Significance
Polymorphisms in NOD2, an intracellular sensor of bacterial peptidoglycan, lead to a significantly increased risk for developing Crohn’s disease. However, the mechanisms by which altered NOD2 function leads to overt intestinal inflammation remain poorly described. Here, we demonstrate a function of NOD2, whereby its activation leads to the generation of immunological tolerance to foreign antigens by inducing tolerant dendritic cells both systemically and within the intestine. This occurs via the generation of the cytokine GM-CSF, a factor that itself has previously been linked to Crohn’s disease development. The identification of a common immunological pathway shared by these two factors will open up new avenues for therapy and further research to identify the precise etiology of this chronic, debilitating condition.
Keywords: dendritic cells, Tregs, NOD2, Crohn’s disease, GM-CSF
Abstract
Four decades ago, it was identified that muramyl dipeptide (MDP), a peptidoglycan-derived bacterial cell wall component, could display immunosuppressive functions in animals through mechanisms that remain unexplored. We sought to revisit these pioneering observations because mutations in NOD2, the gene encoding the host sensor of MDP, are associated with increased risk of developing the inflammatory bowel disease Crohn’s disease, thus suggesting that the loss of the immunomodulatory functions of NOD2 could contribute to the development of inflammatory disease. Here, we demonstrate that intraperitoneal (i.p.) administration of MDP triggered regulatory T cells and the accumulation of a population of tolerogenic CD103+ dendritic cells (DCs) in the spleen. This was found to occur not through direct sensing of MDP by DCs themselves, but rather via the production of the cytokine GM-CSF, another factor with an established regulatory role in Crohn’s disease pathogenesis. Moreover, we demonstrate that populations of CD103-expressing DCs in the gut lamina propria are enhanced by the activation of NOD2, indicating that MDP sensing plays a critical role in shaping the immune response to intestinal antigens by promoting a tolerogenic environment via manipulation of DC populations.
Crohn’s disease (CD) is an inflammatory bowel disease (IBD) marked by severe, debilitating inflammation of the gastrointestinal tract. While the precise etiology of CD remains elusive, single-nucleotide polymorphisms (SNPs) in more than 160 genes have been identified as probable risk factors for the development of the disease (1). Of these, mutations in NOD2 were the first discovered and most strongly linked to the risk of development of CD (2, 3). Three major coding variant SNPs—R702W, G908R, and L1007fs—have been identified that together increase the risk of an individual developing CD by 2–4× for heterozygotes, and 20–40× for homozygotes and compound heterozygotes (3), while other, less frequent risk-associated variants of NOD2 with similar odds ratio values are continually being discovered (4). NOD2 encodes an intracellular protein expressed by a variety of cells of hematopoietic origin, as well as a subset of nonhematopoietic cells such as intestinal epithelial cells (5, 6). There, it serves as an intracellular bacterial sensor via a leucine-rich repeat domain (LRR)-dependent detection of muramyl dipeptide (MDP), a fragment of the peptidoglycan polymer that is the primary component of the bacterial cell wall (7). This leads to the formation of a multiprotein complex containing a host of ubiquitin ligases and the essential scaffolding protein Receptor-interacting Kinase 2 (RIPK2) (8), which in many cell types is known to lead to the activation of downstream proinflammatory pathways such as NF-κB and MAPK (9, 10). Notably, this function is lost in cells harboring the major CD susceptibility SNPs (11), as these SNPs are all found within the MDP-sensing LRR domain of the protein (3).
While these functions play a vital role in the defense against a number of pathogenic microorganisms (10, 12), it is counterintuitive to link a loss of proinflammatory signaling to the development of overt inflammation seen in patients with CD harboring NOD2 mutations. This raises the possibility that unexplored functions for NOD2 activation exist that better explain its role in the development of CD. Interestingly, long prior to its identification as the activating ligand of NOD2, MDP was identified by a number of groups as an in vivo immunomodulatory compound that was found to be immunosuppressive when delivered at certain doses (13), routes of administration (14), and later time-points following injection (15). Moreover, this suppression appeared to be dependent on T cells, suggesting that MDP may be involved in the generation of a regulatory T cell response (16, 17).
In this study, we set out to reexplore these findings in order to clarify whether NOD2 activation can indeed lead to immunological tolerance and to further explore the underlying mechanisms behind this regulation. We demonstrate that activation of NOD2 via systemic administration of MDP leads to an increase in splenic regulatory T cells (Tregs) and a concomitant inhibition of the immune response, but only after sufficient time has elapsed to drive this response, as this phenotype is only apparent at as many as 7 d postinjection. We provide evidence that this is driven by the NOD2-dependent generation of immature classical dendritic cells (cDC1) marked by the expression of the surface marker CD103, which have been widely associated with the generation of a tolerogenic environment (18–20). Interestingly, we show that this phenotype requires the NOD2-dependent production of the cytokine GM-CSF, which has also been implicated in CD pathogenesis in a number of ways, in particular the emergence of its receptor as a CD genetic risk factor itself (21, 22). This is a study that discovers a functional link between NOD2 and GM-CSF, and these findings will open up further avenues of exploration that could eventually lead to the cure of this debilitating condition.
Results
Systemic Activation of NOD2 Leads to Subsequent Immunosuppression via the Late Induction of Regulatory T Cells.
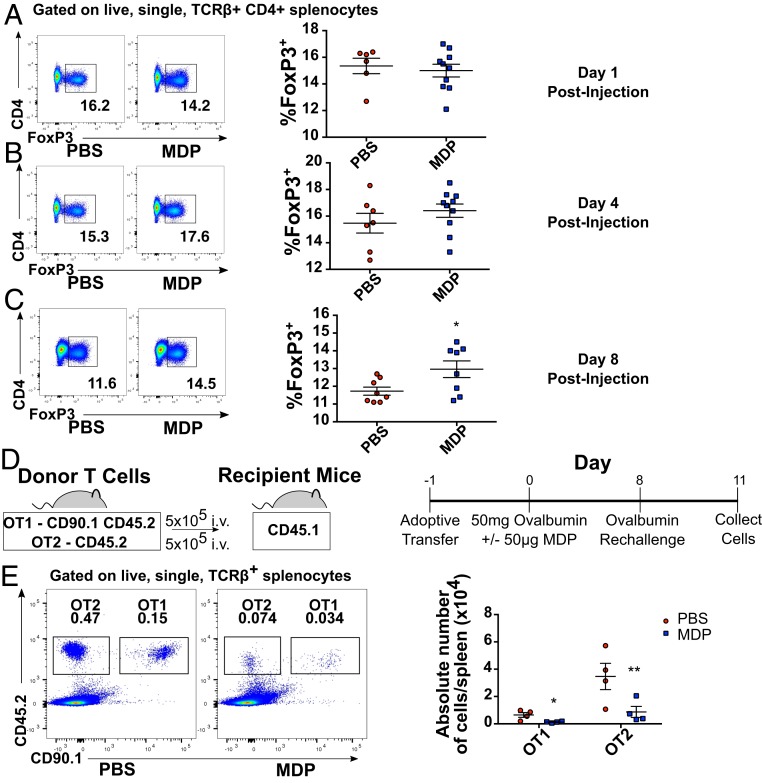
To reexplore the previously described kinetics of NOD2-dependent immunosuppression, we chose to assess whether MDP can induce a time-dependent appearance of regulatory T cells (Tregs). Notably, we found no differences in splenic Treg proportions at early time points postinjection of MDP (days 1 or 4, Fig. 1 A and B), but observed a statistically significant increase in FOXP3-expressing CD4 T cells in the spleens of mice 8 d postinjection with 50 μg of MDP as compared to their vehicle-injected littermate controls (Fig. 1C). Although this increase was marginal, these experiments represented an assessment of the entire Treg repertoire, making the contribution of an increase in a particular subset of Tregs directed toward a specific antigen difficult to detect. We therefore chose to determine the ability of MDP to affect antigen-specific immune responses using the OT-1 and OT-2 transgenic mouse systems. We adoptively transferred OT-1 (CD45.2/CD90.1) and OT-2 (CD45.2) splenocytes expressing congenic surface markers into CD45.1 WT recipients (Fig. 1D), vaccinated them with ultrapure ovalbumin in the presence or absence of MDP, and assessed the proliferative T cell response to antigen reexposure 8 d later. We found a significant dampening of the proliferation of both transferred OT1 and OT2 cells, as measured by the absolute number of OT1 and OT2 cells recovered from the spleen 3 d after the secondary administration of ovalbumin (Fig. 1E). This difference was not likely due to an MDP-induced sequestration of transferred cells in other tissues, as we found no accumulation of OT1 or OT2 cells in the MLN, nor an accumulation of transferred cells circulating in the bloodstream (SI Appendix, Fig. S1). Given that we show here that MDP is capable of simultaneously stimulating Treg populations while preventing the reexpansion of antigen-specific T cells at later timepoints, these results support the previously reported data that systemic administration of MDP is capable of driving a time-dependent inhibition of T cell-mediated immune responses.
