Significance
The Arg/N-degron pathway targets proteins for degradation by recognizing their N-terminal residues. In the present study, we used in vivo and in vitro binding assays to identify specific multienzyme targeting complexes of the Arg/N-degron pathway in yeast (Saccharomyces cerevisiae) and human cells. These targeting complexes contain N-terminal amidases, an arginyltransferase, and specific ubiquitin ligases of the Arg/N-degron pathway. Enzymes or assemblies of enzymes that catalyze sequential reactions have been observed to exhibit substrate channeling, in which a reaction intermediate can be transferred between active sites of interacting enzymes without release of intermediate to the bulk solution. It remains to be determined whether targeting complexes discovered in the present work function to enable substrate channeling.
Keywords: degron, channeling, superchanneling, ubiquitin, degradation
Abstract
The Arg/N-degron pathway targets proteins for degradation by recognizing their N-terminal (Nt) residues. If a substrate bears, for example, Nt-Asn, its targeting involves deamidation of Nt-Asn, arginylation of resulting Nt-Asp, binding of resulting (conjugated) Nt-Arg to the UBR1-RAD6 E3-E2 ubiquitin ligase, ligase-mediated synthesis of a substrate-linked polyubiquitin chain, its capture by the proteasome, and substrate’s degradation. We discovered that the human Nt-Asn–specific Nt-amidase NTAN1, Nt-Gln–specific Nt-amidase NTAQ1, arginyltransferase ATE1, and the ubiquitin ligase UBR1-UBE2A/B (or UBR2-UBE2A/B) form a complex in which NTAN1 Nt-amidase binds to NTAQ1, ATE1, and UBR1/UBR2. In addition, NTAQ1 Nt-amidase and ATE1 arginyltransferase also bind to UBR1/UBR2. In the yeast Saccharomyces cerevisiae, the Nt-amidase, arginyltransferase, and the double-E3 ubiquitin ligase UBR1-RAD6/UFD4-UBC4/5 are shown to form an analogous targeting complex. These complexes may enable substrate channeling, in which a substrate bearing, for example, Nt-Asn, would be captured by a complex-bound Nt-amidase, followed by sequential Nt modifications of the substrate and its polyubiquitylation at an internal Lys residue without substrate’s dissociation into the bulk solution. At least in yeast, the UBR1/UFD4 ubiquitin ligase interacts with the 26S proteasome, suggesting an even larger Arg/N-degron–targeting complex that contains the proteasome as well. In addition, specific features of protein-sized Arg/N-degron substrates, including their partly sequential and partly nonsequential enzymatic modifications, led us to a verifiable concept termed “superchanneling.” In superchanneling, the synthesis of a substrate-linked poly-Ub chain can occur not only after a substrate’s sequential Nt modifications, but also before them, through a skipping of either some or all of these modifications within a targeting complex.
Regulated protein degradation protects cells from abnormal (e.g., misfolded or aggregated) proteins, and also mediates the levels of proteins that evolved to be constitutively or conditionally short-lived in vivo. The ubiquitin (Ub)-proteasome system (UPS) and the autophagy-lysosome system comprise two major sets of intracellular proteolytic pathways. Chaperone proteins are a part of both systems (1–10). Ub ligases of the UPS recognize substrate proteins through their degradation signals (degrons) and conjugate the 9-kDa Ub (usually a poly-Ub chain) to cognate amino acid residues (usually specific internal lysines) of targeted substrates. Deubiquitylases, another major class of UPS enzymes, catalyze deubiquitylation of Ub-conjugated proteins (2–4, 9–13). A substrate-linked poly-Ub chain is recognized by the ATP-dependent protease called the 26S proteasome. This protease unfolds the captured protein and processively converts it to ∼10-residue peptides (14–19).
Several proteolytic systems, called N-degron pathways (previously called “N-end rule pathways”), have in common their ability to recognize proteins that contain N-terminal (Nt) degrons called N-degrons. This recognition causes degradation of targeted proteins by the 26S proteasome and autophagy in eukaryotes, and by the proteasome-like ClpAP protease in bacteria (Fig. 1 and SI Appendix, Fig. S1) (2, 9, 10, 20–48). An N-degron comprises, in particular, a destabilizing Nt-residue of a protein and its internal Lys residue(s) that acts as a site of polyubiquitylation (2, 21, 49).
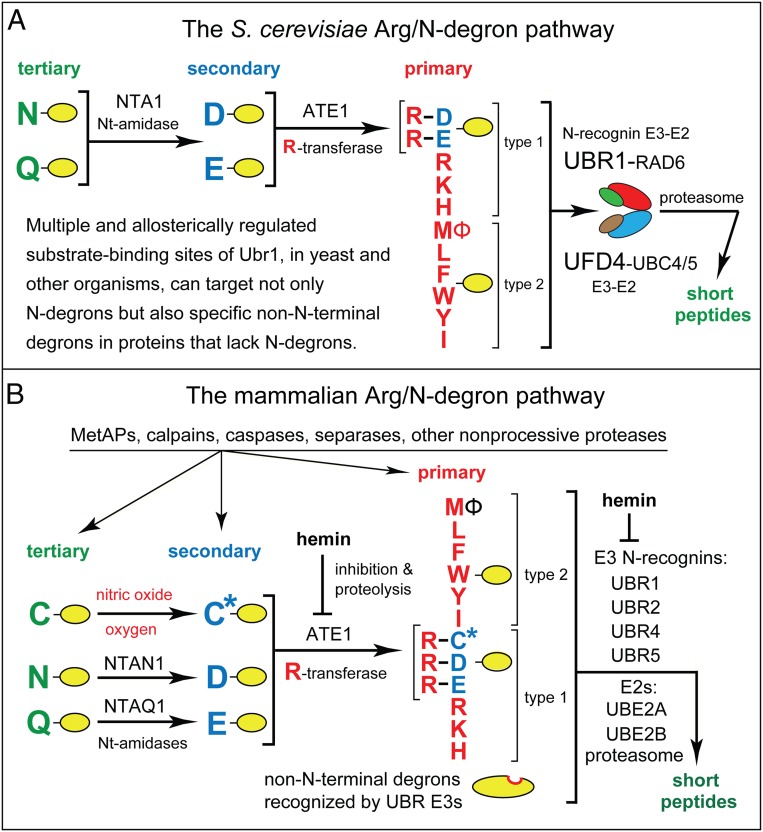
Fig. 1.
(A) The S. cerevisiae Arg/N-degron pathway. Nt-residues are denoted by single-letter abbreviations. Yellow ovals denote the rest of a protein substrate. “Primary,” “secondary,” and “tertiary” refer to mechanistically distinct classes of destabilizing Nt-residues. “Type 1” and “type 2” refer, respectively, to two sets of primary destabilizing Nt-residues, basic (Arg, Lys, His) and bulky hydrophobic (Leu, Phe, Trp, Tyr, Ile, and also Met, if the latter is followed by a bulky hydrophobic residue [Ф]). These Nt-residues are recognized by two substrate-binding sites (type 1 and type 2) of scUBR1, the pathway’s E3 N-recognin. (B) The mammalian Arg/N-degron pathway. Hemin (Fe3+-heme) inhibits the activity of R-transferase and accelerates its degradation in vivo. Hemin also binds to UBR1/UBR2 E3s and inhibits specific aspects of their activity (2, 22, 24). Besides recognizing Arg/N-degrons, E3s of the Arg/N-degron pathway contain, in addition, specific binding sites that are exposed conditionally and recognize non-Nt degrons of proteins that lack Arg/N-degrons (2, 9, 22, 24). See Introduction for references and other details. See also SI Appendix, Fig. S1 for other N-degron pathways.
Eukaryotes contain the Arg/N-degron pathway (it recognizes, in particular, specific unacetylated Nt-residues) (Fig. 1), the Ac/N-degron pathway (it recognizes, in particular, the Nα-terminally acetylated [Nt-acetylated] Nt-residues), the Pro/N-degron pathway (it recognizes, in particular, the Nt-Pro residue or a Pro at position 2), the Gly/N-degron pathway (it recognizes Nt-Gly), and the fMet/N-degron pathway (it recognizes proteins that had been “pretranslationally” Nt-formylated) (SI Appendix, Fig. S1) (2, 9, 10, 22–24, 26, 27, 46–48, 50).
Most N-degrons are initially cryptic (pro-N-degrons). They become active N-degrons either constitutively (e.g., cotranslationally) or via regulated steps. Nonprocessive proteases, including caspases, calpains, separases, and cathepsins, act as specific components of N-degron pathways, since a cleavage of a protein can generate a C-terminal (Ct) fragment bearing an N-degron (9, 10, 33, 46, 51, 52). Active N-degrons can also be formed through enzymatic Nt-acetylation, Nt-deamidation, Nt-oxidation, Nt-arginylation, Nt-leucylation, and Nt-formylation of specific proteins (Fig. 1 and SI Appendix, Fig. S1). Recognition components of N-degron pathways are called N-recognins. They are E3 Ub ligases or other proteins, for example, mammalian p62 or bacterial ClpS, that can recognize N-degrons (2, 6, 9, 10, 23, 24, 41). In cognate sequence contexts, all 20 amino acids of the genetic code are capable of functioning as destabilizing Nt-residues (SI Appendix, Fig. S1). Consequently, many proteins in a cell are constitutive or conditional N-degron substrates, either as full-length proteins or as Ct-fragments.
Regulated degradation of proteins or their natural fragments by N-degron pathways has been shown to function in a large number of biological processes, including the sensing of short peptides, heme, oxygen, and nitric oxide (NO); the regulation of subunit stoichiometries in oligomeric proteins; the elimination of misfolded proteins; the control of DNA repair, transcription, replication, and chromosome cohesion/segregation; the regulation of G proteins, chaperones, cytoskeletal proteins, gluconeogenesis, autophagy, peptide transport, meiosis, circadian rhythms, cell migration, fat metabolism, immunity (including inflammation), cardiovascular system, neurogenesis, and spermatogenesis; a suppression of neurodegeneration and regulation of apoptosis; and also plant cell differentiation, plant defenses against pathogens, the sensing of oxygen and NO, and many other processes in plants (refs. 2, 9, 10, 20, 22–32, 34, 36–47, 50, 53–57, and references therein).
To keep notations uniform, human genetic terms (all-uppercase letters) are used below to denote both human (Homo sapiens, hs) and yeast (Saccharomyces cerevisiae, sc) genes and proteins. scUBR1 encodes the 225-kDa RING-type E3, denoted as scUBR1, that functions as the sole N-recognin of the S. cerevisiae Arg/N-degron pathway. Unmodified Nt-Arg, Nt-Lys, Nt-His, Nt-Leu, Nt-Phe, Nt-Tyr, Nt-Trp, Nt-Ile, and Nt-Met (if Met is followed by a bulky hydrophobic residue) are “primary” destabilizing Nt-residues in that they are directly bound by scUBR1 (Fig. 1A) (2, 20, 22, 33, 34, 36, 51, 58–60). In contrast, Nt-Asp and Nt-Glu are destabilizing because of their Nt-arginylation by scATE1 arginyltransferase (R-transferase). The resulting (conjugated) Nt-Arg is bound by scUBR1. Nt-Asn and Nt-Gln are destabilizing since scNTA1 Nt-amidase converts them to Nt-arginylatable Nt-Asp and Nt-Glu (Fig. 1A) (61, 62). scUBR1 E3 contains multiple binding sites that can target not only N-degrons but also other degrons in specific N-degron–lacking proteins (9, 57, 63, 64). scUBR1 E3 is bound to its cognate E2 (Ub-conjugating) enzyme scRAD6, and also to a complex of scUFD4 E3 with its cognate E2 enzyme (scUBC4 or scUBC5) (Fig. 1A) (65).
