Significance
Direct cell-to-cell spreading of misfolded protein aggregates, such as α-synuclein fibrils (α-Syn), is a pathological hallmark associated with the progression of many neurodegenerative diseases, but it is unclear how mammalian cells take up large protein aggregates to initiate this prion-like protein transmission process. Here we define the endocytosis mechanism of α-Syn preformed fibrils (PFFs) using a combination of genetic, biochemical, and live-cell imaging techniques. Our study shows that α-Syn PFFs enter cells following an entry pathway that is specifically tailored for cargos bearing positive charges. These cargos bind to heparan sulfate proteoglycans on the cell surface via charge–charge interactions, which enable cargo internalization by a previously unknown endocytosis mechanism that requires forces generated by myosin-7B and actin filaments.
Keywords: α-synuclein, clathrin-mediated endocytosis, myosin-7B, heparan sulfate proteoglycan, actin filament
Abstract
Cell-to-cell transmission of misfolding-prone α-synuclein (α-Syn) has emerged as a key pathological event in Parkinson’s disease. This process is initiated when α-Syn–bearing fibrils enter cells via clathrin-mediated endocytosis, but the underlying mechanisms are unclear. Using a CRISPR-mediated knockout screen, we identify SLC35B2 and myosin-7B (MYO7B) as critical endocytosis regulators for α-Syn preformed fibrils (PFFs). We show that SLC35B2, as a key regulator of heparan sulfate proteoglycan (HSPG) biosynthesis, is essential for recruiting α-Syn PFFs to the cell surface because this process is mediated by interactions between negatively charged sugar moieties of HSPGs and clustered K-T-K motifs in α-Syn PFFs. By contrast, MYO7B regulates α-Syn PFF cell entry by maintaining a plasma membrane-associated actin network that controls membrane dynamics. Without MYO7B or actin filaments, many clathrin-coated pits fail to be severed from the membrane, causing accumulation of large clathrin-containing “scars” on the cell surface. Intriguingly, the requirement for MYO7B in endocytosis is restricted to α-Syn PFFs and other polycation-bearing cargos that enter cells via HSPGs. Thus, our study not only defines regulatory factors for α-Syn PFF endocytosis, but also reveals a previously unknown endocytosis mechanism for HSPG-binding cargos in general, which requires forces generated by MYO7B and actin filaments.
In Parkinson’s disease (PD), the major proteinaceous aggregate known as a Lewy body is composed mostly of a synaptic protein, α-synuclein (α-Syn) (1). Coincidentally, genetic mutations in α-Syn–encoding genes were identified as a major contributing factor for Parkinson’s disease (2, 3). α-Syn is a small polypeptide that is aggregation-prone. Clinical studies have shown that α-Syn–positive Lewy bodies can spread in the brain during disease progression (4). Furthermore, following tissue transplantation therapy, grafted tissues can accumulate Lewy body-like aggregates over time, which does not occur if these cells are left intact in the donor (5, 6). These observations prompted the idea that α-Syn might undergo cell-to-cell transmission analogously to a prion protein (7, 8). Indeed, in vitro and animal studies have shown that α-Syn preformed fibrils (α-Syn PFFs) can be readily taken up by various cell types and subsequently transmitted to nearby cells that have not been directly exposed to α-Syn PFFs (9–13).
At the cellular level, the intercellular transmission of neurotoxic proteins like α-Syn comprises two biological events: the release of α-Syn from donor cells via unconventional protein secretion and its internalization by recipient cells via endocytosis (14). The molecular mechanisms of these processes are poorly defined. Recent studies have suggested nanotubes, exosome secretion, or a pathway termed misfolding-associated protein secretion as potential mechanisms for the release of monomeric and oligomerized α-Syn (15, 16). On the uptake side, α-Syn PFFs can enter cells via clathrin-mediated endocytosis (CME) on binding to surface receptors (17), but whether α-Syn endocytosis involves substrate specific endocytosis regulators has not yet been explored. This type of regulator may be particularly relevant to the development of PD drugs that target α-Syn endocytosis. Another unresolved issue is the identity of relevant membrane receptors for α-Syn PFFs; several studies have reported different candidates: heparan sulfate proteoglycans (HSPGs), membrane protein LAG3 for unmodified α-Syn PFFs (18, 19), and a glycoprotein, neurexin 1β, for N-terminally acetylated α-Syn PFFs (20).
HSPGs compose a class of cell surface and extracellular matrix glycoproteins that carry one or more heparan sulfate (HS) chains bearing repeated disaccharide units. This modification is found primarily in the syndecan and glycosylphosphatidylinositol-anchored glypican families (21). The biosynthesis of HSPGs begins in the endoplasmic reticulum, but its completion requires modification of the assembled sugar chains with sulfate using 3′-phosphoadenosine-5′-phosphosulfate (PAPS) as the sulfate donor, which occurs in the Golgi complex (22). Because HS chains carry negative charges, they can interact with a plethora of ligands on the cell surface, either mediating their uptake or activating downstream signal transduction (23, 24). The known HSPG ligands do not share any specific sequence motif for engaging HSPGs; instead, the binding energy seems to be provided by electrostatic interactions, often involving positively charged residues from random sequences in ligands (21). Along with α-Syn PFFs, HSPGs have been implicated in the uptake of tau PFFs and Aβ (18, 25, 26), but how HSPGs interact with these neurotoxic protein aggregates is unclear.
In this study, we used an unbiased CRISPR/Cas9 screen to identify factors that regulate α-Syn PFF endocytosis in mammalian cells. The screen not only isolated known endocytosis regulators of α-Syn PFFs (e.g., HSPGs), but also identified other factors important for α-Syn PFF uptake (e.g., an unconventional myosin and actin). Mechanistically, our study defines the interaction of HSPGs with α-Syn PFFs using biochemical assays and structural modeling. Importantly, we reveal a unique CME mechanism for α-Syn PFFs and other polycation-bearing cargos, which, unlike conventional CME, requires myosin 7B (MYO7B) and actin filaments.
Results
A CRISPR Screen Identifies Proteins Involved in α-Syn PFF Endocytosis.
To dissect the mechanism of α-Syn PFF endocytosis, we labeled purified α-Syn with either a stable fluorophore (Alexa Fluor 594) or a pH-sensitive dye (pHrodo). The pHrodo dye becomes activated only on its arrival at the acidic late endosome/lysosome compartment, emitting red fluorescence (SI Appendix, Fig. S1A). We used labeled proteins to prepare α-Syn PFFs following a well-established protocol (27). Electron microscopy (EM) analysis of sonicated α-Syn PFF revealed 20- to 100-nm-long particles (SI Appendix, Fig. S1B), consistent with the reported α-Syn PFFs bearing pathogenic activities (27). We tested α-Syn PFF uptake in various cell types and found that both neuronal and nonneuronal cells could efficiently internalize α-Syn PFF (SI Appendix, Fig. S1C), suggesting that the core endocytosis machinery for α-Syn PFFs is not specific to neurons. Treating cells with dynamin inhibitors, such as dynasore (28) or dynole 34-2 (29), significantly reduced α-Syn PFF uptake (SI Appendix, Fig. S1 D and E). In addition, α-Syn PFF uptake was also dramatically attenuated in HeLa cells lacking the σ2 subunit of AP-2 (encoded by the AP2S1 gene), a cargo adaptor in CME (SI Appendix, Fig. S1F). These findings confirm CME-dependent α-Syn PFF endocytosis (17, 30).
To search for α-Syn PFF endocytosis regulators, we performed a pooled CRISPR/Cas9 screen (Fig. 1A). We chose HEK293T cells because their fast-growing and high-DNA recombination properties make them well suited for a CRISPR screen. Since pooled CRISPR screens often suffer from poor specificity and reproducibility, we used a strategy akin to the classical genetics approach. We treated HEK293T cells with a lentiviral GeCKO library expressing single guide RNAs (sgRNAs) targeting all human genes. We then incubated mutagenized cells with pHrodo-labeled α-Syn PFFs. Florescence-activated cell sorting (FACS) was used to sort α-Syn PFF–negative cells into 96-well plates at single-cell resolution. When these cells formed colonies, we rescreened them to identify cells defective in α-Syn PFF uptake. Among the 1,248 clones screened, one clone (C10) showed almost no α-Syn PFF uptake (SI Appendix, Fig. S2A), whereas 27 other clones showed reduced α-Syn PFF uptake. In this study, we characterized two clones, which revealed an unexpected requirement for the endocytosis of α-Syn PFF and other polycation-bearing cargos.
