Abstract
In a small fraction of Xenopus tadpoles, a single retinal ganglion cell (RGC) axon misprojects to the ipsilateral optic tectum. Presenting flashes of light to the ipsilateral eye causes that ipsilateral axon to fire, whereas stimulating the contralateral eye excites all other RGC inputs to the tectum. We performed time-lapse imaging of individual ipsilaterally projecting axons while stimulating either the ipsilateral or contralateral eye. Stimulating either eye alone reduced axon elaboration by increasing branch loss. New branch additions in the ipsi axon were exclusively increased by contralateral eye stimulation, which was enhanced by expressing tetanus neurotoxin (TeNT) in the ipsilateral axon, to prevent Hebbian stabilization. Together, our results reveal the existence of a non−cell-autonomous “Stentian” signal, engaged by activation of neighboring RGCs, that promotes exploratory axon branching in response to noncorrelated firing.
Keywords: activity-dependent, axon, retinotectal, Hebbian, Xenopus laevis
Patterned neural activity instructs refinement of axons and dendrites in developing circuits (1). How activity patterns translate into structural remodeling remains an important question. Hebbian plasticity, in which synaptic contacts are strengthened and maintained when the presynaptic cell participates in firing the postsynaptic cell, has been proposed as a mechanism to fine-tune connections (2–6).
A recent study in the developing retinotectal system of Xenopus tadpoles used patterned visual stimulation to drive individual retinal ganglion cell (RGC) axons to fire either synchronously or asynchronously relative to other inputs (7). Consistent with Hebbian plasticity, synchronous firing resulted in synaptic strengthening and structural stabilization of the axon arbor. Furthermore, asynchronous, alternating activation of inputs caused synaptic depression and up-regulated axonal exploratory branch dynamics. This phenomenon of “fire out-of-sync, lose your link” had been predicted as an extension of Hebb’s rule in an influential monograph by Gunther Stent (8).
Alternating stimulation involves two parts: 1) axon activation while surrounding inputs are silent and 2) axon inactivity while surrounding inputs are stimulated. The previous study did not resolve which of these two components induced the exploratory axonal growth. Using the same experimental preparation, we now independently assess the effects of activating either the axon of interest or neighboring inputs. We found that axonal branch losses increase when either the axon of interest or its neighbors are driven, but the increase in new branch additions is exclusively induced by a non−cell-autonomous signal associated with the firing of neighboring cells.
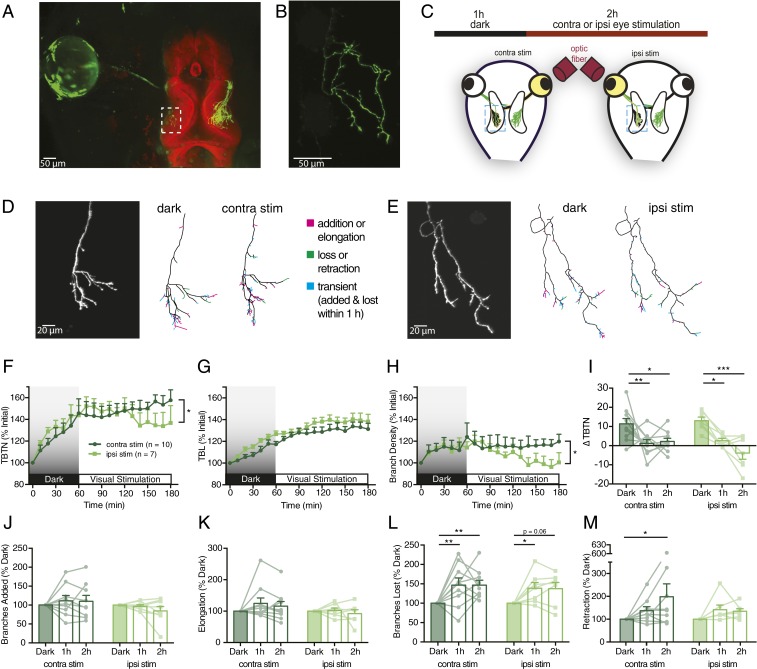
The developing retinotectal system of the translucent albino Xenopus laevis tadpole provides a useful model for in vivo imaging (9). Although nearly all Xenopus RGC axons project contralaterally, a single ectopic ipsilateral (ipsi) axon, targeted to the wrong hemisphere, is found in about 20% of animals (7) (Fig. 1 A and B). We exploited ipsi RGC axons to visually stimulate single retinotectal axons independently of their neighboring inputs.