In contrast to S. cerevisiae, the human genome encodes at least four N-recognin E3s, the 200-kDa hsUBR1 and hsUBR2, the 570-kDa hsUBR4 (p600, BIG), and the 300-kDa hsUBR5 (EDD1, HYD) (Fig. 1B) (2, 24, 66–68). Yet another N-recognin of the human Arg/N-degron pathway is p62, an autophagy-regulating protein distinct from E3s (41, 69, 70). hsUBR1 and hsUBR2 E3s are sequelogous (similar in sequence) (71) (they are 47% identical) to each other and to S. cerevisiae scUBR1 (6). In contrast, sequelogies (sequence similarities†) between hsUBR1/hsUBR2 and hsUBR4 or hsUBR5 are confined largely to their ∼80-residue UBR domains, which recognize N-degrons that bear N-terminal Arg, Lys, or His (Fig. 1B) (2, 59, 60). Animals and plants contain two Nt-amidases, the Nt-Asn-specific NTAN1, and the Nt-Gln-specific NTAQ1 (47, 50, 72). At least in multicellular eukaryotes, Nt-arginylation encompasses not only Nt-Asp and Nt-Glu but also Nt-Cys, after its conditional (oxygen/NO-dependent) oxidation to Nt-Cys-sulfinate or Nt-Cys-sulfonate (Fig. 1B) (44, 47, 55, 73–75).
Degradation, by the human Arg/N-degron pathway, of a substrate bearing, for example, Nt-Asn involves deamidation of Nt-Asn by the Asn/Nt-amidase hsNTAN1; Nt-arginylation of resulting Nt-Asp by the hsATE1 R-transferase; the binding of resulting (conjugated) Nt-Arg to the hsUBR1 (or hsUBR2) E3 component of the E3-E2 Ub ligase; the ligase-mediated synthesis of a poly-Ub chain linked to a substrate’s internal Lys residue; the capture of substrate (at least in part through its poly-Ub) by the 26S proteasome; and the substrate’s processive degradation by the proteasome (Fig. 1B).
In the present study, we discovered that the Asn/Nt-amidase hsNTAN1, the Gln/Nt-amidase hsNTAQ1, the R-transferase hsATE1, and the E3-E2 Ub ligase hsUBR1–hsUBE2A/B (or hsUBR1–hsUBE2A/B) (Fig. 1B) form a targeting complex of the human Arg/N-degron pathway. In addition, the yeast Nt-amidase scNTA1, the R-transferase scATE1, and the double-E3 Ub ligase scUBR1–scRAD6/scUFD4–scUBC4/5 (Fig. 1A) were found to form an analogous targeting complex of the S. cerevisiae Arg/N-degron pathway. Previous work by this laboratory showed that scUBR1 and scUFD4 E3s, which bind to each other (65), also bind to the 26S proteasome (76). That evidence, if viewed together with results of the present study, suggests an even larger N-degron–targeting complex that contains the proteasome as well.
Enzymes or assemblies of enzymes that catalyze sequential reactions have been observed to exhibit substrate channeling, in which a reaction intermediate can be transferred between active sites of interacting enzymes without release of intermediate to the bulk solution (77–85). It remains to be determined whether targeting complexes identified in the present work function to enable substrate channeling.
Specific features of protein-sized Arg/N-degron substrates and targeting complexes that capture them led us to a verifiable concept, termed “superchanneling.” This mechanism is mutually nonexclusive vis-à-vis substrate channeling. In superchanneling, the last modification of a substrate, i.e., the synthesis of a substrate-linked poly-Ub chain can bypass sequential Nt modifications of the substrate by skipping, within a targeting complex, some or all of these modifications. We discuss predictions of the superchanneling model and approaches to its verification.
Results and Discussion
Protein Binding Assays.
Our previous (28, 86) and present work employed the yeast-based two-hybrid (Y2H) method of Fields and colleagues (87–89) for detecting in vivo protein interactions. In assays with Y2H-based protein fusions, we varied locations (Nt or Ct) of a fusion’s activation domain (AD) or DNA-binding domain (DBD), particularly if an initial Y2H assay did not detect an interaction. Controls included immunoblotting to examine Y2H-based fusions, and also verifying that binding-positive Y2H fusions were not autoactivating, i.e., that none of them were positive in Y2H assays that contained just one of two fusions. The results summarized below passed all of these controls. This study also employed split-Ub, glutathione transferase (GST)-pulldown, and coimmunoprecipitation (co-IP) binding assays (Figs. 2–5 and SI Appendix, Figs. S2–S10).
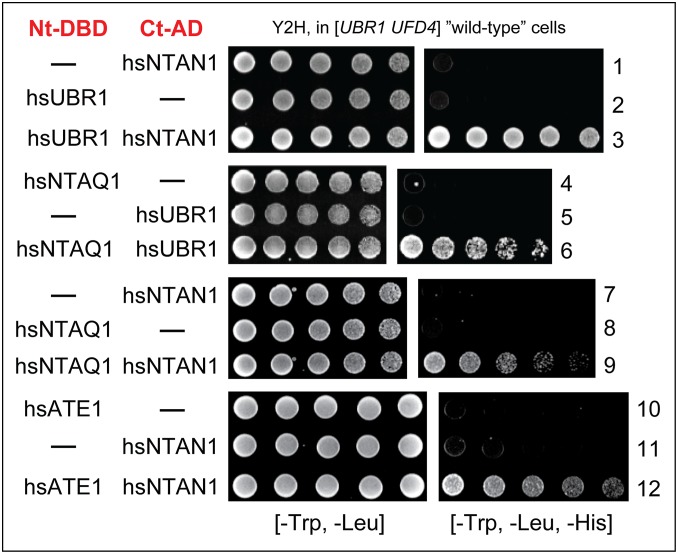
Fig. 2.
Y2H binding assays, in wild-type (UBR1 UFD4) S. cerevisiae, with enzymes of the human Arg/N-degron pathway. Expression of HIS3, the assays’ readout in otherwise His− cells, is a function of binding affinity between test proteins (87). Histidine-lacking plates (shown on the right) were incubated for ∼2 d at 30 °C to detect the growth of His+ cells. Nt and Ct Y2H-specific domains in Y2H-based protein fusions are marked in red. Row 1, hsNTAN1 vs. vector alone. Row 2, hsUBR1 vs. vector alone. Row 3, hsUBR1 vs. hsNTAN1. Row 4, hsNTAQ1 vs. vector alone. Row 5, hsUBR1 vs. vector alone. Row 6, hsNTAQ1 vs. hsUBR1. Row 7, hsNTAN1 vs. vector alone. Row 8, hsNTAQ1 vs. vector alone. Row 9, hsNTAN1 vs. hsNTAQ1. Row 10, hsATE1 vs. vector alone. Row 11, hsNTAN1 vs. vector alone. Row 12, hsATE1 vs. hsNTAN1. See also SI Appendix, Materials and Methods.
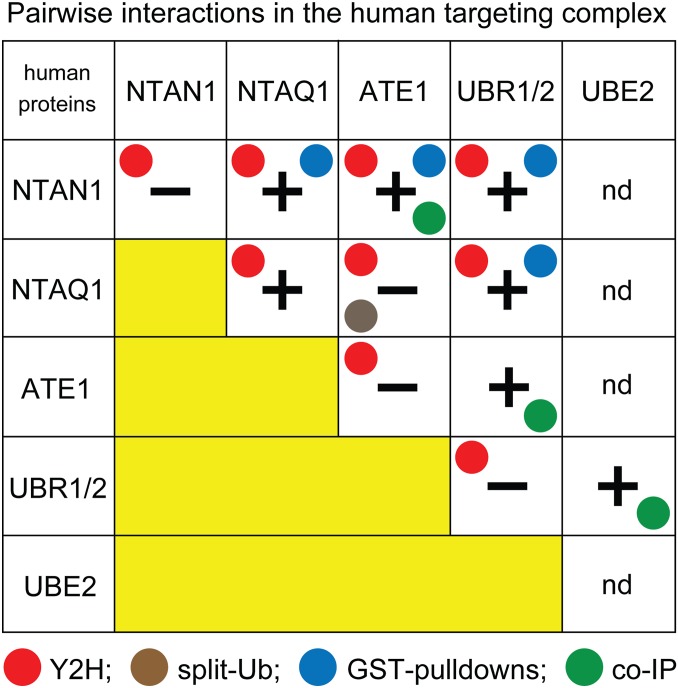
Fig. 5.
Summary of binding assays and detected pairwise interactions among five enzymes of the human Arg/N-degron pathway. Plusses and minuses indicate, respectively, a detected interaction between two indicated human proteins, and the apparent absence of binding. nd, not determined. Colored circles denote in vivo Y2H assays, in vivo split-Ub assays, in vitro GST-pulldown assays, and in vitro co-IP assays, used with the indicated pairs of proteins. In the sole case of disagreement between results of two different assays, the binding of hsATE1 to hsUBR2 was detected by co-IP but was not detected by Y2Hs (Fig. 4C and SI Appendix, Fig. S4, rows 1 to 6). The use, in this study, of more than one binding assay vis-à-vis a given pair of proteins was extensive but not exhaustive.
Human hsUBR1 and hsUBR2 E3s Bind to the Nt-Asn–Specific Nt-Amidase hsNTAN1.
Y2H assays with the full-length 200-kDa hsUBR1 E3 N-recognin vs. the Asn/Nt-amidase hsNTAN1 revealed their interaction (Figs. 1B and 2, rows 1 to 3). Y2Hs with hsUBR2 E3 yielded similar results, in that hsUBR2 also interacted with hsNTAN1 (SI Appendix, Fig. S2, rows 1 to 3).
Human hsUBR1 and hsUBR2 E3s Bind to the Nt-Gln–Specific Nt-Amidase hsNTAQ1.
Nt-amidases catalyze similar reactions (Fig. 1). Nevertheless, the human Asn/Nt-amidase hsNTAN1 is not sequelogous to either the human Gln/Nt-amidase hsNTAQ1 or the S. cerevisiae Nt-amidase scNTA1, which can deamidate both Nt-Asn and Nt-Gln (47, 50, 62, 72). The absence of sequelogy suggests independent evolutionary origins of the three Nt-amidases. Y2Hs indicated the binding of both hsNTAQ1 and hsNTAN1 to both hsUBR1 E3 and hsUBR2 E3 (Figs. 1B and 2, rows 1 to 6, and SI Appendix, Fig. S2, rows 1 to 9, SI Appendix, Fig. S5, row 1, and SI Appendix, Fig. S6, row 17).