Fig. 1.
SLC35B2 is required for endocytosis of α-Syn PFF. (A) The CRISPR screen strategy. (B–D) SLC35B2-KO cells are defective in endocytosis of α-Syn PFF but not monomeric α-Syn. (B) Validation of SLC35B2 (35B2)-KO cells by qRT-PCR. mRNA levels were normalized to the level in control cells. The error bar represents SEM. n = 3. (C) Control, SLC35B2-KO, or SLC35B2-KO cells stably expressing FLAG-SLC35B2 were incubated with pHrodo-labeled α-Syn PFF (200 nM for 4 h) and analyzed by FACS. FL, fluorescence. (D) Control and SLC35B2-KO cells were treated with labeled α-Syn monomer at 800 nM overnight before FACS analysis. (E–G) Knockdown (KD) of SLC35B2 attenuates α-Syn PFF uptake in primary neurons. (E) Primary neurons were infected with lentivirus expressing the indicated shRNAs together with EGFP (driven by the synapsin promoter) at day in vitro (DIV) 3. mRNA was purified from cells at DIV8 for qRT-PCR analysis. Error bar represents SEM. n = 2 biological repeats, each with triplicate PCR analyses. (F) Control or SLC35B2-KD neurons expressing EGFP (green) at DIV8 were incubated with α-Syn PFF Alexa Fluor 594 (200 nM) (red) for 4 h, stained with DAPI (blue), and analyzed by confocal microscopy (Scale bar: 5 µm.) (G) Quantification of α-Syn PFF level in individual cells (indicated by dots) from two independent experiments. Error bar represents SEM. P value from a two-tailed t test. A.U., arbitrary unit. (H and I) SLC35B2 is required for α-Syn PFF binding to the plasma membrane. (H) Control, SLC35B2-KO, or SLC35B2-KO cells reexpressing WT SLC35B2 were incubated with α-Syn PFF Alexa Fluor 594 (200 nM) (magenta) on ice for 30 min, stained with DAPI (blue), and imaged by confocal microscopy (Scale bar: 5 µm.) (I) Quantification of α-Syn PFF surface level in individual cells from two independent experiments.
HSPGs Are Required for α-Syn PFF Endocytosis.
Sequencing identified a sgRNA in one of the clones (C10) targeting SLC35B2. Analysis of SLC35B2-knockout (KO) cells confirmed a specific requirement for SLC35B2 in endocytosis of α-Syn PFFs, but not monomeric α-Syn (mSyn) (Fig. 1 B–D). Furthermore, shRNA-mediated knockdown of SLC35B2 in primary neurons also reduced α-Syn PFF uptake (Fig. 1 E–G), but the uptake defect was less dramatic compared with that in SLC35B2 KO cells, likely because of partial gene silencing. The SLC35B2 gene contains four exons. The identified sgRNA targets a sequence in exon 2 (SI Appendix, Fig. S2B). SLC35B2 is known as a Golgi-localized membrane protein required for transporting PAPS into the Golgi complex for protein sulfation (SI Appendix, Fig. S2C) (31). We confirmed the Golgi localization of SLC35B2 by FLAG antibody immunostaining of SLC35B2-KO cells stably expressing FLAG-tagged SLC35B2 (SI Appendix, Fig. S2D). The functionality of ectopically expressed SLC35B2 was confirmed by its ability to rescue α-Syn PFF uptake in SLC35B2-KO cells (Fig. 1C). Collectively, these results suggest a role for Golgi-localized protein sulfation in α-Syn PFF endocytosis.
Cell surface binding experiments showed that SLC35B2 is required for the recruitment of α-Syn PFFs to the plasma membrane (Fig. 1 H and I). Given the known role of SLC35B2 in the biosynthesis of HSPGs (31) and recent studies suggesting HSPGs as a potential receptor for α-Syn PFFs (18, 32), we presumed that α-Syn PFFs failed to bind SLC35B2-KO cells, because these cells lacked HSPGs. Indeed, immunostaining with an antibody to HSPGs stained the plasma membrane of WT, but not that of SLC35B2-KO cells (SI Appendix, Fig. S2 E and F). Furthermore, KO of XYLT2, another key HSPG biosynthetic enzyme (33), diminished both cell surface HSPG signals (SI Appendix, Fig. S2G) and α-Syn PFF binding (SI Appendix, Fig. S2 H and I). Thus, our unbiased genetic screen confirms HSPGs as critical mediators of α-Syn PFF–membrane interaction, which is essential for α-Syn PFF endocytosis.
Electrostatic Interactions Recruit α-Syn PFF to the Plasma Membrane.
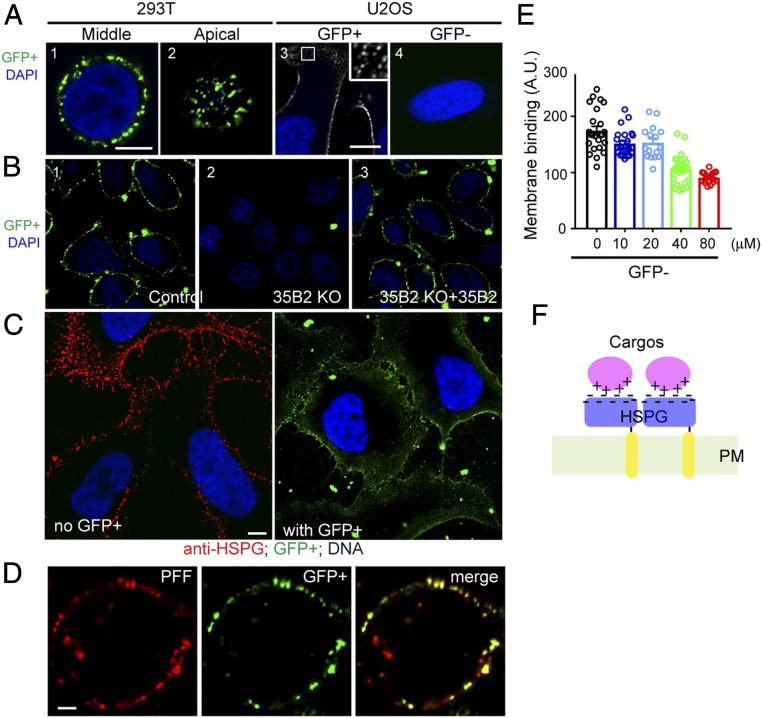
To better characterize the α-Syn PFF–HSPG interaction, we developed a fluorescence probe that can track HSPGs in live cells. Since the oligosaccharide chains in HSPGs are enriched in negative charges (23), we reasoned that an engineered GFP variant carrying 36 net positive charges (GFP+) (34) might be a specific probe for HSPGs. Indeed, several lines of evidence support this notion. First, live-cell confocal microscopy showed that GFP+, but not a GFP variant bearing extra negative charges (GFP−) bound efficiently to the cell surface, initially forming a smooth profile, which was rapidly converted to a punctate pattern reminiscent of cells treated with α-Syn PFF Alexa Fluor 594 (Fig. 2A). The interaction of GFP+ with the cell surface was completely dependent on SLC35B2, as no GFP+ staining was detected in SLC35B2-KO cells (Fig. 2B, compare panel 2 with panels 1 and 3). Furthermore, when cells were pretreated with GFP+, cell surface HSPGs could not be stained by antibodies to HSPG (Fig. 2C), suggesting that GFP+ competes with HSPG antibodies for the same binding site. Because cells treated with GFP+ and α-Syn PFF Alexa Fluor 594 showed extensive colocalization of these two cargos on the cell surface (Fig. 2D), and because pretreating α-Syn PFF with GFP− reduced the binding of α-Syn PFF to cells (Fig. 2E), electrostatic interactions must provide the major energy that recruits α-Syn PFFs to the cell surface (Fig. 2F).
Fig. 2.