Fig. 1.
Systemic administration of MDP leads to the delayed generation of Tregs. Flow cytometry analysis describing the proportion of FOXP3-expessing TCRβ+CD4+ splenocytes at 1 (A), 4 (B), and 8 (C) days postinjection of MDP (50 μg/mL). Two pooled littermate experiments are presented for each time point. (D) Graphical representation of the experimental procedure for experiments performed using adoptive transfer of ovalbumin-specific transgenic T cells. (E) Proportions of adoptively transferred OT1 (CD45.2+CD90.1+) and OT2 (CD45.2+) cells found in the spleen of CD45.1 recipient mice 3 d following rechallenge with ultrapure ovalbumin as assessed by flow cytometry. One representative littermate experiment is shown. For all images, horizontal bars denote the mean, and error bars denote SEM. Asterisks denote significantly different from PBS control as calculated by Student’s t test; *P ≤ 0.05, **P ≤ 0.01.
Systemic Activation of NOD2 Up-Regulates CD103 Expression on Splenic Dendritic Cells.
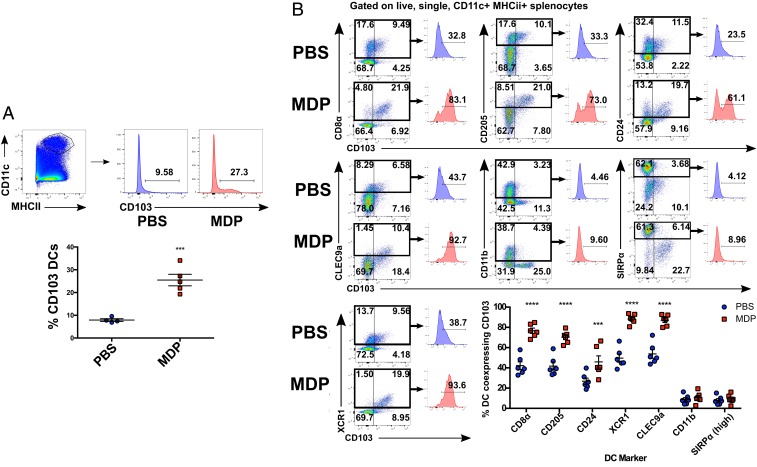
The emergence of peripherally induced Tregs in nonlymphoid tissues such as the intestine has been widely attributed to signals derived from migratory dendritic cells (DCs) expressing the αE integrin CD103 (20, 23). Similarly, lymphoid-resident DCs marked by CD8α and CD205, which are developmentally related to peripheral CD103+ DCs (24), have been shown to have similar properties with respect to their ability to generate Tregs (19) and limit antigen-specific immune responses (25). Thus, we chose to examine the effects of intraperitoneal (i.p.) administration of MDP on the proportions and surface phenotypes of DCs in various immunologic compartments to determine whether NOD2 activation promotes a protolerant DC phenotype. Interestingly, we found that expression of CD103 was significantly up-regulated on DCs collected from the spleen at 16 h after i.p. administration of 50 μg of MDP (Fig. 2A). No such increases in CD103 expression were observed in DCs collected from the thymus or MLN of the same mice (SI Appendix, Fig. S2). Detailed analysis of the surface phenotype of splenic DCs collected from mice injected with MDP revealed that CD103 expression was up-regulated on DCs that coexpress the surface molecules CD8α, CD205, CD24, XCR1, and CLEC9a (a subset commonly referred to as cDC1), while expression of CD103 on DCs expressing CD11b and SIRPα (cDC2) remained unchanged (Fig. 2B). Moreover, mice deficient in the transcription factor Batf3, which is required for the differentiation of the cDC1 lineage (26), were found to have drastically lower levels of CD103 expression on splenic DCs at baseline, and MDP injection failed to induce any changes in CD103 status (SI Appendix, Fig. S3), indicating that MDP modulated CD103 expression on true cDC1 cells. Importantly, the CD103+ subset of cDC1 in particular has been found to be specifically crucial for the development of peripheral self-tolerance (18). As has previously been described by Yamazaki et al. (19), we found that CD205+CD8α+ DCs purified by FACS and loaded with ovalbumin spontaneously converted naïve OT2 T cells into Tregs in a TGF-β–dependent manner (SI Appendix, Fig. S4). While this ability was not further augmented by MDP treatment and the resulting up-regulation of CD103, these data reveal a method by which MDP is capable of obviously modulating the phenotype of a tolerance-inducing cell type, which warranted our further exploration.
Fig. 2.
Systemic activation of NOD2 leads to increased CD103 expression on splenic cDC1s. (A) Flow cytometry analysis of CD103 expression on CD11c+MHCII+ cells from murine spleen following injection with MDP (50 μg i.p.). (B) Flow cytometry analysis of CD103 expression on DCs displaying specific subset markers. Individual mice from one representative experiment are denoted on the plots. Horizontal bars denote the mean, and error bars denote SEM. Asterisks denote significantly different from PBS treated as calculated by two-way ANOVA followed by Tukey’s multiple comparisons test; *P < 0.05; ***P ≤ 0.001; ****P ≤ 0.0001.
The Induction of CD103 on the Surface of Splenic cDC1s Is Dependent on Nod-Like Receptor Activation.
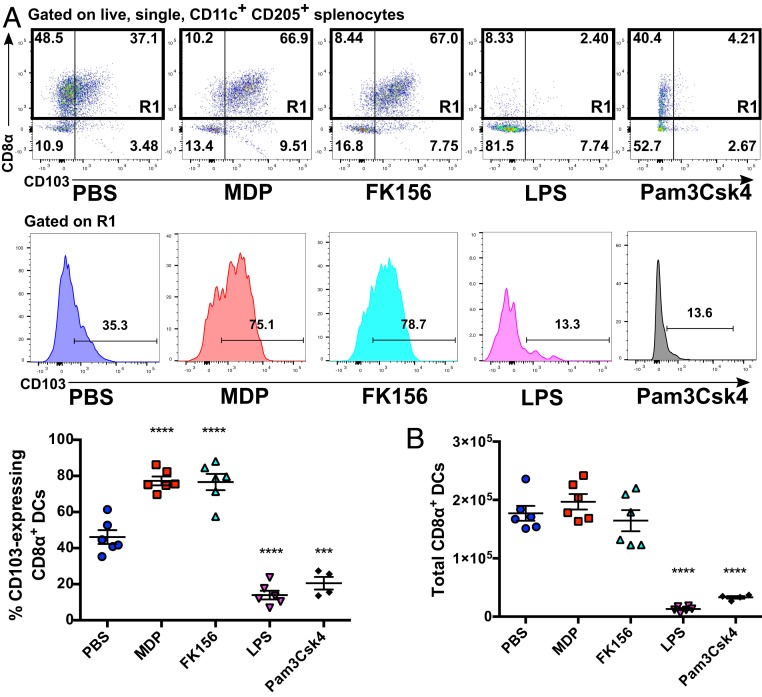
Our next aim was to determine whether this phenotype was a hallmark of DC activation by microbial products in general, or if it was specific to activation of peptidoglycan-sensing NOD-like receptors (NLRs). We found that i.p. injection with 50 μg of FK156, a synthetic agonist of NOD1, induced a similar induction of CD103 expression on CD205+/CD8α+ DCs (Fig. 3A, see SI Appendix, Fig. S5 for gating strategy). Conversely, injection of the Toll-like receptor (TLR) ligands LPS (TLR4, 1 μg) and Pam3Csk4 (TLR2/1, 20 μg) induced a significant decrease in not only the proportions of cDC1s expressing CD103, but also the absolute number of cDC1 that could be found in the spleen 16 h postinjection (Fig. 3A), a phenomenon that has previously been noted by Qiu et al. (18). In the case of LPS, the disappearance of cDC1s could be induced by doses as low as 100 ng (SI Appendix, Fig. S6). Similarly, no significant up-regulation in CD103 expression was seen following injection with flagellin (TLR5, 20 μg) or zymozan (TLR2/6, 10 μg), suggesting that this phenomenon is indeed specific to NOD1/2, rather than TLR activation (SI Appendix, Fig. S7).