To address these interactions in a different way, we also used GST-pulldown assays (64, 90). GST alone as well as hsNTAN1–GST and GST–hsNTAQ1 fusions were expressed in Escherichia coli, purified, and immobilized on glutathione-Sepharose beads (SI Appendix, Fig. S9 A and B). Equal amounts of extract from S. cerevisiae that expressed the N-terminally flag-tagged human fhsUBR2 E3 were incubated with the above (preloaded on beads) GST or GST fusions, followed by washes, elution of beads-associated proteins, their SDS/PAGE, and immunoblotting for fhsUBR2 with anti-flag antibody. In agreement with the above Y2H results, fhsUBR2 interacted with both hsNTAN1–GST and GST–hsNTAQ1, but did not bind to GST alone (Fig. 4D).
Fig. 4.
Co-IPs and GST-pulldown assays with five enzymes of the human Arg/N-degron pathway. (A) Lane 1, kilodalton markers. Lanes 2 to 9, immunoblotting (using a mixture of relevant antibodies) of extracts from eight S. cerevisiae strains that stably coexpressed specific combinations of five human proteins (indicated on top), including a strain that coexpressed all five proteins (lane 9). Epitope tags, indicated on the right, were used to mark these proteins either N terminally or C terminally. The asterisk denotes a yeast protein that cross-reacted with an antibody in the mix. Untagged hsUBE2B E2 was detected by an antibody to hsUBE2B. Of the two highly sequelogous (95% identical) E2s, hsUBE2A and hsUBE2B, only the latter was analyzed in this study. The indicated yeast strains were constructed as described in SI Appendix, Materials and Methods and Fig. S10, through site-specific integrations, into the S. cerevisiae genome, of human cDNAs. (B) The same as the lower part of immunoblot in A, but a higher fluorescence intensity, to increase the brightness of the hsUBE2B bands in lanes 8 and 9. (C) Lane 1, kilodalton markers. Lane 2, detection by immunoblotting, using anti-flag, of 3fhsUBR2 in an input sample of extract from S. cerevisiae that coexpressed 3fhsUBR2 and 3hsvhsATE1 (see lane 3 in A). Lanes 3 and 4, anti-hsv antibody-lacking (lane 3) and anti-hsv–containing beads (lane 4) were used to immunoprecipitate proteins in the extract, followed by SDS/PAGE and immunoblotting with anti-flag antibody. Note the band of coimmunoprecipitated 3fhsUBR2 in lane 4 but not in lane 3. Lanes 5 to 7, same as lanes 2 to 4 but with S. cerevisiae that coexpressed all five human proteins (see also A, lane 9). The asterisk denotes a flag-retaining proteolytic fragment of 3fhsUBR2. (D) GST-pulldown assays, with immunoblottings using anti-flag antibody. Lane 1, kilodalton markers. Lane 2, input sample of extract from S. cerevisiae that expressed the human fhsUBR2 E3. Lanes 3 to 5, GST pulldowns with test proteins immobilized on glutathione beads: GST alone, GST–hsNTAQ1, and hsNTAN1–GST. Note the absence of interaction between fhsUBR2 and GST. (E) Lane 1, kilodalton markers. Lane 2, an input sample of extract from S. cerevisiae that coexpressed all five human proteins, including 3hsvhsATE1 (see also A, lane 9, and C, lanes 5 to 7), was fractionated by SDS/PAGE and analyzed by immunoblotting with anti-ha antibody to hsNTAN13ha. Lanes 3 and 4, anti-hsv antibody-lacking (lane 3) and anti-hsv–containing beads (lane 4) were used, respectively, to immunoprecipitate proteins in the extract, followed by SDS/PAGE and immunoblotting with anti-ha antibody. Note the band of co-IP hsNTAN13ha in lane 4 but not in lane 3.
The Human Asn/Nt-Amidase hsNTAN1 Binds to the Gln/Nt-Amidase hsNTAQ1.
In addition to their binding to the hsUBR1 (and hsUBR2) E3 Ub ligases, the hsNTAN1 and hsNTAQ1 Nt-amidases were also found to interact with each other, in the absence of both hsUBR1 and hsUBR2 (Fig. 2, rows 7 to 9). On the assumption (it remains to be verified) that pairwise interactions among hsNTAN1, hsNTAQ1, and hsUBR1 (or hsUBR2) are mutually nonexclusive, these binding modes would be multivalent and would be expected, therefore, to increase the overall stability of the tripartite hsNTAN1–hsNTAQ1–hsUBR1 complex, in comparison to pairwise interactions within the complex (Fig. 6A).
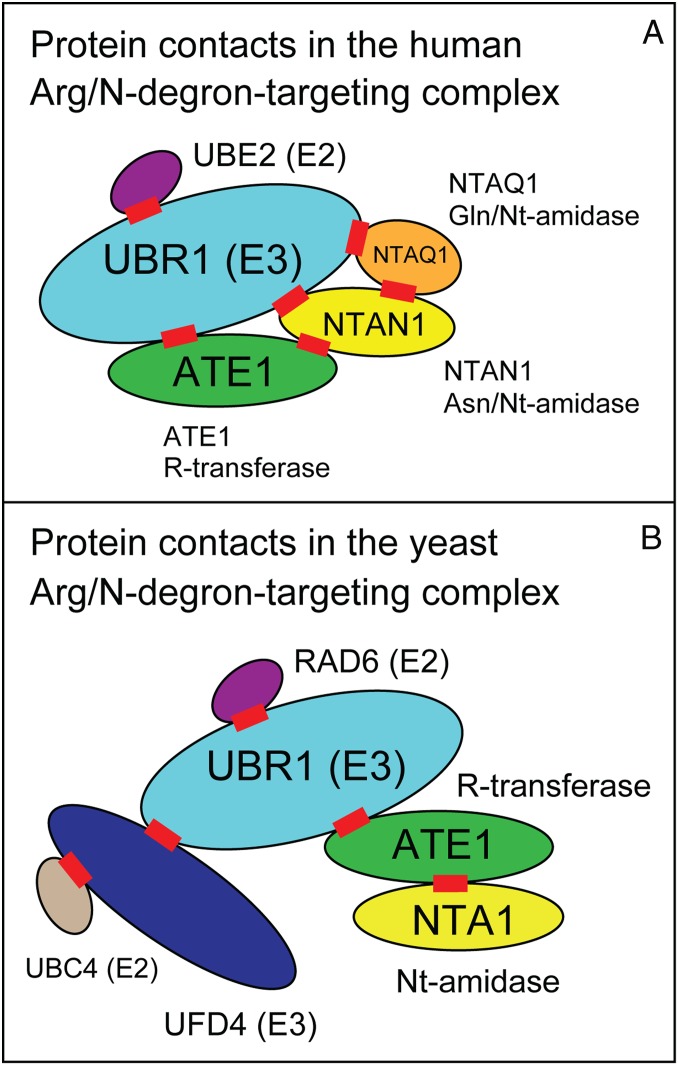
Fig. 6.
Diagram of detected contacts (interactions) among subunits of the human and S. cerevisiae targeting complexes in the Arg/N-degron pathway. In A and B, each red rectangle denotes a detected interaction between a pair of subunits. All subunits are shown as ovals whose relative sizes are roughly proportional to subunits’ molecular masses. Specific locations of protein contacts (red rectangles) within subunits [including E3-E3 contacts in B (65)] are largely unknown and are placed arbitrarily. [One partially defined interaction interface is between the polyacidic Ct-region of scRAD6 E2 and the basic residue-rich region of scUBR1 E3 (109).] Since some proteins in these complexes were found to self-interact in pairwise binding assays (see Results and Discussion), the stoichiometries of subunits in these complexes are known only in part. The depicted pairwise interactions in A and B are also underlain by the assumption that none of these contacts are mutually exclusive. (A) The human targeting complex: The 200-kDa hsUBR1 (or hsUBR2) E3 enzyme, the 17-kDa hsUBE2B (or hsUBE2A) E2 enzyme, the 57-kDa hsATE1 R-transferase, the 34-kDa hsNTAN1 Asn/Nt-amidase, and the 23-kDa hsNTAQ1 Gln/Nt-amidase. Pairwise interactions identified in the present study are those of hsUBR1 (or hsUBR2) with hsNTAN1, hsNTAQ1, hsATE1, and hsUBE2B, as well as those of hsNTAN1 with hsNTAQ1 and hsATE1 (see Results and Discussion and Fig. 5). (B) The S. cerevisiae targeting complex: The 50-kDa scNTA1 Asn/Gln/Nt-amidase, the 55-kDa scATE1 R-transferase, the 225-kDa scUBR1 E3, the 168-kDa scUFD4 E3, the 19-kDa scRAD6 E2, and the 16-kDa scUBC4 (or scUBC5) E2. Pairwise interactions identified in the present study are those of scUBR1 with scNTA1, and of scNTA1 with scATE1 (see Results and Discussion). Three-dimensional crystal structures are known for hsNTAQ1 Nt-amidase (72, 75), hsNTAN1 Nt-amidase (47), scNTA1 Nt-amidase (62), scRAD6 E2 (110), and scUBC4 E2 (111).
The Human hsATE1 R-Transferase Binds to hsNTAN1 Asn/Nt-Amidase.
ATE1 R-transferase conjugates Arg to Nt-Asp, Nt-Glu, or oxidized Nt-Cys. The R-transferase branch of the Arg/N-degron pathway is a sensor of oxygen and NO in animals and plants (Introduction and Fig. 1B) (44, 73, 74, 91, 92). Using Y2H-based hsATE1 fusions (tagged with Nt-DBD or Ct-DBD domains), we found that hsATE1 R-transferase binds to hsNTAN1 Asn/Nt-amidase (Fig. 2, rows 10 to 12, and SI Appendix, Fig. S2, rows 13 to 15).
The Human hsATE1 R-Transferase Does Not Bind to hsNTAQ1 Gln/Nt-Amidase.
In contrast to the binding of hsATE1 R-transferase to Asn/Nt-amidase hsNTAN1 (see above), Y2Hs were negative with hsATE1 vs. Gln/Nt-amidase hsNTAQ1. These results are shown here for Nt-DBD/hsNTAQ1 vs. Nt-AD/hsATE1 (SI Appendix, Fig. S2, rows 10 to 12), and similar results (no interaction) were obtained with all eight Nt/Ct permutations of the DBD and AD domains linked to hsNTAQ1 and hsATE1.
To address this question in a different way, we used a yeast-based split-Ub assay (93, 94). In split-Ub, proteins are expressed as fusions, respectively, to a Ct-half of Ub (CUb) and to its mutant Nt-half (NUb). Interactions between test proteins would reconstitute a quasi-native Ub moiety from CUb and mutant NUb, causing the cleavage of a CUb-containing fusion by deubiquitylases downstream from (reconstituted) Ub moiety. Through additional steps, this cleavage acts as readout of split-Ub assays (93, 94). In agreement with Y2H assays (SI Appendix, Fig. S2, rows 10 to 12), split-Ub (using two orientations of test proteins vis-à-vis NUb and CUb) was negative with hsATE1 vs. hsNTAQ1 (SI Appendix, Fig. S8).
Engineered Yeast Strains That Coexpress Human Proteins: hsATE1 R-Transferase Binds to hsUBR2 E3.