α-Syn PFF interacts with cells via electrostatic interactions. (A) GFP+ binds to the plasma membrane. Panels 1 and 2 show two confocal sections of a HEK293T cell stained with 200 nM GFP+ (green) and DAPI (blue) for 5 min. (Scale bar: 5 µm.) Panels 3 and 4 show U2OS cells incubated with 200 nM of GFP+ (panel 3) or GFP− (panel 4) and then stained with DAPI. (Inset) Enlarged view of the box in panel 3 (Scale bars: 10 µm.) (B) Interaction of GFP+ with the plasma membrane depends on SLC35B2. Control (panel 1) or SLC35B2-KO HEK293T cells with (panel 3) or without (panel 2) the reexpression of WT SLC35B2 were stained with GFP+ and DAPI (Scale bar: 5 µm.) (C) GFP+ inhibits the binding of HSPG antibodies to the cell surface. U2OS cells were either pretreated with GFP+ (200 nM) (Right) for 10 min or left untreated (Left) and then stained with antibodies to HSPG (red) and DAPI (blue) (Scale bar: 5 µm.) (D) Colocalization of GFP+ with α-Syn PFF in cells. Cells incubated with α-Syn PFF Alexa Fluor 594 and GFP+ were imaged. (E) GFP− attenuates α-Syn PFF binding to the plasma membrane. Cells were incubated with α-Syn PFF Alexa Fluor 594 (200 nM) in the presence of the indicated concentrations of GFP−. α-Syn PFF binding to the cell surface was quantified by confocal imaging. (F) An HSPG–cargo interaction model.
Two K-T-K Motifs in Oligomerized α-Syn Enable HSPG Interactions.
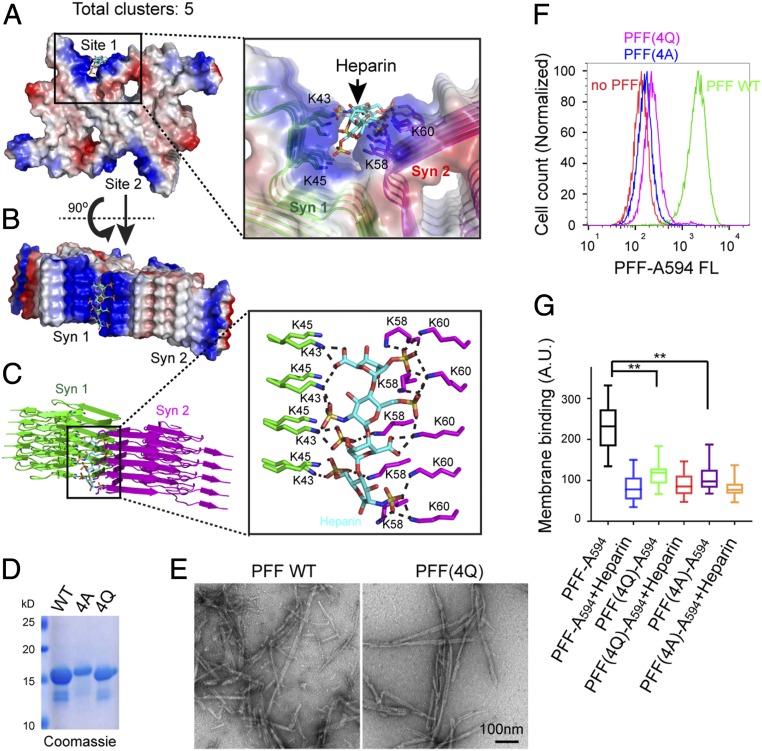
Previous studies have shown that two α-Syn monomers could form either a face-to-face or back-to-back dimer. Many dimers then stack together to form long fibrils of “rod” or “twisted” conformers (35, 36). To elucidate how these α-Syn PFF conformers interact with HSPGs, we used the ClusPro program to model the interaction of α-Syn PFF conformers with heparin, an HS analog that competitively inhibits the binding of α-Syn PFF to cells (18). The modeling resulted in 14 clusters falling into 3 groups, as shown in Fig. 3A and SI Appendix, Fig. S3 A and B. Interestingly, in all clusters, the interactions between α-Syn PFF and heparin were mediated by two K-T-K motifs, K43-T-K45 and K58-T-K60, in α-Syn fibrils. In the twisted conformer, each of these lysine-rich motifs lined up to form a shallow concave surface that accommodates a di-heparin molecule bearing six sulfate groups (SI Appendix, Fig. S3 A and B). In contrast, in the rod conformation, two K-T-K motifs from distinct α-Syn molecules in a dimeric fibril joined together to form one deep binding groove for di-heparin (Fig. 3 A and B). As a result, each fibril could offer only two binding grooves, but within each binding site the heparin molecule was sandwiched by four rows of lysine residues, forming extensive electrostatic interactions as well as hydrogen bonds with α-Syn (Fig. 3C and SI Appendix, Fig. S3C). Thus, α-Syn fibrils in the rod conformer are expected to have a much higher affinity for HSPGs than the twisted conformer. These models explain why HSPGs are only required for the uptake of α-Syn fibrils but not monomeric α-Syn. This result also suggests that a twisted α-Syn oligomer containing three protomers is the minimum requirement for binding one heparin unit; in contrast, for the rod conformer, five or six α-Syn protomers are required to form a higher-affinity heparin-binding site.
Fig. 3.
Two K-T-K motifs in α-Syn PFF enable specific interactions with heparin. (A–C) A model of the α-Syn PFF rod conformer in complex with heparin. (A and B) A model representing five clusters. In this model, each α-Syn PFF dimeric fibril contains two heparin-binding pockets (sites 1 and 2) that each forms a long groove to accommodate a heparin molecule. (C) Heparin is sandwiched by four rows of lysine residues in α-Syn fibril. Dashed lines in C label complementarily charged atoms within 3 Å. (D) Purified WT α-Syn and charge mutants (4A and 4Q) analyzed by SDS/PAGE and Coomassie blue staining. (E) Representative negative stained EM images showing PFFs formed by WT α-Syn and the 4Q α-Syn mutant. (F and G) α-Syn PFFs Alexa Fluor 594 formed by the charge mutants are defective in endocytosis (F) and cell surface binding (G). (F) HEK293T cells were treated with α-Syn PFF Alexa Fluor 594 for 3 h before FACS analysis. (G) Whisker plot showing the relative level of α-Syn PFF binding to cells as determined by confocal imaging of U2OS cells treated with α-Syn PFF Alexa Fluor 594 at 400 nM in the presence or absence of heparin (4 µM) on ice. **P < 0.01, two-tailed Student’s t test.
To validate our models, we generated α-Syn mutants with the four lysine residues K43, K45, K58, and K60 substituted to either glutamine (4Q) or alanine (4A) (Fig. 3D). These mutants could form long fibrils similarly to WT α-Syn (Fig. 3E); however, compared with WT α-Syn PFFs, the uptake of these mutant α-Syn PFFs was dramatically reduced (Fig. 3F). In contrast, the uptake of monomeric α-Syn was not affected by these mutations (SI Appendix, Fig. S3D). Furthermore, cell-binding experiments showed that the interaction of α-Syn PFF with the plasma membrane was significantly inhibited by these mutations (Fig. 3G). These results strongly suggest that α-Syn binds HSPGs via a specific sequence motif that forms binding sites only on α-Syn oligomerization.
HSPG-Mediated Endocytosis Requires MYO7B and Actin Filaments.