Fig. 3.
Increased CD103 expression on splenic cDC1s is dependent on the activation of Nod-like but not Toll-like innate immune receptors. (A) Flow cytometry analysis of CD103 expression on splenic CD8α+ cDC1s (denoted by gate R1) following injection with the NOD2 agonist MDP (50 μg), the NOD1 agonist FK156 (50 μg), the TLR4 agonist LPS (1 μg), or the TLR1/2 agonist Pam3CSK4 (20 μg). Individual mice from one representative experiment are shown on each graph. (B) The total number of CD8α+ cDC1 cells per spleen as treated in A. Horizontal bars denote the mean, and error bars denote SEM. Asterisks denote significantly different from PBS treatment (A and B) as determined by one-way ANOVA followed by Tukey’s multiple comparisons test; ***P ≤ 0.001; ****P ≤ 0.0001.
To verify the specific requirement of NLRs for this phenomenon, we injected Nod1−/− and Nod2−/− mice with either MDP or FK156 and compared the resulting level of CD103 expression on splenic cDC1s to that found in heterozygote littermates treated in the same manner. We found that NOD1 and NOD2 activation of cDC1s operates selectively and independently, as MDP fails to induce CD103 expression on splenic cDC1s in Nod2−/− mice but is active upon injection into Nod1−/− mice, while the inverse is true for FK156 (SI Appendix, Fig. S8 A and B). Additionally, mice that are deficient in RIP2—a serine-threonine kinase required for the downstream signaling of both NOD1 and NOD2—failed to induce CD103 expression upon injection with MDP, providing further support that this phenomenon occurs via canonical NLR signaling (SI Appendix, Fig. S8C).
The Up-Regulation of CD103 on Splenic cDC1s Is Dependent on Activation of NOD2 in Nonhematopoietic Cells.
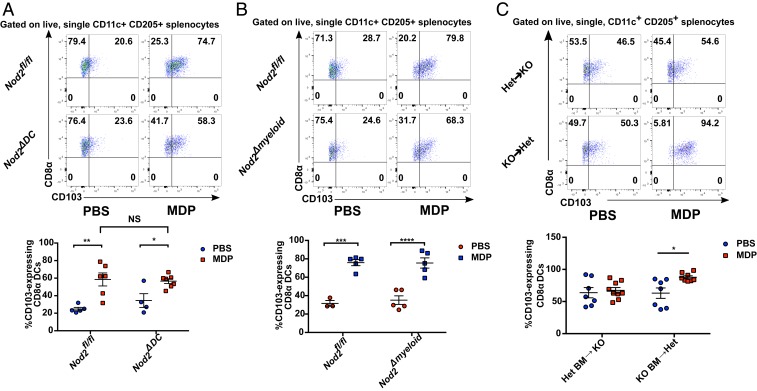
To determine the role that DCs themselves play in this phenomenon, we employed the Cre-lox system to generate DC-specific Nod2 knockout mice as described and characterized previously (27). To our surprise, there was no difference in CD103 up-regulation on splenic cDC1s in response to MDP injection between CD11ccreNod2fl/fl (Nod2ΔDC) mice and their Nod2fl/fl littermate counterparts (Fig. 4A), indicating that this phenotype is not a result of recognition of MDP by DCs themselves, but rather by an intermediary cell type that produced an additional factor in order to signal to the DCs. We then modified our conditional knockout model to include cells of the myeloid immune lineage by employing the Lys2cre mouse, which has been shown to induce genetic knockout in macrophages, neutrophils, and some monocytes and DCs (28). Once again, Lyz2creNod2fl/fl (Nod2Δmyeloid) mice displayed no difference from their Nod2fl/fl littermates in the up-regulation of CD103 on cDC1s following administration of MDP (Fig. 4B). We did, however, observe a failure to recruit neutrophils to the peritoneal cavity following MDP administration in the same cohorts of Nod2Δmyeloid mice (SI Appendix, Fig. S9), verifying that the NOD2Δmyeloid mouse was indeed functional. Neutrophil recruitment upon activation by MDP is a hallmark of NOD2 signaling (29), and the loss of this phenotype in the NOD2Δmyeloid mouse despite the persistence of up-regulated cDC1 CD103 indicates that these phenomena are occurring via divergent biological pathways. To support this, and to clarify whether bone marrow-derived cells are at all involved in the CD103 phenotype that we observed, we performed bone marrow chimera studies in which bone marrow from Nod2-sufficient mice was transferred into lethally irradiated Nod2-knockout mice, and vice versa, before injection with MDP or vehicle. This resulted in elevated and variable levels of CD103 on cDC1s in all treatment groups, likely as a result of the irradiation procedure itself. However, only Nod2-sufficient mice receiving Nod2-knockout bone marrow responded to MDP injection with a clear and statistically significant up-regulation of CD103 on cDC1s (Fig. 4D), indicating that this phenotype was largely dependent on NOD2 activity in nonhematopoietic, radioresistant stromal cells.
Fig. 4.
The induction of CD103 on splenic cDC1s is dependent on activation of NOD2 in nonhematopoietic stromal cells. (A and B) Flow cytometry analysis of CD103 expression on splenic CD8α+ cDC1s isolated from DC-specific (CD11ccreNod2fl/fl; “Nod2ΔDC”) and myeloid lineage-specific (Lyz2creNod2fl/fl; “Nod2ΔMyeloid”) Nod2 mouse lines following injection with MDP (50 μg). (C) Flow cytometry analysis of CD103 expression on splenic CD8α+ cDC1s collected from bone marrow chimeras created by transferring bone marrow from Nod2-sufficient heterozygote mice (Het) into lethally irradiated Nod2-knockout mice (KO), and vice versa following injection of MDP. Individual mice from two (A and B) or three (C) pooled litters of recipient mice are shown on each graph. For each image, horizontal bars denote the mean and error bars denote SEM. Asterisks denote significantly different from Nod2fl/fl littermate-matched PBS control (A and B) or chimera-matched PBS control (C) as determined by two-way ANOVA followed by Tukey’s multiple comparisons test; *P < 0.05; **P < 0.01; ***P ≤ 0.001; ***P ≤ 0.0001. NS, no statistical significance between linked groups.
The Up-Regulation of CD103 on cDC1s in Response to NOD2 Activation Is Dependent on the Cytokine GM-CSF.
Given that these effects of MDP on DCs required the activation of NOD2 by a non-DC cell type, we set out to identify an intermediate messenger that could transduce this signal from the detecting cell to DCs. The cytokine GM-CSF represented an excellent candidate for this function, as it has been previously shown to affect the levels of CD103 expression on splenic cDC1s (30), as well as to induce CD103 on Batf3-dependent DCs generated in culture from bone marrow (31). Accordingly, we found that i.p. injection with GM-CSF produced a splenic cDC1 phenotype very similar to that induced by activation of NOD2 (Fig. 5A). To determine if these two factors are involved in the same biological pathway, we injected Csf2−/− (GM-CSF knockout) mice with MDP and found that these mice failed to up-regulate CD103 on splenic cDC1s (Fig. 5B), indicating that NOD2 and GM-CSF are indeed acting in concert to impact the activation status of splenic cDC1s. Moreover, in agreement with previous reports (30), Csf2−/− mice displayed a consistently diminished proportion of CD103-expressing splenic cDC1s, indicating that this factor is vital for cDC1 homeostasis in the spleen. While the precise cellular source of GM-CSF remains to be determined, qPCR analysis revealed an immediate and robust induction of Csf2 expression within the parietal peritoneum, the membrane encapsulating the peritoneal cavity, following injection with MDP into the peritoneal cavity (Fig. 5C). This induction of gene expression was absent in the spleen, suggesting that GM-CSF is produced at the site of NOD2 activation and not in the vicinity of the affected cDC1s.
Fig. 5.