Y2Hs with hsATE1 vs. full-length hsUBR1 E3 or Nt-half of hsUBR2 (hsUBR21–1040) did not detect their binding to hsATE1 (SI Appendix, Fig. S4, rows 1 to 6). Given these findings, and given Y2H-positive (i.e., the opposite) results with S. cerevisiae scATE1 vs. scUBR1 (see below), we used co-IP to address a possible interaction between hsATE1 and hsUBR2 that might have escaped detection by Y2H.
To carry out co-IP assays, we constructed S. cerevisiae strains in which cDNAs encoding five components of the human Arg/N-degron pathway were integrated into the yeast genome downstream from constitutive or galactose-inducible promoters (SI Appendix, Fig. S10). These strains expressed specific combinations of five human proteins, tagged with different epitopes (3fhsUBR2, 3hsvhsATE1, hsNTAN13ha, 3mychsNTAQ1, hsUBE2B), and one strain expressed all five human proteins, as determined by SDS/PAGE and immunoblotting with five different antibodies (Fig. 4 A and B and SI Appendix, Table S1). Untagged hsUBE2B E2 was detected using an antibody to hsUBE2B (Fig. 4B).
In one example of this approach, we used S. cerevisiae that expressed two (and only two) human proteins, 3fhsUBR2 E3 and 3hsvhsATE1 R-transferase (Fig. 4A, lane 3). IP of extract from these cells was carried out using anti-hsv antibody to 3hsvhsATE1. SDS/PAGE of anti-hsv–precipitated proteins, followed by immunoblotting with anti-flag antibody (specific, in this setting, for 3fhsUBR2), detected coimmunoprecipitated 3fhsUBR2 (Fig. 4C, lanes 2 to 4). This assay was also used with an S. cerevisiae strain that expressed all five components of the human Arg/N-degron pathway, with the same results (Fig. 4A, lane 9, and Fig. 4C, lanes 5 to 7).
Note that while the results of co-IP with yeast expressing solely 3fhsUBR2 and 3hsvhsATE1 (Fig. 4C, lanes 2 to 4) could be interpreted straightforwardly as evidence for interaction between hsUBR2 and hsATE1, a similar co-IP finding with yeast that expressed all five human proteins (Fig. 4C, lanes 5 to 7) could also be interpreted (in the absence of evidence with the strain that expressed only two proteins) as a result that had been caused by “hitchhiking” of 3fhsUBR2 within a larger complex that contained both 3hsvhsATE1 and other coexpressed human proteins, cited in Fig. 4 A and B.
A negative Y2H result, such as the one about hsATE1 vs. hsUBR2 (see above), does not prove the absence of binding (87–89). Therefore, we made a working assumption that a robustly positive result with co-IP assays (Fig. 4A, lane 3, and Fig. 4C, lanes 2 to 4) was likely to signify a bona fide interaction between hsATE1 and hsUBR2, while noting the absence of agreement, in this (and only this) case, between Y2H and co-IP assays.
Another example of this co-IP approach, with the yeast strain that expressed just two human proteins, 3fhsUBR2 E3 and its cognate E2 enzyme hsUBE2B, was detection of interaction between these enzymes (SI Appendix, Fig. S9C).
Yet another example of co-IP approach was IP of extract from the yeast strain that coexpressed all five human proteins (Fig. 4A, lane 9). The result was detection, in an 3hsvhsATE1-specific anti-hsv immunoprecipitate, of the coimmunoprecipitated hsNTAN13ha Asn/Nt-amidase (Fig. 4E). Considered together, co-IP data indicated that the 3hsvhsATE1-specific anti-hsv immunoprecipitate contained at least 3hsvhsATE1, hsNTAN13ha, 3fhsUBR2, and hsUBE2B, a glimpse of binding patterns beyond pairwise interactions (Figs. 4 A–C and E and 6A and SI Appendix, Fig. S9C). A “complete” targeting complex remains to be characterized in vivo and reconstituted in vitro.
hsTRIP12, a Human Sequelog of the Yeast scUFD4 E3, Does Not Bind to hsUBR1.
Previous work has shown that scUBR1, the 225-kDa RING-type E3 N-recognin, binds to scUFD4, a 168-kDa HECT-type E3 (Fig. 1A) (65). scUBR1 and scUFD4 function as the double-E3 scUBR1/scRAD6–scUFD4/scUBC4/5 E3-E2 Ub ligase (65). One function of scUFD4 (which is not an N-recognin) is to increase the length of a substrate-linked poly-Ub chain that has been initiated by scUBR1 (65, 95).
The closest human sequelog of the S. cerevisiae scUFD4 E3 is hsTRIP12 E3 (96–99). scUFD4 and hsTRIP12 are 23% identical and 53% similar. However, Y2Hs did not detect an interaction between hsTRIP12 and hsUBR1 (SI Appendix, Fig. S4, rows 7 to 9). Since negative Y2H results are not definitive (87–89), a potential role of hsTRIP12 in the human Arg/N-degron pathway remains to be addressed. With this caveat, hsTRIP12 is apparently not a part of the human targeting complex, in contrast to S. cerevisiae, in which scUBR1 binds to scUFD4 (65) (Fig. 3, rows 1 to 3).
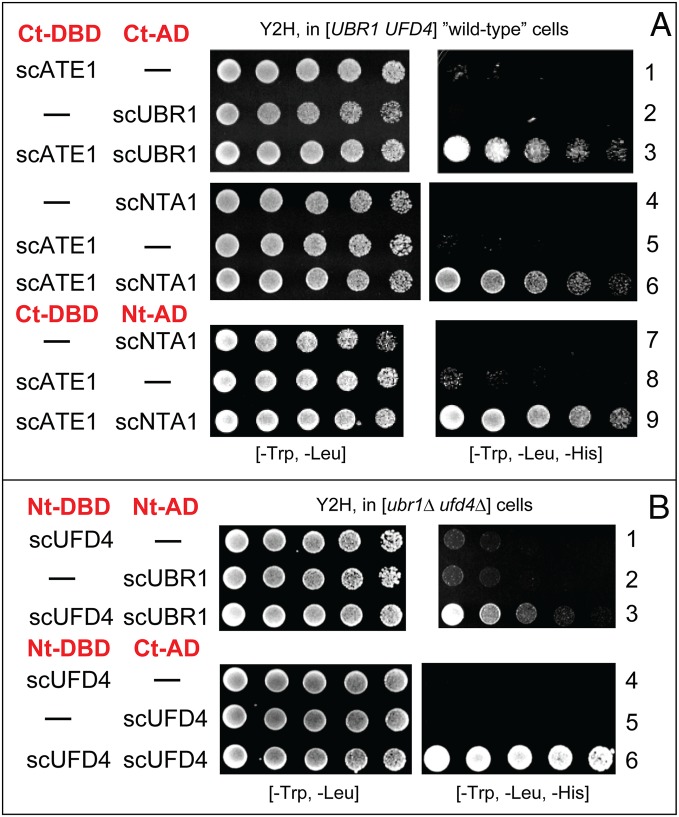
Fig. 3.
Y2H binding assays with enzymes of the S. cerevisiae Arg/N-degron pathway. (A) Y2Hs in wild-type (UBR1 UFD4) S. cerevisiae. Row 1, scATE1 vs. vector alone. Row 2, scUBR1 vs. vector alone. Row 3, scATE1 vs. scUBR1. Row 4, scNTA1 vs. vector alone. Row 5, scATE1 vs. vector alone. Row 6, scATE1 vs. scNTA1. Row 7, scNTA1 vs. vector alone. Row 8, scATE1 vs. vector alone. Row 9, scATE1 vs. scNTA1. (B) Y2Hs in [ubr1Δ ufd4Δ] S. cerevisiae. Row 1, scUFD4 \vs. vector alone. Row 2, scUBR1 vs. vector alone. Row 3, scUFD4 vs. scUBR1. Row 4, scUFD4 (Nt-DBD–tagged) vs. vector alone. Row 5, scUFD4 (Ct-AD–tagged) vs. vector alone. Row 6, scUFD4 vs. scUFD4. See also the legend to Fig. 2 and SI Appendix, Materials and Methods.
The Human Cys-Dioxygenase hsADO Does Not Bind to hsUBR1, hsUBR2, and hsATE1.
In animals and plants, Nt-Cys of some Nt-Cys–bearing proteins can be oxidized through oxygen/NO-dependent reactions, yielding Nt-Cys-sulfinate or Nt-Cys-sulfonate (Nt-Cys*). Nt-arginylation, by ATE1 R-transferase, of (oxidized) Nt-Cys* in mammalian proteins that include G protein regulators RGS4, RGS5, and RGS16, and in specific transcription factors of plants results in degradation of these proteins by the Arg/N-degron pathway (2, 44, 73, 74, 100, 101). This pathway is thus a sensor of oxygen and NO and regulator of responses to these compounds (2, 100). Oxidation of Nt-Cys is mediated, at least in part, by Nt-Cys dioxygenases (2-aminoethandiol dioxygenases) such as hsADO (44, 101). We used Y2H to ask whether hsADO Nt-Cys-dioxygenase might interact with hsUBR1 or hsUBR2 E3s, or with hsATE1 R-transferase. The results were negative (SI Appendix, Fig. S5), suggesting (but not proving) that hsADO dioxygenase is not a part of the human Arg/N-degron–targeting complex.
The Human Arg-tRNA Synthetase Does Not Bind to hsUBR2 and hsATE1.
R-transferase is a part of the human Arg/N-degron–targeting complex (Figs. 5 and 6). Since Arg-tRNA is a cosubstrate of hsATE1 (2), and since fractionations of mammalian cell extracts suggested the possibility of a complex between R-transferase and Arg-tRNA synthetase (102), we used Y2Hs to ask whether the cytosolic Arg-tRNA synthetase hsRARS might bind to hsATE1 R-transferase or hsUBR2 E3. Negative results of these Y2H assays (SI Appendix, Fig. S6) suggested (but did not prove) that there is no interaction between hsRARS and either hsATE1 or hsUBR2.
Self-Interactions Among Components of the Human Targeting Complex.
We also asked whether hsNTAQ1, hsNTAN1, and hsATE1 (Fig. 1B) might self-interact (e.g., homodimerize). The results were positive for Gln/Nt-amidase hsNTAQ1, but negative for Asn/Nt-amidase hsNTAN1 and hsATE1 R-transferase (Fig. 5 and SI Appendix, Fig. S7). As described above, hsNTAQ1 and hsNTAN1 bind to each other, and both of them also bind to hsUBR1 (and hsUBR2) E3 (Figs. 2 and 4–7, SI Appendix, Fig. S2 and SI Appendix, S5, row 1). In the crystal structure of human hsNTAQ1, an extended Nt-region of one hsNTAQ1 molecule in a crystal interacts with the substrate-binding cleft of adjacent hsNTAQ1 molecule (75), suggesting a homodimer. Given multiple pairwise interactions among specific enzymes of the present study, a functional understanding of hsNTAQ1 self-binding (SI Appendix, Fig. S7, rows 1, 2, and 6 to 8) would require structural analyses of hsNTAQ1 as a part of the Arg/N-degron–targeting complex. One possibility is that hsNTAQ1 forms a homodimer in the absence of hsNTAN1, whereas the presence of hsNTAN1 may lead, preferentially, to the also observed hsNTAQ1–hsNTAN1 heterodimer (Fig. 2, rows 7 to 9).