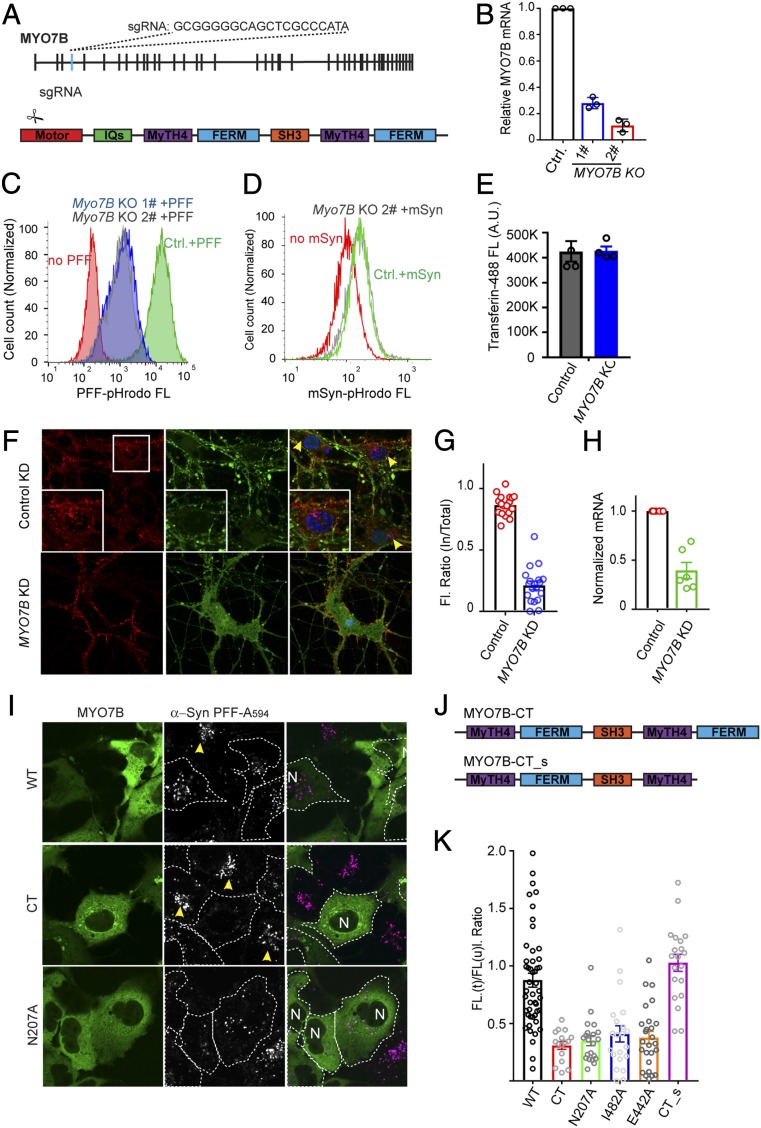
Using a similar approach, we identified a sgRNA from a clone partially defective in α-Syn PFF endocytosis, which targeted MYO7B, an unconventional myosin widely expressed in various human tissues (37). Like for SLC35B2, the sgRNA targeting MYO7B mapped to an early exon (Fig. 4A). Reconstructed MYO7B-KO HEK293T cells or cells treated with MYO7B-specific small interfering RNA (siRNA) showed that depletion of MYO7B diminished endocytosis of α-Syn PFF (Fig. 4 B and C and SI Appendix, Fig. S4A), while reexpression of EGFP-MYO7B in MYO7B-KO cells largely rescued the α-Syn PFF uptake phenotype (SI Appendix, Fig. S4B). An α-Syn PFF uptake defect was similarly observed in primary neurons expressing MYO7B-specific shRNA (Fig. 4 F–H). Strikingly, the function of MYO7B in endocytosis appeared to be restricted to HSPG-dependent cargos, because like HSPG-deficient cells (23), MYO7B-KO cells were also defective in the uptake of GFP+ (SI Appendix, Fig. S4 C and D) and of DNA in complex with a polycation carrier (SI Appendix, Fig. S4E). Furthermore, HSPG-dependent uptake of a lentiviral reporter expressing GFP was significantly reduced in MYO7B-KO cells (SI Appendix, Fig. S4 F and G). On the other hand, endocytosis of monomeric α-Syn or transferrin was not affected in MYO7B-KO cells (Fig. 4 D and E).
Fig. 4.
HSPG-mediated endocytosis requires MYO7B and actin filaments. (A) Mapping the identified MYO7B sgRNA. (B) Validation of MYO7B-KO cells by qRT-PCR. mRNAs extracted from two clones of MYO7B KO cells and control (Ctrl.) clone were analyzed by qRT-PCR. Error bars indicate SEM. n = 3. (C) MYO7B KO cells are defective in α-Syn PFF uptake. Control (Ctrl.) and MYO7B-KO clones were incubated with pHrodo-labeled α-Syn PFF for 4 h before FACS analyses. (D) MYO7B is not required for endocytosis of α-Syn monomer. Control or MYO7B-KO cells were incubated with the α-Syn monomer (800 nM) overnight before FACS analysis. (E) MYO7B is not required for transferrin uptake. Control or MYO7B-KO cells were incubated with fluorescein-labeled transferrin (50 µg/mL for 3 h), washed, and analyzed with a fluorometer. Error bars represent SEM. n = 3. (F–H) KD of MYO7B in primary neurons reduces α-Syn PFF endocytosis without affecting its binding to the plasma membrane. (F) Primary neurons infected with the indicated shRNA-expressing lentivirus together with Synapsin_EGFP lentivirus at DIV3 were incubated with α-Syn PFF Alexa Fluor 594 (200 nM) for 4 h and then imaged at DIV8. (Insets) Enlarged views of the box. Arrows indicate the perinuclear enrichment of α-Syn PFF–positive vesicles in control cells. (G) The ratio of internalized α-Syn PFF relative to total α-Syn PFF in individual cells. (H) qRT-PCR evaluation of MYO7B mRNA levels using the same batch of cells. Error bars represent SEM. n = two biological repeats with triplicated PCR analysis. (I–K) MYO7B dominant-negative mutants inhibit α-Syn PFF uptake. (I) Representative confocal images of COS7 cells transfected with the indicated MYO7B plasmids and treated with α-Syn PFF Alexa Fluor 594 (400 nM) for 3 h. Arrows indicate normal α-Syn PFF uptake in untransfected cells, which was used to normalize uptake. N, nuclei. (J) Graph showing the domain structure of the truncated MYO7B mutants. (K) Quantification of α-Syn PFF fluorescence (FL) in individual cells transfected (t) with the indicated MYO7B mutants normalized by the signal in untransfected cells (u).
To test whether HSPG-mediated endocytosis involves the motor activity of MYO7B, we ectopically expressed MYO7B mutants that either lack the motor domain (CT) or carry a mutation that disrupts the ATPase cycle of the motor domain (N207A, I482A, or E442A) (38). If coupling the motor activity to cargo binding were required for α-Syn PFF uptake, then these mutants should inhibit α-Syn PFF uptake in a dominant negative manner. Indeed, cells expressing these mutants showed significantly reduced α-Syn PFF uptake compared with neighboring untransfected cells or cells transfected with WT MYO7B (Fig. 4 I–K). Furthermore, deleting the C-terminal membrane-binding FERM domain abolished the dominant negative activity of MYO7B-CT, suggesting that membrane binding by a motor-defective MYO7B mutant accounts for the observed endocytosis defect. These results, together with the observation that actin-binding compounds (e.g., jasplakinolide, latrunculin A) that interfere with the function of F-actin also inhibited α-Syn PFF endocytosis (SI Appendix, Fig. S5 A and B), strongly suggest that optimal α-Syn PFF endocytosis requires a membrane-associated functional interplay between MYO7B and actin filaments.
MYO7B Maintains Membrane Dynamics at Clathrin-Enriched Membrane Domains.
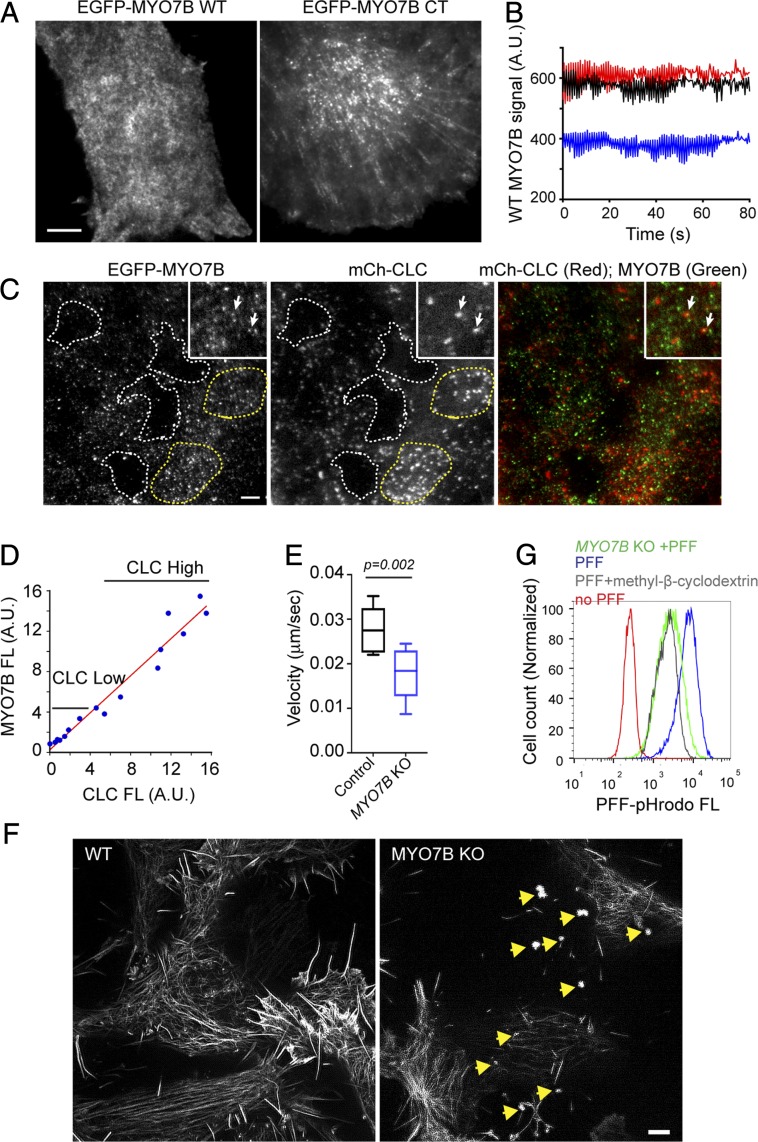
To elucidate the mechanism of MYO7B-mediated endocytosis, we used total internal reflection fluorescence microscopy (TIRF) to examine the localization of MYO7B in U2OS cells stably expressing mCherry-tagged clathrin light chain (mCh-CLC) to label clathrin-coated pits (CCPs) together with either GFP-WT MYO7B or GFP-MYO7B-CT. Consistent with the reported localization of MYO7B to plasma membrane-derived microvilli (38), GFP-MYO7B also bound to the plasma membrane in U2OS cells, forming discrete dots (Fig. 5A). Time-lapse microscopy showed that MYO7B-positive signals fluctuated rapidly, displaying a flickering pattern (Fig. 5B and Movie S1). Interestingly, the MYO7B-CT mutant appeared to form more stable interactions with the membranes (Fig. 5A), resulting in bright foci that remained constant for a prolonged period (Movie S2). We concluded that the MYO7B C-tail domain can bind to the plasma membrane, while its actin-binding domain contributes to the dynamic property of this interaction.
