Significance
Since the start of the HIV/AIDS epidemic, approximately 32 million people have died from the infection. Although antiretroviral therapy slows disease progression by preventing virus replication, it does not eliminate latently infected cells, which can resupply active virus, necessitating lifelong treatment. Latency-reversing agents (LRAs), such as protein kinase C (PKC) modulators, present a strategy to cure the disease by activating and enabling immunological clearance of latent viral reservoirs. A major challenge with the clinical use of LRAs is sustaining therapeutic levels of the active agent while minimizing side effects. To address this problem, we report prodrugs of PKC modulators for use in HIV eradication. Relative to the parent, select prodrugs show delayed activation and significantly better efficacy and tolerability.
Keywords: HIV/AIDS, protein kinase C, bryostatin, prostratin, ingenol
Abstract
AIDS is a pandemic disease caused by HIV that affects 37 million people worldwide. Current antiretroviral therapy slows disease progression but does not eliminate latently infected cells, which resupply active virus, thus necessitating lifelong treatment with associated compliance, cost, and chemoexposure issues. Latency-reversing agents (LRAs) activate these cells, allowing for their potential clearance, thus presenting a strategy to eradicate the infection. Protein kinase C (PKC) modulators—including prostratin, ingenol esters, bryostatin, and their analogs—are potent LRAs in various stages of development for several clinical indications. While LRAs are promising, a major challenge associated with their clinical use is sustaining therapeutically meaningful levels of the active agent while minimizing side effects. Here we describe a strategy to address this problem based on LRA prodrugs, designed for controllable release of the active LRA after a single injection. As intended, these prodrugs exhibit comparable or superior in vitro activity relative to the parent compounds. Selected compounds induced higher in vivo expression of CD69, an activation biomarker, and, by releasing free agent over time, significantly improved tolerability when compared to the parent LRAs. More generally, selected prodrugs of PKC modulators avoid the bolus toxicities of the parent drug and exhibit greater efficacy and expanded tolerability, thereby addressing a longstanding objective for many clinical applications.
HIV/AIDS is a global health problem, with more than 38 million people currently infected worldwide, including ∼1.7 million children (1). Since the start of this epidemic, roughly 75 million people have been infected by HIV and ∼32 million have died (1). The causative virus, HIV, replicates primarily within CD4+ T cells and depletes these cells over time, leading to acquired immunodeficiency syndrome (AIDS). Although the introduction of antiretroviral therapy (ART) has proved successful in preventing virus replication, halting CD4+ T cell depletion and progression of the infection to AIDS, the latent virus persists in reservoir cells (2–4). These latently infected cells survive despite years or even decades of ART and episodically resupply active virus (5). Therefore, to prevent the resurgence of active virus, patients require lifelong ART treatment, which has associated cost and compliance issues as well as health concerns associated with chronic chemotherapy (6–8). Furthermore, while the accessibility of ART continues to rise, nearly half of HIV-positive adults do not have access to medication (53% of adults living with HIV in 2016 had access to antiretroviral treatment), and even those with access often opt out of treatment due to side effects or compliance issues (1, 9–12). Shortages of antiretrovirals and erratic supply further emphasize the need for research directed at disease eradication (13–15).
A leading strategy for HIV/AIDS eradication is based on elimination of the latent virus through a so-called “kick and kill” approach. This strategy involves the use of latency-reversing agents (LRAs) to activate the HIV provirus in latent viral reservoir cells (16–18). These activated cells can then be cleared through viral cytopathic effects, immune-mediated clearance, and/or exogenous cytotoxins capable of targeting productively infected cells. The majority of the LRAs in clinical studies are histone deacetylase (HDAC) inhibitors, which up-regulate viral protein expression by keeping chromatin in its transcriptionally active state, but they have thus far not shown sufficient efficacy (19–21). Other notable classes of LRAs include cytokines, bromodomain inhibitors, toll-like receptor agonists, histone crotonylation agents, and protein kinase C (PKC) modulators (22–27).
PKC modulators, among the most promising agents for latency reversal, act by binding to and modulating the activity of PKC in competition with its endogenous ligand, diacylglycerol (28–30). Ligand binding to PKC enables cell membrane association of the complex and subsequent signal transduction via phosphorylation of downstream targets (31). Modulation of PKC by certain ligands has been demonstrated to activate the canonical NF-κB transcription factor pathway, leading to transcription of viral RNA and thus reactivation of latent viral reservoirs. NF-κB also has other concomitant effects on T cell gene and protein expression, including up-regulation of the early activation marker CD69 (a useful biomarker to track PKC modulator activity in vivo) (32, 33). Thus, combinatorial treatment of patients with HIV/AIDS with LRAs and ART could reduce or potentially eliminate the latent virus, allowing for longer times between ART treatments or ultimately virus eradication.
A number of PKC modulators have been surveyed ex vivo, and one of the most promising, bryostatin 1 (1), has been entered in a phase I clinical trial for HIV/AIDS eradication, although at a dose which proved insufficient to detect PKC activation or transcription effects (34–38). In addition to extensive studies of bryostatin itself, synthetic bryostatin analogs have recently been introduced as preclinical candidates for HIV eradication, with several demonstrating improved pharmacological utility compared to the natural product itself (39). Combinatorial treatments of PKC modulators and other LRAs have shown synergistic effects, providing an increase in HIV latency reversal while also permitting lower doses of the individual agents and thus better tolerability (37, 40–42).
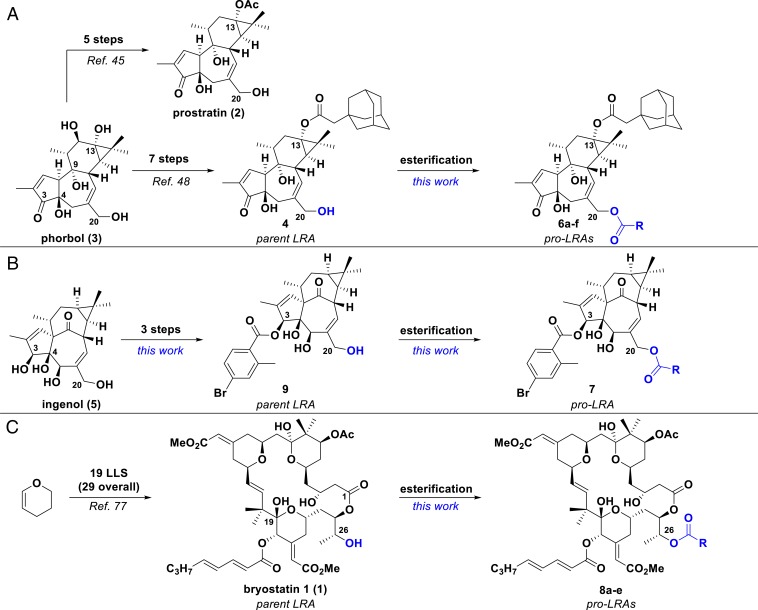
Although bryostatin 1 remains the most studied PKC modulator in the clinic, various tigliane and ingenane natural products, as well as their unnatural derivatives, have also been identified as potent PKC modulators and LRAs. The most notable LRA of the tigliane family is prostratin (2), which has been advanced preclinically (43). Prostratin elicits two distinct effects relevant to HIV treatment in addition to reversal of HIV from latency: protection of CD4+ T cells from HIV-1 entry by down-regulating HIV entry receptors (CD4, CCR5, and CXCR4) and enhancement of cell survival possibly due to cytostatic effects (42, 44). Previously, our group developed a practical five-step synthesis of prostratin from phorbol (3), a readily available and sustainable natural product isolated as a major constituent of croton oil (45). As is necessary for clinical and research studies, this synthesis has allowed for facile access to large quantities of prostratin and its analogs and a lead analog has recently been used in ex vivo reservoir studies of perinatally and acutely HIV-infected children and adults, respectively (46, 47). Significantly, many C13 analogs of prostratin accessible with this synthesis displayed higher potency (up to two orders of magnitude greater) than the natural product itself in binding affinity to PKC δ, in vitro CD69 activation, and viral induction in primary cells from HIV-positive donors (48). Adamantyl ester C13 analog 4 was among the most potent of the C13 prostratin derivatives disclosed. We have previously shown that 4 is able to activate latent HIV both in vitro and ex vivo, demonstrating the value of 4 as an LRA candidate. Ingenol esters—esterified derivatives of the natural product ingenol (5)—which are finding clinical value in treating actinic keratosis and basal cell carcinoma, have also been shown to activate latent HIV reservoirs at a level that equals or exceeds the current best candidates, including prostratin (49–54). The HIV latency reversal activity of extracts from Euphorbia kansui is also thought to arise from ingenol-derived constituents (55, 56).
While the LRA activities of PKC modulators including bryostatin 1, the adamantyl prostatin analog 4, and various reported ingenol esters are encouraging, concerns have been raised about dose-related side effects. For example, the bolus administration of a single dose of bryostatin in oncology trials has been associated with myalgia as a dose-limiting side effect (57). While reversible, this problem has been ameliorated by the slow administration of bryostatin 1 over extended times. Although this maintains the drug at the minimum effective concentration, it places a burden on those administering and receiving treatment often over a period of days. For example, in a clinical study for advanced malignancies, bryostatin 1 required a multiday infusion to achieve the desired concentration of drug in the blood while minimizing side effects (58). These prolonged administration times could also arise for certain clinical applications of tigliane or ingenane PKC modulators.