Fig. 7.
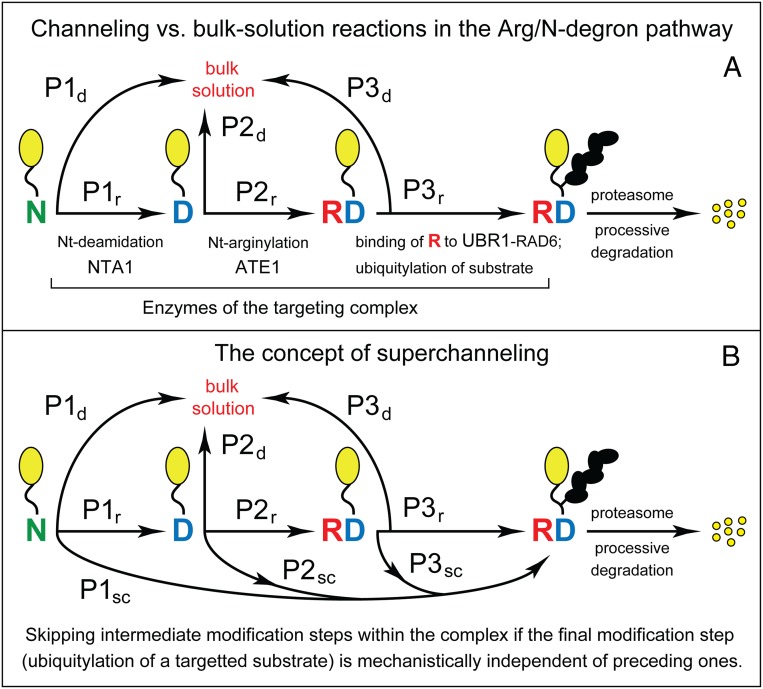
The possibilities of substrate channeling and superchanneling by targeting complexes of the Arg/N-degron pathway. Shown here are diagrams for reactions mediated by the S. cerevisiae Arg/N-degron–targeting complex (Fig. 6B). The setting of the human complex (Fig. 6A) is similar. To simplify diagrams, we left aside (as discussed in Results and Discussion) the scUFD4–scUBC4/5 part of the yeast targeting complex (scUFD4 E3 is not an N-recognin). (A) Substrate channeling. Nt-residues are denoted by single-letter abbreviations. Yellow ovals denote the rest of a protein substrate. A chain of black ovals is a substrate-linked poly-Ub chain. The hypothetical possibility of substrate channeling is diagrammed as a sequence of reaction/relocation steps for an (initially) Nt-Asn-bearing substrate. The substrate is sequentially Nt-deamidated and Nt-arginylated, then relocates and binds, via its conjugated Nt-Arg residue, to scUBR1–scRAD6 E3-E2, and is polyubiquitylated by this Ub ligase, followed by the proteasome-mediated degradation of substrate. The diagram uses notations of transition probabilities, at each reaction/relocation step, for a molecule of an (initially) Nt-Asn-bearing substrate. P1r, P2r, and P3r denote the probabilities of substrate moving through each reaction/relocation step while being retained (“r”) within the targeting complex (Fig. 6B). P1d, P2d, and P3d denote the “alternative” probabilities of substrate’s dissociation (”d”), at each reaction/relocation step, into the bulk solution. An efficacious (as distinguished from weak or nonexistent) substrate channeling would obtain, in this setting, if P1d << P1r, P2d << P2r, and P3d << P3r. (B) Superchanneling. In the hypothetical concept of superchanneling (see Results and Discussion), the transitions (and associated probabilities) that are shown in A are “supplemented” with probabilities of superchanneling (“sc”) transitions (P1sc, P2sc, and P3sc) at each reaction/relocation step. These transitions would result, at each step, in a bypass of downstream steps and direct polyubiquitylation of the substrate. In the language of probability-based transitions, an efficacious superchanneling would obtain, for example, if P1r << P1sc and P1d << P1sc; that is, if the initial binding of an Nt-Asn–bearing substrate to the complex-bound scNTA1 Nt-amidase would be rapidly followed by substrate’s polyubiquitylation, without the necessity of (mechanistically independent) Nt modifications and accompanying physical relocations of the substrate. Superchanneling would not occur if P1sc, P2sc, and P3sc are negligible in comparison to corresponding P1r and P1d probabilities.
The Yeast Nt-Amidase scNTA1 Binds to scATE1 R-Transferase but Not to scUBR1 E3.
We asked whether the S. cerevisiae Arg/N-degron–targeting complex may be larger than the previously identified scUBR1/scRAD6–scUFD4/scUBC4/5 complex, in which two interacting E3s are bound to their respective E2 enzymes (65, 103). scNTA1, encoding the Asn/Gln/Nt-amidase, contains Met AUG codons at positions 1 and 11. The sequence of scNTA1 before Met11 is similar to a typical mitochondrial presequence, in agreement with the presence of scNTA1 in both the cytosol/nucleus and mitochondrial matrix (61, 62, 104). Y2Hs detected the binding of scATE1 R-transferase to scNTA1 Nt-amidase (the cytosolic version of it, initiated at Met11). This interaction was observed with two configurations of Y2H-based scNTA1 fusions (Fig. 3A, rows 4 to 9). Y2Hs did not detect the binding of scNTA1 to scUBR1 E3, in all eight Nt/Ct permutations of the DBD and AD domains linked to scNTA1 and scUBR1 (SI Appendix, Fig. S3A, rows 1 to 6).
The Yeast scATE1 R-Transferase Binds to scUBR1 E3.
Y2Hs detected the binding of scATE1 to scUBR1 (Fig. 3A, rows 1 to 3). Thus, although the scNTA1 Nt-amidase apparently does not interact directly with scUBR1 (see above), scNTA1 would still be expected to be a part of the targeting complex, since scNTA1 binds to scATE1 R-transferase (Fig. 3A, rows 4 to 9), and the latter, in turn, binds to scUBR1 (Fig. 3A, rows 1 to 3, and Fig. 6B).
The Yeast scUFD4 E3 Binds to the scUBR1 E3 N-Recognin.
Previous work, mentioned above, used split-Ub and co-IP binding assays to discover the functionally relevant double-E3 complex scUBR1–scRAD6/scUFD4–scUBC4/5 (65). We used Y2H assays [not employed in the earlier study (65)] to confirm the binding of scUBR1 to scUFD4 in double-mutant [ubr1Δ ufd4Δ] S. cerevisiae that lacked the endogenous forms of these yeast E3s, thereby precluding the possibility of effects by endogenous scUBR1/scUFD4 (Fig. 3B, rows 1 to 3).
The Yeast scUBR1 E3 N-Recognin Does Not Self-Interact.
Studies that used gel-filtration assays indicated that the 225-kDa scUBR1 E3 N-recognin does not homodimerize at least in vitro (105, 106). In agreement with these results, Y2Hs with scUBR1 vs. itself in [ubr1Δ ufd4Δ] cells also did not detect a self-interaction of scUBR1 (SI Appendix, Fig. S3B, rows 10 to 12).
The Yeast scUFD4 E3 Self-Interacts.
Y2Hs with scUFD4 vs. itself in [ubr1Δ ufd4Δ] S. cerevisiae indicated a self-interaction (presumably homodimerization) of scUFD4 E3 (Fig. 3B, rows 4 to 6, and SI Appendix, Fig. S3B, rows 1 to 3). At the same time, both Y2Hs, split-Ub, and co-IP assays indicated that scUFD4 E3 also binds to scUBR1 E3 (Fig. 3B, rows 1 to 3) (65). It is unknown whether self-binding by scUFD4 is mutually exclusive with the scUFD4–scUBR1 interaction. Given the apparently monomeric state of scUBR1 in the absence of scUFD4 (105, 106) (SI Appendix, Fig. S3B, rows 10 to 12), the in vivo stoichiometry of these (interacting) E3s in the yeast Arg/N-degron–targeting complex remains to be determined (Fig. 6B).
The Yeast scUFD4 E3 Does Not Bind to Nt-Amidase scNTA1 and R-Transferase scATE1.
We also used Y2Hs to ask whether scUFD4 E3 (which binds to scUBR1, forming a double-E3 complex; see above) might interact with scNTA1 Nt-amidase and scATE1 R-transferase. Negative results (SI Appendix, Fig. S3B, rows 4 to 9) suggested (but did not prove) that there is no interaction between scUFD4 E3 and either scNTA1 or scATE1. In contrast, scUBR1 interacts with scATE1 (Fig. 3A, rows 1 to 3), and is therefore expected to be indirectly bound to scNTA1 as well, since the latter interacts with scATE1 (Fig. 3A, rows 4 to 9, and Fig. 6B).
On Substrate Channeling in the Arg/N-Degron Pathway.
A likely function of the human and yeast Arg/N-degron–targeting complexes is substrate channeling, in which a reaction intermediate can be transferred between active sites of interacting enzymes without release of intermediate to the bulk solution (Figs. 6 and 7A). Potential advantages of channeling include its higher kinetic efficacy as well as protection/shielding of reactive and toxic intermediates (77–85). An example of direct channeling is tryptophan (Trp) synthase. Its two active sites are linked by an intraprotein tunnel. The product of the first catalyzed reaction, indole, diffuses through the tunnel from the first to the second active site, where Trp is formed from indole and a derivative of serine (83).
In a different setting, called proximity channeling, and possibly relevant to multienzyme complexes of Arg/N-degron pathways, two or more enzymes are spatially close (e.g., through their physical interaction), so that an intermediate produced by the first enzyme can be captured (most of the time or some of the time) by the second enzyme before the intermediate can diffuse away into the bulk solution. In some settings, a proximity channeling would be kinetically superior to a nonchanneled succession of the same reactions only if many quasi-identical complexes are spatially clustered together (81).
Aggregates of complexes are indeed observed with some multienzyme assemblies (77–85). Such aggregates include natural and dynamic associations of multiple 26S proteasome particles that occur in the bulk cytosol, inside the nucleus, and at the nuclear envelope (ref. 107 and references therein). Interestingly, the Arg/N-degron–targeting complexes described in the present study (Fig. 6) apparently interact, through their E3s, with the 26S proteasome (76) (Introduction and Concluding Remarks). If Arg/N-degron–targeting complexes associate with naturally aggregated 26S proteasomes in vivo (this remains to be determined), such multienzyme assemblies may at least facilitate and possibly enable the still hypothetical substrate channeling function of targeting complexes in the Arg/N-degron pathway.
The Concept of Superchanneling.
Specific features of targeting complexes and Arg/N-degron substrates (Figs. 1, 6, and 7) led us to a novel possibility vis-à-vis these complexes. The envisioned mechanism, termed “superchanneling,” is mutually nonexclusive vis-à-vis substrate channeling (Fig. 7B). We begin description of superchanneling by noting that previously characterized cases of substrate channeling are quite distinct from transitions that involve Nt modifications of Asn, Gln, Asp, Glu, or (oxidized) Cys, followed by downstream reactions (Fig. 1). First, an Arg/N-degron substrate is protein-sized (i.e., it’s large and conformationally dynamic). Second, modification of a substrate that immediately precedes (and causes) its capture by the 26S proteasome is the formation of a poly-Ub linked to a substrate’s cognate internal Lys residue. This reaction is not inherently sequential vis-à-vis the obligatorily sequential Nt modifications of, for example, an Nt-Asn–bearing substrate (Figs. 1 and 7).