Fig. 5.
MYO7B maintains plasma membrane dynamics at domains enriched in clathrin. (A) TIRF microscopy analysis of plasma membrane binding by EGFP-WT MYO7B and EGFP-CT MYO7B in cells stably expressing low levels of these proteins (Scale bar: 5 µm.) (B) Quantification of representative EGFP-WT MYO7B signal on the plasma membrane in a time-lapse video. Shown are fluorescence intensities of three randomly chosen spots measured by Fiji. A.U., arbitrary unit. (C and D) MYO7B binds to the plasma membrane in clathrin-enriched domains. (C) TIRF microscopy analysis of cells stably expressing EGFP-WT MYO7B and mCh-CLC. (Insets) Close-up views of cells revealing limited colocalization between MYO7B and CLC. The dashed lines indicate surface regions either lacking clathrin (white) or enriched in clathrin (yellow). (D) Correlation between CLC and MYO7B fluorescence (FL) intensity in cell surface domains as outlined in Fig. 5C (R2 = 0.956 by linear regression). (E) MYO7B inactivation affects plasma membrane dynamics. Control and MYO7B-KO cells were stained with GFP+ and imaged by time-lapse confocal microscopy (SI Appendix, Fig. S5C and Movies S3 and S4). The whisker graph shows the plasma membrane ruffling velocity determined by the Nikon Element analysis of videos taken from three independent experiments. Control, n = 6 cells; MYO7B-KO, n = 12 cells. P values by two-tailed unpaired Student’s t test. (F) MYO7B-KO cells have reduced actin fibers under the plasma membrane. TIRF-SIM analysis of WT or MYO7B-KO cells transfected with EGFP-tractin. Note the presence of actin-containing aggregates in MYO7B-KO cells (indicated by arrows) (Scale bar: 10 µm.) (G) Depletion of cholesterol inhibits α-Syn PFF endocytosis. Cells were treated with methyl-β-cyclodextrin (5 mM) or DMSO for 30 min before incubation with α-Syn PFF pHrodo and FACS analysis.
When colocalization of MYO7B with CCPs was examined by two-color TIRF, we found limited overlapping signals (Fig. 5C, Insets). However, we noticed that MYO7B was not uniformly distributed on the plasma membrane. Instead, certain areas had more MYO7B dots than others (Fig. 5C). Intriguingly, MYO7B-high plasma membrane domains were often enriched in CCPs (Fig. 5 C and D). Therefore, we postulated that dynamic interactions of MYO7B within certain plasma membrane domains enable membrane flexibility and deformability, which might in turn facilitate the maturation of CCPs located in the same membrane domain.
To measure plasma membrane dynamics, we used GFP+ to label the cell surface and then took time-lapse videos. In WT cells, the plasma membrane usually undergoes dynamic expansion and retraction, generating a ruffling motion (SI Appendix, Fig. S5C and Movie S3). In contrast, the membranes of MYO7B-KO cells had significantly reduced mobility (Movie S4 and Fig. 5E). Furthermore, TIRF microscopy showed that MYO7B-KO cells had a significantly reduced amount of actin filaments underneath the plasma membranes (Fig. 5F). These results suggest that MYO7B may regulate actin assembly to maintain membrane dynamics.
If MYO7B promotes endocytosis via membrane dynamics at cargo-binding sites, other agents capable of increasing membrane stiffness should similarly inhibit α-Syn PFF uptake. To test this idea, we treated cells with the cholesterol-depleting drug methyl-β-cyclodextrin, which increases membrane stiffness in mammalian cells (39). Consistent with our hypothesis, methyl-β-cyclodextrin also reduced α-Syn PFF uptake to a similar degree as depletion of MYO7B (Fig. 5G).
MYO7B Facilitates the Maturation of Clathrin-Coated Pits.
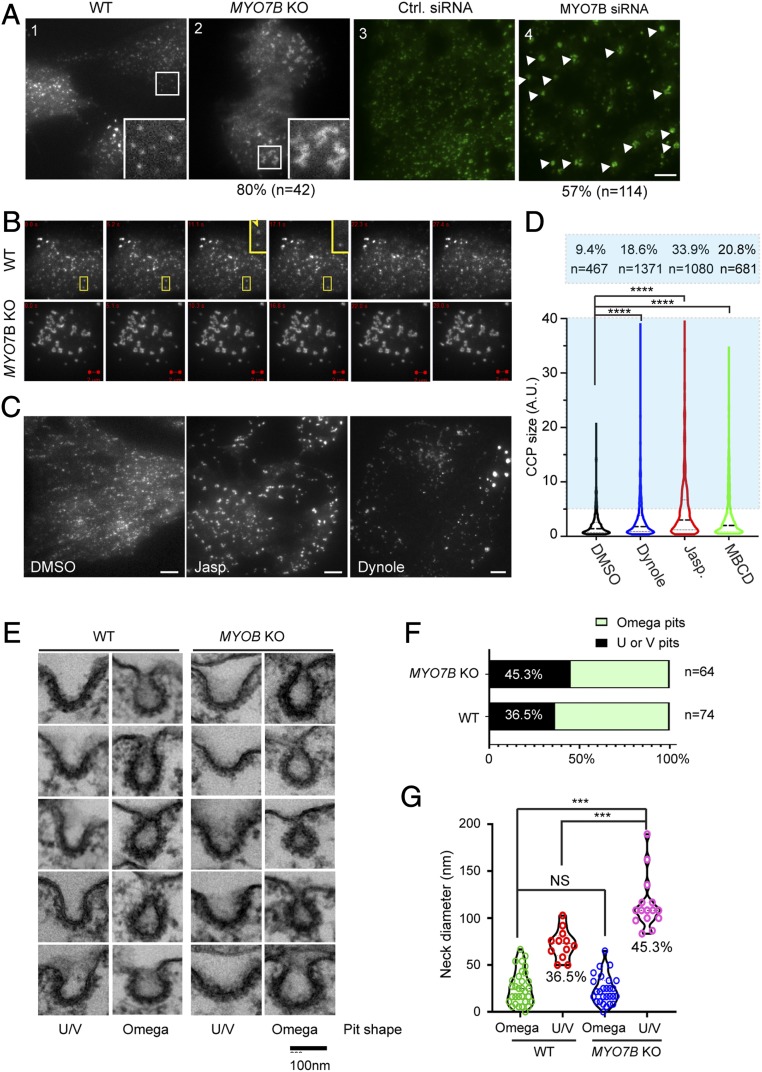
Since α-Syn PFF endocytosis requires CME, we characterized the effect of MYO7B inactivation on CCP morphology using TIRF microscopy. In WT cells transfected with GFP-CLC, TIRF detected small CLC-positive fluorescence puncta at the basal membranes (Fig. 6A, panel 1). In time-lapse videos, these CLC-positive puncta frequently detached from the membranes, vanishing into the cytosol due to endocytosis (Fig. 6B, Upper). Intriguingly, in MYO7B-KO cells, only a fraction of CCPs behaved like those in WT cells, while in ∼80% of the cells, we detected large CCP clusters that were completely immobile (Fig. 6A, panel 2 and B, Lower). The CCP clustering phenotype was also observed in GFP-CLC–stable U2OS cells transfected with a MYO7B-specific siRNA (Fig. 6A, panel 4 vs. panel 3). In addition, cells treated with jasplakinolide, dynole 34-2, or methyl-β-cyclodextrin (MBCD) also accumulated clustered CCP puncta at the basal membranes, coincident with α-Syn PFF endocytosis inhibition (Fig. 6 C and D). These results suggest that MYO7B-dependent membrane dynamics might facilitate CCP function in HSPG-mediated endocytosis. When CCPs fail to mature, these endocytosis-defective pits accumulate at the basal plasma membranes and form clusters.