An alternative approach is a prodrug strategy, which utilizes chemical or enzymatic transformations of a LRA prodrug (pro-LRA) in vivo to allow for controllable release of the active compound over time (59–62). This strategy offers the advantage of a single short injection time while allowing for gradual payout of the active drug over time and thus maintenance of the concentration of the active compound within the desired therapeutic window (63). In addition, while not the focus of our initial proof-of-concept studies, such an approach could also be used to beneficially impact formulation, biodistribution, metabolism and clearance, and thus efficacy and tolerability. Despite the appeal of this strategy, it has not yet been applied to LRAs, although control of LRA dosing is of major interest in the field of AIDS eradication and likely to figure in other therapeutic indications involving PKC modulators, including their use in enhancing cell surface target antigen/neoantigen density and persistence in targeted immunotherapies using monoclonal antibodies, bispecific antibodies, or chimeric antigen receptor (CAR) T and natural killer (NK) cell therapies (64).
Here we report the syntheses of prodrugs of therapeutic leads of the tigliane (6a–f), ingenane (7), and bryostatin 1 scaffolds (8a–e) via reversible modification at a hydroxyl group essential for their biological activity (Fig. 1). Our approach is based on a function-oriented synthesis strategy and our seminal computer-based pharmacophore analysis which hypothesizes that ligand binding to PKC, and thus its subsequent cellular function, is attributable in part to the presence of a hydrogen bond-donating OH group at C20 in the tiglianes and ingenanes and at C26 in the bryostatins (65–68). It follows that derivatization of this OH group would reduce or prevent PKC binding and thus abrogate activity, while reversal of this derivatization in vivo would allow for release of the free PKC-binding agent over time. While there are examples of naturally occurring tiglianes and ingenanes with functionality other than an OH group at C20, there has not been a systematic evaluation of their use as prodrugs of LRAs, nor have synthetic bryostatin analogs with C26 modification been studied for this purpose (69–72). In this study, we have prepared examples of prodrug derivatives of three classes of lead PKC modulators that render the compounds inactive until the masking group is cleaved to yield the parent LRA compound. We show that these pro-LRAs are gradually converted to their active counterparts in vitro and in vivo and that selected pro-LRAs exhibit improved efficacy and a significantly expanded therapeutic window.
Fig. 1.
Synthetic strategies for prodrugs of PKC modulators. (A) Synthesis of prostratin (2), adamantyl prostratin analog (4), and corresponding prodrugs (6a–f) from phorbol (3). (B) Synthesis of 4-bromo-2-methyl-benzoate ingenol ester (9) and corresponding prodrug (7) from ingenol (5). (C) Synthesis of bryostatin 1 (1) and corresponding prodrugs (8a–e) from dihydropyran. LLS, longest linear sequence. R groups are defined in Figs. 2 and 3.
Prodrug Design, Synthesis, and Stability.
Drawing on structure–function studies from various sources, our computer-based pharmacophore analyses have indicated that ligand binding to PKC is minimally due to the spatial colocation of lipid domains and hydrogen-bonding triads involving the oxygenation at C3/4, C9, and C20 in the tiglianes and ingenanes and to a spatially similar oxygenation triad at C1, C19, and C26 in bryostatin (65, 66). It follows and is supported by prior studies and by results described herein that derivatization of the relevant tigliane/ingenane C20 OH or bryostatin C26 OH would produce “prodrug” compounds with diminished or no affinity to PKC while reversion to the corresponding free OH under biological or abiological conditions would restore PKC binding and thus function. We initiated our work by modifying the tigliane scaffold of a prostratin analog as it is readily available from our prior scalable synthesis (45). Accordingly, the C20 hydroxyl group of the adamantyl prostratin analog 4 was capped with a range of promoieties to study esterase susceptibility and release rates. Carbonate and carbamate derivatives were also prepared with the expectation that they would exhibit increased stability against esterases and therefore further control of release rates (73). As with prostratin, we have previously proposed that ingenol’s PKC affinity is also influenced by its C20 hydroxyl group and thus applied our prodrug strategy by capping this functionality (65, 74).
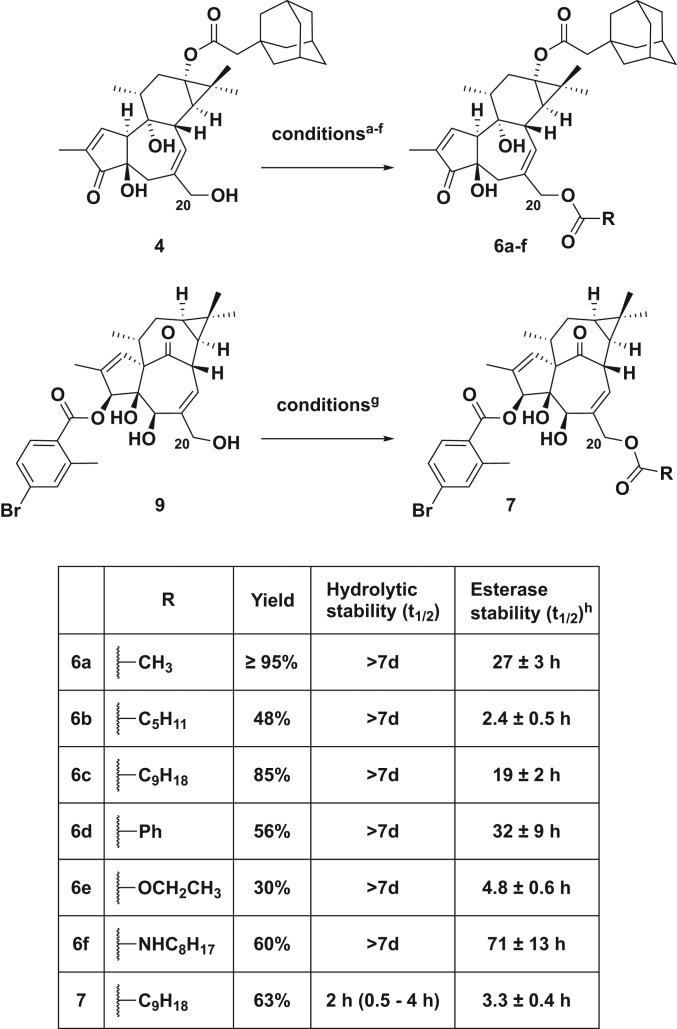
Both parent LRA compounds 4 and 9 can be prepared in only a few steps from the starting natural products, phorbol and ingenol, respectively. Adamantyl prostratin (4) was synthesized as previously reported (45, 48) and 4-bromo-2-methyl-benzoate ingenol ester (9) was synthesized in three steps from ingenol (SI Appendix). The C20 derivatives (pro-LRAs) were then prepared in a single step from the parent compounds (4 or 9) via modification with the corresponding acid chloride, anhydride, chloroformate, or isothiocyanate at room temperature (RT) in dichloromethane (Fig. 2).
Fig. 2.
Summary of tigliane and ingenane prodrugs synthesized and their hydrolytic and PLE stability. aConditions for synthesis of 6a, acetic anhydride, DMAP, NEt3, RT, overnight; bconditions for synthesis of 6b, hexanoyl chloride, NEt3, RT, 2 h; cconditions for synthesis of 6c, decanoic anhydride, DMAP, NEt3, RT, overnight; dconditions for synthesis of 6d, benzoyl chloride, NEt3, RT, overnight; econditions for synthesis of 6e, ethyl chloroformate, DMAP, NEt3, RT, 8 h; fconditions for synthesis of 6f, octyl isothiocyanate, NEt3, RT, 96 h; gconditions for synthesis of 7, decanoic anhydride, DMAP, NEt3, RT, overnight. hReported as average of three runs.
The hydrolytic stabilities of these pro-LRAs were evaluated in 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid (Hepes)-buffered saline (pH 7.4) at 37 °C, with 2,4,6-trimethylphenol as an internal standard for quantitative high-performance liquid chromatography (HPLC) analysis. The half-lives of all of the tigliane compounds extended beyond 7 days in the absence of esterase. Conversely, their half-lives in the presence of pig liver esterase (PLE), selected as a model esterase, varied greatly from hours to days, depending on the C20 substituent, as is desired for tunable control over the release rate. While the more sterically inaccessible C13-adamantyl ester on 4 had a PLE half-life of 18 h, the C20 esters showed varied labilities. Notably, the hexanoate derivative 6b cleaved rapidly (PLE t1/2 = 2.4 h), while the acetate 6a and decanoate 6c had intermediate PLE half-lives of 27 and 19 h, respectively. The benzoate derivative 6d was the most stable ester linkage with a PLE half-life over 30 h. As expected, the carbamate derivative 6f cleaved slowly in the presence of PLE; however, the carbonate 6e had a short half-life of ∼5 h. Others have also observed relatively rapid cleavage of carbonate groups in the presence of esterases, especially when the carbonate is sterically accessible (75). The stabilities of the ingenane derivatives were considerably different. As has been noted with other C3 derivatives of ingenol, the benzoate ester of 9 was somewhat labile even in the hydrolytic stability assay, with a half-life of 18 h (76). Cleavage was accelerated in the presence of PLE, regenerating ingenol (5) with a half-life of 2 h. Ingenane pro-LRA 7 exhibited rapid hydrolytic cleavage (H2O t1/2 < 4 h) to the parent C3-benzoate ester 9, confirming that a primary alkyl ester (C20 decanoate) is indeed more labile than a sterically hindered secondary aryl ester (C3 benzoate). Interestingly, the decanoate ester exhibited similar stability to the parent compound in the PLE assay (PLE t1/2 = 3 h), which is comparable to its hydrolytic stability, potentially due to the increased lipophilicity of the prodrug compound relative to 9. Due to limited supply further derivatization of ingenol analogs was not explored.