Consider the S. cerevisiae targeting complex (Fig. 6B). [The same arguments are relevant to the human complex (Fig. 6A).] To simplify discussion, we leave aside the scUFD4–scUBC4/5 part of the yeast complex (scUFD4 E3 is not an N-recognin) (65). Suppose that in the (currently unknown) 3D structure of this complex, the substrate-binding sites of scNTA1 Nt-amidase, scATE1 R-transferase, and scUBR1–scRAD6 E3-E2 face each other in such a way that movements of the N terminus of an (initially) Nt-Asn–bearing substrate between these sites upon a substrate’s successive Nt modifications involve short distances, equal to or smaller than the sizes of protein molecules involved. These movements would culminate in relocation of the substrate’s newly acquired (conjugated) Nt-Arg residue from the active site of scATE1 R-transferase to the complex-bound scUBR1 N-recognin E3. We suggest that this setting may enable a process termed superchanneling (Fig. 7B).
Specifically, an Nt-Asn–bearing substrate would be captured, initially, from the bulk solution by the targeting complex-bound scNTA1 Nt-amidase (Figs. 1A and 7B). Depending on the spatial localization and conformational mobility of a substrate’s cognate internal lysine (i.e., the Lys residue to be polyubiquitylated), this lysine may find itself, upon substrate’s capture by Nt-amidase, in a stereo/catalytically favorable position vis-à-vis the active site of the scRAD6 Ub-conjugating (E2) enzyme, which is bound to scUBR1 E3 in the targeting complex (Fig. 7B). If so, successive Nt modifications of the captured substrate can proceed on their own, while the (initially) Nt-Asn–bearing substrate may be polyubiquitylated (at the above internal lysine) either immediately or at any later time after substrate’s capture by the complex-bound Nt-amidase. In this case, a polyubiquitylation of the substrate would be able to bypass (i.e., would not have to wait for) either Nt modifications of the substrate or its relocation (after Nt-arginylation) to the Nt-Arg–binding domain of scUBR1 E3 (Figs. 1A and 7B): Hence, the term superchanneling. It refers to the capture of, for example, an Nt-Asn–bearing substrate by Nt-amidase, to a bypass of Nt modifications/relocations of this substrate, to a bypass of relocation-mediated capture of Nt-arginylated substrate by E3, and also to channeling, for example, to the final modification (polyubiquitylation) of the substrate without its dissociation into the bulk solution.
In this hypothetical mechanism, a major role of the targeting complex-bound scNTA1 Nt-amidase and scATE1 R-transferase would be their physical affinity for their cognate Arg/N-degron substrates. This affinity may allow the complex-bound scUBR1–scRAD6 E3-E2 to begin producing a substrate-linked poly-Ub without (necessarily) waiting for Nt modifications and relocations of a bound substrate to run their course.
The actual disposition, to be addressed experimentally, can range (depending on a specific Nt-Asn–bearing protein substrate) from complete absence of superchanneling to efficacious superchanneling. In the former case, Nt modifications of a substrate and relocation of the resulting Nt-Arg to E3 N-recognin would have to precede the substrate’s polyubiquitylation. In the latter case, polyubiquitylation of a substrate may become stereo/catalytically feasible immediately after its capture by the complex-bound Nt-amidase (Fig. 7B).
The efficacy of classic substrate channeling would be determined by the rates of individual reactions within the targeting complex vis-à-vis the rates of substrate’s escape into the bulk solution at each reaction/relocation step. In this setting, superchanneling may either obtain or not obtain essentially independently of whether or not a targeting complex exhibits an efficacious substrate channeling. The envisioned, and so far hypothetical, processes of substrate channeling and superchanneling are diagrammed in Fig. 7, using notations of transition probabilities for each reaction step in these mutually compatible mechanisms.
For now, we leave aside approaches to measuring the extent of substrate channeling in these settings and consider a way to address the existence and efficacy of superchanneling (Fig. 7B). Molar ratios of enzymes that reside in an Arg/N-degron–targeting complex to their “free” counterparts in the bulk solution are unknown, awaiting measurements of this partitioning in unperturbed in vivo settings. Outside of a targeting complex, in the bulk solution, the enzymatic activities of scNTA1 Nt-amidase and scATE1 R-transferase, and the Nt-Arg–binding capability of scUBR1 E3 would be strictly required for a (later) polyubiquitylation of, for example, an initially Nt-Asn–bearing substrate. That is so because scUBR1 E3 would be unable to recognize (bind to) the above substrate in the bulk solution until after a substrate’s Nt modifications have run their course and yielded the (conjugated) Nt-Arg residue that can be (noncovalently) captured by scUBR1 (Figs. 1A and 7).
In contrast, the enzymatic activities of Nt-amidase and R-transferase might be dispensable for superchanneling, which can occur only within a targeting complex. Specifically, if superchanneling is efficacious, the Nt-deamidation activity of the complex-bound scNTA1 Nt-amidase would not be strictly required (or may not be required at all) for polyubiquitylation of an (initially) Nt-Asn–bearing substrate. A nearly wild-type level of physical affinity of scNTA1 for Nt-Asn would still be essential, so that a substrate could be (reversibly) captured by the complex-bound Nt-amidase and thereby (possibly) placed spatially close to the complex-bound scUBR1–RAD6 E3-E2 Ub ligase (Figs. 1A and 7). But Nt-deamidation activity of Nt-amidase may be dispensable in this setting, given the logic of superchanneling model (Fig. 7B).
The crystal structure of the S. cerevisiae scNTA1 Nt-amidase was determined by Song and colleagues (62), who also characterized the active site of scNTA1. To address the hypothesis of superchanneling, one could replace, using reverse genetics, the scNTA1 gene with its allele that encodes a missense active-site mutant of scNTA1 that is inactive as Nt-amidase but is essentially unchanged in its affinity for Nt-Asn–bearing substrates. Whether an enzymatically inactive scNTA1 mutant that retains affinity for Nt-Asn is a technically realistic proposition remains to be seen. If the Arg/N-degron pathway exhibits superchanneling, yeast cells expressing wild-type levels of mutant scNTA1 (enzymatically inactive but binding, in particular, to Nt-Asn) might continue to polyubiquitylate and degrade an Nt-Asn–bearing substrate despite the absence of Nt-deamidation in these cells. In contrast, nta1Δ cells, which lack the entire scNTA1 protein, would be unable to target and degrade such a substrate, as has been shown previously (61).
Even a partial retention of polyubiquitylation and degradation of an Nt-Asn–bearing substrate in a missense nta1 mutant that satisfies the above constraints would suggest the existence of superchanneling. Conversely, if an enzymatically inactive scNTA1 protein (that retains the affinity for Nt-Asn) would be unable to support degradation of an Nt-Asn–bearing substrate, it would be evidence for a weak or absent superchanneling of that substrate.
A general notion of superchanneling is as follows. Consider a multienzyme complex that catalyzes modifications of a protein substrate. Some of these modifications are obligatorily sequential, some are not. At least one (“last”) enzyme of the complex can modify the substrate independently of other enzymes. However, the last enzyme cannot bind to (i.e., does not have affinity for) the initial substrate, whereas the first, “upstream” enzyme of the multienzyme complex does. In such a setting, the binding of upstream enzyme to the initial substrate may bring the latter into a stereo/catalytically favorable proximity to the last enzyme of the complex, thereby enabling a substrate’s modification by the last enzyme. That modification would bypass, either entirely or in part, the intermediate modification steps. Such a bypass (referred to as superchanneling) may be possible within a proximity-enabling targeting complex, but would be impossible with free enzymes in the bulk solution. In the latter case, intermediate modification steps would be essential for a productive binding of the last enzyme to the (properly modified) substrate. We are working to verify the idea of superchanneling and to also determine the extent of substrate channeling in the yeast and human Arg/N-degron pathways.
Concluding Remarks.
Three-dimensional structures of Arg/N-degron–targeting complexes are unknown. This information would be essential for a deeper understanding of these multienzyme assemblies. A previous study has shown that scUBR1 and scUFD4 E3s, which bind to each other (65), also bind to the 26S proteasome (76). That evidence, if viewed together with the present results (Fig. 6), suggests an even larger juggernaut of an Arg/N-degron–targeting complex that contains the proteasome as well.
Now that targeting complexes of the Arg/N-degron pathway have been found to exist but remain to be understood, one set of salient questions is about their dynamics and composition. How stable (or fleeting) are these complexes in living cells? How to detect and monitor a “complete” targeting complex in vivo? Does assembly of these complexes require chaperones? Do these complexes form constitutively or conditionally, depending on cell’s metabolic state? Do they contain other components? [An example of an untested candidate component is hsLIAT1, a human protein of unknown function that specifically binds to R-transferase hsATE1 (108).] It also remains to be determined whether UBR4 (570 kDa) and UBR5 (300 kDa), two other E3 N-recognins of the Arg/N-degron pathway in animals and plants (Fig. 1B) (2, 24, 66–68), function as subunits of multienzyme complexes that are analogous to those containing UBR1 or UBR2.
Materials and Methods
For further information, see SI Appendix, SI Materials and Methods.
Yeast Strains and Genetic Techniques.
S. cerevisiae strains used in this work are described on SI Appendix, Table S1. Standard techniques were used for strain construction and transformation.
Construction of Plasmids.
The plasmids are described in SI Appendix, Table S2. Construction details are described in SI Appendix, Materials and Methods.
Two-Hybrid Assays.
Y2H assays (87) were carried out as described previously (9, 10, 28, 86). In both Y2H and split-Ub assays, the expression of HIS3 (a readout of both assays), in otherwise His− cells, was a function of affinity between test proteins.
Split-Ub Assays.
Yeast-based split-Ub binding assays (93, 94) were carried out largely as described previously (9, 28).
GST-Pulldowns.
These binding assays were carried out with yeast extracts largely as described previously (90), using, in particular, E. coli-expressed, purified fusions of human test proteins with GST.
Co-IP Assays.
These binding assays were carried out with yeast extracts largely as described previously (90), using engineered S. cerevisiae strains that stably coexpressed specific epitope-tagged human test proteins from their integrated cDNAs (SI Appendix, Materials and Methods).
Data Availability.
All relevant data in the paper are entirely available through both text and figures, in the main text and SI Appendix.
Supplementary Material
Acknowledgments
We thank current and former members of the A.V. laboratory for their advice and assistance. This work was supported by the NIH Grants 1R01DK039520 and 1R01GM031530 (to A.V.).
Footnotes
The authors declare no competing interest.
†“Sequelog” denotes a sequence that is similar, to a specified extent, to another sequence (71). Derivatives of sequelog include “sequelogy” (sequence similarity) and “sequelogous” (similar in sequence). The usefulness of sequelog and derivative notations stems from the rigor and clarity of their evolutionary neutrality. In contrast, in settings that use “homolog,” “ortholog,” and “paralog” (they denote, respectively, common descent and functional similarity/dissimilarity), these terms are often interpretation-laden and can be imprecise. Homolog, ortholog, and paralog are compatible with the sequelog terminology. The former terms can be used to convey understanding about common descent and biological functions, if this additional information, distinct from sequelogy per se, is actually present (71).