Fig. 1.
Ipsi RGC axons respond differentially to simulation of the ipsi and contra eyes. (A) EGFP-electroporated eye (green) projecting to BODIPY-labeled tectum (red). (B) Ipsi RGC axon from rectangle in A. (C) Experimental protocol: ipsi axon imaged every 10 min over 1-h baseline in darkness and 2-h visual stimulation of the ipsi or contra eye via optical fiber. (D and E) Ipsi RGC axon arbors undergoing (D) contra eye stimulation or (E) ipsi eye stimulation in the tectum. Reconstructed arbors show cumulative changes during 1 h in darkness and the last hour of stimulation, indicating added or elongated (magenta), lost or retracted (green), and transient (both added or elongated and lost or retracted within 1 h, blue) branch tips. (F) Total branch tip number (TBTN) normalized to initial number. (G) Total branch length (TBL) normalized to initial length. (H) Branch density (branch number/total length) normalized to initial density. (I) Net change in TBTN every 10 min binned by hour. (J) Mean number of branch additions every 10 min normalized to mean additions in darkness. (K−M) Data binned as in J for (K) elongation (mean length added every 10 min of growing branches, normalized to elongation in darkness), (L) losses, and (M) retraction. All graphs present ipsi axons from contra-eye-stimulated (n = 10, dark green) and ipsi-eye-stimulated (n = 7, light green) animals. Mixed-design two-way ANOVA, Bonferroni’s post hoc test to compare hours is indicated above bar graphs, and interaction of time vs. eye stimulated is indicated to the right of time plots (*P < 0.05, **P < 0.01, ***P < 0.001).
We screened tadpoles, electroporated to express EGFP in one eye, for the presence of an ipsi RGC axon and performed in vivo two-photon imaging every 10 min while presenting visual stimuli. The experimental paradigm consisted of 1 h of darkness followed by 2 h of monocular light flashes (10 ms at 0.5 Hz) to either the contralateral (contra) or ipsi eye (Fig. 1C). Stimulating the contra eye alone activates many axons, and postsynaptic partners, around the single ipsi axon being imaged, and thus tests how activity of surrounding inputs modulates axonal growth and dynamics. Conversely, ipsi eye stimulation activates just the single axon without its neighbors.
Ipsi eye stimulation resulted in simpler arbors (Fig. 1 D–H), with fewer branch tips (Fig. 1F) and reduced branch density (Fig. 1H) after 2 h compared to contra eye stimulation. While stimulation of either eye reduced the rate of axon branch accumulation compared to RGC axon growth in darkness, activation of the ipsi eye caused a greater reduction in branch elaboration, resulting in a loss of branch tip number over time (Fig. 1I).
While visual stimulation caused no significant differences in branch addition (mean number of new branch tips every 10 min, normalized to 1 h of darkness) or elongation (mean length added every 10 min on growing branches, normalized to 1 h of darkness) (Fig. 1 J and K), visual stimulation through either eye significantly increased rates of branch loss and retraction relative to baseline in darkness (Fig. 1 L and M). Thus, activating neighboring inputs increased addition and loss rates comparably, maintaining arbor complexity, whereas stimulation of the axon of interest reduced arbor complexity by shifting the balance to favor branch loss over addition (Fig. 1 F and H).
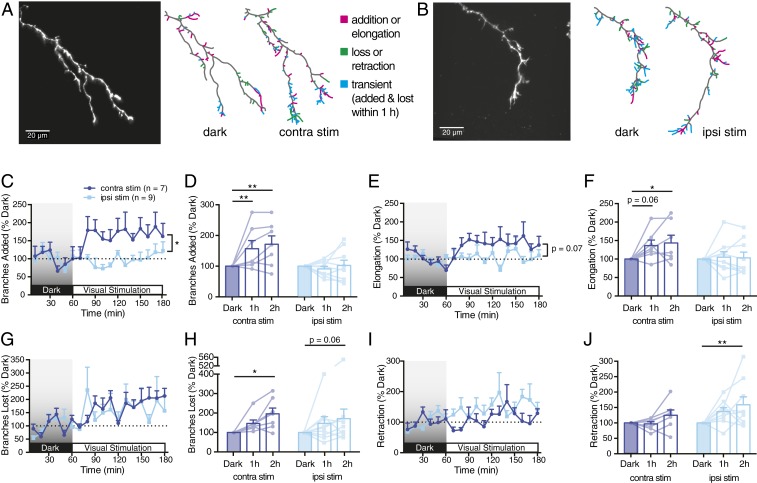
In prior studies, synaptic activation of N-methyl-D-aspartate receptors decreased axon branch addition rates, suggesting Hebbian stabilization (7, 10). Expressing tetanus neurotoxin (TeNT) to block transmission from RGC axons eliminated this branch suppression and unmasked an activity-dependent up-regulation in axon branch additions (7, 11). To remove confounding influences of the stabilization signal, we tested the effects of ipsi versus contra eye stimulation on TeNT-expressing axons.