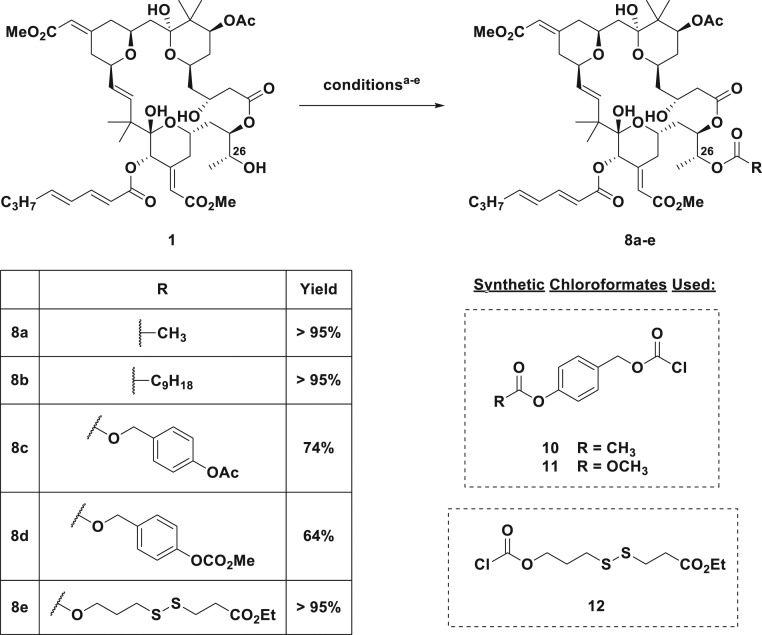
Having synthesized LRA prodrugs based on the tigliane and ingenane scaffolds, we next turned to modification of synthetic bryostatin 1, which is available in sufficient quantity for synthetic studies through our recently reported scalable total synthesis (77). Anticipating analogous activity between bryostatin esters at C26 and C20 esters of the tigliane/ingenane scaffolds, we synthesized bryostatin prodrugs 8a and 8b, bearing a C26 acetate and decanoate, respectively (Fig. 3). However, unlike the tigliane and ingenane prodrugs for which ester attachment is to a primary OH group, the esterase-labile carbonyl of 8a and 8b is attached to a more sterically encumbered secondary OH group. Thus, we also designed prodrugs bearing more accessible esterase-labile groups at the terminus of the self-immolative linkers. Toward this end, benzyl chloroformates 10 and 11, bearing a p-OAc and p-OCOCH3, respectively, were reacted with bryostatin 1 to provide p-acetate and p-carbonate prodrugs 8c and 8d, respectively, which we anticipated would undergo cleavage at the more accessible phenolic ester and subsequent 1,6-elimination to provide the parent LRA compounds (78). Finally, as a proof of concept, we synthesized redox-labile disulfide bryostatin prodrug 8e using chloroformate 12 as the coupling partner. Whereas the ester pro-LRAs (8a–8d) were designed to be cleaved by both intra- and extracellular esterases, the disulfide pro-LRA was designed to be stable until cell entry and cleavage in the reducing environment of the cytosol (79). Due to the high value of these bryostatin compounds, they were not evaluated in the PLE assay and instead were carried forward to further in vitro analyses.
Fig. 3.
Summary of bryostatin prodrugs synthesized. aConditions for synthesis of 8a, acetic anhydride, DMAP, pyridine, RT, 2 h; bconditions for synthesis of 8b, decanoic anhydride, pyridine, DMAP, RT, 2 h; cconditions for synthesis of 8c, 10, DMAP, RT, overnight; dconditions for synthesis of 8d, 11, DMAP, RT, overnight; econditions for synthesis of 8e, 12, DMAP, RT, overnight.
PKC Binding Affinity.
Because activation of latent HIV is dependent on an initial PKC binding event (80), we determined the PKC binding affinities of both the prodrugs and the parent compounds using a radiolabeled ligand displacement assay (Table 1). As the C20 (for tiglianes and ingenanes) or C26 (for bryostatins) hydroxyl group is capped in the prodrugs, minimal PKC binding was expected based on our computer modeling which indicates that this OH is required for hydrogen bonding to PKC. The pro-LRAs were evaluated against the full-length novel isoform PKC δ to explore this point. Additionally, some were also evaluated against the conventional isoform βI, and the parent compounds were evaluated against the full panel of seven conventional and novel human PKC isoforms (SI Appendix, Table S1). As expected from our pharmacophore analysis and prodrug design, the pro-LRAs displayed orders of magnitude weaker affinities (308 nM to > 5 μM) than the respective parent compounds 1, 4, and 9 (0.5 to 2.2 nM) against all tested PKC isoforms.
Table 1.
Binding affinities to PKC δ and PKC βI for tigliane, ingenane, and bryostatin analogs and prodrugs
| PKC binding affinity, Ki | |
| 4 (parent) | 0.5 nM, 1.4 nM* |
| 6a | >2.5 µM† |
| 6b | >2.5 µM, >2.5 µM* |
| 6c | >2.5 µM† |
| 6d | >2.5 µM, >0.5 µM* |
| 6e | 380 nM† |
| 6f | >2.5 µM† |
| 9 (parent) | 1.1 nM, 0.85 nM* |
| 7 | >2.5 µM, >2.5 µM* |
| 1 (parent) | 1.1 nM, 2.2 nM* |
| 8a | >1 µM‡ |
| 8b | >5 µM‡ |
| 8c | 308 nM‡ |
| 8d | >5 µM‡ |
| 8e | >5 µM‡ |
Ki values are from a heterogeneous competitive binding assay against [3H]-phorbol dibutyrate.
Binding affinity to PKC δ and βI, respectively.
Binding affinity to PKC δ.
Binding affinity to PKC βI.
In Vitro HIV Latency Reversal.
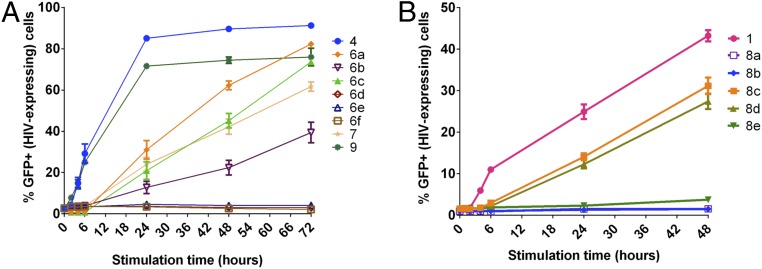
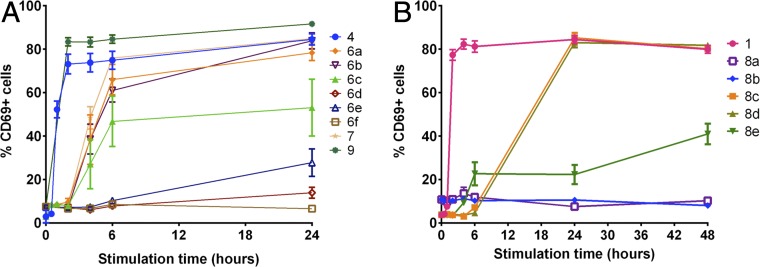
We next evaluated the ability of the pro-LRAs to induce latent HIV expression in vitro. We first tested their efficacy in J-Lat cells, which are genetically modified to express an HIV-GFP fusion protein under control of the viral promoter upon LRA activation (81), allowing correlation between HIV activation levels and GFP fluorescence readout. We hypothesized that gradual conversion of the LRA prodrugs would result in a delayed biological activity readout and slower HIV latency reversal relative to the parent LRA. In the assay, J-Lat cells were treated with the parent LRAs or molar equivalents of the corresponding pro-LRAs. As designed, we found that the aliphatic ester prodrugs of the tigliane and ingenane scaffolds exhibited delayed activation compared to the parent compounds (prostratin scaffold, 6a–6c relative to parent 4; ingenane scaffold, 7 relative to parent 9; Fig. 4A). While the parent compounds displayed robust activation at the 4-h time point and by 24 h over 70% of the cells were expressing GFP/HIV, the corresponding prodrugs did not significantly induce expression of HIV until the 24-h timepoint and then showed a steady increase in percentage of GFP+ cells over the subsequent 48 h. As expected, compounds 6d–f, possessing less labile promoieties, showed no activity in this assay.
Fig. 4.
HIV latency reversal in J-Lat cell line by prodrug PKC modulators. J-Lat 10.6 cells were stimulated with 100 nM (for 4 and 9, and prodrugs thereof, A) or 1,000 nM (for 1 and prodrugs thereof, B) concentration of parent compounds or prodrug versions of the compounds. Cells were assessed for GFP expression (latent HIV expression) by flow cytometry after 0, 2, 4, 6, 24, 48, and 72 h. Mean results ± 1 SE from a total of three to nine replicates (derived from three independent experiments) are shown. P < 0.05 for compounds 4 and 9 (t ≥ 2 h) and 6a, 6b, 6c, and 7 (t ≥ 24 h) vs. unstimulated (two-sided equal variance t test).