The up-regulation of CD103 on splenic cDC1s in response to activation of NOD2 is dependent on GM-CSF. (A) Flow cytometry analysis of CD103 expression on splenic CD8α+ cDC1s collected from C57BL/6 littermates 16 h postinjection with GM-CSF (200 ng i.p.). (B) Flow cytometry analysis of CD103 expression on splenic CD8α+ cDC1s collected from GM-CSF–deficient mice (Csf2−/−) and their heterozygote littermates (Csf2+/−) 16 h postinjection with PBS or MDP (50 μg i.p.). (C) qPCR analysis of Csf2 gene expression in the parietal peritoneum and spleen of C57BL/6 mice 4 or 24 h post-MDP treatment (50 μg). (D) CD90.1+ OT1 cells were adoptively transferred into CD90.2+Csf2−/− mice and their CD90.2Csf2+/− littermate controls and treated as in the scheme outlined in Fig. 1D. The proportion of donor-derived CD8 T cells (D), as well as the proportion of donor-derived T cells expressing markers of short-lived effector cells (E; CD127−KLRG1+) was determined by flow cytometry. Individual mice from one representative experiment (A; C, Right; and D) or two pooled litters (B and C, Left) are shown. Horizontal bars denote the mean, and error bars denote SEM. Asterisks denote significantly different from PBS control as determined by Student’s t test (A), genotype-matched PBS-treated littermate control as determined by two-way ANOVA followed by Tukey’s multiple comparisons test (B), or naïve mice as determined by one-way ANOVA followed by Tukey’s multiple comparisons test (C). Asterisks and horizontal brackets denote significant genotype-dependent differences regardless of treatment as calculated by two-way ANOVA; *P ≤ 0.05, **P ≤ 0.01, ***P ≤ 0.001, ****P ≤ 0.0001; NS, no significant difference between linked groups.
If GM-CSF–dependent induction of CD103+ DCs is indeed required for the development of MDP-mediated tolerance to antigen reexposure, then it would follow that Csf2−/− mice injected with MDP would fail to suppress the secondary T cell expansion as we had seen previously. We therefore subjected these mice to the same treatment protocol outlined in Fig. 1 and compared the results to Csf2+/− littermate controls. We found that CD8 T cell expansion in response to a second exposure to ovalbumin was highly augmented in the Csf2−/− mice, regardless of treatment with MDP, suggesting that these mice are deficient in mechanisms that negatively regulate T cell activation (Fig. 5D). Moreover, the transplanted OT1 cells were more likely to take on a KLRG1+CD127− surface phenotype (Fig. 5D), which is typically associated with a short-lived effector cell (SLEC), a cytotoxic T cell phenotype typically associated with highly inflammatory environments such as acute viral or bacterial infection (32). Thus, our data support the idea that NOD2 activation can boost an immunosuppressive environment through the induction of GM-CSF, thereby limiting the immune response to self and innocuous foreign antigens.
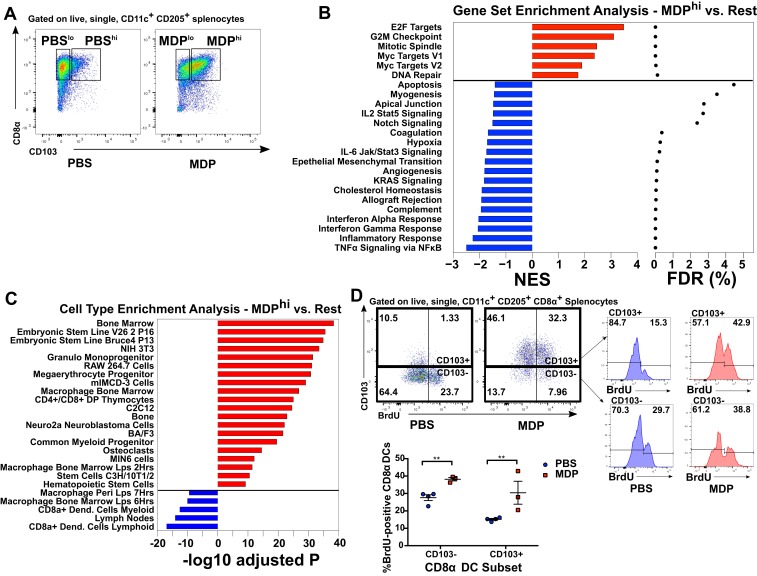
cDC1s Activated by the NOD2/GM-CSF Axis Resemble Newly Differentiated and Immature DCs.
To better understand the precise function of CD103-expressing cDC1s stimulated by NOD2 activation through GM-CSF, we performed microarray transcriptome profiling analysis on purified splenic cDC1s from PBS and MDP-treated mice. The DCs were further subdivided into CD103-high and CD103-low expressing populations by FACS in order to determine whether the CD103-expressing DCs present at baseline were similar to those induced by MDP injection (see Fig. 6A for gating scheme). In total, we found 302 genes that varied significantly between any of the four groups according to ANOVA followed by the Holm–Bonferroni method to correct for multiple testing. These changes were only modest, with only a small number of genes exhibiting changes of greater than 1.6-fold when comparing MDPhi to the other groups (SI Appendix, Fig. S10A). Gene ontology enrichment analysis suggested that when omitting multiple testing correction, genes found to be up-regulated in the MDPhi group tended to be involved in the regulation of cell division and the cell cycle, while genes found to be decreased in the MDPhi group tended to be involved in immunity and inflammation (SI Appendix, Fig. S10B). With this in mind, we performed a more thorough gene-set enrichment analysis using the expression level of all transcripts assessed by the microarray and found that a number of cell cycle-associated pathways were significantly enriched (false discovery rate [FDR] < 0.05) in MDPhi DCs as compared to DCs from the other three groups (Fig. 6B). Similarly, a number of gene-set pathways typically associated with the immune system and inflammatory signaling were significantly decreased in the same cells (Fig. 6B). We then took the top 100 positively and negatively ranked genes according to their enrichment score and performed cell-type enrichment analysis to determine whether the modifications in gene expression induced in MDPhi DCs resembled the expression signature of a non-cDC1 cell type. We found that the genes that tended to be up-regulated in MDPhi DCs were typically expressed in a number of immature, progenitor-type cells, while the genes that tended to be down-regulated were those associated with the classical cDC1 signature (Fig. 6C). Taken together, these two analyses indicated that the CD103hi cDC1s induced by injection with MDP were immature as compared to the cDC1s found at the steady state and had likely recently differentiated from progenitor cells under the control of GM-CSF. To test this, we administered the nucleotide analog BrdU to PBS- and MDP-treated mice to assess whether their splenic cDC1s had undergone cell division during the period after treatment. We found that the splenic CD103+ cDC1s that can be found under homeostatic conditions were largely BrdU-negative, indicating that these cells are stably nonproliferative, while CD103− cells exhibited moderate levels of proliferation (Fig. 6D). After MDP injection, however, ∼50% of CD103+ cDC1s were positive for BrdU, suggesting that MDP injection leads to the generation of new cDC1s. This is in line with our data suggesting that MDP leads to the up-regulation of GM-CSF expression, as this cytokine is known to induce the expansion of DCs in vivo (33)
Fig. 6.
NOD2 activation induces the differentiation of newly divided, immature splenic cDC1s. (A) Gating strategy for FACS-sorting of splenic CD8α+ cDC1s into high and low CD103-expressing subtypes from PBS- and MDP-treated mice. (B) Gene set enrichment analysis of RNA isolated from cells sorted as above and subjected to whole-transcriptone microarray analysis. Gene sets that are positively enriched in the MDPhi group compared to the others are denoted in red, while negative enrichment is denoted in blue. The size of the bars is indicative of the normalized enrichment score, and the FDR q-value shown on the right. (C) Cell type enrichment analysis for the top 100 positively and negatively enriched genes expressed by MDPhi cells. Cell types associated with up-regulated genes are shown in blue, while cell types associated with down-regulated genes are shown in red. (D) Flow cytometry analysis of splenic CD8α+ cDC1s isolated from mice injected with the proliferation tracking agent BrdU. Individual mice from one representative experiment are shown. Horizontal bars denote the mean, and error bars denote SEM. Asterisks denote significantly different from CD103 expression-matched PBS control as calculated by ANOVA followed by Tukey’s multiple comparisons test; **P ≤ 0.01.
NOD2 Activation Manipulates DC Populations in the Intestine.