Fig. 6.
MYO7B inactivation affects the maturation of CCPs. (A and B) MYO7B depletion causes CCPs to cluster. (A) TIRF microscopy analysis of CCPs in control (panel 1) or MYO7B-KO (panel 2) HEK293T cells transiently transfected with GFP-CLC, or in GFP-CLC–stable U2OS cells transfected with control (panel 3) or MYO7B (panel 4) siRNA. Approximately 80% of MYO7B-KO cells show the phenotype shown in panel 2. (Insets) Enlarged views of the boxed regions. (Scale bar: 5 µm.) (B) Images from time-lapse videos showing CCP dynamics on the cell surface. Boxes and Insets show examples of CCP (arrow) vanishing into the cytosol in a WT cell (Scale bars: 2 µm.) (C and D) Effect of jasplakinolide (JASP), dynole 34-2 (Dynole), and MBCD on CCP morphology analyzed by TIRF in GFP-CLC stable U2OS cells. (C) Representative images of DMSO-treated, JASP-treated (100 nM for 1 h), and dynole-treated (18 µM for 2 h) cells. (Scale bar: 5 µm.) (D) Violin plot showing the relative CCP sizes under different drug-treated conditions. The numbers indicate the percentage of clustered CCPs. n, number of pits analyzed. ****P < 0.0001, one-way ANOVA. (E–G) Transmission EM analysis of CCP morphology in control and MYO7B-KO cells. Control and MYO7B-KO cells were incubated with α-Syn PFF (400 nM for 1 h), and then fixed for EM analysis. (E) Representative CCPs. (F) The relative abundance of U- and V-shaped pits vs. omega-shaped pits in control and MYO7B-KO cells. n, number of pits analyzed. (G) Violin plot showing the neck length of CCPs measured from two independent experiments. ***P < 0.001, unpaired Student’s t test. NS, not significant.
To test the foregoing hypothesis, we used transmission EM to examine the morphology of CCPs at the apical and lateral membranes where HSPG-mediated endocytosis occurs. To capture defects associated with α-Syn PFF uptake, we first incubated cells with α-Syn PFF at 37 °C for 1 h to initiate PFF uptake. EM analyses identified approximately four CCPs per cell section in WT cells, but only one CPP per section on the plasma membrane of SLC35B2-KO cells (SI Appendix, Fig. S6 A and B), suggesting that the detected CCPs were mostly linked to HSPG-mediated endocytosis. Although the number of CCPs in MYO7B-KO cells was comparable to that of WT cells, we found that compared with WT cells, MYO7B-KO cells had a ∼9% increase in the number of U- or V-shaped pits and a concomitant reduction of omega-shaped pits (Fig. 6 E and F). In addition, for the U- or V-shaped pits, the average neck diameter was significantly increased in MYO7B-KO cells, whereas the neck diameter for omega-shaped pits was comparable in WT and MYO7B-KO cells (Fig. 6G). Since U- and V-shaped CCPs are thought to be precursors of omega-shaped CCPs, these results support the notion that MYO7B is required for optimal CCP function.
Discussion
Role of HSPGs in Endocytosis.
HSPGs have been implicated in endocytosis of a variety of cargos (23), and the substrate diversity has been attributed to nonspecific electrostatic interactions between cargos and HSPGs. Consistent with this notion, in this study, we have shown that a GFP variant bearing net positive charges (GFP+) can be used as a probe to label HSPGs in mammalian cells. However, our study also shows that certain cargos like α-Syn PFFs can interact with HSPGs via specific sequence motifs. Interestingly, among the 15 lysine residues in α-Syn, 4 have been confirmed to mediate the interaction with HSPGs. These residues by themselves do not form the binding site, but when α-Syn assembles into stacked oligomers, they can line up to form long binding grooves that accommodate the linear oligosaccharide chains of HSPGs. Interaction with α-Syn PFFs does not require the protein core of HSPGs, meaning that any of the 16 members of the HSPG family could possibly serve as a recruiter for α-Syn PFFs. Importantly, our findings also suggest that the two previously established α-Syn PFF conformers have different affinities to HSPGs, which may correspond to the reported α-Syn PFF strains bearing different pathogenic activities (40, 41). Although we cannot exclude the involvement of other α-Syn motifs in cell surface binding, identification of the K-T-K motifs in α-Syn as a major binding element for HSPGs may help design specific inhibitors that target α-Syn PFF entry for PD.
Despite its essential function in recruiting α-Syn PFFs to the cell surface, HSPGs alone might not be sufficient for linking HSPG cargos to downstream CME effectors such as clathrin, because many HSPG family members do not have a cytoplasmic domain that is required for recruiting cytosolic endocytosis effectors. Another membrane protein capable of interacting with clathrin or other endocytosis regulators may assist HSPGs by providing the missing link. Along this line, a recent study suggested LAG3 as a neuronal-specific membrane receptor for α-Syn PFFs (19). We propose that HSPGs may cooperate with distinct membrane receptors in a tissue-specific manner to jointly mediate α-Syn PFF endocytosis. Recruitment of α-Syn PFFs to the plasma membrane by HSPGs should increase their local concentration, and thus the avidity for a downstream coreceptor.
MYO7B and Actin Filaments in HSPG-Mediated Endocytosis.
It is well established that mechanical force generated by actin polymerization can drive membrane invagination in CME in yeast and in mammalian cells bearing high membrane tension (42–44). However, the function of myosin motors in CME is much less clear, particularly in higher eukaryotes. In Saccharomyces cerevisiae, the type I myosins (Myo3p and Myo5p) can promote the recruitment of actin assembly factors to the site of endocytosis, causing spatially restricted actin polymerization to deform the membranes (45, 46). The mammalian class I myosin-1E was also found at CCPs, coincident with a burst of actin assembly; however, depletion of myosin-1E had no discernible effect on transferrin uptake under normal growth conditions (47). Another study suggested a role for a class VI myosin in CME based on its localization to CCPs/vesicles (48); however, a more recent study showed that myosin-6 is localized only to uncoated vesicles (49). On a related note, genetic studies have implicated MYO7A as a regulator of Listeria phagocytosis (50, 51), but this process is independent of CME. Thus, whether mammalian CME involves myosin(s) and if so, in what capacity, has been unclear.
Our study now provides compelling evidence that MYO7B serves a specific function in CME, at least for certain polycation-bearing cargos that enter cells via HSPGs. Live-cell imaging showed that on binding to the cell surface, GFP+ is rapidly clustered into patches (Fig. 2), suggesting that HSPGs may undergo oligomerization on ligand binding. Because membrane invagination during CME is expected to bring cargos and HSPGs close to each other, which would increase the repellent force between molecules bearing the same charge, we reason that MYO7B and actin filaments may provide the extra energy to overcome this membrane-bending barrier. Because actin was found both under the plasma membrane and on the surface of some α-Syn PFF–bearing endocytic vesicles (SI Appendix, Fig. S6C), we propose that MYO7B either promotes actin assembly or stabilizes actin filaments at membranes near oligomerized HSPG patches, which in turn facilitates membrane protrusions. These protrusions eventually wrap around cargos, forming omega-shaped CCPs with actin filaments surrounding the CCP neck. Membrane severance by dynamin subsequently releases the vesicles into the cytoplasm, leaving an F-actin “scar” attached to it (SI Appendix, Fig. S6D). Additional studies are needed to reveal the mechanistic details of MYO7B-mediated CME, as well as additional mechanisms that contribute to α-Syn PFF endocytosis. Nevertheless, our study has linked the function of MYO7B and actin filaments to HSPG-mediated endocytosis of α-Syn PFF and other polycation-bearing cargos. This unique feature may be exploited to develop drugs that prevent the transmission of α-Syn pathology in PD.