In contrast to the tigliane and ingenane prodrugs, but consistent with anticipated steric congestion at the site of ester attachment, bryostatin prodrugs possessing simple aliphatic esters at C26 (8a,b) displayed no activity in this assay, while bryostatin 1 elicited a robust response within hours (Fig. 4B). Significantly, prodrugs 8c and 8d, in which the hydrolyzable moiety was positioned farther out from the sterically congested macrolactone, demonstrated prodrug behavior. Compared to the relatively rapid HIV expression induced by bryostatin 1, pro-LRAs 8c and 8d induced delayed HIV reactivation, with marked signal present only after 24 h of stimulation. Disulfide prodrug 8e showed a more diminished response, eliciting minimal detectable signal after 48 h.
Primary Immune Cell Activation.
Since these prodrugs performed well in the J-Lat cell assays, we next evaluated their ability to activate primary peripheral blood mononuclear cells (PBMCs) from HIV-uninfected individuals. Upon activation of the NF-κB pathway, such as by PKC modulators, CD4+ cells rapidly display cell surface expression of the early T cell activation biomarker CD69 (82). The promoter for CD69 shares many transcription factor binding sites with the HIV long terminal repeat promoter, and the compound concentrations required to induce up-regulation of CD69 are highly correlated with those needed to induce HIV from latency (39). Thus, observation of CD69 up-regulation is correlated with HIV latency reversal.
Although the response times of CD69 expression in this primary cell assay were far more rapid than those of GFP expression in the J-Lat assay, analogous trends of activity were observed. For the tigliane and ingenane compounds, aliphatic ester prodrugs showed a slower onset of CD69 up-regulation (significant cell surface expression after 4 h) compared to the parent compounds (expression evident after only 1 h), whereas pro-LRAs with more stable C20-protecting groups showed limited activation even after 72 h. (Fig. 5A and SI Appendix, Fig. S1). Bryostatin prodrugs also displayed the anticipated prodrug activity in this assay (Fig. 5B). Consistent with the J-Lat assay, aliphatic bryostatin prodrugs 8a and 8b did not induce a cellular response, while the p-acetate and p-carbonate derivatives 8c and 8d elicited a delayed, robust signal only after 24 h. Surprisingly, disulfide prodrug 8e, which was a poor performer in the J-Lat assay, induced up to 40% CD69 activation. Taken together, these two in vitro studies demonstrate that designed prodrugs 6a–c, 7, and 8c–e exhibit the behavior anticipated of LRA prodrugs, that different LRA prodrugs exhibit different release rates, and that this strategy is compatible with the three most clinically relevant classes of LRAs that proceed through a PKC-activation pathway.
Fig. 5.
Up-regulation of CD69 (biomarker for activated cells) in primary human PBMCs indicates delayed activity of pro-LRAs. A shows tigliane and ingenane-related compounds, and B shows bryostatin 1-related compounds. PBMCs were stimulated with 100 nM (with the exception of 8e, dosed at 1,000 nM) concentration of parent compounds or prodrug versions of the compounds. Cell surface expression of the early T cell activation marker CD69 was evaluated by flow cytometry at the indicated times postcompound addition. Mean results ± 1 SE from a total of three to nine replicates per data point (derived from three independent experiments using different human donor cells) are shown.
Cytokine Induction Profiles in Uninfected PBMCs.
To further define the downstream effects of prodrug exposure to human immune cells, we also conducted experiments examining how prodrugs influence a broader range of cell surface activation marker expression levels and cytokine/chemokine production. PBMCs from four non–HIV-infected donors were individually exposed to media only; anti-CD3+anti-CD28 costimulation (positive control); or compounds 1, 4, 6a, 8c, or 8d for 48 h. Cells were analyzed for the cell surface activation markers CD69, CD25, and human leukocyte antigen DR-isotype (HLA-DR) by flow cytometry, and levels of 38 different human cytokines or chemokines in the supernatants were quantified by Luminex assay. CD69 levels were highly elevated in cultures exposed to either parent compound (1 and 4) or active prodrugs (6a, 8c, and 8d), whereas CD25 and HLA-DR levels were more modestly affected or unaffected by these compounds (SI Appendix, Fig. S2). All cytokines except monocyte chemotactic protein-3 (MCP-3) and macrophage-derived chemokine (MDC) were up-regulated in the costimulation conditions (SI Appendix, Tables S2 and S3). Cytokines produced in response to PKC modulators or prodrugs partitioned into those showing limited or no evidence of induction (SI Appendix, Table S2) and those displaying greater elevation over negative controls (SI Appendix, Table S3). Prodrugs 8c and 8d induced similar cytokine levels to the parent compound 1 (bryostatin 1), whereas compound 6a induced lower levels of most cytokines than parent compound 4, perhaps due to the delay in prodrug-induced stimulation leading to insufficient time for cytokines to accumulate in the culture supernatants. Cytokines of relevancy to HIV that were substantially up-regulated by PKC modulators included the beta-chemokines macrophage inflammatory protein (MIP)-1-α and MIP-1-β, together with tumor necrosis factor α (TNF-α) and interleukin (IL)-7. MIP-1-α and MIP-1-β are each able to inhibit spread of R5-tropic strains of HIV that use CCR5 in conjunction with CD4 to enter cells (83), and induction of these cytokines may therefore contribute to the inhibition of HIV spread that occurs in mixed PBMC culture exposed to PKC modulators (42). Conversely, TNF-α and IL-7 induction may contribute to the HIV latency reversal properties of the PKC modulators as these cytokines can induce expression of latent HIV (84, 85). Together, these data indicate that the parent compounds and prodrugs do induce some cytokine up-regulation as expected from prior studies of PKC modulators (42, 86) but to a much lower extent and with less breadth than maximal T cell activation mediated through anti-CD3+anti-CD28 costimulation.
Latency Reversal in CD4+ T Cells from HIV-Infected Patients.
To verify that the pro-LRAs could also activate latent HIV in primary patient-derived latently infected cells, we tested several compounds for their ability to reverse HIV from latency ex vivo using CD4+ T cells isolated from five aviremic ART-suppressed HIV-infected individuals (Fig. 6). Cells were incubated in antiretroviral drugs to prevent virus spread and then were left unstimulated, costimulated with anti-CD3 + anti-CD28 antibodies, treated with 1 μM bryostatin 1 (1), or exposed to one of the bryostatin prodrugs 8c or 8d. After 2 days the culture supernatant was harvested, and HIV RNA copies were quantified. One of five donors produced a positive signal in the media-only condition, versus four of five for bryostatin 1, three of five for prodrug 8c, and five of five for prodrug 8d. The results for compound 8d were not statistically significant from those of anti-CD3 + anti-CD28, indicating that this compound performed similarly to the costimulation-positive control in inducing high-level virus expression.
Fig. 6.
Latency reversal in CD4+ T cells obtained from HIV-infected ART-treated patients. CD4+ T cells from aviremic ART-treated patients were isolated and then stimulated with 1 μM of compound for 48 h in the presence of antiretroviral drugs. Virion-associated HIV RNA in supernatant was quantified. Cells from each patient are represented with a different symbol. Compound 1, compound 8d, and anti-CD3+anti-CD28 conditions were all significantly increased compared with media control (P < 0.05, two-tailed, unpaired Mann–Whitney test). Compound 8d was not significantly different from the anti-CD3+anti-CD28 condition, indicating similar HIV latency reversal efficacy to T cell costimulation.
In Vivo Studies in C57/BL6 Mice.
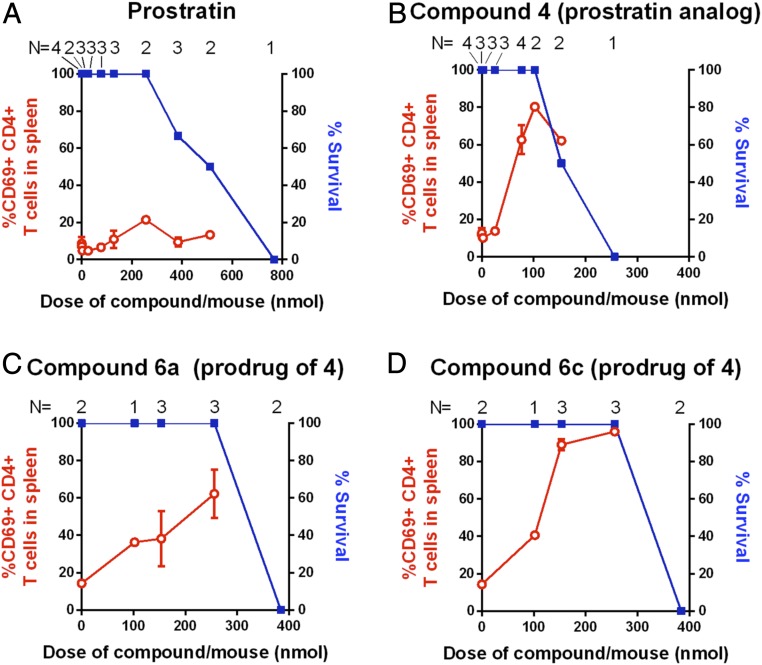
The delayed CD69 expression in PBMCs as well as the low binding affinity to PKC of tigliane pro-LRAs 6a and 6c compared to the parent compound led us to advance these two derivatives forward into animal studies. Previously, prostratin itself was found to exhibit low potency in cellular assays (maximum activation of CD69 expression at 1 µM) and the adamantyl prostratin analog 4 was able to significantly increase potency (48). Here we sought not only to evaluate the potency of these compounds in a live animal, but also to gauge whether our prodrug strategy would lead to diminished toxicity levels and an increased therapeutic window relative to the parent compound. In performing these experiments, C57/BL6 mice were dosed intraperitoneally (i.p.) with varying amounts of adamantyl prostratin analog 4 or molar equivalents of prodrug 6a or 6c to determine their maximum tolerated doses as well as CD69 expression. We evaluated acute toxicities over 24 h to identify the minimal doses that either were lethal or necessitated animal euthanasia due to signs of ill health (such as low body temperatures, lethargy, hunched posture, ruffled fur, etc.). Mice were killed 24 h after treatment, and CD69 levels were quantified in spleen CD4+ T cells. Prostratin induced CD69 expression in fewer than 25% of the CD4+ T cells at all tested doses and was toxic at doses between 150 and 300 µg per mouse, corresponding to 384 to 768 nmol per mouse (Fig. 7A). Parent LRA 4 displayed the expected increased potency (maximal expression of ∼80% CD69+ at 102 nmol) and tolerability at maximum CD69 induction, although doses above that level were not well tolerated (Fig. 7B). Significantly, all mice treated with either pro-LRA 6a or 6c survived at a 256-nmol per mouse dose and showed strong stimulation of CD69 in splenocytes (Fig. 7 C and D). Decanoate pro-LRA 6c even produced a higher percentage of stimulated CD4+ T cells than parent compound 4, with almost 100% of these cells expressing CD69 24 h after the 256-nmol per mouse injection. The pro-LRAs did not affect survival until beyond the 256-nmol dose, displaying significantly better tolerability than the parent LRA compound coupled with equivalent or improved efficacy.