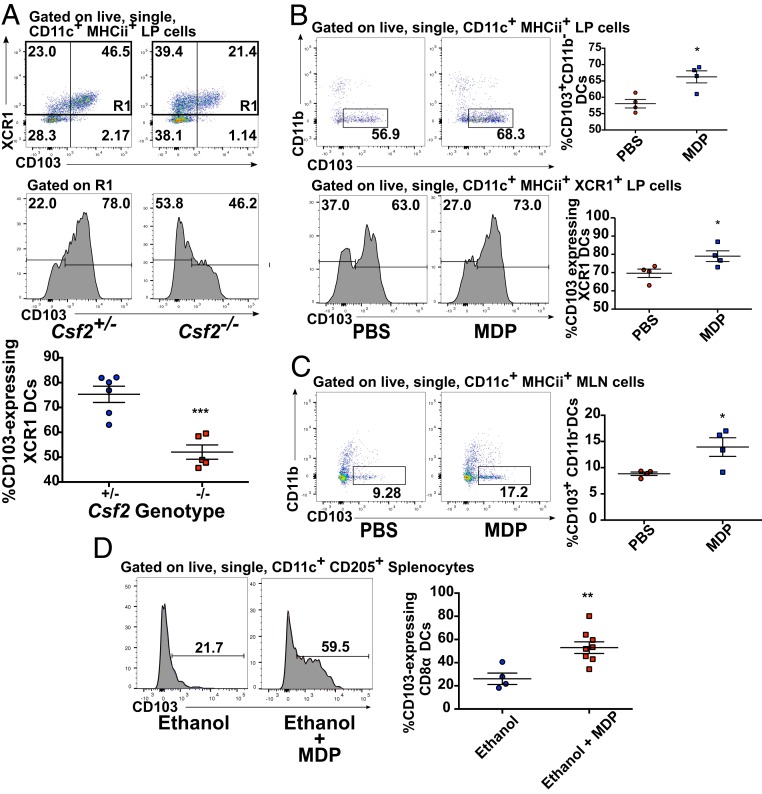
While the spleen remains a useful model organ in which to characterize this phenomenon, examining the effects of NOD2 activation on DC populations in the intestine is of utmost importance given the known contribution of NOD2 polymorphisms to the development of CD. We therefore chose to determine how MDP and GM-CSF could act in concert to manipulate cDC1 populations in the gut, in particular due to the known role of CD103-expressing cDC1s in intestinal tolerance (34). GM-CSF has been shown to be required for optimal overall proportions of CD103 DCs in both the small and large intestine (35), but the specific contributions of cDC1s and cDC2s to this deficit in Csf2−/− mice is unclear. We found that in the colonic lamina propria (LP), CD103 expression was nearly entirely restricted to XCR1+ cDCs1, and this expression was significantly diminished on XCR1+ DCs in Csf2−/− mice (Fig. 7A), illustrating the importance of this cytokine on cDC1 CD103 expression in peripheral tissues as well. Given the link we identified between MDP and GM-CSF for splenic DCs, we next chose to see if boosting levels of available MDP was capable of modulating proportions of colonic CD103-expressing DCs. Indeed, instillation of 100 μg of MDP directly into the colon of WT mice via rectal catheter led to a significant increase in the proportion of CD103+CD11b− DCs (Fig. 7B) in the colonic LP. Notably, this increase was largely explained by an increase in the proportion of XCR1+ DCs expressing CD103, verifying that this was indeed due to the effects of MDP exposure on cDC1s (Fig. 7B). Accordingly, and similar to what was observed in the parietal peritoneum following i.p. injection of MDP, we observed a strong but not statistically significant trend toward increased Csf2 expression in whole-thickness colonic tissue sampled from the site of exposure 2 h postadministration (SI Appendix, Fig. S11). We saw similar effects in the MLN following rectal MDP exposure, suggesting either that these cells can migrate to local lymphoid organs or that the signal resulting in increased CD103 expression can be transduced from the colon to the MLN (Fig. 7C). Additionally, when an intrarectal bolus of MDP was delivered into mice in 50% ethanol, we observed a significant up-regulation of surface CD103 expression on splenic cDC1s as well (Fig. 7D). As this delivery method is likely to induce a breach of natural barrier mechanisms of the intestine, thereby increasing exposure of the body proper to MDP, these results suggest that activation of the NOD2/GM-CSF axis can be limited to local immune compartments or systemically widespread depending on the route of exposure to MDP.
Fig. 7.
NOD2 activation alters the balance of DC subsets in the large intestine. (A) Flow cytometry analysis of XCR1 and CD103 expression on colonic LP DCs isolated from untreated Csf2+/− and Csf2−/− littermate mice. (B) Flow cytometry analysis of colonic LP DCs collected from C57BL/6J assessing the proportions of CD103+CD11b− DCs (Upper) or CD103-expressing XCR1+ DCs (Lower) 24 h postadministration of 100 μg of MDP via intrarectal catheter. (C) Flow cytometry analysis of MLN DCs collected from C57BL/6J assessing the proportions of CD103+CD11b− DCs 24 h postadministration of 100 μg of MDP via intrarectal catheter. (D) Flow cytometry analysis of CD103 expression on splenic cDC1s isolated 24 h postadministration from mice treated with 300 μg of MDP in 50% ethanol or 50% ethanol alone delivered via an intrarectal catheter. For all images, individual mice from one representative experiment are shown. Horizontal bars denote the mean, and error bars denote SEM. Asterisks denote significantly different from heterzygote control (A), PBS control (B and C), or ethanol control (D) as calculated by Student’s t test. *P ≤ 0.05, *P ≤ 0.01, ***P ≤ 0.001.
In addition to administering purified exogenous MDP to modulate intestinal DC populations, we further examined this event in a more physiologically relevant manner by inducing a transient breach in the intestinal barrier via rectal administration of 50% ethanol. This treatment has long been recognized to lead to increased permeability of the intestine to macromolecules, including microbes and their potentially immunomodulatory products, such as peptidoglycan (36). Thus, we chose to assess the effects of peptidoglycan sensing following barrier breach on gut DC populations by administering ethanol to NOD1 and NOD2 signaling-deficient Rip2−/−mice. We found that ethanol administration resulted in a significant increase in the proportion of total DCs in the colon 3 d posttreatment in both Rip2−/− mice and their heterozygote littermate controls, although the increase was less drastic in the Rip2−/− group (Fig. 8A). To examine if this difference was due to differential changes in specific subtypes, we subdivided these cells into CD103-expressing cDC1s (CD103+CD11b−) and cDC2s (CD11b+) and found that Rip2+/− mice exhibited a trend toward increased proportions of CD103+ cDC1s, while Rip2-deficient animals exhibited a significant preferential recruitment of cDC2s (Fig. 8B), leading to a significant decrease in the ratio of CD103/CD11b DCs in the colonic LP of these mice following a breach in the intestinal barrier (Fig. 8C). We then chose to determine if these changes in intestinal DC populations were associated with any notable alterations in the composition of the intestinal Treg milleu. Indeed, we found that intrarectal ethanol administration led to a notable increase in the proportion of colonic FoxP3+ CD4 T cells 3 d postinjection in Rip2-sufficient animals, while this increase was not apparent in Rip2-deficient mice (Fig. 8D). This phenotype mirrors the changes that were observed for colonic cDC1s following exposure to ethanol, suggesting that these phenotypes may be related. These data suggest that NOD1 and NOD2 signaling are vital for the maintenance of cDC1 populations in the gut following a period of increased permeability, and a loss of this balance could potentially lead to the skewing of the immune environment toward an aberrant inflammatory state.
Fig. 8.
A transient breach in the intestinal barrier leads to RIP2-dependent increases in proportions of both CD103-expressing cDC1s and FoxP3-expressing Tregs. (A and B) Flow cytometry analysis of colonic LP cells isolated from Rip2+/−mice and their Rip2−/− littermates assessing the proportion of total DCs (A), CD11b+ or CD103+CD11b− DCs (B), or Foxp3+ CD4 T cells (D) 3 d after administration of 100 μL of 50% ethanol via an intrarectal catheter. Individual mice from two (A and B) or three (D) pooled litters are shown. (C) The ratio of CD103+CD11b− to CD11b+ DCs 3 d following administration of 100 μL of 50% ethanol via an intrarectal catheter. Horizontal bars denote the mean, and error bars denote SEM. Asterisks and brackets denote significantly different groups as calculated by two-way ANOVA followed by Tukey’s multiple comparisons test. *P ≤ 0.05, ***P ≤ 0.001. &P < 0.05, significantly different by t test alone. NS, nonstatistically significant differences between groups.