Materials and Methods
Cell Lines and DNA Transfection.
All reagents are listed in SI Appendix, Table S1. HEK293T, U2OS, COS7, and HeLa cells were purchased from American Type Culture Collection. HEK293FT cells were obtained from Invitrogen. Cells were maintained in Dulbecco's Modified Eagle’s Medium (DMEM; Corning) containing 10% FBS and antibiotics (penicillin/streptomycin, 10 U/mL). All cell lines were maintained at 37 °C in a 5% CO2 humidified atmosphere. Cells stably expressing GFP-CLC or mCh-CLC or GFP-tagged MYO7B WT and the CT mutant were generated using U2OS cells. Specifically, these cells were seeded in a six-well plate and transfected with the reporter constructs using Lipofectamine 2000. At 2 wk posttransfection, cells stably expressing the reporters were sorted by FACS. Stable KO cells were generated by infecting HEK293T cells with lentivirus expressing sgRNAs targeting the gene of interest. Cells were selected by 0.35 µg/mL puromycin for 4 d to generate cells stably expressing the sgRNAs. Single cell-derived KO clones were obtained by sequential dilution or by FACS sorting.
Cell transfection was performed using TransIT-293 reagent (Mirus) for HEK293T cells or Lipofectamine 2000 (Invitrogen) for COS7, HeLa, and U2OS cells following the manufacturer’s instructions. For RNAi-mediated gene silencing, Lipofectamine RNAiMAX (Invitrogen) was used according to the manufacturer’s protocol. U2OS cells were seeded in four-well chambers and transfected with 60 pmol siRNA per well in antibiotic-free medium for 48 h before imaging analyses.
Plasmid DNA, Molecular Biology, and Virus Production.
A CRISPRv2 construct bearing sgRNA-targeting genes of interest was generated as follows. First, 5 µg of CRISPRv2 was digested with BsmBI at 37 °C for 30 min. The digested vector was then gel-purified and eluted in pure water. Each pair of sgDNA oligos was phosphorylated and annealed by incubating 1 µL of sgRNA oligo 1 or 2 (100 µM) with 1 µL of 10× T4 ligation buffer in the presence of 0.5 µL of T4 polynucleotide kinase in 10 µL. The reaction was placed in a thermocycler using the following parameters: 37 °C for 30 min, 95 °C for 5 min, and then a ramp down to room temperature at 5 °C/min. The annealed sgDNA oligo was then ligated with the BsmBI-digested CRISPRv2 to create CRISPRv2-sgDNA constructs targeting SLC35B2 and MYO7B. KO lentivirus was produced by transfecting 1 million HEK293FT cells in a 3.5-cm dish with 0.4 µg of pVSV-G, 0.6 µg of psPAX2, and 0.8 µg of CRISPRv2-sgRNA. Transfected cells were incubated with 3 mL of fresh DMEM for 72 h, followed by harvesting of virus. shRNA knockdown virus was produced in a similar manner, except that a pair of shRNA-coding oligos were annealed and ligated with Age1-EcoR1–digested pLKO.1 construct. SLC35B2 rescue plasmid was generated by PCR-amplifying the SLC35B2 cDNA sequence with a FLAG tag-coding sequence and a NheI site appended at the 5′ end and an EcoRI site at the 3′ end. The PCR fragment was digested with NheI and EcoRI and further purified. The purified DNA fragment was then inserted into pLJM1. Five nonsense mutations were introduced at the sgRNA targeting sequence by site-directed mutagenesis to avoid Cas9 recognition of the rescue DNA. PCR mutagenesis was performed using the QuikChange II site-directed mutagenesis kit (Agilent) according to the manufacturer’s protocol.
Protein Purification, Labeling, and PFF Production.
Human α-Syn protein was expressed in BL21(DE3)RIL-competent Escherichia coli and purified following an established protocol (27). In brief, 1 L of E. coli culture was induced to express α-Syn with 0.5 mM isopropyl β-d-1-thiogalactopyranoside at 20 °C overnight. Cells were pelleted down at 5,000 rpm for 20 min, then resuspended with high-salt buffer (750 mM NaCl, 10 mM Tris pH 7.6, and 1 mM EDTA) with protease inhibitors and 1 mM PMSF (50 mL for 1 L of culture). Resuspended cells were sonicated with a 0.25-inch probe tip at 60% power for a total time of 10 min. Sonicated cell lysate was boiled for 15 min to precipitate unwanted proteins and then cooled on ice for 20 min. The lysate was then centrifuged at 6,000 × g for 20 min. The supernatant containing α-Syn was collected and dialyzed in TNE buffer (10 mM Tris pH 7.6, 25 mM NaCl, and 1 mM EDTA). Isolated proteins were further fractionated with a Bio-Rad NGS chromatography system first through a size exclusion column in TNE buffer, followed by an ion-exchange Mono-Q column. Protein was eluted in TNE buffer containing increasing concentrations of NaCl (25 mM to 1 M). Purified monomeric α-Syn was dialyzed extensively in PBS, followed by labeling with a threefold molar excess of pH-Rodo Red or Alexa Fluor 596 succinimidyl ester fluorescence dye (Thermo Fisher Scientific) for 4 h at room temperature. Unconjugated dye was removed by dialysis in PBS. The labeled α-Syn was adjusted to 5 mg/mL in a volume of 500 µL and then placed in a ThermoMixer shaker (Eppendorf) at 1,000 rpm at 37 °C for 7 d to generate protein fibrils. Preformed α-Syn PFFs were stained with 3% uranyl acetate and examined by EM to verify the formation of α-Syn PFF, then stored in a −80 °C freezer in small aliquots. GPF+ was expressed and purified according to a previously published protocol (34). Additional experimental procedures and methods are presented in SI Appendix.
Data Availability.
All data, associated protocols, methods, and sources of materials are available in the main text or SI Appendix.
Supplementary Material
Acknowledgments
We thank Drs. R. Tycko and U. Ghosh (National Institute of Diabetes and Digestive and Kidney Diseases [NIDDK]) for assistance with protein negative staining, J. Reece at the NIDDK imaging core for imaging assistance, D. Lu for assistance with data processing, H. Meyer (University of Duisburg Essen) for critical reading of the manuscript, and M. Tyska (Vanderbilt University) for MYO7B WT and mutant cDNAs. Q.Z., Y.X., J.L., and Y.Y. are supported by the NIDDK intramural research program (DK075143); X.W. is supported by the National Heart, Lung, and Blood Institute intramural research program; M.J. and J.S.B. are supported by the Eunice Kennedy Shriver National Institute of Child Health and Human Development intramural research program (ZIA HD001607); and J.S. is supported by NIH Grants AG061829 and GM126960.
Footnotes
The authors declare no competing interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at https://www.pnas.org/lookup/suppl/doi:10.1073/pnas.1918617117/-/DCSupplemental.