Fig. 7.
In vivo bioactivity and acute toxicity of tigliane PKC modulators. C57/BL6 mice were injected i.p. with the indicated compound, and the frequency of CD69+CD4+ T cells in the spleen of the animals was evaluated at 24 h (red lines). The health of the mice was monitored and the percentage of animals that survived the treatment without requiring premature euthanasia is indicated (blue lines). Numbers of individual animals tested (N) are provided. (A) Prostratin 2 (natural product). (B) Parent adamantyl prostratin analog 4. (C) Pro-LRA 6a (C20-acetate prodrug of 4). (D) Pro-LRA 6c (C20-decanoate prodrug of 4). Mean results ± 1 SE are shown.
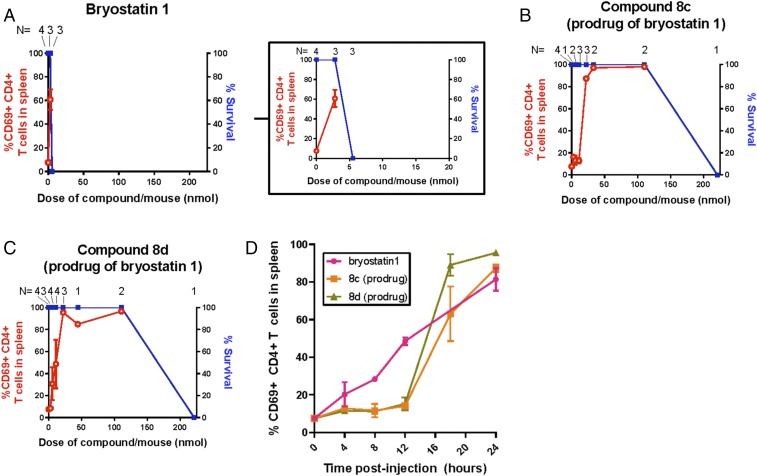
Given their excellent activity in all cell assays, we next investigated bryostatin prodrugs 8c and 8d in live mice in comparison to their parent compound bryostatin 1. Treatment of mice with bryostatin 1 led to animal death or required euthanasia at a 5.5-nmol dose, just one doubling beyond its maximum effective dose (60% CD69 activation) (Fig. 8A). In sharp contrast to this bolus toxicity, bryostatin prodrugs 8c and 8d showed no toxicity until the 221-nmol dose, at which point mouse death was observed. Notably, in addition to improved tolerability, these prodrugs elicited robust activation of CD69 in splenocytes at 22.1 nmol (8c) and 5.5 to 11.1 nmol (8d), significantly better than the parent compound while providing a 10- to 40-fold increase in the therapeutic window for these compounds relative to the parent (Fig. 8 B and C). Remarkably, nearly 100% CD69 activation was observed at the 22.1-nmol doses for bryostatin prodrugs 8c and 8d, significantly outperforming prostratin prodrugs 6a and 6c at this same dose as well as bryostatin 1 itself. We also compared the in vivo CD4+ T cell activation kinetics following administration of bryostatin 1 or prodrugs 8c and 8d (Fig. 8D). As expected, activation of CD69 expression in the spleens of the animals was faster after injection of bryostatin 1 than with pro-LRAs 8c or 8d, with expression of CD69 in CD4+ T cells significantly activated at 8 h postinjection with bryostatin 1, but only after 18 h postinjection with pro-LRAs. Notably, by the 24-h timepoint the bryostatin 1-treated mice required euthanasia due to signs of ill health including lethargy, whereas the mice receiving the pro-LRAs showed no such signs. To determine whether any extended toxicity would become evident over longer time periods after prodrug administration, we also i.p. injected groups of three mice with 22.1 nmol of either pro-LRA 8c or 8d and then observed them for 4 wk before euthanasia and evaluation of CD69 expression in spleen. These mice did not show signs of ill health throughout the observation period and upon euthanasia (4 wk after compound administration) they did not have elevated levels of CD69 expression in splenocytes, indicating that the observed activation at 24 h was transient (SI Appendix, Fig. S3).
Fig. 8.
In vivo bioactivity and acute toxicity of bryostatin PKC modulators. (A–C) Mice were injected i.p. with bryostatin 1 or pro-LRAs 8c or 8d and 24 h later analyzed for CD69 activation as described in Fig. 7. Prodrugs of bryostatin retained bioactivity but were better tolerated than bryostatin 1. Inset in A shows bryostatin 1 data with shortened x-axis scale for clarity. (D) Mice were injected with 5.5 nmol of bryostatin 1 or 22.1 nmol of compound 8c or 8d and euthanized at the indicated postinjection timepoint. The percentage of CD69-expressing CD4+ T cells in the spleen was quantified by flow cytometry. Bryostatin 1-treated animals were significantly different from mock at times ≥8 h postinjection, whereas 8c- and 8d-treated animals were not significantly different from mock until ≥18 h postinjection. N = 3 to 4 mice per condition and timepoint. Mean results ±1 SE are shown.
Discussion
LRAs are promising preclinical leads for the activation and elimination of HIV proviral reservoirs and thus for eradication of HIV/AIDS. The most promising LRA leads that operate through PKC are bryostatin 1, prostratin, ingenol esters, and increasingly some of their analogs. While highly potent, these agents would require careful administration and dosing to optimize efficacy and tolerability (58, 87). By releasing LRAs over extended times controlled by design, LRA prodrugs would allow for sustained exposure after a single injection, thereby avoiding treatment problems associated with prolonged LRA administration times or peak/trough effects associated with bolus administration of the free LRA. This is likely to be of general benefit to existing and projected clinical applications of PKC modulators (88–91). As bryostatin 1 has already been entered into a phase I clinical trial for latency reversal (38), it was a natural candidate to explore a pro-LRA strategy. We also applied this pro-LRA strategy to the tigliane and ingenane scaffolds, as prostratin and ingenol esters have been extensively investigated as LRAs, with ingenol derivatives currently in clinical investigation.
To design an LRA prodrug we drew on our earlier computer studies, identifying pharmacophoric features of PKC modulators required for their binding and function. This seminal analysis identified the C20 OH group of the tigliane/ingenane scaffolds or the bryostatin C26 OH group as being a key requirement for PKC binding. Thus, we hypothesized that its reversible capping would serve to create a prodrug whose uncapping under biological or abiological conditions would liberate a free PKC modulator at a rate controlled by design.
We synthesized a series of pro-LRAs stemming from three different parent LRA compounds: adamantyl prostratin (4), an ingenol C3-benzoate (9), and bryostatin 1 (1). From readily available 4, we made several aliphatic and aromatic esters as well as carbonate and carbamate derivatives to serve as controls. We also synthesized a C20-decanoate ingenol ester from active analog 9 and several C26 prodrugs (aliphatic esters, self-immolative carbonates, disulfide promoieties) of clinical candidate bryostatin 1. Because prostratin analogs were readily available from our reported semisynthesis, we were able to assess the stability (enzymatic and hydrolytic) of these compounds in greater detail (45). All of the prostratin derivatives studied were hydrolytically stable, with no apparent degradation after 7 d in buffered saline at physiological temperature. While the lesser hydrolytic stability of parent ingenol ester 9 was initially a point of concern, it exhibited comparable activity to parent adamantyl prostratin 4 in the subsequent cellular assays.
To determine whether these compounds were indeed performing as prodrugs, rather than intrinsically active, they were assayed for affinity to their parental cellular targets, PKC isoforms. As expected, while the parent leads of the bryostatin, prostratin, and ingenol scaffolds exhibited high PKC affinities, all prodrug derivatives were at best weak binders to representative PKC isoforms, with Ki values greater than the parent systems by two to four orders of magnitude. These studies further support the view that the free alcohol at C20 in the tigliane and ingenane scaffolds, and C26 in the bryostatin scaffold, is essential for binding to PKC and that any bioactivity in subsequent cellular assays can be attributed to release of parent compound rather than directly induced by the prodrug.