This article contains supporting information online at https://www.pnas.org/lookup/suppl/doi:10.1073/pnas.2003043117/-/DCSupplemental.
References
- 1.Hershko A., Ciechanover A., Varshavsky A., Basic Medical Research Award. The ubiquitin system. Nat. Med. 6, 1073–1081 (2000). [DOI] [PubMed] [Google Scholar]
- 2.Varshavsky A., N-degron and C-degron pathways of protein degradation. Proc. Natl. Acad. Sci. U.S.A. 116, 358–366 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Finley D., Ulrich H. D., Sommer T., Kaiser P., The ubiquitin-proteasome system of Saccharomyces cerevisiae. Genetics 192, 319–360 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Vittal V., Stewart M. D., Brzovic P. S., Klevit R. E., Regulating the regulators: Recent revelations in the control of E3 ubiquitin ligases. J. Biol. Chem. 290, 21244–21251 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pohl C., Dikic I., Cellular quality control by the ubiquitin-proteasome system and autophagy. Science 366, 818–822 (2019). [DOI] [PubMed] [Google Scholar]
- 6.Ji C. H., Kwon Y. T., Crosstalk and interplay between the ubiquitin-proteasome system and autophagy. Mol. Cells 40, 441–449 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Balchin D., Hayer-Hartl M., Hartl F. U., In vivo aspects of protein folding and quality control. Science 353, aac4354 (2016). [DOI] [PubMed] [Google Scholar]
- 8.Sun Z., Brodsky J. L., Protein quality control in the secretory pathway. J. Cell Biol. 218, 3171–3187 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Oh J. H., Hyun J. Y., Varshavsky A., Control of Hsp90 chaperone and its clients by N-terminal acetylation and the N-end rule pathway. Proc. Natl. Acad. Sci. U.S.A. 114, E4370–E4379 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chen S. J., Melnykov A., Varshavsky A., Evolution of substrates and components of the pro/N-degron pathway. Biochemistry 59, 582–593 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zheng N., Shabek N., Ubiquitin ligases: Structure, function, and regulation. Annu. Rev. Biochem. 86, 129–157 (2017). [DOI] [PubMed] [Google Scholar]
- 12.Watson E. R., Brown N. G., Peters J. M., Stark H., Schulman B. A., Posing the APC/C E3 ubiquitin ligase to orchestrate cell division. Trends Cell Biol. 29, 117–134 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schapira M., Calabrese M. F., Bullock A. N., Crews C. M., Targeted protein degradation: Expanding the toolbox. Nat. Rev. Drug Discov. 18, 949–963 (2019). [DOI] [PubMed] [Google Scholar]
- 14.Bard J. A. M. et al., Structure and function of the 26S proteasome. Annu. Rev. Biochem. 87, 697–724 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schweitzer A. et al., Structure of the human 26S proteasome at a resolution of 3.9 Å. Proc. Natl. Acad. Sci. U.S.A. 113, 7816–7821 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Finley D., Prado M. A., The proteasome and its network: Engineering for adaptability. Cold Spring Harb. Perspect. Biol. 12, a033985 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Collins G. A., Goldberg A. L., The logic of the 26S proteasome. Cell 169, 792–806 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Budenholzer L., Cheng C. L., Li Y., Hochstrasser M., Proteasome structure and assembly. J. Mol. Biol. 429, 3500–3524 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yu H., Matouschek A., Recognition of client proteins by the proteasome. Annu. Rev. Biophys. 46, 149–173 (2017). [DOI] [PubMed] [Google Scholar]
- 20.Bachmair A., Finley D., Varshavsky A., In vivo half-life of a protein is a function of its amino-terminal residue. Science 234, 179–186 (1986). [DOI] [PubMed] [Google Scholar]
- 21.Bachmair A., Varshavsky A., The degradation signal in a short-lived protein. Cell 56, 1019–1032 (1989). [DOI] [PubMed] [Google Scholar]
- 22.Varshavsky A., The N-end rule pathway and regulation by proteolysis. Protein Sci. 20, 1298–1345 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dougan D. A., Micevski D., Truscott K. N., The N-end rule pathway: From recognition by N-recognins, to destruction by AAA+proteases. Biochim. Biophys. Acta 1823, 83–91 (2012). [DOI] [PubMed] [Google Scholar]
- 24.Tasaki T., Sriram S. M., Park K. S., Kwon Y. T., The N-end rule pathway. Annu. Rev. Biochem. 81, 261–289 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Aksnes H., Drazic A., Marie M., Arnesen T., First things first: Vital protein marks by N-terminal acetyltransferases. Trends Biochem. Sci. 41, 746–760 (2016). [DOI] [PubMed] [Google Scholar]
- 26.Gibbs D. J., Bacardit J., Bachmair A., Holdsworth M. J., The eukaryotic N-end rule pathway: Conserved mechanisms and diverse functions. Trends Cell Biol. 24, 603–611 (2014). [DOI] [PubMed] [Google Scholar]
- 27.Dissmeyer N., Rivas S., Graciet E., Life and death of proteins after protease cleavage: Protein degradation by the N-end rule pathway. New Phytol. 218, 929–935 (2018). [DOI] [PubMed] [Google Scholar]
- 28.Chen S. J., Wu X., Wadas B., Oh J.-H., Varshavsky A., An N-end rule pathway that recognizes proline and destroys gluconeogenic enzymes. Science 355, eaal3655 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dong C. et al., Molecular basis of GID4-mediated recognition of degrons for the pro/N-end rule pathway. Nat. Chem. Biol. 14, 466–473 (2018). [DOI] [PubMed] [Google Scholar]
- 30.Dougan D. A., Varshavsky A., Understanding the pro/N-end rule pathway. Nat. Chem. Biol. 14, 415–416 (2018). [DOI] [PubMed] [Google Scholar]
- 31.Kim J. M. et al., Formyl-methionine as an N-degron of a eukaryotic N-end rule pathway. Science 362, eaat0174 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hwang C. S., Shemorry A., Varshavsky A., N-terminal acetylation of cellular proteins creates specific degradation signals. Science 327, 973–977 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Piatkov K. I., Brower C. S., Varshavsky A., The N-end rule pathway counteracts cell death by destroying proapoptotic protein fragments. Proc. Natl. Acad. Sci. U.S.A. 109, E1839–E1847 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Brower C. S., Piatkov K. I., Varshavsky A., Neurodegeneration-associated protein fragments as short-lived substrates of the N-end rule pathway. Mol. Cell 50, 161–171 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kasu Y. A. T., Alemu S., Lamari A., Loew N., Brower C. S., The N-termini of TAR DNA-binding protein 43 (TDP43) C-terminal fragments influence degradation, aggregation propensity, and morphology. Mol. Cell. Biol. 38, e00243-18 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kim H. K. et al., The N-terminal methionine of cellular proteins as a degradation signal. Cell 156, 158–169 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nguyen K. T. et al., N-terminal acetylation and the N-end rule pathway control degradation of the lipid droplet protein PLIN2. J. Biol. Chem. 294, 379–388 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Shemorry A., Hwang C. S., Varshavsky A., Control of protein quality and stoichiometries by N-terminal acetylation and the N-end rule pathway. Mol. Cell 50, 540–551 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Shearer R. F., Iconomou M., Watts C. K., Saunders D. N., Functional roles of the E3 ubiquitin ligase UBR5 in cancer. Mol. Cancer Res. 13, 1523–1532 (2015). [DOI] [PubMed] [Google Scholar]
- 40.Rivera-Rivera I., Román-Hernández G., Sauer R. T., Baker T. A., Remodeling of a delivery complex allows ClpS-mediated degradation of N-degron substrates. Proc. Natl. Acad. Sci. U.S.A. 111, E3853–E3859 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yoo Y. D. et al., N-terminal arginylation generates a bimodal degron that modulates autophagic proteolysis. Proc. Natl. Acad. Sci. U.S.A. 115, E2716–E2724 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Vicente J. et al., The Cys-Arg/N-end rule pathway is a general sensor of abiotic stress in flowering plants. Curr. Biol. 27, 3183–3190.e4 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Oh J. H., Chen S. J., Varshavsky A., A reference-based protein degradation assay without global translation inhibitors. J. Biol. Chem. 292, 21457–21465 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Masson N. et al., Conserved N-terminal cysteine dioxygenases transduce responses to hypoxia in animals and plants. Science 365, 65–69 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gao X., Yeom J., Groisman E. A., The expanded specificity and physiological role of a widespread N-degron recognin. Proc. Natl. Acad. Sci. U.S.A. 116, 18629–18637 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Timms R. T. et al., A glycine-specific N-degron pathway mediates the quality control of protein N-myristoylation. Science 365, eaaw4912 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Park J. S. et al., Structural analyses on the deamidation of N-terminal Asn in the human N-degron pathway. Biomolecules 10, 163 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Qiao S. et al., Interconversion between anticipatory and active GID E3 ubiquitin ligase conformations via metabolically driven substrate receptor assembly. Mol. Cell 77, 150–163.e9 (2020). [DOI] [PubMed] [Google Scholar]
- 49.Inobe T., Fishbain S., Prakash S., Matouschek A., Defining the geometry of the two-component proteasome degron. Nat. Chem. Biol. 7, 161–167 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kwon Y. T. et al., Altered activity, social behavior, and spatial memory in mice lacking the NTAN1p amidase and the asparagine branch of the N-end rule pathway. Mol. Cell. Biol. 20, 4135–4148 (2000). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Piatkov K. I., Oh J.-H., Liu Y., Varshavsky A., Calpain-generated natural protein fragments as short-lived substrates of the N-end rule pathway. Proc. Natl. Acad. Sci. U.S.A. 111, E817–E826 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Eldeeb M. A., Fahlman R. P., The anti-apoptotic form of tyrosine kinase Lyn that is generated by proteolysis is degraded by the N-end rule pathway. Oncotarget 5, 2714–2722 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Graciet E. et al., Aminoacyl-transferases and the N-end rule pathway in a human pathogen. Proc. Natl. Acad. Sci. U.S.A. 103, 3078–3083 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Piatkov K. I., Colnaghi L., Békés M., Varshavsky A., Huang T. T., The auto-generated fragment of the Usp1 deubiquitylase is a physiological substrate of the N-end rule pathway. Mol. Cell 48, 926–933 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Gibbs D. J., Holdsworth M. J., Every breath you take: New insights into plant and animal oxygen sensing. Cell 180, 22–24 (2020). [DOI] [PubMed] [Google Scholar]
