., Taxon sampling and inferred community phylogenies: R replication code and data. (2017). https://zenodo.org/record/1095663. Accessed 12 December 2017.
- 59.Park D. S., Worthington S., Xi Z., Taxon sampling effects on the quantification and comparison of community phylogenetic diversity. Mol. Ecol. 27, 1296–1308 (2018). [DOI] [PubMed] [Google Scholar]
- 60.Smith S. A., Brown J. W., Constructing a broadly inclusive seed plant phylogeny. Am. J. Bot. 105, 302–314 (2018). [DOI] [PubMed] [Google Scholar]
- 61.Martins W. S., Carmo W. C., Longo H. J., Rosa T. C., Rangel T. F., SUNPLIN: Simulation with uncertainty for phylogenetic investigations. BMC Bioinformatics 14, 324 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Boyle B., et al. , The taxonomic name resolution service: An online tool for automated standardization of plant names. BMC Bioinformatics 14, 16 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Tsirogiannis C., Sandel B., PhyloMeasures: A package for computing phylogenetic biodiversity measures and their statistical moments. Ecography 39, 709–714 (2016). [Google Scholar]
- 64.Bates D., Maechler M., Bolker B., Walker S., Fitting linear mixed-effects models using ime4. J. Stat. Softw. 67, 1–48 (2015). [Google Scholar]
- 65.Hayes K. R., Barry S. C., Are there any consistent predictors of invasion success? Biol. Invasions 10, 483–506 (2008). [Google Scholar]
- 66.Stohlgren T. J., et al. , Assessing vulnerability to invasion by nonnative plant species at multiple spatial scales. Environ. Manage. 29, 566–577 (2002). [DOI] [PubMed] [Google Scholar]
- 67.Pavoine S., Bonsall M. B., Measuring biodiversity to explain community assembly: A unified approach. Biol. Rev. Camb. Philos. Soc. 86, 792–812 (2011). [DOI] [PubMed] [Google Scholar]
- 68.Willis C. G., et al. , Phylogenetic community structure in Minnesota oak savanna is influenced by spatial extent and environmental variation. Ecography 33, 565–577 (2010). [Google Scholar]
- 69.Gilbert G. S., Webb C. O., Phylogenetic signal in plant pathogen-host range. Proc. Natl. Acad. Sci. U.S.A. 104, 4979–4983 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Cavender-Bares J., Ackerly D. D., Baum D. A., Bazzaz F. A., Phylogenetic overdispersion in Floridian oak communities. Am. Nat. 163, 823–843 (2004). [DOI] [PubMed] [Google Scholar]
- 71.Macarthur R., Levins R., The limiting similarity, convergence, and divergence of coexisting species. Am. Nat. 101, 377–385 (1967). [Google Scholar]
- 72.Cadotte M. W., Tucker C. M., Should environmental filtering be abandoned? Trends Ecol. Evol. 32, 429–437 (2017). [DOI] [PubMed] [Google Scholar]
- 73.Simberloff D. S., Taxonomic diversity of island biotas. Evolution 24, 23–47 (1970). [DOI] [PubMed] [Google Scholar]
- 74.Grant P. R., Ecological compatibility of bird species on islands. Am. Nat. 100, 451–462 (1966). [Google Scholar]
- 75.Elton C., Competition and the structure of ecological communities. J. Anim. Ecol. 15, 54–68 (1946). [Google Scholar]
- 76.Hawkins B. A., et al. , Community phylogenetics at the biogeographical scale: Cold tolerance, niche conservatism and the structure of North American forests. J. Biogeogr. 41, 23–38 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Funk J. L., Vitousek P. M., Resource-use efficiency and plant invasion in low-resource systems. Nature 446, 1079–1081 (2007). [DOI] [PubMed] [Google Scholar]
- 78.Lembrechts J. J., et al. , Disturbance is the key to plant invasions in cold environments. Proc. Natl. Acad. Sci. U.S.A. 113, 14061–14066 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Gallien L., Carboni M., Münkemüller T., Identifying the signal of environmental filtering and competition in invasion patterns–A contest of approaches from community ecology. Methods Ecol. Evol. 5, 1002–1011 (2014). [Google Scholar]
- 80.Miller E. T., Farine D. R., Trisos C. H., Phylogenetic community structure metrics and null models: A review with new methods and software. Ecography 40, 461–477 (2017). [Google Scholar]
- 81.Woodward F. I., Williams B. G., Climate and plant distribution at global and local scales. Vegetatio 69, 189–197 (1987). [Google Scholar]
- 82.Ricklefs R. E., Environmental heterogeneity and plant species diversity: A hypothesis. Am. Nat. 111, 376–381 (1977). [Google Scholar]
- 83.MacArthur R. H., Patterns of species diversity. Biol. Rev. Camb. Philos. Soc. 40, 510–533 (1965). [Google Scholar]
- 84.Watt A. S., Pattern and process in the plant community. J. Ecol. 35, 1–22 (1947). [Google Scholar]
- 85.Folk R. A., et al. , Challenges of comprehensive taxon sampling in comparative biology: Wrestling with rosids. Am. J. Bot. 105, 433–445 (2018). [DOI] [PubMed] [Google Scholar]
- 86.Qian H., Sandel B., Phylogenetic relatedness of native and exotic plants along climate gradients in California, USA. Divers. Distrib. 23, 1323–1333 (2017). [Google Scholar]
- 87.Wiens J. J., Graham C. H., “Niche conservatism: Integrating evolution, ecology, and conservation biology” Annu. Rev. Ecol. Evol. Syst. 36, 519–539 (2005). [Google Scholar]
- 88.van Kleunen M., Weber E., Fischer M., A meta-analysis of trait differences between invasive and non-invasive plant species. Ecol. Lett. 13, 235–245 (2010). [DOI] [PubMed] [Google Scholar]
- 89.Kunstler G., et al. , Competitive interactions between forest trees are driven by species’ trait hierarchy, not phylogenetic or functional similarity: Implications for forest community assembly. Ecol. Lett. 15, 831–840 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Meynard C. N., et al. , Disentangling the drivers of metacommunity structure across spatial scales. J. Biogeogr. 40, 1560–1571 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Belmaker J., Jetz W., Spatial scaling of functional structure in bird and mammal assemblages. Am. Nat. 181, 464–478 (2013). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data discussed in the paper are publicly available through the USDA Forest Inventory and Analysis website (https://www.fia.fs.fed.us), the National Ecological Observatory Network data portal (https://data.neonscience.org), and the WorldClim database (https://www.worldclim.org). Replication code is available in a Zenodo repository (doi: 10.5281/zenodo.3710499).