In both the J-Lat and PBMC assays, rapid activation was observed following treatment with the parent LRA compounds, while the activation was delayed, as expected, upon treatment with the lead pro-LRAs. This trend is consistent with slow release of the active compound via esterase cleavage. Interestingly, while aliphatic ester prodrugs of the tigliane and ingenane scaffolds showed the desired delayed activation in vitro, aliphatic ester derivatives of bryostatin (8a,b) proved inactive in cellular assays, presumably due to increased steric hindrance around the site of esterification which prevents access to the esterase active site. However, bryostatin prodrugs bearing self-immolative linkers, in which the esterase- or redox-labile moiety was positioned at least four atoms removed from the C26-ester linkage, demonstrated the expected prodrug-like behavior. While benzylic acetate and methyl carbonate derivatives 8c and 8d showed slow payout over time in both J-Lat and PBMC assays, with high levels of signal reached in each case, disulfide 8e was a modest performer, providing minimal signal in the J-Lat experiment and attenuated CD69 up-regulation in PBMCs relative to 8c and 8d. While 8c and 8d might be cleaved by both intra- and extracellular esterases, disulfide 8e must come in contact with the reducing environment of its target cell’s cytosol to be converted to bryostatin 1. Because it is plausible that bryostatin prodrug 8e spends the majority of its postcellular uptake time in the cell membrane, it might not readily come in contact with the reducing agents present in the cellular milieu (e.g., glutathione). It is also possible that disulfide exchange with glutathione leads to a glutathione drug conjugate and not free agent because of the expectedly similar reactivity of the disulfide sulfurs. Most importantly though, we found that payout and activation in vitro can be delayed after a single administration with pro-LRAs 8c and 8d, thus allowing these LRA prodrugs to function like slow and continuously administered agents. Our finding that compound 8d can reverse HIV latency with similar efficacy to maximal T cell activation in patient-derived CD4+ T cells (Fig. 6) while inducing substantially lower levels of most cytokines tested (SI Appendix, Tables S2 and S3) indicated that this compound was particularly worthy of advancing to in vivo testing.
The in vivo activation and tolerability profiles of the top pro-LRAs (6a and 6c for the tigliane scaffold, 8c and 8d for the bryostatin scaffold) in C57/BL6 mice were again consistent with slow release of the active LRA over time. For the prostratin scaffold, decanoate derivative 6c outperformed acetate derivative 6a in overall activity, eliciting nearly 100% CD69 expression in isolated splenocytes. However, both compounds demonstrated improved tolerability relative to the parent compound, with no mouse death observed until the 256-nmol dose. These trends were further reinforced for the bryostatin scaffold. Pro-LRAs 8c and 8d achieved nearly 100% CD69 expression at 22.1-nmol doses, with no animal lethality until the 221-nmol dose. This is notable, as bryostatin 1, the parent compound for 8c and 8d, had shown much lower tolerability than the parent prostratin analog 4. It is plausible that the release of bryostatin is much slower than release of the corresponding prostratin analogs, thus improving the toxicity profile of the bryostatin prodrugs relative to those of the tigliane scaffold. This is either due to the specific reactivity of the bryostatin C26-OH versus the tigliane C20-OH or due to slower release kinetics of the self-immolative linker compared to a simple aliphatic ester hydrolysis. The kinetics of drug release and activity in live animals of the better-performing LRAs are currently being pursued.
Conclusion
The results from all cellular assays and an animal model strongly support the further exploration and possible use of an LRA prodrug strategy in efforts to reverse latency for the eradication of HIV/AIDS. Significantly, not only are these derivatives better tolerated, but also they are more effective than the parent scaffolds. This is especially significant in the case of bryostatin prodrugs, as slow infusion has been adopted as the most effective strategy to minimize dose-limiting toxicity, placing a burden on both the patient and the practitioner. A prodrug, which in itself would provide a slow payout after a single injection, would avoid this problem and could also be used to enhance formulation, distribution, and metabolism and thus efficacy and tolerability. We are currently investigating the application of this highly effective prodrug strategy to some of our most potent bryostatin analogs, which have already been shown to be more effective and better tolerated than the natural product in all comparative assays. Importantly, while our focus herein has been on latency-reversing agents, this prodrug strategy and the agents studied extend to other clinical applications of PKC modulators, including their use in treating neurological disorders (e.g., Alzheimer’s disease, Niemann–Pick disease, fragile X syndrome), as agents to enhance cell surface antigen/neoantigen density in monoclonal and CAR T and NK cell therapies for cancer and as leads for the treatment of multiple sclerosis, among other indications. This prodrug approach significantly enhances the activity and expands the therapeutic window of PKC modulators, addressing a longstanding concern for many clinical applications and providing potentially immediate clinical benefits.
Methods
Synthesis.
Full synthetic procedures and characterization data are described in SI Appendix. Due to the value of the parent compounds and their potencies, syntheses were conducted on scales sufficient to supply assay needs.
HIV Latency Reversal in J-Lat Cells.
J-Lat cells were incubated in 200 μL RF10 media and the indicated concentration of the parent compounds or molar equivalents of the corresponding prodrugs. Cells were seeded at a starting cell density of 20,000 cells per well in u-bottomed 96-well tissue culture plates. Cells were incubated for 2, 4, 6, 24, 48, and 72 h posttreatment and then harvested by washing with 2% fetal bovine serum (FBS) in phosphate buffered saline (PBS) and resuspending pellets in 2% paraformaldehyde. The percentage of GFP+ cells was quantified by flow cytometry as described in SI Appendix.
Noninfected Primary Cell Activation Procedures.
PBMCs were cultured in u-bottomed 96-well tissue culture plates with a starting cell density of 20,000 to 100,000 cells per well in 200 μL of RF10 media containing the indicated equimolar concentration of parent compound or corresponding prodrug. For the costimulation positive control 2 µL per well of anti-CD3/anti-CD28 Dynabeads (Gibco 11132D) was added. Cells were exposed to compound for either 0.5, 1, 2, 4, 6, 24, or 48 h before staining and flow cytometric analysis.
Ex Vivo Assay Using Cells from HIV-Infected Patients.
CD4+ T cells were isolated from HIV-infected individuals who were receiving antiretroviral therapy. The level of inducible HIV was determined by incubating 6 to 8 × 106 CD4+ T cells with indicated compounds for 48 h followed by quantitation of cell-free HIV RNA using the Cobas Ampliprep/Cobas Taqman HIV-1 Test, version 2.0 (Roche Diagnostics). Leukapheresed products were collected from HIV-infected individuals in accordance with the clinical protocol approved by the Institutional Review Board of the National Institute of Allergy and Infectious Diseases, National Institutes of Health. All study subjects provided informed consent. All subjects’ cells were deidentified prior to use in this study.
In Vivo C57/BL6 Mouse CD69 Stimulation Assay.
Animal experiments conformed to all local and national guidelines and regulations, and procedures were approved by the University of California, Los Angeles (UCLA) animal research committee. C57/BL6 mice were obtained from the UCLA Department of Radiation Oncology or from Charles River Laboratories. Compounds were suspended in dimethyl sulfoxide (DMSO) and then further diluted in PBS to produce a final volume of 500 μL (containing a maximum 4% DMSO), which were administered by i.p. injection into mice. Mice were monitored for acute toxicities and then euthanized at the indicated times. After necropsy, spleens were removed and disaggregated by forcing tissue through a 40-μm filter and then filtered through a second 40-μm filter to produce a single-cell suspension. Splenocytes were then stained with fluorescent antibodies and analyzed by flow cytometry to quantify cell surface expression of the early T cell activation marker CD69 as described below.
Data Availability.
Data are available in the main text and SI Appendix.
Supplementary Material
Acknowledgments
This work was supported by grants from the National Institutes of Health (NIH) (AI124743 to J.A.Z. and P.A.W.; AI124763 and CA031845 to P.A.W.), the National Center for Advancing Translational Sciences UCLA Clinical and Translational Science Institute (CTSI) Grants UL1TR001881 and UL1TR000124, and the UCLA Center for AIDS Research Grant AI28697. M.S.A.S. is a postdoctoral fellow/trainee supported by a UCLA Tumor Immunology training grant (US Department of Health and Human Services (USHHS) Ruth L. Kirschstein Institutional National Research Service Award T32-CA009120). This research was supported in part by the Intramural Research Program of the National Institute of Allergy and Infectious Diseases, NIH. Cytokine analysis was performed by the Immune Assessment Core at UCLA. We acknowledge the use of instruments from the Prof. C. Bertozzi laboratory and the Prof. M. Kanan laboratory.
Footnotes
Competing interest statement: Stanford University has filed patent applications on this and related technology, which has been licensed by Neurotrope BioScience for the treatment of neurological disorders and by Bryologyx Inc. for use in HIV/AIDS eradication and in enhanced cancer immunotherapy. P.A.W. is an adviser to both companies and a cofounder of the latter. J.A.Z. is on the scientific advisory board for BryoLogyx and a founder of CDR3 Therapeutics.
This article contains supporting information online at https://www.pnas.org/lookup/suppl/doi:10.1073/pnas.1919408117/-/DCSupplemental.
References
- 1.UNAIDS , Fact sheet - World AIDS Day 2019. Global HIV Statistics (2018). https://www.unaids.org/sites/default/files/media_asset/UNAIDS_FactSheet_en.pdf. Accessed 30 April 2020.