When blocking vesicular release in the ipsi axon, contra eye stimulation increased the rate of new branch additions and elongation compared to darkness, whereas ipsi eye stimulation had little impact (Fig. 2 A–F). Branch loss was significantly up-regulated with contra eye stimulation, and, to a lesser extent, with ipsi eye stimulation (Fig. 2 G and H), which enhanced branch retraction (Fig. 2 I and J). In summary, axon branching and elongation were up-regulated by stimulating the surrounding contra eye axons and not by stimulating the ipsi axon. Branch elimination was increased by stimulating either eye, with retractions significantly enhanced by ipsi eye stimulation.
Fig. 2.
TeNT expression in ipsi RGC axon enhances dynamic branch additions in response to contra, but not ipsi, eye stimulation. (A and B) TeNT-expressing ipsi RGC axon arbors undergoing (A) contra eye stimulation or (B) ipsi eye stimulation. Reconstructed arbors show cumulative changes during 1 h in darkness and the last hour of stimulation. (C and D) Mean branch additions every 10 min, (C) normalized to mean additions in darkness and (D) binned by hour. (E–J) Corresponding graphs for (E and F) branch elongation, (G and H) losses, and (I and J) retraction. All graphs present TeNT-expressing ipsi axons from contra-eye-stimulated (n = 7, dark blue) and ipsi-eye-stimulated animals (n = 9, light blue). Mixed-design two-way ANOVA to compare hours is indicated above bar graphs, and interaction of time vs. eye stimulated is indicated to the right of time plots (*P < 0.05, **P < 0.01).
These experimental findings support a model in which firing up-regulates branch loss in general, but activity of nearby axons also generates a signal that promotes new branch formation in noncoactive axons. Thus, axons exhibit exploratory branch dynamics (both formation and elimination of branches) under conditions of uncorrelated firing.
In an earlier study when contra and ipsi eyes were synchronously stimulated, synaptic strength was maintained and exploratory branch remodeling was down-regulated, indicative of a Hebbian stabilization mechanism (7). In contrast, asynchronous stimulation of the eyes weakened synaptic inputs and destabilized axon branches. Such findings support Stent’s corollary that synaptic efficacy may be reduced when there is postsynaptic activity without concurrent presynaptic firing (8, 12, 13). Our results provide direct evidence for an intercellular “Stentian” signal that promotes an axon’s elaboration when other inputs repeatedly and persistently fire without it, forcing it to seek out more appropriate contacts elsewhere through exploratory growth.
Methods
Animals were maintained at 18 °C to 21 °C with a 12-h/12-h light−dark cycle.
We performed plasmid electroporation as described previously (7), using 1.5 μg/μL to 2 µg/µL pEGFP-N1 or 5UAS-TeNT-Lc:EGFP (TeNT) + pbGAL4-VP16 injected into the eyes of stages 39 to 40 albino X. laevis tadpoles. Animals were anesthetized in 0.02% MS-222 before electroporation.
Electroporated tadpoles were screened for single ipsi RGC axons 3 d to 4 d later around stages 46 to 48 and immobilized for imaging by intraperitoneal injection of d-tubocurarine (2.5 mM). They were placed into a custom polydimethylsiloxane chamber or embedded in 1.8% [wt/vol] low-melt agarose under a coverslip and perfused with oxygenated 0.1× modified Barth's solution with Hepes. Animals stabilized for 30 min in darkness before imaging. A BFL48-400 optical fiber (Thorlabs) was used to stimulate the ipsi or contra eye with Red Rebel LEDs (Luxeon Star) controlled by a STG4002 stimulus generator (Multichannel Systems). Multiphoton z series were collected at 1-µm intervals every 10 min using an Olympus 20× immersion objective (1.0 numerical aperture) on a Thorlabs resonant scanning two-photon microscope. The 910-nm excitation was provided by a Spectra Physics Maitai BB Ti:sapphire pulsed laser.