Discussion
The generation of an appropriate immune response requires a delicate balance of response and regulation in order to eliminate threats while at the same time avoiding long-lasting and unnecessary damage to the self. To manage this balance, a number of regulatory mechanisms have evolved in living systems that prevent perpetual responses to foreign material that is constantly present, and also to assist in the return of the system to its homeostatic state once the foreign material has been removed. In many cases, these mechanisms are dependent on the products of inflammation itself, which results in the production of signals that act as a rheostat or brake, instructing the system to begin contracting the inflammatory machinery (37). In this study, we describe one such system where a stimulus that is generally considered to be proinflammatory—the detection of invading bacteria by the intracellular pattern receptor NOD2—leads to the eventual generation of an immunosuppressive response in the form of increased regulatory T cell activity.
NOD2 activity is closely associated with aberrant inflammation, as mutations in NOD2 confer the strongest statistical association with the development of CD of any known locus (38). This presents somewhat of a paradox, as these disease-associated alleles are known to result in an inability of NOD2 to generate a response to ligation by MDP and should therefore be antiinflammatory themselves. However, it is important to note that the types of immune responses generated by activation of NOD2 vary significantly depending on the nature of the activation being observed (39, 40). It is likely that different doses of MDP, time periods of incubation, or experimental settings can result in seemingly highly differential roles in the adaptive immune response for NOD2 activation in vivo. High doses of MDP at early time points are able to drive proinflammatory responses driven by cytokine production and antimicrobial effector responses. At later time points, once the majority of the invading threat has been cleared from the environment, the previous NOD2 activation sets the environment up for resolution, potentially via the generation of tolerance-mediating DCs.
Interestingly, the observations we present here are in agreement with what has been previously reported for injection with Complete Freund’s Adjuvant (CFA), which induces an early Th1-type response that closely models autoimmunity but eventually leads to the generation of a late suppressor-cell response (41). In fact, CFA injection has been shown to prevent the onset of the T cell-dependent Non-Obese Diabetic model of diabetes via the generation of a robust regulatory T cell response (42, 43) and can do so with as little as a single administration (44). Notably, the primary bioactive component of CFA is heat-killed Mycobacterium tuberculosis particles, and the minimal structure required to replicate the adjuvant activity of CFA has long been recognized to be MDP (45), suggesting that NOD2 activation may be the driving mechanism behind this phenomenon.
Similarly, GM-CSF signaling appears to be contextual with respect to its effects on the immune response. On the proinflammatory side, GM-CSF is thought to play a critical, driving role in the pathogenesis of multiple models of experimental disease, including multiple sclerosis (46) and glomerulonephritis (47). However, GM-CSF is also widely reported to be immunosuppressive via the generation of tolerogenic DCs (48) and is capable of significantly ameliorating experimental colitis (49). Similarly, knockout models show that GM-CSF deficiency leads to a significant exacerbation of intestinal disease (50). This can potentially be explained by its role in the maintenance of the DC/Treg axis in the gut, as Csf2−/− mice have been shown to display decreased proportions of intestinal CD103+ DCs, leading to decreased numbers of Tregs and a loss of tolerance to orally-delivered antigens (35). Enticingly, much like what was seen with CFA, injection with GM-CSF has been shown to be capable of delaying the onset of diabetes in the Non-Obese Diabetic model in a DC and Treg-dependent fashion (51), providing more evidence that NOD2 and GM-CSF act in concert to promote immunological tolerance.
Along those lines, we have shown here that the decreased numbers of CD103-expressing DCs in the gut of Csf2−/− mice can be explained by a decrease in the overall proportion of XCR1+ DCs expressing CD103. While we did not see a similar phenotype in Rip2−/− mice, it is likely that there are multiple pathways that can lead to GM-CSF production in the gut, and it is possible that the pathways required for homeostatic maintenance of GM-CSF and, therefore, DC CD103 expression, are NOD2 signaling-independent. In fact, our data does not necessarily indicate that GM-CSF production is being directly induced by NOD2 activation, as we did not observe a definitive, NOD2-dependent stimulation of GM-CSF production by a specific cell type in our model, and it may be that stromal cell-based NOD2 activation leads to the production of an unidentified messenger that stimulates GM-CSF production by an additional cell type. That said, stromal cells isolated from the intestinal lamina propria express both NOD1 and NOD2 (52), and similar cells produce GM-CSF in a microbiota-dependent manner (53). Thus, GM-CSF–mediated modulation of intestinal DCs likely occurs through signal input from multiple cell types, depending on the source and nature of the microbial challenge that is encountered. We hypothesize that NOD2-mediated GM-CSF becomes of greater importance in response to situations that require an emergency restoration of intestinal DC populations, such as after a breach in intestinal barrier. This is particularly true if strong microbial signaling through TLRs leads to the deletion of cDC1s, as it would be vital to replenish DCs in the affected tissue cells once the primary threat has been cleared. Indeed, we have shown here that a breach in the intestinal barrier leads to the recruitment of all types of DCs into the colonic LP in RIP2-sufficient mice, but a preferential recruitment of CD11b+CD103− DCs occurs in RIP2-deficient mice, suggesting that NOD signaling drives the recovery of the intestinal DC landscape back to a CD103+ DC balanced permissive state. As the currently accepted paradigm states that the DCs marked by CD103 are better suited for the generation of Tregs (20), while DCs marked by CD11b are better suited for the generation of a Th17 response (54), maintaining a balance between DC subtypes is essential for gut homeostasis, and restoration of this balance is critical following an inflammatory episode.
With the maintenance of this balance in mind, it may be the case that the GM-CSF signaling pathway is an outright hallmark of CD, as multiple studies have reported that mutations in the GM-CSF receptor genes CSF2RA and CSF2RB themselves represent genetic risk factors for developing the disease (21, 22, 55). Moreover, autoantibodies capable of neutralizing free circulating GM-CSF are increased 5- to 30-fold in CD patients, resulting in an impairment of the GM-CSF signaling pathway (56, 57). Interestingly, the NOD2 variants and GM-CSF autoantibodies are both associated with an ileal, stricturing presentation of CD (57, 58), suggestive of similar pathogenic mechanisms between both factors.
Our results indicate that NOD2 feeds directly into this pathway, which not only further supports the role of GM-CSF signaling as an important player in the maintenance of intestinal immune homeostasis but also has immediate implications in the treatment of CD. Injection with sargramostim, a recombinant form of GM-CSF, has been used as a treatment for CD in the past, although a meta-analysis of three clinical trials showed that sargramostim was not statistically more effective than placebo in the induction of remission or clinically relevant responses (59). It is important to note, however, that these studies were not stratified by genotype, and it is possible that patients with NOD2 mutations specifically would benefit significantly from sargramostim injections if they are incapable of generating the appropriate GM-CSF–mediated response to a particular NOD2-dependent peptidoglycan challenge. Notably, of these three clinical trials described in the meta-analysis, two displayed significant efficacy, while the one reporting the least clinical difference between sargramostim and placebo was conducted with an international cohort of patients from centers around the globe (60). This is potentially significant because while NOD2 mutations are significantly associated with CD in Caucasian populations, the same cannot be said for a number of international cohorts, including those from Japan (61) and India (62). Thus, the lesser prevalence of patients with nonfunctional NOD2 in the third study could explain the lack of statistical efficacy in this group, and our results indicate that for sargramostim may still be a viable therapy option if the genotype of individual patients is taken into consideration.
In summary, we have described here a mechanism by which detection of peptidoglycan by NOD1 and NOD2 expressed in nonhematopoietic cells leads to the production of GM-CSF, which, in turn, leads to the generation of Treg-promoting DCs. Depending on the route of stimulation, this phenomenon can occur either systemically or locally within the intestinal LP. This knowledge provides an insight into the biological function of NOD proteins, and we are excited about the potential for exploiting this pathway in the generation of immunosuppressive adjuvants. Moreover, we hope that this research will provide a better understanding of the pathophysiological mechanisms that drive CD, and this understanding will eventually lead to a cure for this debilitating condition.
Methods
Mice.
All animals were maintained under specific pathogen-free conditions at the University of Toronto Department of Comparative Medicine, and experiments were conducted as approved by the University of Toronto Animal Care Committee in accordance with the regulations set by the Canadian Council of Animal Care. In some cases, age- and sex-matched C57BL/6J WT mice were purchased from Jackson Laboratories and used at 6–10 wk of age. Otherwise, mice were obtained from their respective suppliers and bred in-house to control for littermate effects. Mice were maintained on a +/− by −/− or Cre+ by Cre- breeding system and littermate mice subjected to analysis at 6–10 wk of age.