- 2.Chun T.-W., et al. , Presence of an inducible HIV-1 latent reservoir during highly active antiretroviral therapy. Proc. Natl. Acad. Sci. U.S.A. 94, 13193–13197 (1997). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wong J. K., et al. , Recovery of replication-competent HIV despite prolonged suppression of plasma viremia. Science 278, 1291–1295 (1997). [DOI] [PubMed] [Google Scholar]
- 4.Finzi D., et al. , Identification of a reservoir for HIV-1 in patients on highly active antiretroviral therapy. Science 278, 1295–1300 (1997). [DOI] [PubMed] [Google Scholar]
- 5.Coiras M., López-Huertas M. R., Pérez-Olmeda M., Alcamí J., Understanding HIV-1 latency provides clues for the eradication of long-term reservoirs. Nat. Rev. Microbiol. 7, 798–812 (2009). [DOI] [PubMed] [Google Scholar]
- 6.Sued O., Figueroa M. I., Cahn P., Clinical challenges in HIV/AIDS: Hints for advancing prevention and patient management strategies. Adv. Drug Deliv. Rev. 103, 5–19 (2016). [DOI] [PubMed] [Google Scholar]
- 7.Ryom L., et al. ; D:A:D Study Group , Use of antiretroviral therapy and risk of end-stage liver disease and hepatocellular carcinoma in HIV-positive persons. AIDS 30, 1731–1743 (2016). [DOI] [PubMed] [Google Scholar]
- 8.Antiretroviral Therapy Cohort Collaboration , Life expectancy of individuals on combination antiretroviral therapy in high-income countries: A collaborative analysis of 14 cohort studies. Lancet 372, 293–299 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ammassari A., et al. ; AdICONA Study Group , Self-reported symptoms and medication side effects influence adherence to highly active antiretroviral therapy in persons with HIV infection. J. Acquir. Immune Defic. Syndr. 28, 445–449 (2001). [DOI] [PubMed] [Google Scholar]
- 10.Estrada V., Portilla J., Dyslipidemia related to antiretroviral therapy. AIDS Rev. 13, 49–56 (2011). [PubMed] [Google Scholar]
- 11.Deeks S. G., Phillips A. N., HIV infection, antiretroviral treatment, ageing, and non-AIDS related morbidity. BMJ 338, a3172 (2009). [DOI] [PubMed] [Google Scholar]
- 12.Nel A., Kagee A., Common mental health problems and antiretroviral therapy adherence. AIDS Care 23, 1360–1365 (2011). [DOI] [PubMed] [Google Scholar]
- 13.Rosen S., Sanne I., Collier A., Simon J. L., Rationing antiretroviral therapy for HIV/AIDS in Africa: Choices and consequences. PLoS Med. 2, e303 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Meloni S. T., et al. , Drug resistance patterns following pharmacy stock shortage in Nigerian antiretroviral treatment program. AIDS Res. Ther. 14, 58 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schouten E. J., et al. , Antiretroviral drug supply challenges in the era of scaling up ART in Malawi. J. Int. AIDS Soc. 14 (suppl. 1), S4 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Marsden M. D., Zack J. A., Experimental approaches for eliminating latent HIV. For. Immunopathol. Dis. Therap. 6, 91–99 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Marsden M. D., Zack J. A., HIV/AIDS eradication. Bioorg. Med. Chem. Lett. 23, 4003–4010 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Margolis D. M., Garcia J. V., Hazuda D. J., Haynes B. F., Latency reversal and viral clearance to cure HIV-1. Science 353, aaf6517 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Archin N. M., et al. , Administration of vorinostat disrupts HIV-1 latency in patients on antiretroviral therapy. Nature 487, 482–485 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rasmussen T. A., et al. , Panobinostat, a histone deacetylase inhibitor, for latent-virus reactivation in HIV-infected patients on suppressive antiretroviral therapy: A phase 1/2, single group, clinical trial. Lancet HIV 1, e13–e21 (2014). [DOI] [PubMed] [Google Scholar]
- 21.Søgaard O. S., et al. , The depsipeptide romidepsin reverses HIV-1 latency in vivo. PLoS Pathog. 11, e1005142 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tsai A., et al. , Toll-like receptor 7 agonist GS-9620 induces HIV expression and HIV-specific immunity in cells from HIV-infected individuals on suppressive antiretroviral therapy. J. Virol. 91, e02166-16 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lim S. Y., et al. , TLR7 agonists induce transient viremia and reduce the viral reservoir in SIV-infected rhesus macaques on antiretroviral therapy. Sci. Transl. Med. 10, eaao4521 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jiang G., et al. , HIV latency is reversed by ACSS2-driven histone crotonylation. J. Clin. Invest. 128, 1190–1198 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shang H. T., et al. , Progress and challenges in the use of latent HIV-1 reactivating agents. Acta Pharmacol. Sin. 36, 908–916 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhao M., et al. , T cell toxicity of HIV latency reversing agents. Pharmacol. Res. 139, 524–534 (2019). [DOI] [PubMed] [Google Scholar]
- 27.Borducchi E. N., et al. , Antibody and TLR7 agonist delay viral rebound in SHIV-infected monkeys. Nature 563, 360–364 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kazanietz M. G., et al. , Characterization of ligand and substrate specificity for the calcium-dependent and calcium-independent protein kinase C isozymes. Mol. Pharmacol. 44, 298–307 (1993). [PubMed] [Google Scholar]
- 29.Bertolini T. M., Giorgione J., Harvey D. F., Newton A. C., Protein kinase C translocation by modified phorbol esters with functionalized lipophilic regions. J. Org. Chem. 68, 5028–5036 (2003). [DOI] [PubMed] [Google Scholar]
- 30.Newton A. C., Protein kinase C: Perfectly balanced. Crit. Rev. Biochem. Mol. Biol. 53, 208–230 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Williams S. A., et al. , Prostratin antagonizes HIV latency by activating NF-kappaB. J. Biol. Chem. 279, 42008–42017 (2004). [DOI] [PubMed] [Google Scholar]
- 32.Genot E. M., Parker P. J., Cantrell D. A., Analysis of the role of protein kinase C-α, -ε, and -ζ in T cell activation. J. Biol. Chem. 270, 9833–9839 (1995). [DOI] [PubMed] [Google Scholar]
- 33.López-Cabrera M., et al. , Transcriptional regulation of the gene encoding the human C-type lectin leukocyte receptor AIM/CD69 and functional characterization of its tumor necrosis factor-α-responsive elements. J. Biol. Chem. 270, 21545–21551 (1995). [DOI] [PubMed] [Google Scholar]
- 34.Kulkosky J., et al. , Prostratin: Activation of latent HIV-1 expression suggests a potential inductive adjuvant therapy for HAART. Blood 98, 3006–3015 (2001). [DOI] [PubMed] [Google Scholar]
- 35.Pérez M., et al. , Bryostatin-1 synergizes with histone deacetylase inhibitors to reactivate HIV-1 from latency. Curr. HIV Res. 8, 418–429 (2010). [DOI] [PubMed] [Google Scholar]
- 36.Darcis G., et al. , An in-depth comparison of latency-reversing agent combinations in various in vitro and ex vivo HIV-1 latency models identified bryostatin-1+JQ1 and ingenol-B+JQ1 to potently reactivate viral gene expression. PLoS Pathog. 11, e1005063 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bullen C. K., Laird G. M., Durand C. M., Siliciano J. D., Siliciano R. F., New ex vivo approaches distinguish effective and ineffective single agents for reversing HIV-1 latency in vivo. Nat. Med. 20, 425–429 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gutiérrez C., et al. , Bryostatin-1 for latent virus reactivation in HIV-infected patients on antiretroviral therapy. AIDS 30, 1385–1392 (2016). [DOI] [PubMed] [Google Scholar]
- 39.Marsden M. D., et al. , In vivo activation of latent HIV with a synthetic bryostatin analog effects both latent cell “kick” and “kill” in strategy for virus eradication. PLoS Pathog. 13, e1006575 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Albert B. J., et al. , Combinations of isoform-targeted histone deacetylase inhibitors and bryostatin analogues display remarkable potency to activate latent HIV without global T-cell activation. Sci. Rep. 7, 7456 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Laird G. M., et al. , Ex vivo analysis identifies effective HIV-1 latency-reversing drug combinations. J. Clin. Invest. 125, 1901–1912 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Marsden M. D., et al. , Characterization of designed, synthetically accessible bryostatin analog HIV latency reversing agents. Virology 520, 83–93 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Trushin S. A., et al. , Human immunodeficiency virus reactivation by phorbol esters or T-cell receptor ligation requires both PKCalpha and PKCtheta. J. Virol. 79, 9821–9830 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gustafson K. R., et al. , A nonpromoting phorbol from the Samoan medicinal plant Homalanthus nutans inhibits cell killing by HIV-1. J. Med. Chem. 35, 1978–1986 (1992). [DOI] [PubMed] [Google Scholar]