- 56.Weaver B. P., Weaver Y. M., Mitani S., Han M., Coupled caspase and N-end rule ligase activities allow recognition and degradation of pluripotency factor LIN-28 during non-apoptotic development. Dev. Cell 41, 665–673.e6 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Nillegoda N. B. et al., Ubr1 and Ubr2 function in a quality control pathway for degradation of unfolded cytosolic proteins. Mol. Biol. Cell 21, 2102–2116 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Yamano K., Youle R. J., PINK1 is degraded through the N-end rule pathway. Autophagy 9, 1758–1769 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Choi W. S. et al., Structural basis for the recognition of N-end rule substrates by the UBR box of ubiquitin ligases. Nat. Struct. Mol. Biol. 17, 1175–1181 (2010). [DOI] [PubMed] [Google Scholar]
- 60.Matta-Camacho E., Kozlov G., Li F. F., Gehring K., Structural basis of substrate recognition and specificity in the N-end rule pathway. Nat. Struct. Mol. Biol. 17, 1182–1187 (2010). [DOI] [PubMed] [Google Scholar]
- 61.Baker R. T., Varshavsky A., Yeast N-terminal amidase. A new enzyme and component of the N-end rule pathway. J. Biol. Chem. 270, 12065–12074 (1995). [DOI] [PubMed] [Google Scholar]
- 62.Kim M. K., Oh S. J., Lee B. G., Song H. K., Structural basis for dual specificity of yeast N-terminal amidase in the N-end rule pathway. Proc. Natl. Acad. Sci. U.S.A. 113, 12438–12443 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Turner G. C., Du F., Varshavsky A., Peptides accelerate their uptake by activating a ubiquitin-dependent proteolytic pathway. Nature 405, 579–583 (2000). [DOI] [PubMed] [Google Scholar]
- 64.Du F., Navarro-Garcia F., Xia Z., Tasaki T., Varshavsky A., Pairs of dipeptides synergistically activate the binding of substrate by ubiquitin ligase through dissociation of its autoinhibitory domain. Proc. Natl. Acad. Sci. U.S.A. 99, 14110–14115 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Hwang C. S., Shemorry A., Auerbach D., Varshavsky A., The N-end rule pathway is mediated by a complex of the RING-type Ubr1 and HECT-type Ufd4 ubiquitin ligases. Nat. Cell Biol. 12, 1177–1185 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kim S. T. et al., The N-recognin UBR4 of the N-end rule pathway is required for neurogenesis and homeostasis of cell surface proteins. PLoS One 13, e0202260 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Rinschen M. M. et al., The ubiquitin ligase Ubr4 controls stability of podocin/MEC-2 supercomplexes. Hum. Mol. Genet. 25, 1328–1344 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Flack J. E., Mieszczanek J., Novcic N., Bienz M., Wnt-dependent inactivation of the Groucho/TLE co-repressor by the HECT E3 ubiquitin ligase Hyd/UBR5. Mol. Cell 67, 181–193.e5 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Cha-Molstad H. et al., Regulation of autophagic proteolysis by the N-recognin SQSTM1/p62 of the N-end rule pathway. Autophagy 14, 359–361 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Kwon D. H. et al., Insights into degradation mechanism of N-end rule substrates by p62/SQSTM1 autophagy adapter. Nat. Commun. 9, 3291 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Varshavsky A., “Spalog” and “sequelog”: Neutral terms for spatial and sequence similarity. Curr. Biol. 14, R181–R183 (2004). [DOI] [PubMed] [Google Scholar]
- 72.Wang H., Piatkov K. I., Brower C. S., Varshavsky A., Glutamine-specific N-terminal amidase, a component of the N-end rule pathway. Mol. Cell 34, 686–695 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Hu R.-G. et al., The N-end rule pathway as a nitric oxide sensor controlling the levels of multiple regulators. Nature 437, 981–986 (2005). [DOI] [PubMed] [Google Scholar]
- 74.Lee M. J. et al., RGS4 and RGS5 are in vivo substrates of the N-end rule pathway. Proc. Natl. Acad. Sci. U.S.A. 102, 15030–15035 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Park M. S. et al., Crystal structure of human protein N-terminal glutamine amidohydrolase, an initial component of the N-end rule pathway. PLoS One 9, e111142 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Xie Y., Varshavsky A., Physical association of ubiquitin ligases and the 26S proteasome. Proc. Natl. Acad. Sci. U.S.A. 97, 2497–2502 (2000). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Wheeldon I. et al., Substrate channelling as an approach to cascade reactions. Nat. Chem. 8, 299–309 (2016). [DOI] [PubMed] [Google Scholar]
- 78.Raushel F. M., Thoden J. B., Holden H. M., Enzymes with molecular tunnels. Acc. Chem. Res. 36, 539–548 (2003). [DOI] [PubMed] [Google Scholar]
- 79.Welch G. R., Easterby J. S., Metabolic channeling versus free diffusion: Transition-time analysis. Trends Biochem. Sci. 19, 193–197 (1994). [DOI] [PubMed] [Google Scholar]
- 80.Sathyanarayanan N. et al., Molecular basis for metabolite channeling in a ring opening enzyme of the phenylacetate degradation pathway. Nat. Commun. 10, 4127 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Castellana M. et al., Enzyme clustering accelerates processing of intermediates through metabolic channeling. Nat. Biotechnol. 32, 1011–1018 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Zhao X. et al., Substrate-driven chemotactic assembly in an enzyme cascade. Nat. Chem. 10, 311–317 (2018). [DOI] [PubMed] [Google Scholar]
- 83.Dunn M. F., Niks D., Ngo H., Barends T. R., Schlichting I., Tryptophan synthase: The workings of a channeling nanomachine. Trends Biochem. Sci. 33, 254–264 (2008). [DOI] [PubMed] [Google Scholar]
- 84.Lee H., DeLoache W. C., Dueber J. E., Spatial organization of enzymes for metabolic engineering. Metab. Eng. 14, 242–251 (2012). [DOI] [PubMed] [Google Scholar]
- 85.Pedley A. M., Benkovic S. J., A New view into the regulation of purine metabolism: The purinosome. Trends Biochem. Sci. 42, 141–154 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Melnykov A., Chen S. J., Varshavsky A., Gid10 as an alternative N-recognin of the pro/N-degron pathway. Proc. Natl. Acad. Sci. U.S.A. 116, 15914–15923 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Vidal M., Fields S., The yeast two-hybrid assay: Still finding connections after 25 years. Nat. Methods 11, 1203–1206 (2014). [DOI] [PubMed] [Google Scholar]
- 88.Brückner A., Polge C., Lentze N., Auerbach D., Schlattner U., Yeast two-hybrid, a powerful tool for systems biology. Int. J. Mol. Sci. 10, 2763–2788 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Stellberger T. et al., Improving the yeast two-hybrid system with permutated fusions proteins: The Varicella Zoster virus interactome. Proteome Sci. 8, 8 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Ausubel F. M., Ed. et al., Current Protocols in Molecular Biology, (Wiley-Interscience, New York, 2017). [Google Scholar]
- 91.Kwon Y. T. et al., An essential role of N-terminal arginylation in cardiovascular development. Science 297, 96–99 (2002). [DOI] [PubMed] [Google Scholar]
- 92.Kwon Y. T., Kashina A. S., Varshavsky A., Alternative splicing results in differential expression, activity, and localization of the two forms of arginyl-tRNA-protein transferase, a component of the N-end rule pathway. Mol. Cell. Biol. 19, 182–193 (1999). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Johnsson N., Varshavsky A., Split ubiquitin as a sensor of protein interactions in vivo. Proc. Natl. Acad. Sci. U.S.A. 91, 10340–10344 (1994). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Dünkler A., Müller J., Johnsson N., Detecting protein-protein interactions with the split-Ubiquitin sensor. Methods Mol. Biol. 786, 115–130 (2012). [DOI] [PubMed] [Google Scholar]
- 95.Xia Z., Turner G. C., Hwang C. S., Byrd C., Varshavsky A., Amino acids induce peptide uptake via accelerated degradation of CUP9, the transcriptional repressor of the PTR2 peptide transporter. J. Biol. Chem. 283, 28958–28968 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Gudjonsson T. et al., TRIP12 and UBR5 suppress spreading of chromatin ubiquitylation at damaged chromosomes. Cell 150, 697–709 (2012). [DOI] [PubMed] [Google Scholar]
- 97.Park Y., Yoon S. K., Yoon J. B., The HECT domain of TRIP12 ubiquitinates substrates of the ubiquitin fusion degradation pathway. J. Biol. Chem. 284, 1540–1549 (2009). [DOI] [PubMed] [Google Scholar]
- 98.Liu X. et al., Trip12 is an E3 ubiquitin ligase for USP7/HAUSP involved in the DNA damage response. FEBS Lett. 590, 4213–4222 (2016). [DOI] [PubMed] [Google Scholar]
- 99.Hanoun N. et al., The E3 ubiquitin ligase thyroid hormone receptor-interacting protein 12 targets pancreas transcription factor 1a for proteasomal degradation. J. Biol. Chem. 289, 35593–35604 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Holdsworth M. J., Vicente J., Sharma G., Abbas M., Zubrycka A., The plant N-degron pathways of ubiquitin-mediated proteolysis. J. Integr. Plant Biol. 62, 70–89 (2020). [DOI] [PubMed] [Google Scholar]
- 101.Weits D. A. et al., Plant cysteine oxidases control the oxygen-dependent branch of the N-end-rule pathway. Nat. Commun. 5, 3425 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Ciechanover A. et al., Purification and characterization of arginyl-tRNA-protein transferase from rabbit reticulocytes. Its involvement in post-translational modification and degradation of acidic NH2 termini substrates of the ubiquitin pathway. J. Biol. Chem. 263, 11155–11167 (1988). [PubMed] [Google Scholar]
- 103.Dohmen R. J., Madura K., Bartel B., Varshavsky A., The N-end rule is mediated by the UBC2(RAD6) ubiquitin-conjugating enzyme. Proc. Natl. Acad. Sci. U.S.A. 88, 7351–7355 (1991). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Varshavsky A., The N-end rule: Functions, mysteries, uses. Proc. Natl. Acad. Sci. U.S.A. 93, 12142–12149 (1996). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Bartel B., Wünning I., Varshavsky A., The recognition component of the N-end rule pathway. EMBO J. 9, 3179–3189 (1990). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Du F., “Allosteric activation of the ubiquitin ligase UBR1 by short peptides: Molecular mechanisms and physiological functions,” PhD thesis, California Institute of Technology, Pasadena, CA (2001).
- 107.Albert S. et al., Direct visualization of degradation microcompartments at the ER membrane. Proc. Natl. Acad. Sci. U.S.A. 117, 1069–1080 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Brower C. S. et al., Liat1, an arginyltransferase-binding protein whose evolution among primates involved changes in the numbers of its 10-residue repeats. Proc. Natl. Acad. Sci. U.S.A. 111, E4936–E4945 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Xie Y., Varshavsky A., The E2-E3 interaction in the N-end rule pathway: The RING-H2 finger of E3 is required for the synthesis of multiubiquitin chain. EMBO J. 18, 6832–6844 (1999). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Worthylake D. K., Prakash S., Prakash L., Hill C. P., Crystal structure of the Saccharomyces cerevisiae ubiquitin-conjugating enzyme Rad6 at 2.6 A resolution. J. Biol. Chem. 273, 6271–6276 (1998). [DOI] [PubMed] [Google Scholar]
- 111.Cook W. J., Jeffrey L. C., Xu Y., Chau V., Tertiary structures of class I ubiquitin-conjugating enzymes are highly conserved: Crystal structure of yeast Ubc4. Biochemistry 32, 13809–13817 (1993). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All relevant data in the paper are entirely available through both text and figures, in the main text and SI Appendix.