Injections.
Ligands were purchased from their respective suppliers and prepared at the doses indicated in the text in sterile PBS prior to being administered to mice via injection into the peritoneal cavity. Intrarectal administration of MDP and 50% ethanol was performed by anesthetizing animals under gaseous isofluorane before inserting a plastic catheter 3 cm into the anus and slowly injecting 100 μL of fluid directly into the colon lumen.
Tissue Collection.
Single-cell suspensions were obtained by enzymatic digestion of harvested tissues followed by the mechanical disruption of remaining solid tissue through a 70 μM nylon filter with the plunger of a 30-mL syringe.
Detailed methods can be found in SI Appendix.
Data Availability Statement.
Complete Microarray dataset (RMA Normalized, Log2 transformed intensities) has been deposited in the Gene Expression Omnibus (GEO) database (https://www.ncbi.nlm.nih.gov/geo/) under accession no. GSE145280 (63). All other raw data from this study is available upon request from D.P. or D.J.P.
Supplementary Material
Acknowledgments
We thank Dr Philip Rosenstiel for providing the Nod2fl/fl mice, Drs. Marco Giovannini and Jean-Pierre Hugot for providing the Nod2 knockout mice, Dr. Richard Flavell for the Rip2 knockout mice, Dr. Jennifer Gommerman for the Batf3 knockout mice, Dr. Philippe Poussier for providing the FLT3L-secreting B16 cells, and the University of Toronto Faculty of Medicine Flow Cytometry Facility and the Division of Comparative Medicine for their assistance throughout this study. This work was funded by Canadian Institutes of Health Research Grants FDN-14333 (to D.J.P.) and MOP-12353 (to S.E.G.), and their generous support is greatly appreciated.
Footnotes
The authors declare no competing interest.
This article is a PNAS Direct Submission.
Data deposition: The microarray data reported in this paper have been deposited in the Gene Expression Omnibus (GEO) database, https://www.ncbi.nlm.nih.gov/geo (accession no. GSE145280).
This article contains supporting information online at https://www.pnas.org/lookup/suppl/doi:10.1073/pnas.1912866117/-/DCSupplemental.
References
- 1.Jostins L., et al. ; International IBD Genetics Consortium (IIBDGC) , Host-microbe interactions have shaped the genetic architecture of inflammatory bowel disease. Nature 491, 119–124 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ogura Y., et al. , A frameshift mutation in NOD2 associated with susceptibility to Crohn’s disease. Nature 411, 603–606 (2001). [DOI] [PubMed] [Google Scholar]
- 3.Hugot J. P., et al. , Association of NOD2 leucine-rich repeat variants with susceptibility to Crohn’s disease. Nature 411, 599–603 (2001). [DOI] [PubMed] [Google Scholar]
- 4.Rivas M. A., et al. ; National Institute of Diabetes and Digestive Kidney Diseases Inflammatory Bowel Disease Genetics Consortium (NIDDK IBDGC); United Kingdom Inflammatory Bowel Disease Genetics Consortium; International Inflammatory Bowel Disease Genetics Consortium , Deep resequencing of GWAS loci identifies independent rare variants associated with inflammatory bowel disease. Nat. Genet. 43, 1066–1073 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zanello G., et al. , Nod2 activates NF-kB in CD4+ T cells but its expression is dispensable for T cell-induced colitis. PLoS One 8, e82623 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Berrebi D., et al. , Card15 gene overexpression in mononuclear and epithelial cells of the inflamed Crohn’s disease colon. Gut 52, 840–846 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Girardin S. E., et al. , Nod2 is a general sensor of peptidoglycan through muramyl dipeptide (MDP) detection. J. Biol. Chem. 278, 8869–8872 (2003). [DOI] [PubMed] [Google Scholar]
- 8.Hrdinka M., et al. , Small molecule inhibitors reveal an indispensable scaffolding role of RIPK2 in NOD2 signaling. EMBO J. 37, e99372 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ogura Y., et al. , Nod2, a Nod1/Apaf-1 family member that is restricted to monocytes and activates NF-kappaB. J. Biol. Chem. 276, 4812–4818 (2001). [DOI] [PubMed] [Google Scholar]
- 10.Kobayashi K. S., et al. , Nod2-dependent regulation of innate and adaptive immunity in the intestinal tract. Science 307, 731–734 (2005). [DOI] [PubMed] [Google Scholar]
- 11.Inohara N., et al. , Host recognition of bacterial muramyl dipeptide mediated through NOD2. Implications for Crohn’s disease. J. Biol. Chem. 278, 5509–5512 (2003). [DOI] [PubMed] [Google Scholar]
- 12.Petnicki-Ocwieja T., et al. , Nod2 is required for the regulation of commensal microbiota in the intestine. Proc. Natl. Acad. Sci. U.S.A. 106, 15813–15818 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Leclerc C., Juy D., Bourgeois E., Chedid L., In vivo regulation of humoral and cellular immune responses of mice by a synthetic adjuvant, N-acetyl-muramyl-L-alanyl-D-isoglutamine, muramyl dipeptide for MDP. Cell. Immunol. 45, 199–206 (1979). [DOI] [PubMed] [Google Scholar]
- 14.Souvannavong V., Adam A., Opposite effects of the synthetic adjuvant N-acetyl-muramyl-L-alanyl-D-isoglutamine on the immune response in mice depending on experimental conditions. Eur. J. Immunol. 10, 654–656 (1980). [DOI] [PubMed] [Google Scholar]
- 15.Zídek Z., Capková J., Boubelík M., Masek K., Opposite effects of the synthetic immunomodulator, muramyl dipeptide, on rejection of mouse skin allografts. Eur. J. Immunol. 13, 859–861 (1983). [DOI] [PubMed] [Google Scholar]
- 16.Leclerc C., Bourgeois E., Chedid L., Demonstration of muramyl dipeptide (MDP)-induced T suppressor cells responsible for MDP immunosuppressive activity. Eur. J. Immunol. 12, 249–252 (1982). [DOI] [PubMed] [Google Scholar]
- 17.Kishimoto T., et al. , Regulation of antibody response in different immunoglobulin classes. VI. Selective suppression of IgE response by administration of antigen-conjugated muramylpeptides. J. Immunol. 123, 2709–2715 (1979). [PubMed] [Google Scholar]
- 18.Qiu C. H., et al. , Novel subset of CD8alpha+ dendritic cells localized in the marginal zone is responsible for tolerance to cell-associated antigens. J. Immunol. 182, 4127–4136 (2009). [DOI] [PubMed] [Google Scholar]
- 19.Yamazaki S., et al. , CD8+ CD205+ splenic dendritic cells are specialized to induce Foxp3+ regulatory T cells. J. Immunol. 181, 6923–6933 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Coombes J. L., et al. , A functionally specialized population of mucosal CD103+ DCs induces Foxp3+ regulatory T cells via a TGF-beta and retinoic acid-dependent mechanism. J. Exp. Med. 204, 1757–1764 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chuang L.-S., et al. , A frameshift in CSF2RB predominant among ashkenazi jews increases risk for Crohn’s disease and reduces monocyte signaling via GM-CSF. Gastroenterology 151, 710–723.e2 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Levine A. P., et al. ; NIDDK Inflammatory Bowel Disease Genetics Consortium , Genetic complexity of Crohn’s disease in two large ashkenazi jewish families. Gastroenterology 151, 698–709 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sun C. M., et al. , Small intestine lamina propria dendritic cells promote de novo generation of Foxp3 T reg cells via retinoic acid. J. Exp. Med. 204, 1775–1785 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Crozat K., et al. , Cutting edge: Expression of XCR1 defines mouse lymphoid-tissue resident and migratory dendritic cells of the CD8α+ type. J. Immunol. 187, 4411–4415 (2011). [DOI] [PubMed] [Google Scholar]
- 25.Bonifaz L., et al. , Efficient targeting of protein antigen to the dendritic cell receptor DEC-205 in the steady state leads to antigen presentation on major histocompatibility complex class I products and peripheral CD8+ T cell tolerance. J. Exp. Med. 196, 1627–1638 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hildner K., et al. , Batf3 deficiency reveals a critical role for CD8alpha+ dendritic cells in cytotoxic T cell immunity. Science 322, 1097–1100 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kim D., et al. , Nod2-mediated recognition of the microbiota is critical for mucosal adjuvant activity of cholera toxin. Nat. Med. 22, 524–530 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Abram C. L., Roberge G. L., Hu Y., Lowell C. A., Comparative analysis of the efficiency and specificity of myeloid-Cre deleting strains using ROSA-EYFP reporter mice. J. Immunol. Methods 408, 89–100 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shimada K., et al. , The NOD/RIP2 pathway is essential for host defenses against Chlamydophila pneumoniae lung infection. PLoS Pathog. 5, e1000379 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhan Y., et al. , GM-CSF increases cross-presentation and CD103 expression by mouse CD8+ spleen dendritic cells. Eur. J. Immunol. 41, 2585–2595 (2011). [DOI] [PubMed] [Google Scholar]