Statistical tests were performed using Prism 7.0 (Graphpad). Normality of the data distributions was confirmed using the Shapiro−Wilk test. Error bars indicate SEM. All animal experiments were approved by the Animal Care Committee of the Montreal Neurological Institute-Hospital.
Data Availability.
Original data and detailed methods have been uploaded, for public access, to https://figshare.com/projects/Stentian_structural_plasticity_in_the_developing_visual_system/78012 (14).
Acknowledgments
Funding sources are as follows: Natural Sciences and Engineering Research Council Canada Graduate Scholarship (T.N.R.), Sievers Award (M.M.), Jeanne-Timmins Costello and Molson awards (E.K.), and Canadian Institutes of Health Research Grant FDN-143238 (E.S.R.). We thank Martin Meyer for TeNT-Lc:EGFP, and Hollis Cline for pbGal4-VP16.
Footnotes
Data deposition: Morphometric analysis data have been made available at https://figshare.com/projects/Stentian_structural_plasticity_in_the_developing_visual_system/78012.
References
- 1.Cang J., Feldheim D. A., Developmental mechanisms of topographic map formation and alignment. Annu. Rev. Neurosci. 36, 51–77 (2013). [DOI] [PubMed] [Google Scholar]
- 2.Hebb D., The Organization of Behavior (John Wiley, New York, NY, 1949). [Google Scholar]
- 3.Constantine-Paton M., Cline H. T., Debski E., Patterned activity, synaptic convergence, and the NMDA receptor in developing visual pathways. Annu. Rev. Neurosci. 13, 129–154 (1990). [DOI] [PubMed] [Google Scholar]
- 4.Katz L. C., Shatz C. J., Synaptic activity and the construction of cortical circuits. Science 274, 1133–1138 (1996). [DOI] [PubMed] [Google Scholar]
- 5.Assali A., Gaspar P., Rebsam A., Activity dependent mechanisms of visual map formation—From retinal waves to molecular regulators. Semin. Cell Dev. Biol. 35, 136–146 (2014). [DOI] [PubMed] [Google Scholar]
- 6.Kutsarova E., Munz M., Ruthazer E. S., Rules for shaping neural connections in the developing brain. Front. Neural Circuits 10, 111 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Munz M., et al. , Rapid Hebbian axonal remodeling mediated by visual stimulation. Science 344, 904–909 (2014). [DOI] [PubMed] [Google Scholar]
- 8.Stent G. S., A physiological mechanism for Hebb’s postulate of learning. Proc. Natl. Acad. Sci. U.S.A. 70, 997–1001 (1973). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Harris W. A., Holt C. E., Bonhoeffer F., Retinal axons with and without their somata, growing to and arborizing in the tectum of Xenopus embryos: A time-lapse video study of single fibres in vivo. Development 101, 123–133 (1987). [DOI] [PubMed] [Google Scholar]
- 10.Rajan I., Witte S., Cline H. T., NMDA receptor activity stabilizes presynaptic retinotectal axons and postsynaptic optic tectal cell dendrites in vivo. J. Neurobiol. 38, 357–368 (1999). [DOI] [PubMed] [Google Scholar]
- 11.Ben Fredj N., et al. , Synaptic activity and activity-dependent competition regulates axon arbor maturation, growth arrest, and territory in the retinotectal projection. J. Neurosci. 30, 10939–10951 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Oh W. C., Parajuli L. K., Zito K., Heterosynaptic structural plasticity on local dendritic segments of hippocampal CA1 neurons. Cell Rep. 10, 162–169 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Winnubst J., Cheyne J. E., Niculescu D., Lohmann C., Spontaneous activity drives local synaptic plasticity in vivo. Neuron 87, 399–410 (2015). [DOI] [PubMed] [Google Scholar]
- 14.Ruthazer E., Stentian structural plasticity in the developing visual system. Figshare. https://figshare.com/projects/Stentian_structural_plasticity_in_the_developing_visual_system/78012. Deposited 31 March 2020. [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Original data and detailed methods have been uploaded, for public access, to https://figshare.com/projects/Stentian_structural_plasticity_in_the_developing_visual_system/78012 (14).