References
- 1.Spillantini M. G., et al. , Alpha-synuclein in Lewy bodies. Nature 388, 839–840 (1997). [DOI] [PubMed] [Google Scholar]
- 2.Krüger R., et al. , Ala30Pro mutation in the gene encoding alpha-synuclein in Parkinson’s disease. Nat. Genet. 18, 106–108 (1998). [DOI] [PubMed] [Google Scholar]
- 3.Polymeropoulos M. H., et al. , Mutation in the alpha-synuclein gene identified in families with Parkinson’s disease. Science 276, 2045–2047 (1997). [DOI] [PubMed] [Google Scholar]
- 4.Irwin D. J., Lee V. M., Trojanowski J. Q., Parkinson’s disease dementia: Convergence of α-synuclein, tau and amyloid-β pathologies. Nat. Rev. Neurosci. 14, 626–636 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kordower J. H., Chu Y., Hauser R. A., Freeman T. B., Olanow C. W., Lewy body-like pathology in long-term embryonic nigral transplants in Parkinson’s disease. Nat. Med. 14, 504–506 (2008). [DOI] [PubMed] [Google Scholar]
- 6.Li J. Y., et al. , Lewy bodies in grafted neurons in subjects with Parkinson’s disease suggest host-to-graft disease propagation. Nat. Med. 14, 501–503 (2008). [DOI] [PubMed] [Google Scholar]
- 7.Frost B., Diamond M. I., Prion-like mechanisms in neurodegenerative diseases. Nat. Rev. Neurosci. 11, 155–159 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Karpowicz R. J. Jr, Trojanowski J. Q., Lee V. M., Transmission of α-synuclein seeds in neurodegenerative disease: Recent developments. Lab. Invest. 99, 971–981 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Desplats P., et al. , Inclusion formation and neuronal cell death through neuron-to-neuron transmission of alpha-synuclein. Proc. Natl. Acad. Sci. U.S.A. 106, 13010–13015 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Volpicelli-Daley L. A., et al. , Exogenous α-synuclein fibrils induce Lewy body pathology leading to synaptic dysfunction and neuron death. Neuron 72, 57–71 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Luk K. C., et al. , Exogenous alpha-synuclein fibrils seed the formation of Lewy body-like intracellular inclusions in cultured cells. Proc. Natl. Acad. Sci. U.S.A. 106, 20051–20056 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Luk K. C., et al. , Pathological α-synuclein transmission initiates Parkinson-like neurodegeneration in nontransgenic mice. Science 338, 949–953 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kim S., et al. , Transneuronal propagation of pathologic alpha-synuclein from the gut to the brain models Parkinson’s disease. Neuron 103, 627–641.e7 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ye Y., Regulation of protein homeostasis by unconventional protein secretion in mammalian cells. Semin. Cell Dev. Biol. 83, 29–35 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lee J., Ye Y., The roles of endo-lysosomes in unconventional protein secretion. Cells 7, E198 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lee J. G., Takahama S., Zhang G., Tomarev S. I., Ye Y., Unconventional secretion of misfolded proteins promotes adaptation to proteasome dysfunction in mammalian cells. Nat. Cell Biol. 18, 765–776 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Oh S. H., et al. , Mesenchymal stem cells inhibit transmission of α-synuclein by modulating clathrin-mediated endocytosis in a parkinsonian model. Cell Rep. 14, 835–849 (2016). [DOI] [PubMed] [Google Scholar]
- 18.Holmes B. B., et al. , Heparan sulfate proteoglycans mediate internalization and propagation of specific proteopathic seeds. Proc. Natl. Acad. Sci. U.S.A. 110, E3138–E3147 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mao X., et al. , Pathological α-synuclein transmission initiated by binding lymphocyte-activation gene 3. Science 353, aah3374 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Birol M., Wojcik S. P., Miranker A. D., Rhoades E., Identification of N-linked glycans as specific mediators of neuronal uptake of acetylated α-synuclein. PLoS Biol. 17, e3000318 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sarrazin S., Lamanna W. C., Esko J. D., Heparan sulfate proteoglycans. Cold Spring Harb. Perspect. Biol. 3, a004952 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Carlsson P., Presto J., Spillmann D., Lindahl U., Kjellén L., Heparin/heparan sulfate biosynthesis: Processive formation of N-sulfated domains. J. Biol. Chem. 283, 20008–20014 (2008). [DOI] [PubMed] [Google Scholar]
- 23.Christianson H. C., Belting M., Heparan sulfate proteoglycan as a cell-surface endocytosis receptor. Matrix Biol. 35, 51–55 (2014). [DOI] [PubMed] [Google Scholar]
- 24.Condomitti G., de Wit J., Heparan sulfate proteoglycans as emerging players in synaptic specificity. Front. Mol. Neurosci. 11, 14 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rauch J. N., et al. , Tau internalization is regulated by 6-O sulfation on heparan sulfate proteoglycans (HSPGs). Sci. Rep. 8, 6382 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Liu C. C., et al. , Neuronal heparan sulfates promote amyloid pathology by modulating brain amyloid-β clearance and aggregation in Alzheimer’s disease. Sci. Transl. Med. 8, 332ra44 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Volpicelli-Daley L. A., Luk K. C., Lee V. M., Addition of exogenous α-synuclein preformed fibrils to primary neuronal cultures to seed recruitment of endogenous α-synuclein to Lewy body and Lewy neurite-like aggregates. Nat. Protoc. 9, 2135–2146 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Macia E., et al. , Dynasore, a cell-permeable inhibitor of dynamin. Dev. Cell 10, 839–850 (2006). [DOI] [PubMed] [Google Scholar]
- 29.Hill T. A., et al. , Inhibition of dynamin mediated endocytosis by the dynoles—Synthesis and functional activity of a family of indoles. J. Med. Chem. 52, 3762–3773 (2009). [DOI] [PubMed] [Google Scholar]
- 30.Rodriguez L., Marano M. M., Tandon A., Import and export of misfolded α-synuclein. Front. Neurosci. 12, 344 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kamiyama S., et al. , Molecular cloning and identification of 3′-phosphoadenosine 5′-phosphosulfate transporter. J. Biol. Chem. 278, 25958–25963 (2003). [DOI] [PubMed] [Google Scholar]
- 32.Stopschinski B. E., et al. , Specific glycosaminoglycan chain length and sulfation patterns are required for cell uptake of tau versus α-synuclein and β-amyloid aggregates. J. Biol. Chem. 293, 10826–10840 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cuellar K., Chuong H., Hubbell S. M., Hinsdale M. E., Biosynthesis of chondroitin and heparan sulfate in Chinese hamster ovary cells depends on xylosyltransferase II. J. Biol. Chem. 282, 5195–5200 (2007). [DOI] [PubMed] [Google Scholar]
- 34.McNaughton B. R., Cronican J. J., Thompson D. B., Liu D. R., Mammalian cell penetration, siRNA transfection, and DNA transfection by supercharged proteins. Proc. Natl. Acad. Sci. U.S.A. 106, 6111–6116 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Li B., et al. , Cryo-EM of full-length α-synuclein reveals fibril polymorphs with a common structural kernel. Nat. Commun. 9, 3609 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Guerrero-Ferreira R., et al. , Cryo-EM structure of alpha-synuclein fibrils. eLife 7, e36402 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nagase T., et al. , Prediction of the coding sequences of unidentified human genes, XIX: The complete sequences of 100 new cDNA clones from brain which code for large proteins in vitro. DNA Res. 7, 347–355 (2000). [DOI] [PubMed] [Google Scholar]
- 38.Weck M. L., Crawley S. W., Stone C. R., Tyska M. J., Myosin-7b promotes distal tip localization of the intermicrovillar adhesion complex. Curr. Biol. 26, 2717–2728 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Byfield F. J., Aranda-Espinoza H., Romanenko V. G., Rothblat G. H., Levitan I., Cholesterol depletion increases membrane stiffness of aortic endothelial cells. Biophys. J. 87, 3336–3343 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bousset L., et al. , Structural and functional characterization of two alpha-synuclein strains. Nat. Commun. 4, 2575 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Peng C., et al. , Cellular milieu imparts distinct pathological α-synuclein strains in α-synucleinopathies. Nature 557, 558–563 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Batchelder E. M., Yarar D., Differential requirements for clathrin-dependent endocytosis at sites of cell-substrate adhesion. Mol. Biol. Cell 21, 3070–3079 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Boulant S., Kural C., Zeeh J. C., Ubelmann F., Kirchhausen T., Actin dynamics counteract membrane tension during clathrin-mediated endocytosis. Nat. Cell Biol. 13, 1124–1131 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kumari A., et al. , Actomyosin-driven force patterning controls endocytosis at the immune synapse. Nat. Commun. 10, 2870 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Pedersen R. T. A., Drubin D. G., Type I myosins anchor actin assembly to the plasma membrane during clathrin-mediated endocytosis. J. Cell Biol. 218, 1138–1147 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Manenschijn H. E., et al. , Type-I myosins promote actin polymerization to drive membrane bending in endocytosis. eLife 8, e44215 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Cheng J., Grassart A., Drubin D. G., Myosin 1E coordinates actin assembly and cargo trafficking during clathrin-mediated endocytosis. Mol. Biol. Cell 23, 2891–2904 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Buss F., Arden S. D., Lindsay M., Luzio J. P., Kendrick-Jones J., Myosin VI isoform localized to clathrin-coated vesicles with a role in clathrin-mediated endocytosis. EMBO J. 20, 3676–3684 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Dance A. L., et al. , Regulation of myosin-VI targeting to endocytic compartments. Traffic 5, 798–813 (2004). [DOI] [PubMed] [Google Scholar]
- 50.Titus M. A., A class VII unconventional myosin is required for phagocytosis. Curr. Biol. 9, 1297–1303 (1999). [DOI] [PubMed] [Google Scholar]
- 51.Sousa S., et al. , Unconventional myosin VIIa and vezatin, two proteins crucial for Listeria entry into epithelial cells. J. Cell Sci. 117, 2121–2130 (2004). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data, associated protocols, methods, and sources of materials are available in the main text or SI Appendix.