- 31.Mayer C. T., et al. , Selective and efficient generation of functional Batf3-dependent CD103+ dendritic cells from mouse bone marrow. Blood 124, 3081–3091 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Joshi N. S., et al. , Inflammation directs memory precursor and short-lived effector CD8(+) T cell fates via the graded expression of T-bet transcription factor. Immunity 27, 281–295 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Vremec D., et al. , The influence of granulocyte/macrophage colony-stimulating factor on dendritic cell levels in mouse lymphoid organs. Eur. J. Immunol. 27, 40–44 (1997). [DOI] [PubMed] [Google Scholar]
- 34.Esterházy D., et al. , Classical dendritic cells are required for dietary antigen-mediated induction of peripheral T(reg) cells and tolerance. Nat. Immunol. 17, 545–555 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mortha A., et al. , Microbiota-dependent crosstalk between macrophages and ILC3 promotes intestinal homeostasis. Science 343, 1249288 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tabata T., Tani T., Endo Y., Hanasawa K., Bacterial translocation and peptidoglycan translocation by acute ethanol administration. J. Gastroenterol. 37, 726–731 (2002). [DOI] [PubMed] [Google Scholar]
- 37.Sugimoto M. A., Vago J. P., Perretti M., Teixeira M. M., Mediators of the resolution of the inflammatory response. Trends Immunol. 40, 212–227 (2019). [DOI] [PubMed] [Google Scholar]
- 38.Franke A., et al. , Genome-wide meta-analysis increases to 71 the number of confirmed Crohn’s disease susceptibility loci. Nat. Genet. 42, 1118–1125 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Magalhaes J. G., et al. , Nucleotide oligomerization domain-containing proteins instruct T cell helper type 2 immunity through stromal activation. Proc. Natl. Acad. Sci. U.S.A. 108, 14896–14901 (2011). Correction in: Proc. Natl. Acad. Sci. U.S.A. 109, 10605 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Saha S., et al. , PGLYRP-2 and Nod2 are both required for peptidoglycan-induced arthritis and local inflammation. Cell Host Microbe 5, 137–150 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Billiau A., Matthys P., Modes of action of Freund’s adjuvants in experimental models of autoimmune diseases. J. Leukoc. Biol. 70, 849–860 (2001). [PubMed] [Google Scholar]
- 42.McInerney M. F., Pek S. B., Thomas D. W., Prevention of insulitis and diabetes onset by treatment with complete Freund’s adjuvant in NOD mice. Diabetes 40, 715–725 (1991). [DOI] [PubMed] [Google Scholar]
- 43.Manirarora J. N., Kosiewicz M. M., Parnell S. A., Alard P., APC activation restores functional CD4(+)CD25(+) regulatory T cells in NOD mice that can prevent diabetes development. PLoS One 3, e3739 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sadelain M. W., Qin H. Y., Lauzon J., Singh B., Prevention of type I diabetes in NOD mice by adjuvant immunotherapy. Diabetes 39, 583–589 (1990). [DOI] [PubMed] [Google Scholar]
- 45.Adam A., Ellouz F., Ciorbaru R., Petit J. F., Lederer E., Peptidoglycan adjuvants: Minimal structure required for activity. Z. Immunitatsforsch. Exp. Klin. Immunol. 149, 341–348 (1975). [PubMed] [Google Scholar]
- 46.McQualter J. L., et al. , Granulocyte macrophage colony-stimulating factor: A new putative therapeutic target in multiple sclerosis. J. Exp. Med. 194, 873–882 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kitching A. R., et al. , The requirement for granulocyte-macrophage colony-stimulating factor and granulocyte colony-stimulating factor in leukocyte-mediated immune glomerular injury. J. Am. Soc. Nephrol. 13, 350–358 (2002). [DOI] [PubMed] [Google Scholar]
- 48.Bhattacharya P., Gopisetty A., Ganesh B. B., Sheng J. R., Prabhakar B. S., GM-CSF-induced, bone-marrow-derived dendritic cells can expand natural Tregs and induce adaptive Tregs by different mechanisms. J. Leukoc. Biol. 89, 235–249 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sainathan S. K., et al. , Granulocyte macrophage colony-stimulating factor ameliorates DSS-induced experimental colitis. Inflamm. Bowel Dis. 14, 88–99 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Xu Y., Hunt N. H., Bao S., The role of granulocyte macrophage-colony-stimulating factor in acute intestinal inflammation. Cell Res. 18, 1220–1229 (2008). [DOI] [PubMed] [Google Scholar]
- 51.Gaudreau S., et al. , Granulocyte-macrophage colony-stimulating factor prevents diabetes development in NOD mice by inducing tolerogenic dendritic cells that sustain the suppressive function of CD4+CD25+ regulatory T cells. J. Immunol. 179, 3638–3647 (2007). [DOI] [PubMed] [Google Scholar]
- 52.Owens B. M. J., et al. , CD90(+) stromal cells are non-professional innate immune effectors of the human colonic mucosa. Front. Immunol. 4, 307 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Vicente-Suarez I., et al. , Unique lamina propria stromal cells imprint the functional phenotype of mucosal dendritic cells. Mucosal Immunol. 8, 141–151 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Scott C. L., et al. , CCR2(+)CD103(-) intestinal dendritic cells develop from DC-committed precursors and induce interleukin-17 production by T cells. Mucosal Immunol. 8, 327–339 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Denson L. A., et al. , Genetic and transcriptomic variation linked to neutrophil granulocyte-macrophage colony-stimulating factor signaling in pediatric Crohn’s disease. Inflamm. Bowel Dis. 25, 547–560 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Han X., et al. , Granulocyte-macrophage colony-stimulating factor autoantibodies in murine ileitis and progressive ileal Crohn’s disease. Gastroenterology 136, 1261–1271.e3 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Gathungu G., et al. , Granulocyte-macrophage colony-stimulating factor autoantibodies: A marker of aggressive Crohn’s disease. Inflamm. Bowel Dis. 19, 1671–1680 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Cleynen I., et al. , Genetic factors conferring an increased susceptibility to develop Crohn’s disease also influence disease phenotype: Results from the IBDchip European project. Gut 62, 1556–1565 (2013). [DOI] [PubMed] [Google Scholar]
- 59.Roth L., Macdonald J. K., McDonald J. W., Chande N., Sargramostim (GM-CSF) for induction of remission in Crohn’s disease. Cochrane Database Syst. Rev. 132, CD008538 (2011). [DOI] [PubMed] [Google Scholar]
- 60.Feagan B., Anderson F., Radford-Smith G., Solovyov O., Zurdel-Dillinger S., Efficacy and safety of sargramostim in moderate to severe Crohn’s disease: Results of N.O.V.E.L. 4, a phase III multicenter study. Gastroenterology 132, A103 (2007). [Google Scholar]
- 61.Hirano A., et al. , Association study of 71 European Crohn’s disease susceptibility loci in a Japanese population. Inflamm. Bowel Dis. 19, 526–533 (2013). [DOI] [PubMed] [Google Scholar]
- 62.Mahurkar S., et al. , Common variants in NOD2 and IL23R are not associated with inflammatory bowel disease in Indians. J. Gastroenterol. Hepatol. 26, 694–699 (2011). [DOI] [PubMed] [Google Scholar]
- 63.Prescott D., Philpott D. J., Gene Expression of purified murine splenic CD205+CD8+ Dendritic Cells. NCBI Gene Expression Omnibus. https://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE145280. Deposited 13 February 2020.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Complete Microarray dataset (RMA Normalized, Log2 transformed intensities) has been deposited in the Gene Expression Omnibus (GEO) database (https://www.ncbi.nlm.nih.gov/geo/) under accession no. GSE145280 (63). All other raw data from this study is available upon request from D.P. or D.J.P.