- 45.Wender P. A., Kee J. M., Warrington J. M., Practical synthesis of prostratin, DPP, and their analogs, adjuvant leads against latent HIV. Science 320, 649–652 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Huiting E. D., et al. , Impact of treatment interruption on HIV reservoirs and lymphocyte subsets in individuals who initiated antiretroviral therapy during the early phase of infection. J. Infect. Dis. 220, 270–274 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bitnun A., et al. , Clinical correlates of human immunodeficiency virus-1 (HIV-1) DNA and inducible HIV-1 RNA reservoirs in peripheral blood in children with perinatally acquired HIV-1 infection with sustained virologic suppression for at least 5 years. Clin. Infect. Dis. 70, 859–866 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Beans E. J., et al. , Highly potent, synthetically accessible prostratin analogs induce latent HIV expression in vitro and ex vivo. Proc. Natl. Acad. Sci. U.S.A. 110, 11698–11703 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Spivak A. M., et al. , Ex vivo bioactivity and HIV-1 latency reversal by ingenol dibenzoate and panobinostat in resting CD4(+) T cells from aviremic patients. Antimicrob. Agents Chemother. 59, 5984–5991 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Spivak A. M., et al. , Synthetic ingenols maximize protein kinase C-induced HIV-1 latency reversal. Antimicrob. Agents Chemother. 62, e01361-18 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Jiang G., et al. , Synergistic reactivation of latent HIV expression by ingenol-3-Angelate, PEP005, targeted NF-kB signaling in combination with JQ1 induced p-TEFb activation. PLoS Pathog. 11, e1005066 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Jiang G., et al. , Reactivation of HIV latency by a newly modified Ingenol derivative via protein kinase Cδ-NF-κB signaling. AIDS 28, 1555–1566 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lebwohl M., et al. , Ingenol mebutate gel for actinic keratosis. N. Engl. J. Med. 366, 1010–1019 (2012). [DOI] [PubMed] [Google Scholar]
- 54.Bettencourt M. S., Treatment of superficial basal cell carcinoma with ingenol mebutate gel, 0.05%. Clin. Cosmet. Investig. Dermatol. 9, 205–209 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Cary D. C., Fujinaga K., Peterlin B. M., Euphorbia kansui reactivates latent HIV. PLoS One 11, e0168027 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wang P., et al. , Reactivation of HIV-1 from latency by an ingenol derivative from Euphorbia kansui. Sci. Rep. 7, 9451 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Jayson G. C., et al. , A phase I trial of bryostatin 1 in patients with advanced malignancy using a 24 hour intravenous infusion. Br. J. Cancer 72, 461–468 (1995). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Marshall J. L., et al. , Phase I study of prolonged infusion Bryostatin-1 in patients with advanced malignancies. Cancer Biol. Ther. 1, 409–416 (2002). [DOI] [PubMed] [Google Scholar]
- 59.Ray A. S., Fordyce M. W., Hitchcock M. J. M., Tenofovir alafenamide: A novel prodrug of tenofovir for the treatment of human immunodeficiency virus. Antiviral Res. 125, 63–70 (2016). [DOI] [PubMed] [Google Scholar]
- 60.Huttunen K. M., Raunio H., Rautio J., Prodrugs–from serendipity to rational design. Pharmacol. Rev. 63, 750–771 (2011). [DOI] [PubMed] [Google Scholar]
- 61.Ettmayer P., Amidon G. L., Clement B., Testa B., Lessons learned from marketed and investigational prodrugs. J. Med. Chem. 47, 2393–2404 (2004). [DOI] [PubMed] [Google Scholar]
- 62.Meng Q., et al. , Logical design and application of prodrug platforms. Polym. Chem. 10, 306–324 (2019). [Google Scholar]
- 63.Rautio J., Meanwell N. A., Di L., Hageman M. J., The expanding role of prodrugs in contemporary drug design and development. Nat. Rev. Drug Discov. 17, 559–587 (2018). [DOI] [PubMed] [Google Scholar]
- 64.Biberacher V., et al. , The cytotoxicity of anti-CD22 immunotoxin is enhanced by bryostatin 1 in B-cell lymphomas through CD22 upregulation and PKC-βII depletion. Haematologica 97, 771–779 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Wender P. A., Koehler K. F., Sharkey N. A., Dell’Aquila M. L., Blumberg P. M., Analysis of the phorbol ester pharmacophore on protein kinase C as a guide to the rational design of new classes of analogs. Proc. Natl. Acad. Sci. U.S.A. 83, 4214–4218 (1986). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Wender P. A., et al. , Modeling of the bryostatins to the phorbol ester pharmacophore on protein kinase C. Proc. Natl. Acad. Sci. U.S.A. 85, 7197–7201 (1988). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Wender P. A., Verma V. A., Paxton T. J., Pillow T. H., Function-oriented synthesis, step economy, and drug design. Acc. Chem. Res. 41, 40–49 (2008). [DOI] [PubMed] [Google Scholar]
- 68.Wender P. A., Quiroz R. V., Stevens M. C., Function through synthesis-informed design. Acc. Chem. Res. 48, 752–760 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Hecker E., Tumour promoters of the irritant diterpene ester type as risk factors of cancer in man. Bot. J. Linn. Soc. 94, 197–219 (1987). [Google Scholar]
- 70.Vasas A., Hohmann J., Euphorbia diterpenes: Isolation, structure, biological activity, and synthesis (2008-2012). Chem. Rev. 114, 8579–8612 (2014). [DOI] [PubMed] [Google Scholar]
- 71.Wang H. B., Wang X. Y., Liu L. P., Qin G. W., Kang T. G., Tigliane diterpenoids from the Euphorbiaceae and Thymelaeaceae families. Chem. Rev. 115, 2975–3011 (2015). [DOI] [PubMed] [Google Scholar]
- 72.Appendino G., Ingenane diterpenoids. Prog. Chem. Org. Nat. Prod. 102, 1–90 (2016). [DOI] [PubMed] [Google Scholar]
- 73.Huang T. L., et al. , Hydrolysis of carbonates, thiocarbonates, carbamates, and carboxylic esters of α-naphthol, β-naphthol, and p-nitrophenol by human, rat, and mouse liver carboxylesterases. Pharm. Res. 10, 639–648 (1993). [DOI] [PubMed] [Google Scholar]
- 74.Nakamura H., Kishi Y., Pajares M. A., Rando R. R., Structural basis of protein kinase C activation by tumor promoters. Proc. Natl. Acad. Sci. U.S.A. 86, 9672–9676 (1989). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Seki H., Kawaguchi T., Higuchi T., Specificity of esterases and structure of prodrug esters: Reactivity of various acylated acetaminophen compounds and acetylaminobenzoated compounds. J. Pharm. Sci. 77, 855–860 (1988). [DOI] [PubMed] [Google Scholar]
- 76.Grue-Sørensen G., et al. , Synthesis, biological evaluation and SAR of 3-benzoates of ingenol for treatment of actinic keratosis and non-melanoma skin cancer. Bioorg. Med. Chem. Lett. 24, 54–60 (2014). [DOI] [PubMed] [Google Scholar]
- 77.Wender P. A., et al. , Scalable synthesis of bryostatin 1 and analogs, adjuvant leads against latent HIV. Science 358, 218–223 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Greenwald R. B., et al. , Drug delivery systems employing 1,4- or 1,6-elimination: Poly(ethylene glycol) prodrugs of amine-containing compounds. J. Med. Chem. 42, 3657–3667 (1999). [DOI] [PubMed] [Google Scholar]
- 79.Jones L. R., et al. , Releasable luciferin-transporter conjugates: Tools for the real-time analysis of cellular uptake and release. J. Am. Chem. Soc. 128, 6526–6527 (2006). [DOI] [PubMed] [Google Scholar]
- 80.Jiang G., Dandekar S., Targeting NF-κB signaling with protein kinase C agonists as an emerging strategy for combating HIV latency. AIDS Res. Hum. Retroviruses 31, 4–12 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Jordan A., Bisgrove D., Verdin E., HIV reproducibly establishes a latent infection after acute infection of T cells in vitro. EMBO J. 22, 1868–1877 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Pitsios C., Dimitrakopoulou A., Tsalimalma K., Kordossis T., Choremi-Papadopoulou H., Expression of CD69 on T-cell subsets in HIV-1 disease. Scand. J. Clin. Lab. Invest. 68, 233–241 (2008). [DOI] [PubMed] [Google Scholar]
- 83.Cocchi F., et al. , Identification of RANTES, MIP-1 α, and MIP-1 β as the major HIV-suppressive factors produced by CD8+ T cells. Science 270, 1811–1815 (1995). [DOI] [PubMed] [Google Scholar]
- 84.Folks T. M., et al. , Tumor necrosis factor alpha induces expression of human immunodeficiency virus in a chronically infected T-cell clone. Proc. Natl. Acad. Sci. U.S.A. 86, 2365–2368 (1989). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Brooks D. G., et al. , Molecular characterization, reactivation, and depletion of latent HIV. Immunity 19, 413–423 (2003). [DOI] [PubMed] [Google Scholar]
- 86.Spivak A. M., et al. , Janus kinase inhibition suppresses PKC-induced cytokine release without affecting HIV-1 latency reversal ex vivo. Retrovirology 13, 88 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Clamp A. R., et al. ; Cancer Research UK Phase I/II Committee , A phase II trial of bryostatin-1 administered by weekly 24-hour infusion in recurrent epithelial ovarian carcinoma. Br. J. Cancer 89, 1152–1154 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Kornberg M. D., et al. , Bryostatin-1 alleviates experimental multiple sclerosis. Proc. Natl. Acad. Sci. U.S.A. 115, 2186–2191 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Sun M.-K., Hongpaisan J., Lim C. S., Alkon D. L., Bryostatin-1 restores hippocampal synapses and spatial learning and memory in adult fragile X mice. J. Pharmacol. Exp. Ther. 349, 393–401 (2014). [DOI] [PubMed] [Google Scholar]
- 90.Sun M.-K., Hongpaisan J., Alkon D. L., Postischemic PKC activation rescues retrograde and anterograde long-term memory. Proc. Natl. Acad. Sci. U.S.A. 106, 14676–14680 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Tan Z., et al. , Bryostatin improves survival and reduces ischemic brain injury in aged rats after acute ischemic stroke. Stroke 44, 3490–3497 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data are available in the main text and SI Appendix.